Introduction
Cells with indefinite proliferation, spreading to
adjacent tissues, regional lymph nodes and distant organs are
characteristics of cancer. Among the oral and maxillofacial
cancers, squamous cell carcinoma is the most common one. Every year
>410,000 new oral squamous cell carcinoma patients are
diagnosed, accounting for 1–5% of all cancers (1). In oral malignant tumors tongue
squamous cell carcinoma (TSCC) is the most common cause of
cancer-related deaths. Although chemotherapy, radiotherapy, and
surgical therapy for TSCC have developed rapidly in the past years,
the 5-year survival rate is still poor (2,3).
Most cancers including TSCC are considered as a gene-related
disease and associated with the activation of oncogenes and
inactivation of tumor-suppressor genes. Hence, finding a safe and
effective therapy to change the abnormal expression of genes and to
improve the rate of survival with TSCC is imperative. RNA
interference (RNAi) has emerged as a powerful method for gene
suppression in molecular medicine. RNAi is the process of silencing
genes by the sequence specific double-stranded RNA (dsRNA). Hence
it is post-transcriptional gene silencing in animals and plants.
Fire and Mello were awarded the Nobel Prize for Medicine in 2006
for discovering RNAi in 1998 (4).
Studies have shown that RNAi is a promising anticancer therapeutic
tool (5,6).
The center of the solid tumor is often in a hypoxic
microenvironment because of its rapid growth (7). The hypoxic conditions can lead to a
more malignant tumor. It can enhance abnormal angiogenesis,
invasion, metastasis of tumors, and result in poor prognosis
(8,9). To adapt to the hypoxic
microenvironment, many normal and abnormal factors are regulated,
including hypoxia-inducible factor-1(HIF-1) which plays an
important role in the process. HIF-1, a transcription factor was
found in 1992 (10). It is
composed of two subunits, a strictly regulated α subunit and a
constitutive β subunit, HIF-1β is also called aryl hydrocarbon
receptor nuclear translocator (ARNT) (11). HIF-1β levels of mRNA and protein
are maintained constant regardless of oxygen tension (12), whereas, HIF-1α is an oxygen-liable
subunit. In normoxia, HIF-1α can be degraded by rapid
ubiquitination [its protein has a short half-life (t1/2~5
min) under normoxia (13)].
However, under hypoxic conditions, the decay of HIF-1α is
suppressed, and then it can translocate into the nucleus and
dimerizes with HIF-1β and forms the active complex HIF-1 (14). The activated complex associate with
hypoxia response element (HRE) to induce expression of its target
genes (15). The target genes,
including erythropoiesis, glycolysis and angiogenesis (16), are essential for tumors to adapt to
and survive in hypoxic conditions. Previous studies have found
overexpression of HIF-1α in various human cancers may play an
important role for cancer progression (17,18),
which implied that HIF-1α is an essential transcriptional regulator
of tumor microenvironment. Therefore, gene silencing HIF-1α by RNAi
may be an effective method to control the malignancy of tumors and
improve the survival of patients.
Previously it was found that HIF-1α might be a
significant prognostic predictor for TSCC patients (19). Another study showed that HIF-1α can
regulate angiogenesis and survival of oral squamous cell carcinoma
(20). Also, we that HIF-1α was
expressed in oral squamous cell carcinoma, and found that the
levels of HIF-1α in human TSCC seemed to be correlated with human
prognosis (21). These findings
implied that HIF-1α is an important factor in development and
treatment of TSCC. In the present study, according to the
principles of RNAi, we constructed lentiviral vector targeting
HIF-1α and infected TSCC cell line SCC-15 cells to investigate the
effect of HIF-1α on the biological behavior of SCC-15 cells.
Materials and methods
Cell lines and reagents
Human TSCC SCC-15 cell line was provided by Dr Huang
Xin, Beijing Stomatological Hospital, Capital Medical University,
Beijing, China. SCC-15 cells were cultured in Dulbecco’s modified
Eagle’s medium/F12 (DMEM/F12=1:1). Medium was supplement with 10%
fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml
streptomycin. The cells were incubated at 37°C in a humidified
atmosphere with 5% CO2. Hypoxia was induced by 100
μmol/l deferoxamine mesylate (DFO), with 1% O2 balanced
with N2.
Construction of lentiviral vector
mediated RNAi
We use siRNA design software (www.ambion.com)
to choose the RNAi target gene sequence. The target gene sequence
of HIF-1α (NM_001530) is GATGAAAGAATTACCGAAT. The control target
sequence is TTCTCCGAACGTGTCACGT. In this experiment, we generated
the double-stranded oligonucleotides targeting the endogenous
HIF-1α gene cloned into GV248 vector
(hU6-MCS-Ubiquitin-EGFP-IRES-puromycin), and named it
lentiviral/shRNA-HIF-1α (LV-shHIF-1α) (Table I). The sequence was not related to
HIF-1α sequence which was designed and used as negative control and
termed letiviral/shRNA-control (LV-shCon) (Table I). The two vectors were confirmed
by DNA sequencing.
 | Table IThe sequence of double-stranded
oligonucleotides for LV-shHIF-1α and LV-shCon. |
Table I
The sequence of double-stranded
oligonucleotides for LV-shHIF-1α and LV-shCon.
