Introduction
The World Health Organization (WHO) reported that in
2012 cancer was a leading cause of death with 8.2 million cancer
deaths, 32.6 million cancer patients, and 14.1 million new cancer
cases (1,2). In many cancers, the nuclear factor-κB
(NF-κB) pathway is one of the most important for carcinogenesis, as
its activation promotes tumor growth and progression (3,4).
Inactive NF-κB is located in cytoplasm; however, when it is
activated by phosphorylation it accumulates in the nucleus
(5). Activated NF-κB transcription
factor can inhibit apoptosis (6)
and has been shown to upregulate expression of cyclooxygenase-2
(COX-2), a critical pro-survival inflammatory signaling molecule
(7). In MDA-MB-231 breast cancer
carcinoma cells, NF-κB and its inhibitor protein IκB are
constitutively phosphorylated (8),
which leads to chronic NF-κB activation and increased COX-2
expression (9).
Many non-specific inhibitors of NF-κB and the IκB
kinase, IKKβ, have been developed and used to inhibit tumor growth
and progression. These agents include anti-inflammatory drugs such
as sulphasalazine and trans-resveratrol, non-steroidal
anti-inflammatory drugs including aspirin, sulindac sulfide,
cyclopentenone prostaglandins, and proteasome inhibitors, and
glucocorticoids (10–12). COX-2 inhibitors have also been used
successfully to slow cancer progression in patients (13,14).
A selective COX-2 inhibitor, celecoxib, induces apoptosis by
inactivating the pro-survival kinase Akt, both in the osteosarcoma
cell line MG63 (15) and the liver
cancer cell lines HepG2 and Hep3B (16). Another COX-2 inhibitor, NS-398,
induces apoptosis in the colon carcinoma cell line HCA-7 (17) and promotes caspase-independent
apoptosis in the hepatocellular carcinoma cell line Hep3B (18).
Epidemiological studies have demonstrated that a
fruit and vegetable-rich diet reduces cancer incidence (19). Additionally, cancer morbidity is
reduced by 50% when smoking cessation is combined with a low-fat
diet rich in fruits and vegetables (20). Although few fruits and vegetables
have been definitively shown to actively prevent or treat cancer,
investigators continue to search for active agents in these food
groups (21). Similarly, the
anticancer benefits of herbal agents are due to their effects on
signal transduction processes including NF-κB inhibition, apoptosis
induction, DNA methylation, antioxidant activity, and metastasis
inhibition. For example, lycopene in tomatoes exerts anticancer
properties that are enhanced by vitamin E (22).
Many members of the Stephania plant family
exhibit pharmacological benefits. For example, biscoclaurine
alkaloid cepharanthine isolated from the herb S. cepharantha
Hayata protects against DNA damage and scavenges free radicals to
prevent lipid peroxidation (23).
In addition, it induces G0/G1 cell cycle
arrest and apoptosis by upregulating p15INK4B and
p21Waf1/Cip1 in 12PE myeloma cells (24). Bis-benzylisoquinoline alkaloid
tetrandrine isolated from the roots of S. tetrandra S. Moore
induces G1 arrest by downregulating E2F1 and
upregulating p53/p21Waf1/Cip1 in human colon carcinoma
HT29 cells (25). In 2011 our
group reported that S. delavayi Diels. inhibits carcinoma
proliferation (26), indicating
that S. delavayi Diels. is a novel anticancer therapeutic
candidate. This herb is already used in traditional Chinese
medicine to relieve pain and cure acute gastroenteritis. However,
the specific anticancer mechanism of action must be elucidated
prior to its wide use in humans.
FK-3000, a component of the S. delavayi
Diels. extract, has been reported to exhibit antiviral effects
against herpes simplex virus type-1 (HSV-1) (27) and human immunodeficiency virus type
1 (HIV-1) (28,29). It also has been shown to
downregulate NF-κB activity (30).
Another extract constituent, sinococuline is an effective inhibitor
of tumor cell growth (31) and
exhibits antimalarial activity (33). Therefore, FK-3000 and sinococuline
are prime candidates for the major active components in S.
delavayi Diels.
In this study, we evaluated the anti-proliferative
effect of 6,7-di-O-acetylsinococuline (FK-3000) isolated from S.
delavayi Diels. against breast carcinoma associated with the
apoptotic pathway via NF-κB and COX-2 in vitro and in
vivo.