| Name | Sequence |
|---|
| LV-shHIF-1α-a |
CCGGGTGATGAAAGAATTACCGAATCTCGAGATTCGGTAATTCTTTCATCACTTTTTG |
| LV-shHIF-1α-b |
AATTCAAAAAGTGATGAAAGAATTACCGAATCTCGAGATTCGGTAATTCTTTCATCAC |
| LV-shCon-a |
CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG |
| LV-shCon-b |
AATTCAAAAATTCTCCGAACGTGTCACGTAAGTTCTCTACGTGACACGTTCGGAGAA |
Lentiviral vector production
According to the manufacturer’s instructions, first,
the packaging 293T cells were trypsinized, collected, and
resuspended at a density of 1.2×107 cells/20 ml in
growth medium containing 10% serum. The cells were seeded in a
15-cm dish. Next, DNA-Lipofectamine 2000 complexes containing 20 μg
pGC-LV vector, 15 μg pHelper 1.0 vector, 10 μg pHelper 2.0 vectors
and 100 μl Lipofectamine 2000 were prepared and mixed with Opti-MEM
medium ≤5 ml, and incubated at room temperature for 20 min. After
the 293T cells reaching 80% confluence, the medium was replaced
with serum-free medium. Two hours later, the DNA-Lipofectamine 2000
complexes were added to the serum-free medium and incubated at 37°C
in a humidified atmosphere with 5% CO2. Eight hours
later, the medium was removed and replaced with the fresh medium
containing 10% serum. Forty-eight hours later, the medium
containing lentivirus was centrifuged at 4000 g for 10 min at 4°C
to pellet cell debris, and to concentrate the lentivirus at 4000 g
for 15 min and stored at −80°C.
Transduction of target cells and
selection
SCC-15 cells (2×105) were collected and
seeded in each well of a 6-well plate with 1 ml of complete media
and transduced by lentiviral vectors at a MOI (Manual Optical
Inspector) 10. Transduction was carried out with 5 μg/ml of
Polybrene. Twelve hours later the medium was removed and replaced
with fresh, complete medium. Seventy-two hours later, the medium
was replaced with fresh, complete medium containing 2 μg/ml of
puromycin to select for stably transduced cells. The medium was
replaced with fresh medium containing puromycin every 3 days until
puromycin-resistant colonies were identified. The cells transducted
with lentiviral vectors targeting HIF-1α were named as RNAi cells.
The LV-shCon affected cells were named as Con cells, and the
untransducted cells were named as Mock.
Real-time RT-PCR analysis for HIF-1α
RNAi, Con and Mock cells were treated with or
without DFO for 24 h. Total RNA was extracted by using TRIzol
reagent (Invitrogen, USA). Additionally, cDNA was reverse
transcripted by Transcriptor First Strand cDNA Synthesis kit
(Roche, USA). HIF-1α mRNA expression was evaluated quantitatively
by real-time RT-PCR Faststart Essential DNA Green Master kit
(Roche) and LightCycler® 96 Instrument system (Roche).
The thermocycler conditions were preincubation at 95°C for 60 sec,
45 cycles of 95°C for 10 sec, 60°C for 10 sec and 72°C for 20 sec,
melting of 95°C for 10 sec, 65°C for 60 sec and 97°C for 1 sec.
Reactions were run in triplicate and repeated three times. As an
internal control of each sample, the β-actin gene was used for
standardization. The relative mRNA expression level of the gene was
calculated using the 2−ΔΔCt method. The primers were
synthesized (Invitrogen) and the sequences were as follows: HIF-1α:
forward, 5′-GTCGCTTCGGCCAGTGTG-3; reverse,
5′-GGAAAGGCAAGTCCAGAGGTG-3′. β-actin: forward,
5′-TGGCACCCAGCACAATGAA-3; reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Western blot analysis
After the three groups of cells were treated with or
without DFO for 24 h. Cells were washed with ice-cold PBS twice,
treated with buffer [50 mmol/l Tris-HCl (pH 7.5), 5 mmol/l EDTA,
150 mmol/l NaCl, 0.5% Triton X-100, 10 mmol/l sodium fluoride, 20
mmol/l h-mercaptoethanol, 250 mmol/l sodium orthovanadate, 1 mmol/l
phenylmethylsulfonyl fluoride], and incubated at 4°C for 30 min.
The lysates were centrifuged at 10,000 g for 10 min, followed by
collecting the supernatants and stored at -80°C. Protein
concentrations were examined by bicinchoninic acid assay methods
(BCA protein assay kit, Thermo, USA). Equivalent amount of protein
were loaded into 8% SDS-PAGE gels and electroblotted onto PVDF
membrane (Sigma, USA). The PVDF membrane was washed three times
with TBST solution (0.1% Tween-20 in TBS, pH 7.5) and blocked it
for 1 h with 5% skim milk in TBST at room temperature.
Subsequently, the membrane was incubated at 4°C overnight with
antibodies against HIF-1α (CST, USA, 1:1,000). Following washing
with TBST three times, for 10 min, the membrane was incubated with
secondary antibody against rabbit at room temperature for 1 h and
washed with TBST three times, 15 min each. Each sample was also
probed with an anti-β-actin antibody (CST, 1:1,000) as a loading
control. Bands were visualized by SmartChem™ Image Analysis System
(Sagecreation, China).