Materials and methods
Isolation of FK-3000 and
sinococuline
S. delavayi Diels. extract (1 g) was
separated into 6 fractions by chromatography on a Sephadex LH-20
column with methanol (860×40 mm i.d., 25–100 μm). Fraction 3 (700
mg) was further purified by C18 high-performance liquid
chromatography (HPLC) (YMC-Pack Pro, S-5 μm, 250×20 mm i.d.; 10–30%
aqueous acetonitrile in 0.05% trifluoroacetic acid for 90 min at 7
ml/min), which yielded compound 1 (sinococuline) (15 mg,
Rt 36.01 min) and compound 2 (FK-3000) (76
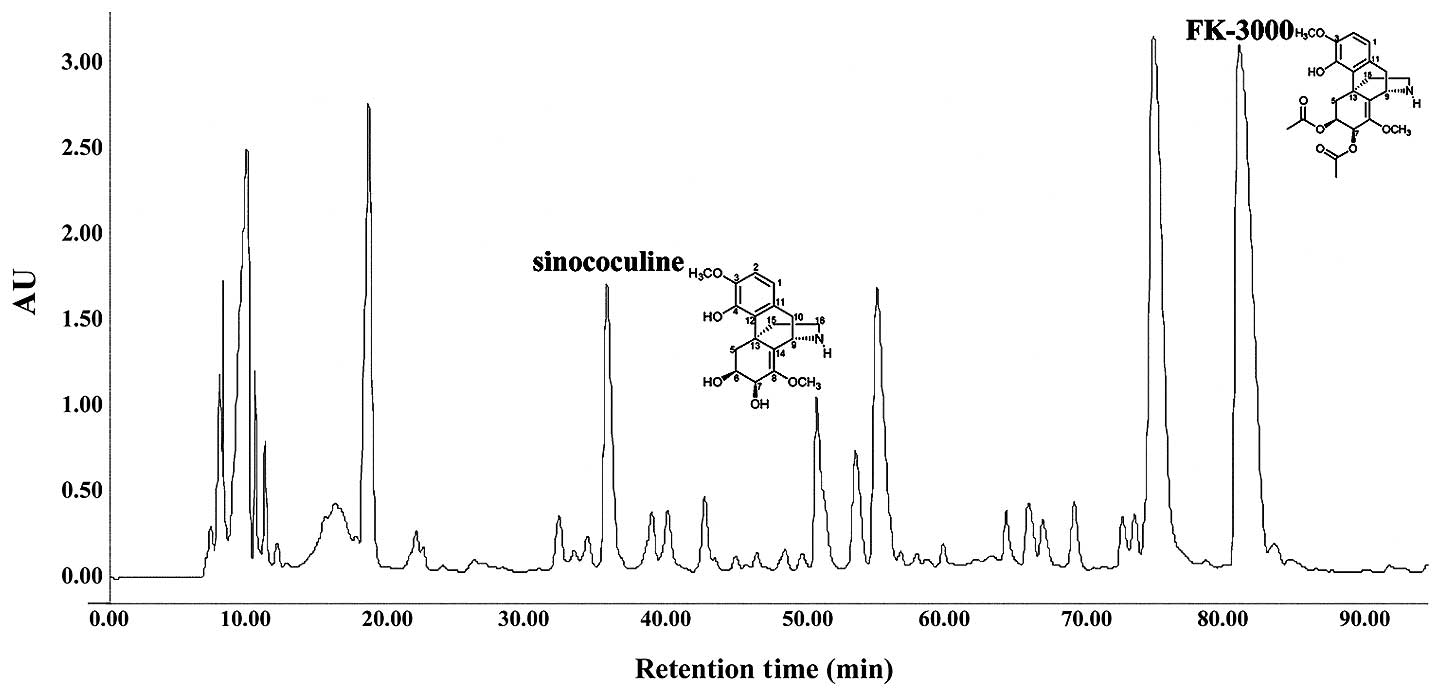
mg, Rt 82.14 min) (Fig. 1). The 1H,
13C, and two-dimensional nuclear magnetic resonance (2D
NMR) spectra of the isolates were in good agreement with
sinococuline and FK-3000 chemical structures (data not shown).