Cell proliferation assay
The proliferation of the cells was assessed by the
CCK-8 (Dojindo, Kumamoto, Japan) assay. The cells were collected
and diluted into 50,000 cells/ml, then plated with 100 μl cell
suspension in each well (n=5) of 96-multiwell plates (Corning,
USA). Cells were treated with or without DFO and then cultured for
24, 48 and 72 h before the addition of 10 μl of CCK-8 to the
culture medium in each well. After 1-h incubation at 37°C, the
optical density of each well was measured with an infinite M200
reader (Tecan, Austria) at 450 nm. Cell viability =
(ODexperiment-ODblank)/(ODcontrol-ODblank). Each experiment was
repeated three times.
Cell apoptosis and cell cycle
analysis
Three groups of cells were treated with or without
DFO for 24 h. After treatment the cells were trypsinized and washed
twice with PBS and resuspended with 500 μl binding buffer. Then 5
μl of Annexin V-KeyFlour647 antibody and 5 μl of 7-ADD was added,
and incubated for 15 min at room temperature in the dark according
to the manufacturer’s protocol prior to FACS analysis. Both early
and late stages of apoptotic cells were detected by a flow
cytometer of FACSVerse™ (BD, USA).
Mock, Con and RNAi cells were plated in 6-well
plates (1×105/well) after reaching 80% confluence; the
cells were treated with or without DFO, and then incubated for 24 h
at 37°C in a humidified atmosphere with 5% CO2. Cells
were washed in ice-cold PBS and harvested by trypsinization. The
cells were washed with ice-cold PBS twice and fixed with 75%
pre-cold ethanol overnight at 4°C. The cells were centrifuged at
2,000 rpm for 5 min, the supernatant was discarded. One hundred
microliters of RNaseA (200 μg/ml) was added to the cells for 30 min
at 37°C, then 400 μl propidium iodine (10 μg/ml) was added to the
cells for 30 min at 4°C in the dark. Flow cytometry was used for
analysis by FACSVerse™ (BD).
Cell invasion assay
Invasion of the three groups of cells were
determined by using transwell chambers (24-well plates, 8-μm pore
size, Corning). After reaching 80% confluence, cells
(1×105) were trypsinized, resuspended and loaded into
the inner chamber containing 200 μl serum-free DMEM/F12 medium with
or without 100 μmol/l DFO. The lower chamber was added with
DMEM/F12 medium (500 μl) containing 10% FBS. The Matrigel gel
(Corning) was diluted by serum-free DMEM/F12 medium and placed on
the surface of filtration membrane of the transwell chambers. After
24 h of incubation at 37°C, the non-invading cells and the Matrigel
gel in the inner chamber were gently removed by a cotton-tipped
swab, and the invasive cells were gently washed by PBS. Then the
invasive cells were fixed with methanol for 20 min and stained with
0.1% crystal violet for 15 min. The number of invasive cells was
counted with five random fields under a light microscope at ×200
magnification. Results were presented as the mean percentage of the
control group. Experiments were done in triplicate and repeated
three times.
Statistical analysis
Statistical analysis was calculated using SPSS 20.0
software. Data are presented as the means ± SD. Student’s t-test
was used for statistical analysis, with significant differences
determined as p<0.05.
Results
Expression of HIF-1α in SCC-15 cells and
regulation by DFO
The level of HIF-1α mRNA in SCC-15 cells under
normoxic or hypoxic condition was analyzed by real-time RT-PCR.
After treated with DFO, no significant change in HIF-1α mRNA
transcript was observed (Fig. 1A).
However, the level of HIF-1α protein tested by western blotting
demonstrated that DFO induced a significant increase of HIF-1α
protein in SCC-15 cells ≤24 h (Fig.
1B). The observed molecular weight of HIF-1α protein was
detected at 120 kDa (Fig. 1B).
Lentiviral vector can effectively
suppress the expression of HIF-1α
Lentiviral vector affected the SCC-15 cell lines
(Fig. 2). Quantitative real-time
RT-PCR (Fig. 3A) and western
blotting (Fig. 3B) results showed
that the mRNA and protein expression levels of HIF-1α in RNAi cells
were significantly inhibited compared to Mock and Con cells under
normoxic and hypoxic condition in vitro.
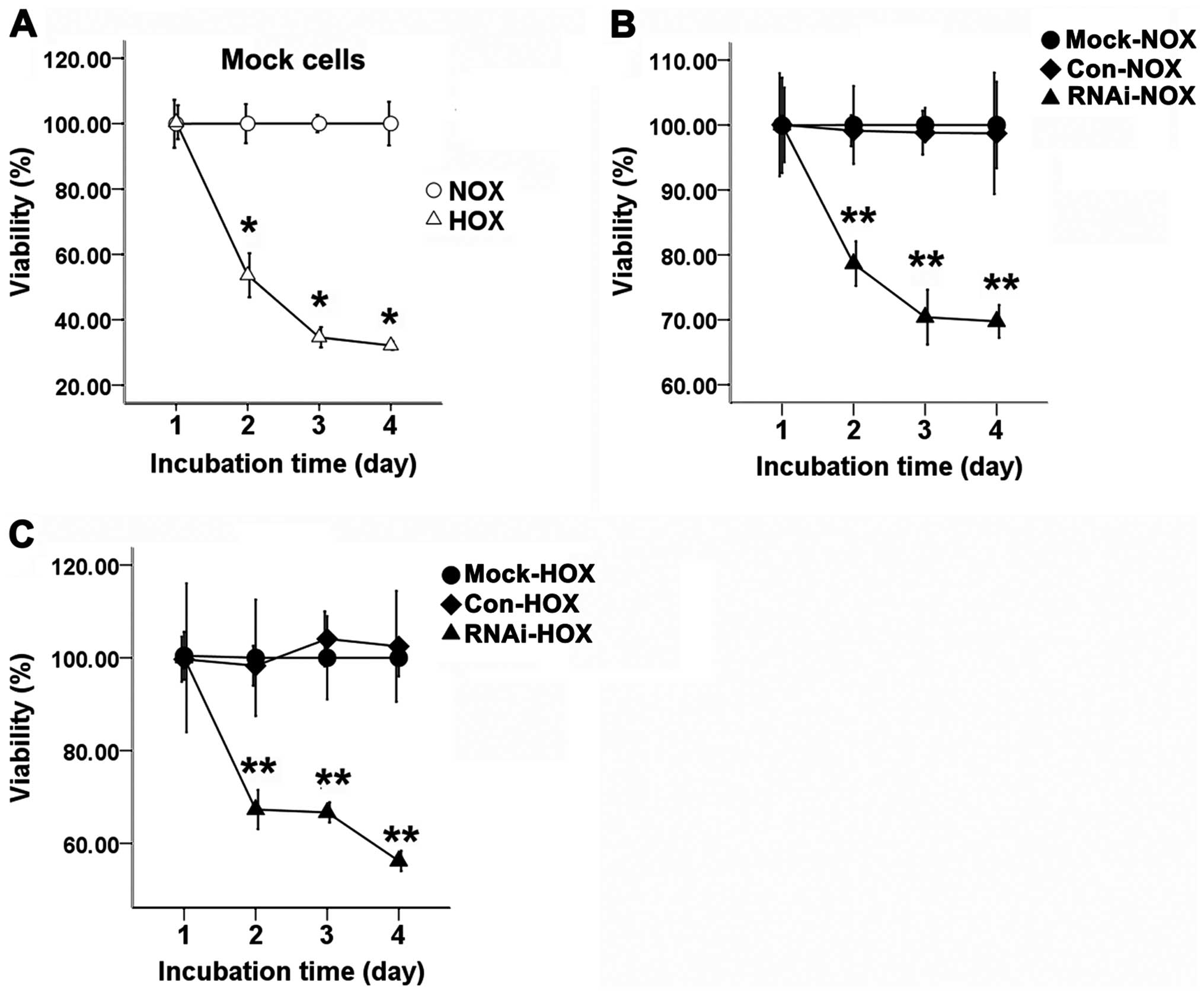
Cell proliferation
The three groups of cells were cultured with or
without DFO for 24, 48, or 72 h. When SCC-15 cells were treated
with DFO, the proliferation of hypoxic cells was significantly
inhibited compared with the normoxic cells (Fig. 4A). The proliferation of RNAi cells
was significantly inhibited compared to the Con and Mock cells
under normoxic (Fig. 4B) and
hypoxic conditions (Fig. 4C).
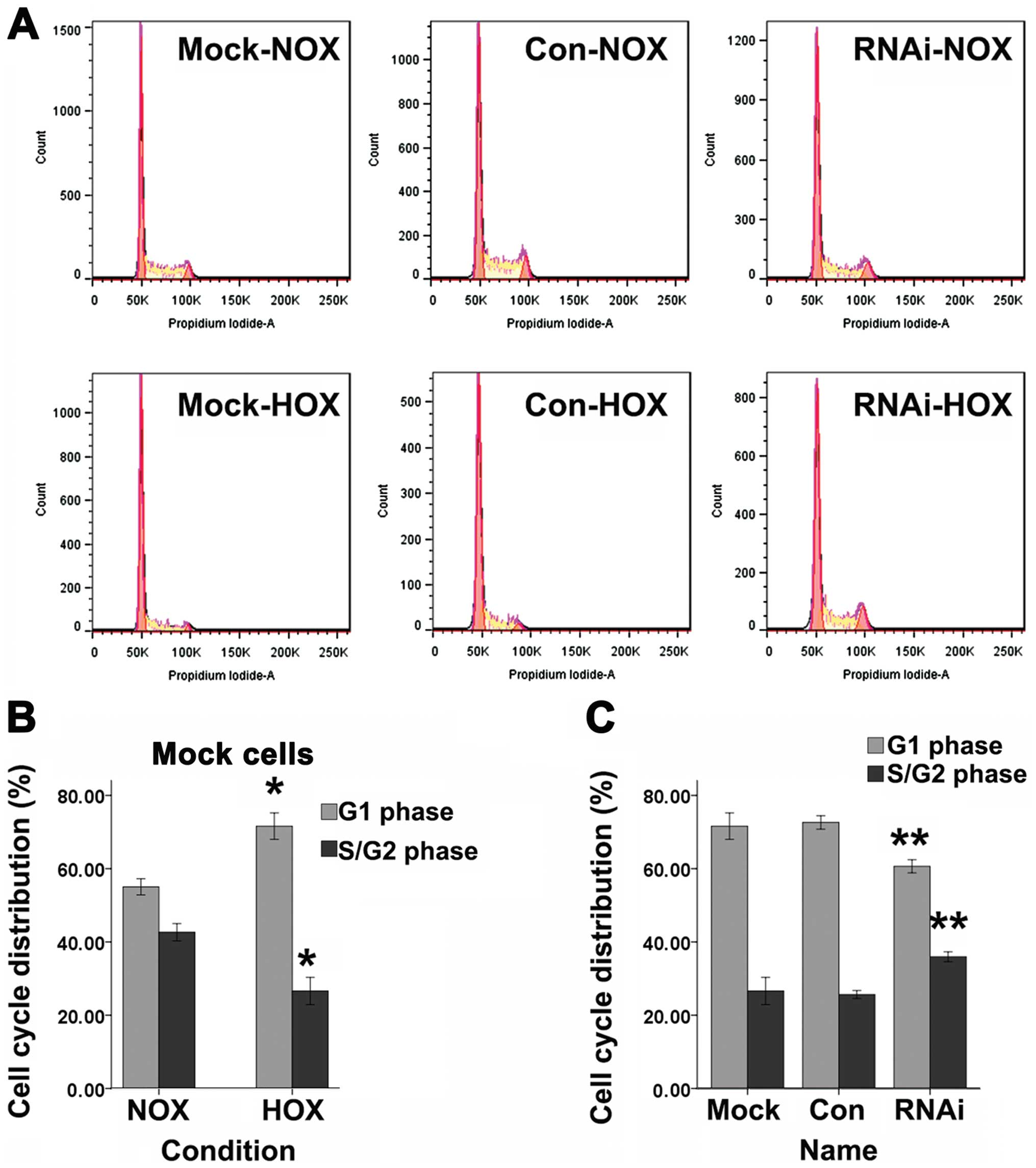
Cell cycle
Cell cycle for cells in G1 phase or S/G2 phase was
tested by FACS with DNA staining by propidium iodide (Fig. 5A). DFO can induce a significant
increase of SCC-15 cells in G1 phase and a decrease of cells in the
S/G2 phase compared to normoxic cells (Fig. 5B). SCC-15 cells treated with
lentiviral vector targeting HIF-1α showed a significant decrease of
G1 phase cells and an increase of S/G2 phase cells compared to Mock
and Con cells after treated with DFO for 24 h (Fig. 5C).
Cell apoptosis
Cell apoptosis was evaluated by FACS with Annexin
V-keyFlour647 Apoptosis Detection kit (Fig. 6A). There was no significant change
in the percentage of apoptotic cells after SCC-15 cells were
cultured with DFO for 24 h when compared with non-treated incubated
cells (Fig. 6B). It was revealed
that the apoptotic cells for RNAi cells were significantly increase
compared to Mock and Con cells when incubated with or without DFO
for 24 h (Fig. 6B).
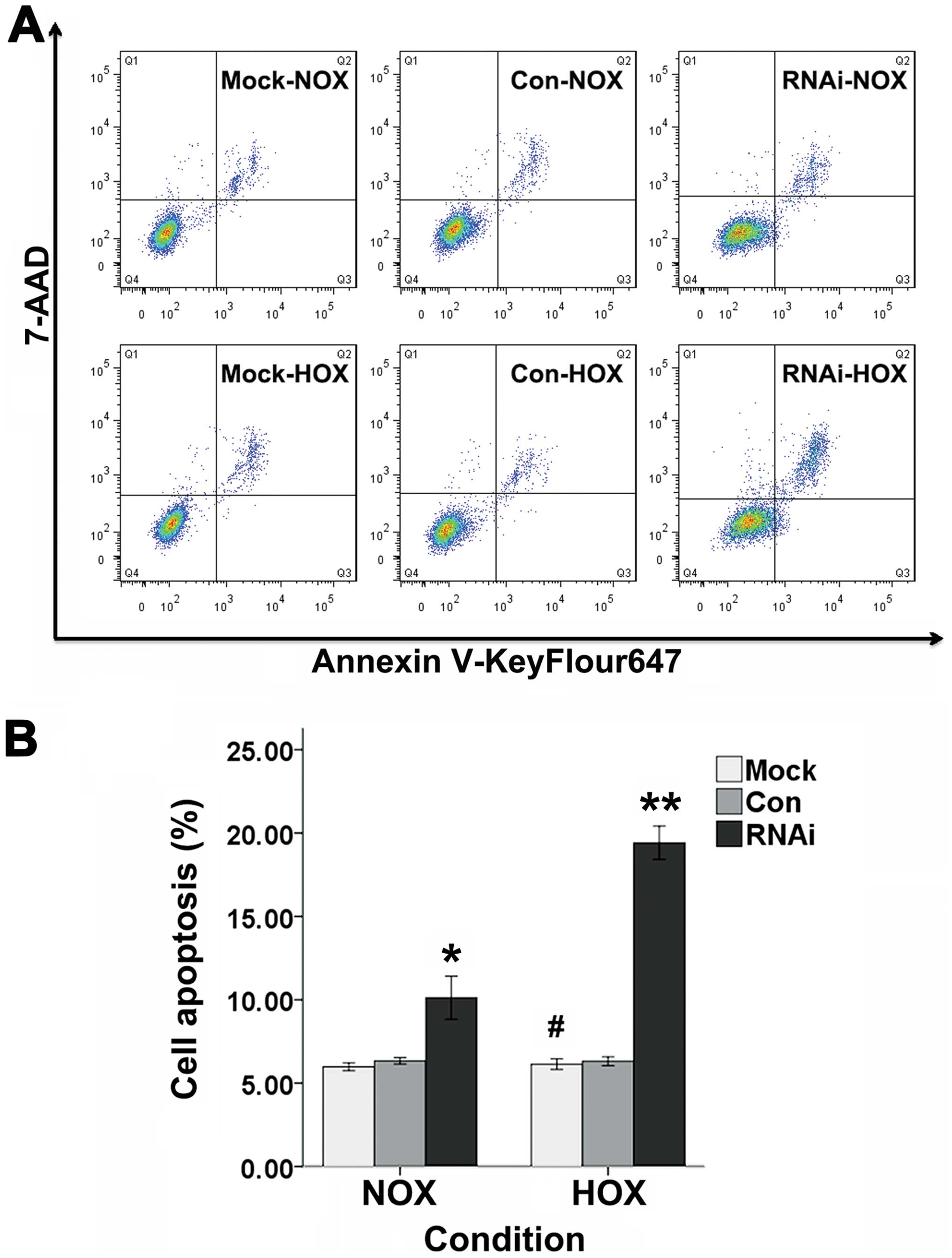 | Figure 6The apoptosis rate of Mock, Con and
RNAi cells were evaluated by FACS. (A) The three groups of cells
were treated with or without DFO for 24 h. Annexin V-KeyFlour647
and 7-AAD-staining of cells were performed and followed by flow
cytometeric analysis. Cells in Q1 quadrant represent cells