Anti-proliferation evaluation
In order to evaluate the proliferation inhibition of
S. delavayi Diels., sinococuline, and FK-3000, we used
several cancer cell lines including MDA-MB-231 (human breast
carcinoma), MCF-7 (human breast carcinoma), PC-3 (human prostate
carcinoma), A-431 (human epidermoid carcinoma), HT-29 (human
colorectal carcinoma), and CT-26 (murine colorectal carcinoma).
These cell lines were obtained from the Korean Cell Line Bank
(Seoul, Korea). Cells were seeded in triplicate into 96-well plates
at a density of 1.5×104 cells/well. Following a 12-h
incubation, cells were treated with 0–16 μg/ml of S.
delavayi Diels., 0–5 μg/ml of FK-3000, or 0–16 μg/ml of
sinococuline. The control cells were treated with 0.1% DMSO alone.
Following 48-h incubation, cell proliferation was analyzed using
the CCK-8 cell counting kit (Dojindo Laboratories, Mashikimachi,
Japan) according to the manufacturer’s instructions.
Apoptosis induction analysis
MDA-MB-231 cells were seeded into 96-well plates as
described above, incubated for 12 h, and treated with 0.5 or 5.0
μg/ml FK-3000. Following 48-h incubation, cells were harvested by
trypsinization, washed in cold PBS, and resuspended in binding
buffer (0.01 M HEPES/NaOH, 0.14 M NaCl, 2.5 mM CaCl2, pH
7.4). Annexin V-FITC (5 μl) (Becton-Dickinson, Franklin Lakes, NJ,
USA) and 5 μl propidium iodide (Becton-Dickinson) were added to the
cells followed by incubation with gentle mixing for 15 min at room
temperature in the dark. Additional binding buffer was added and
the Annexin V-stained cells were analyzed using a BD Model FACScan
(Becton-Dickinson).
Analysis of p-NF-κB localization
We measured activated NF-κB levels in MDA-MB-231
cells using an NF-κB translocation assay. Attached cells were
treated with 5.0 μg/ml FK-3000 and incubated for 120 min in a
Lab-Tek® II Chamber Slide™ system (Nalge Nunc
International). Cells were washed twice in cold PBS, fixed with
cold acetone, blocked with Animal-Free Blocker™ (Vector, SP-5030)
for 1 h, and incubated overnight at 4°C with a rabbit anti-human
NF-κB p65 antibody (Cell Signaling, cat. no. 4764). Cells were
incubated for 1 h with a FITC-conjugated anti-rabbit IgG (Cayman,
cat. no. CAY-10006588), followed by DAPI staining. The cells were
imaged using an IX51 Research Microscope (Olympus, Japan).
Measurement of NF-κB phosphorylation and
COX-2 expression levels
MDA-MB-231 cells were plated, incubated 12 h, then
treated with 0.5 μg/ml or 5.0 μg/ml FK-3000. Following a 60-min to
48-h incubation, the cells were trypsinized, the harvested cells
were washed twice with cold PBS, and total protein lysates were
prepared using PRO-PREP™ (iNtRON Biotechnology, Seongnam, Korea)
according to the manufacturer’s instructions. Cytosolic and nuclear
proteins were separated using a Nuclear Extraction kit (Panomics,
San Francisco, CA, USA) following the manufacturer’s protocol. The
protein content of each sample was measured using the Bio-Rad Dc
protein assay kit (Bio-Rad, Hercules, CA, USA) according to the
manufacturer’s instructions. Equal protein amounts were loaded and
separated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel,
electrophoretically transferred to a nitrocellulose membrane using
Trans-Blot® Transfer Medium (Bio-Rad), and incubated
with the following antibodies: monoclonal mouse anti-human
p-NF-κB antibody (Cell Signaling, cat. no. 3036, Danvers,
MA, USA), polyclonal mouse anti-human COX-2 antibody (Cayman, cat.
no. CAY-160106, Ann Arbor, MI, USA), monoclonal β-actin antibody
(Sigma-Aldrich, Inc., cat. no. A-5316, St. Louis, MO, USA), or
monoclonal PARP antibody (Biomol International, cat. no. SA-250,
Plymouth Meeting, PA, USA). HRP-conjugated goat anti-rabbit IgG
(Cayman, cat. no. 10004301) and antimouse IgG (Cell Signaling, cat.
no. 7076) were used as secondary antibodies. The bands were
visualized using an ECL detection kit (Amersham Biosciences, UK)
according to the manufacturer’s protocol and a LAS 3000 imaging
system (Fuji Film, Japan).
Assessment of tumor growth
The human tumor xenograft study was approved by the
Institute of Animal Care and Use Committee prior to performing the
experiments. Forty, 8-week-old, female BALB/cnu/nu mice were
purchased from OrientBio (Sungnam, Korea) and allowed to acclimate
for 7 days. All animals were housed in a temperature and relative
humidity-controlled environment (22±3°C, 50±5%, 12-h light/dark
cycle) throughout the acclimation and experimental period. The mice
were provided a Purina diet (Purina Korea) and water ad
libitum. Mice were subcutaneously injected with
5×106 of MDA-MB-231 cells in each flank. When the tumor
volumes reached 100–150 mm3, mice were randomly divided
into four groups. The first group (control group, n=7) was
intraperitoneally administered vehicle (0.1% DMSO, once a day). The
second, third, and fourth groups (n=8 each) received Taxol
(Sigma-Aldrich, 10 mg/kg body weight, intraperitoneally once per
week), FK-3000 (1 mg/kg body weight, intraperitoneally daily), or
Taxol (10 mg/kg, intraperitoneally once a week) and FK-3000 (1
mg/kg, intraperitoneally daily) for 24 days. The tumors were
measured by caliper every 3 days and tumor volumes were calculated
using axb2/2 (where a was
the width at the widest tumor point and b was the width
perpendicular to a). The mice were sacrificed at day 25.
Histopathological examination
After all the animals were sacrificed, organ weight
was measured of brain, pituitary gland, liver, spleen, heart,
thymus, salivary gland, kidney, adrenal gland, lung, thyroid
gland/parathyroid gland, seminal vesicle (male only), prostate
(male only), testes (male only), epididymis (male only), ovary
(female only), and uterus/cervix (female only). Histopatholgical
examination was conducted only in the control group (0 mg/kg/day
PbL treatment group) and the high dosing group (3,000 mg/kg/day
treated group) Testes and epididymis were fixed with Bouin solution
and the other tissues were fixed in 10% (v/v) formaldehyde
solution, dehydrated with ethanol (99.9, 90, 80 and 70%) and water,
and embedded in paraffin. Specimens were sliced into sections of
5-μm thickness. The slides were stained with hematoxylin and eosin
(H&E).
Statistical analysis
Results are expressed as mean ± standard deviation
(SD). Groups were compared using Tukey’s studentized range (HSD)
test with SPSS Statics (IBM, Armonk, NY, USA); Statistical
significance *p<0.1; **p<0.05.
Results
FK-3000 has an anti-proliferative effect
against several carcinomas
We chromatographically isolated two structurally
similar compounds from S. delavayi Diels. extract. The
1H, 13C and 2D NMR spectra of these compounds
were consistent with those published previously for FK-3000 and
sinococuline (Fig. 1) (33,34).
We compared the inhibitory effects of S. delavayi Diels.
extract, sinococuline, and FK-3000 on proliferation in several
cancer cell lines (Table I).
FK-3000 more effectively inhibited cell proliferation in the six
carcinomas tested when compared to S. delavayi Diels.
extract or sinococuline. In particular, MDA-MB-231, MCF-7, PC-3,
and HT-29 cell growth was more sensitive to FK-3000. Sinococuline
was less effective than S. delavayi Diels. extract at
inhibiting growth in the six cancer lines tested. In MDA-MB-231
cells at 48 h post-treatment, the IC50 ranges of S.
delavayi Diels. extract, sinococuline, and FK-3000 were
1.20–5.32, 4.49–15.88 and 0.22–2.70 μg/ml, respectively.
 | Table IThe half maximal inhibitory
concentration (IC50) of S. delavayi Diels.,
sinococuline and FK-3000 on six carcinoma cell lines at 48 h. |
Table I
The half maximal inhibitory
concentration (IC50) of S. delavayi Diels.,
sinococuline and FK-3000 on six carcinoma cell lines at 48 h.