undergoing necrosis, Q2 quadrant represent late stage of apoptosis,
Q3 quadrant represent early stage of apoptosis and Q4 quadrant
represent viable cells. (B) The percentage of cell apoptosis for
Mock, Con and RNAi cells in normoxia and hypoxia (bars, ± SD,
#p>0.05, compared to normoxic Mock cells,
*p<0.05, compared to Mock and Con cells in normoxia,
**p<0.01, compared to Mock and Con groups in
hypoxia). NOX, normoxia; HOX, hypoxia. |
Cell invasion
Studies have shown that HIF-1α can regulate the
invasion of tumor cells (22). To
investigate the effect of HIF-1α on cancer cell invasion of SCC-15
cells in vitro, we used the transwell assay (Fig. 7A). After the three groups of cells
were treated with or without DFO and incubated in the transwell
chamber for 24 h, 0.1% crystal violet staining was used to detect
the number of cells to pass through the Matrigel membrane which
represent the invasive abilities. As shown, after treated with DFO,
the number of SCC-15 cells passing through the Matrigel membrane
significantly increased (Fig. 7B).
When HIF-1α was suppressed, the number of cells was significantly
decreased compared to the Mock and Con cells under normoxic or
hypoxic conditions (Fig. 7C).
Discussion
Oxygen is one of essential factors for cells and
tissues to maintain their function. Hypoxia-inducible factor-1
(HIF-1), a transcription factor, is a key regulator for cells and
tissues to adapt to and survive from hypoxia (11,23).
HIF-1 contains HIF-1α and HIF-1β, and HIF-1α is highly regulated by
oxygen (13). DFO has been proved
to induce expression of HIF-1α (24). In the present study, we used DFO to
simulate the hypoxic condition, and analyzed the effect of HIF-1α
on proliferation, cell cycle, apoptosis and invasion of SCC-15
cells which were correlated to the growth, survival and metastasis