| Cell line | S. delavayi
Diels. (μg/ml) | Sinococuline
(μg/ml) | FK-3000
(μg/ml) |
|---|
| MDA-MB-231 | 2.31 | 4.49 | 0.52 |
| MCF-7 | 2.05 | 14.45 | 0.77 |
| PC-3 | 1.20 | 6.81 | 0.22 |
| A-431 | 5.32 | 6.83 | 2.70 |
| HT-29 | 4.57 | 15.88 | 0.40 |
| CT-26 | 3.37 | 11.21 | 1.90 |
| Average | 3.137 | 9.945 | 1.085 |
FK-3000 increases MDA-MB-231 cell
apoptosis in a dose-and time-dependent manner
FACS analysis demonstrated that FK-3000 induced
apoptosis in a dose- and time-dependent manner (Fig. 2). After 24-h treatment with 0.5
μg/ml FK-3000, the percentage of apoptotic cells was ~8.01% and by
48 h it had increased to 21.13%, compared to 7.00 and 11.34%,
respectively, in vehicle-treated cells. At 5.0 μg/ml FK-3000
dosage, the percent of apoptotic cells increased to 12.97% after 24
h and 37.69% at 48 h.
FK-3000 effectively blocks NF-κB nuclear
translocation
In most cancer cells NF-κB proteins are active and
localized to the nucleus, which inhibits apoptosis induction. This
is in contrast to normal cells where it is localized in the
cytoplasm in an inactive form (36). To confirm that FK-3000 inactivates
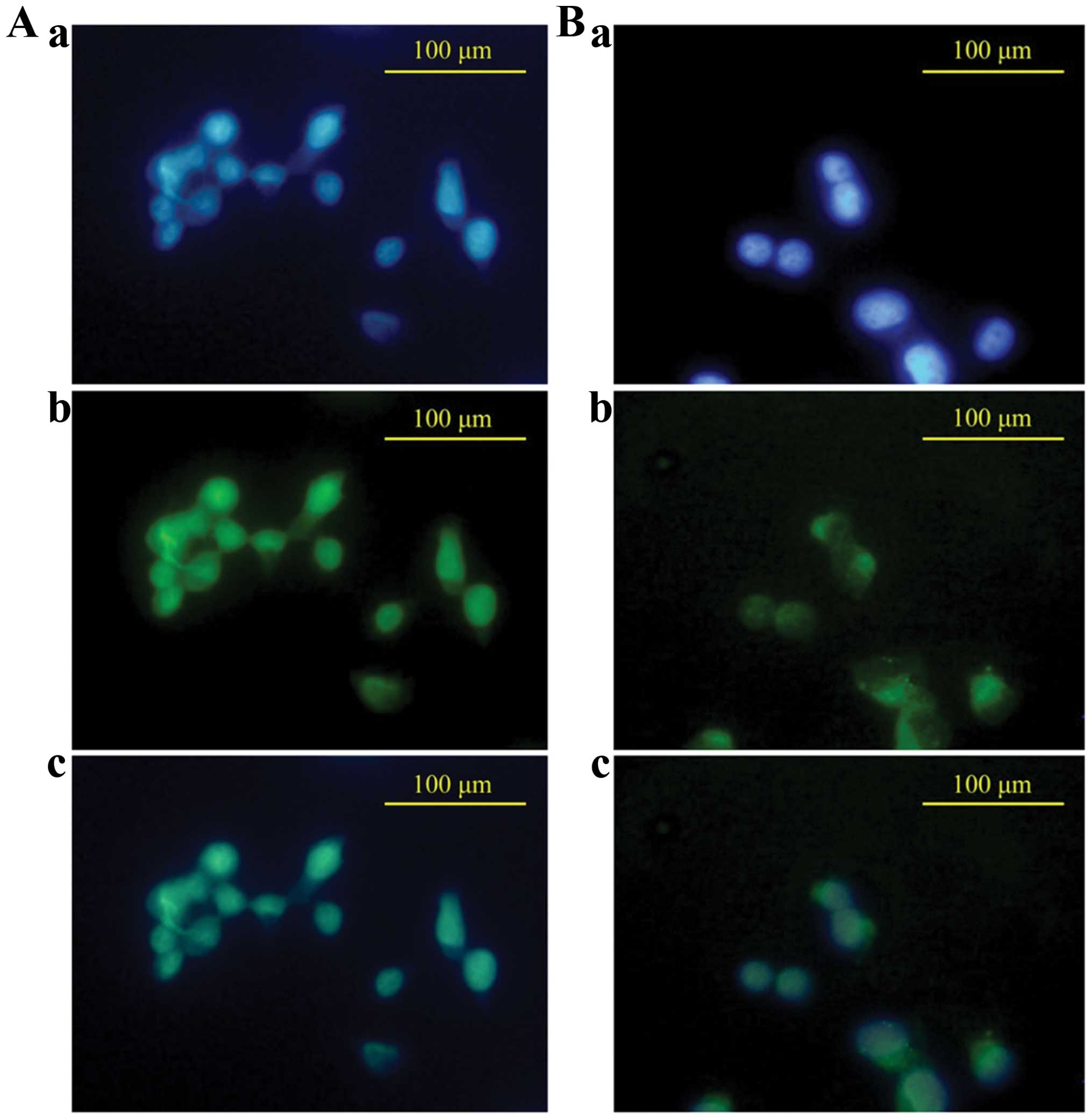
NF-κB, we evaluated whether it blocks NF-κB nuclear localization
(Fig. 3). Untreated MDA-MB-231
cell staining clearly showed that NF-κB p65 proteins were primarily
localized to the nucleus (Fig.