of tumors. When the SCC-15 cells were cultured with DFO (100
μmol/l), the expression of HIF-1α was significantly increased,
which was similar to our previous study in SCC-6 cells (25). After treated with DFO, the
proliferation of SCC-15 cells was inhibited, which was like the
other cell types (26). We
observed a significant increase of cells in G1 phase and a decrease
of cells in the S/G2 phase in DFO-treated SCC-15 cells, which
indicated the G1 arrest. However, there was no significant change
in the percent of apoptotic cells. These findings were similar to
the other cell types in hypoxia (27), which meant a hypoxia-induced growth
arrest in SCC-15 cells. The invasion ability of SCC-15 cells was
significantly increased after treated with DFO. Therefore, HIF-1α
may be an important regulator for the different biological
characteristics of SCC-15 cells.
It has been reported that HIF-1α can be regulated by
DFO (24). In the present study,
we found the level of HIF-1α protein increased after treated with
DFO. Then HIF-1α associates with HIF-1β to form HIF-1, and HIF-1
activates hundreds of target genes to regulate angiogenesis, blood
vessel tone, vascular remodeling; cell proliferation and viability;
erythropoiesis and iron metabolism; glucose transport and
glycolysis (11). Therefore,
HIF-1α is very important for cells to adapt to hypoxia. Studies
found that HIF-1α is overexpressed in most of human tumor cells
(18,28). In head and neck carcinomas, study
showed that the expression of HIF-1α protein is associated with the
microvessel vascular density and vascular endothelial growth factor
(VEGF) expression (28). In our
previous study, we also found HIF-1α was overexpressed in oral
squamous cell carcinoma (21). In
the present study, we found that HIF-1α enhanced malignant
phenotype of SCC-15 cells.
The hypoxia-inducible transcription factor α subunit
was found to be upregulated in DFO-treated SCC-15 cells. We
demonstrated that the level of HIF-1α mRNA was not significantly
changed between DFO treated and normal SCC-15 cells. However the
level of HIF-1α protein was significantly increased after treated
with DFO up to 24 h. Study showed that the level of HIF-1α protein
is essential for tumor cells to adapt to and survive from hypoxic
microenvironment (29). HIF-1α is
a key regulator of hypoxic regulation, and it was upregulated
mainly on protein level in SCC-15 cells after treated with DFO,
indicating that overexpression of HIF-1α was an essential factor
for SCC-15 cells to show aggressive phenotype under hypoxic
condition. We used RNAi technique to suppress the expression of
HIF-1α, and found that silencing HIF-1α can significantly decrease
the aggressive potential for SCC-15 cells in hypoxia.
In the present study, we constructed a lentiviral
vector to suppress the expression of HIF-1α, which helps to
evaluate the function of HIF-1α in progression of SCC-15 cells in
hypoxia. SCC-15 cells were transduced with lentiviral vector
targeting HIF-1α mRNA. The efficacy of interference was assessed by
real-time RT-PCR and western blotting. Results showed that the
level of HIF-1α mRNA and protein was significantly suppressed
compared to Con and Mock cells in both normaxia and hypoxia.
Although our study showed the association between
HIF-1α and proliferation, the mechanism is still not fully
understood (30). Hypoxia can
arrest tumor cells proliferation (31), however, another study showed that
the increased hypoxic stress can accelerate the growth of HIF-1α
tumors (27). In the present
study, we found a significant increase of the G1 phase cells after
treated with DFO. It is suggested that DFO can arrest SCC-15 cells
in G1 phase. We used RNAi to inhibit the expression of HIF-1α
protein, and test the effect of HIF-1α on the cell cycle of SCC-15
cells. We found a significant decrease of G1 phase cells and
increase G2/S phase cells compared to the Mock and Con cells under
hypoxia. Hence, HIF-1α was the master regulator for SCC-15 cells to
adapt to the hypoxic condition.
Furthermore, hypoxia can not only be associated with
cell proliferation but also with cell apoptosis in some
circumstances. Hypoxia can induce apoptosis, where HIF-1 plays a
complex role (27). It was found
that HIF-1α was related to apoptosis (32). It has been reported that severe or
prolonged hypoxic condition can maintain the stabilization of p53
protein by HIF-1α stimulation, and leads to induction of apoptosis
(33). However, another study
showed that HIF-1α might inhibit the hypoxia-induced apoptosis
(29). These studies suggest that
HIF-1α protein plays a key role in mediating apoptosis of tumor
cells. We found that there is no significant change between normal
SCC-15 cells and hypoxic cells. RNAi technique was used to evaluate
the effect of HIF-1α on apoptosis of cultured human SCC-15 cells
under normaxia and hypoxia, and we found the number of apoptotic
cells was significantly increased in RNAi cells compared to that of
Mock or Con cells under normoxic and hypoxic condition. These
results suggested that HIF-1α has an important function in
apoptosis of SCC-15 cells.
Hypoxia is a key factor for cancer metastasis
(34). Cell invasion is involved
in tumor metastasis and progression. In a glioma cell line, gene
silencing of HIF-1α can downregulate MMP-2/MMP-9 to suppress cell
migration and invasion into adjacent normal tissue (35). In the present study, we analyzed
the effect of HIF-1α on cell invasion of SCC-15 cells by a
transwell assay in vitro. In hypoxia, the number of SCC-15
cells passing through the extracellular matrices (ECM)
significantly increases compared with the normoxic cells. Treatment
with RNAi lentiviral vector, decreased significantly the amount of
cells passing through the Matrigel gel. Thus, HIF-1α may play a
major role in regulating the hypoxia-induced invasion of SCC-15
cells to Matrigel in vitro.
In conclusion, our results showed that hypoxic
SCC-15 cells expressed high level of HIF-1α protein, and were
invasive, whereas downregulation of HIF-1α by RNAi technique can
decrease the malignancy of SCC-15 cells. Our study suggests that
lentiviral vector target HIF-1α specifically suppressed HIF-1α gene
expression, induced cell apoptosis, and inhibited the growth and
invasion of SCC-15 cells. Hence gene silencing HIF-1α can
consequently reduce the malignant potential of SCC-15 cells. Our
present study showed the expression of HIF-1α can regulate
proliferation, cell cycle, apoptosis and invasion. Thus, the
expression of HIF-1α is an essential factor for human TSCC to
regulate its malignancy. The RNAi target HIF-1α can be a potential
therapeutic measure and effective tool to improve the survival of
TSCC patients.
Acknowledgements
This study was supported by the grant of Shanghai
Natural Science Funds, Science and Technology Commission of
Shanghai Municipality (grant no. 12ZR1434700). We also thank
Mingyue Chu, Danying Chen and Yi Liu for their excellent technical
and theoretical assistance.
References
|
1
|
Abreu LP, Kruger E and Tennant M: Oral
cancer in Western Australia, 1982–2006: A retrospective
epidemiological study. J Oral Pathol Med. 39:376–381.
2010.PubMed/NCBI
|
|
2
|
Mignogna MD, Fedele S and Lo Russo L: The
World Cancer Report and the burden of oral cancer. Eur J Cancer
Prev. 13:139–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kademani D, Bell RB, Bagheri S, et al:
Prognostic factors in intraoral squamous cell carcinoma: the
influence of histologic grade. J Oral Maxillofac Surg.