3A-c). In the FK-3000 treated cells NF-κB p65 proteins were
localized mainly to the cytoplasm (Fig. 3B-c) This demonstrates that FK-3000
effectively inhibited NF-κB translocation from the cytoplasm to
nucleus.
FK-3000 decreases NF-κB phosphorylation
and COX-2 protein expression
There are several mechanisms to induce cell
apoptosis including the caspase cascade, the Bcl family pathway
(35), or the NF-κB-COX-2 pathway
(36). In our previous study on
S. delavayi Diels., the FK-3000 parental material
demonstrated an anti-proliferative effect via the NF-κB-COX-2
pathway (26). Therefore, we
investigated whether FK-3000 could induce apoptosis in the same
manner using MDA-MB-231 cells. In order to determine FK-3000’s
NF-κB phosphorylation (activation) inhibitive effect, we performed
western blot analyses of the p-NF-κB levels at various time points
from 60–120 min following 5.0 μg/ml FK-3000 treatment (Fig. 4). The phosphorylation of NF-κB
decreased in a time-dependent manner and by 120 min was nearly
undetectable. Therefore, FK-3000 effectively suppressed the
phosphorylation of NF-κB. COX-2 protein can induce apoptosis in
cells and its expression is controlled by NF-κB (36). At 24 h following FK-3000 treatment,
the COX-2 protein level decreased in a dose-dependent manner
(Fig. 4). At 48 h post-treatment
the result was unchanged.
FK-3000 inhibits cancer cell growth in a
mouse xenograft model
To assess whether FK-3000 is a viable candidate for
anticancer therapy, we used an MDA-MB-231 xenografted mouse model
to directly evaluate its antitumor effects (Fig. 5). Mice were treated with vehicle,
FK-3000, Taxol, or FK-3000 in combination with Taxol. At 12 days of
treatment, the tumor volume in the Taxol and FK-3000 co-treatment
group was the smallest among the four groups (p<0.05). Following
21 days of treatment, tumor volumes were significantly different in
all the treatment groups compared to the control (p<0.05).
FK-3000 alone inhibited tumor growth to a similar extent as Taxol.
Additionally, FK-3000 treatment showed no signs of toxicity. There
were no differences in liver function tests, complete blood cell
counts, and serum enzyme levels between any of the drug treatment
groups and the controls (Tables I
and II). There were no
histopathological changes observed in any group (data not shown).
Interestingly, FK-3000 and Taxol co-treatment exhibited a
synergistic effect (Fig. 5).
 | Table IIComplete blood cell counts from
MDA-MB-231 xenografted mice in the treatment and control
groups. |
Table II
Complete blood cell counts from
MDA-MB-231 xenografted mice in the treatment and control
groups.