63:1599–1605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
July LV, Beraldi E, So A, Fazli L, Evans
K, English JC and Gleave ME: Nucleotide-based therapies targeting
clusterin chemosensitize human lung adenocarcinoma cells both in
vitro and in vivo. Mol Cancer Ther. 3:223–232. 2004.PubMed/NCBI
|
|
6
|
Karasarides M, Chiloeches A, Hayward R,
Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D,
Martin J, Marshall CJ, et al: B-RAF is a therapeutic target in
melanoma. Oncogene. 23:6292–6298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
8
|
Höckel M, Schlenger K, Mitze M, Schäffer U
and Vaupel P: Hypoxia and radiation response in human tumors. Semin
Radiat Oncol. 6:3–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.PubMed/NCBI
|
|
11
|
Semenza GL: Hypoxia-inducible factor 1:
Master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kallio PJ, Pongratz I, Gradin K, McGuire J
and Poellinger L: Activation of hypoxia-inducible factor 1alpha:
Posttranscriptional regulation and conformational change by
recruitment of the Arnt transcription factor. Proc Natl Acad Sci
USA. 94:5667–5672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ema M, Hirota K, Mimura J, Abe H, Yodoi J,
Sogawa K, Poellinger L and Fujii-Kuriyama Y: Molecular mechanisms
of transcription activation by HLF and HIF1alpha in response to
hypoxia: Their stabilization and redox signal-induced interaction
with CBP/p300. EMBO J. 18:1905–1914. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lando D, Peet DJ, Whelan DA, Gorman JJ and
Whitelaw ML: Asparagine hydroxylation of the HIF transactivation
domain a hypoxic switch. Science. 295:858–861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
18
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang X, Zheng M, Jiang J, Zhu G, Yang J
and Tang Y: Hypoxia-inducible factor-1 alpha, in association with
TWIST2 and SNIP1, is a critical prognostic factor in patients with
tongue squamous cell carcinoma. Oral Oncol. 47:92–97. 2011.
View Article : Google Scholar
|
|
20
|
Zhou H, Fei W, Bai Y, et al: RNA
interference-mediated downregulation of hypoxia-inducible
factor-1alpha inhibits angiogenesis and survival of oral squamous
cell carcinoma in vitro and in vivo. Eur J Cancer Prev. 21:289–299.
2012. View Article : Google Scholar
|
|
21
|
Kang FW, Gao Y, Que L, Sun J and Wang ZL:
Hypoxia-inducible factor-1α overexpression indicates poor clinical
outcomes in tongue squamous cell carcinoma. Exp Ther Med.
5:112–118. 2013.
|
|
22
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi
P, et al: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
23
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang GL and Semenza GL: Desferrioxamine
induces erythropoietin gene expression and hypoxia-inducible factor
1 DNA-binding activity: Implications for models of hypoxia signal
transduction. Blood. 82:3610–3615. 1993.PubMed/NCBI
|
|
25
|
Kang FW, Que L, Wu M, Wang ZL and Sun J:
Effects of trichostatin A on HIF-1α and VEGF expression in human
tongue squamous cell carcinoma cells in vitro. Oncol Rep.
28:193–199. 2012.PubMed/NCBI
|
|
26
|
Jiang L, Peng WW, Li LF, Du R, Wu TT, Zhou
ZJ, Zhao JJ, Yang Y, Qu DL and Zhu YQ: Effects of deferoxamine on
the repair ability of dental pulp cells in vitro. J Endod.
40:1100–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis,
cell proliferation and tumour angiogenesis. Nature. 394:485–490.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beasley NJ, Leek R, Alam M, Turley H, Cox
GJ, Gatter K, Millard P, Fuggle S and Harris AL: Hypoxia-inducible
factors HIF-1alpha and HIF-2alpha in head and neck cancer:
Relationship to tumor biology and treatment outcome in surgically
resected patients. Cancer Res. 62:2493–2497. 2002.PubMed/NCBI
|
|
29
|
Akakura N, Kobayashi M, Horiuchi I, Suzuki
A, Wang J, Chen J, Niizeki H, Kawamura Ki, Hosokawa M and Asaka M:
Constitutive expression of hypoxia-inducible factor-1alpha renders
pancreatic cancer cells resistant to apoptosis induced by hypoxia
and nutrient deprivation. Cancer Res. 61:6548–6554. 2001.PubMed/NCBI
|
|
30
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hammer S, To KK, Yoo YG, Koshiji M and
Huang LE: Hypoxic suppression of the cell cycle gene CDC25A in
tumor cells. Cell Cycle. 6:1919–1926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goda N, Dozier SJ and Johnson RS: HIF-1 in
cell cycle regulation, apoptosis, and tumor progression. Antioxid
Redox Signal. 5:467–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Graeber TG, Peterson JF, Tsai M, Monica K,
Fornace AJ Jr and Giaccia AJ: Hypoxia induces accumulation of p53
protein, but activation of a G1-phase checkpoint by low-oxygen
conditions is independent of p53 status. Mol Cell Biol.
14:6264–6277. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsai YP and Wu KJ: Hypoxia-regulated
target genes implicated in tumor metastasis. J Biomed Sci.
19:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiwara S, Nakagawa K, Harada H, Nagato
S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S and Ohnishi T:
Silencing hypoxia-inducible factor-1alpha inhibits cell migration
and invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2007.PubMed/NCBI
|