| Control | Taxol | FK-3000 | FK-3000+Taxol |
|---|
| Leukocytes |
| WBC (k/μl) | 3.39±2.54 | 7.12±3.17 | 5.06±2.56 | 3.93±1.47 |
| NE (k/μl) | 1.12±0.78 | 2.24±1.05 | 1.99±1.11 | 1.55±0.95 |
| LY (k/μl) | 1.89±1.55 | 4.03±1.76 | 2.41±1.29 | 2.01±0.45 |
| MO (k/μl) | 0.17±0.13 | 0.42±0.18 | 0.29±0.12 | 0.20±0.07 |
| EO (k/μl) | 0.16±0.13 | 0.33±0.18 | 0.29±0.13 | 0.15±0.10 |
| BA (k/μl) | 0.05±0.03 | 0.10±0.05 | 0.09±0.05 | 0.05±0.05 |
| Erythrocytes |
| RBC (M/μl) | 9.36±0.41 | 9.47±0.42 | 9.53±1.16 | 9.57±0.31 |
| Hb (M/dl) | 12.76±0.42 | 13.14±0.49 | 10.80±5.13 | 13.30±0.63 |
| HCT (%) | 49.84±2.22 | 50.16±1.36 | 50.86±4.94 | 52.00±1.52 |
| MCV (fl) | 53.26±0.91 | 53.54±1.76 | 53.54±1.76 | 54.34±1.69 |
| MCH (pg) | 13.66±0.49 | 13.56±1.31 | 13.56±1.31 | 13.88±0.51 |
| MCHC (g/dl) | 25.62±0.76 | 25.26±1.71 | 25.26±1.71 | 25.58±0.91 |
| RDW (%) | 16.46±0.43 | 17.42±2.30 | 16.88±1.70 | 16.80±0.25 |
| Thrombocyte |
| PLT (k/μl) | 521.8±142.7 | 392.6±57.36 | 234.8±73.58 | 435.0±166.2 |
| MPV (fl) | 4.74±0.17 | 5.10±0.60 | 5.36±0.60 | 4.92±0.40 |
Discussion
Previously, we reported that S. delavayi
Diels. suppressed MDA-MB-231 carcinoma proliferation by inducing
apoptosis (26). In this study
FK-3000 and sinococuline were isolated from S. delavayi
Diels. extract (Fig. 1). FK-3000
and sinococuline inhibited proliferation in several carcinomas
including MDA-MB-231, MCF-7, PC-3, A-431, HT-29, and CT-26
(Table I). The anti-proliferative
effect was greatest using FK-3000, followed by S. delavayi
Diels. extract, and sinococuline. FK-3000 induced dose- and
time-dependent apoptosis in MDA-MB-231 cells and the 5.0 μg/ml
FK-3000 treatment increased the percentage of apoptotic cells from
12.97% at 24 h to 37.69% at 48 h, a 26.35% increase compared to
control cells (Fig. 2). The active
form of NF-κB is phosphorylated and localized to the nucleus. In
MDA-MB-231 cells NF-κB is constitutively active (6). FK-3000 at a 5.0 μg/ml dose
significantly blocked NF-κB translocation from the cytoplasm to the
nucleus (Fig. 3). FK-3000
inhibited both NF-κB phosphorylation and COX-2 protein expression
in a dose- and time-dependent manner (Fig. 4). At 120 min with 5.0 μg/ml
FK-3000, the NF-κB was almost completely dephosphorylated. FK-3000
inhibited tumor growth in the MDA-MB-231 xenograft model. FK-3000
is as effective as Taxol, with daily 1 mg/kg body weight FK-3000
treatments exhibiting similar effects to weekly 10 mg/kg body
weight Taxol administration. Since an overall lower FK-3000 dose (7
mg/kg body weight/week) was able to reduce tumor growth to the same
degree as Taxol (10 mg/kg body weight/week), FK-3000 may be a more
effective antitumor agent. FK-3000 also had a synergistic effect
when used in combination with Taxol (Fig. 5). This may be due to modulation of
different pathways, with FK-3000 targeting NF-κB activation and
Taxol blocking cell mitosis (37).
As a whole, these observations suggest that FK-3000 is a promising
anticancer drug candidate.
Many epidemiological studies report that
vegetable-rich diets reduce both cancer incidence and morbidity,
but the action mechanisms are ambiguous in most cases. Thus,
identifying the specific antitumor effect mechanisms of a plant is
an active area of research. For example, Nexrutine, a
Phellodendron amurense herbal extract, has been investigated
as a prostate cancer treatment (38,39).
In this study we determined that the apoptosis induction effect
seen with S. delavayi Diels. extract is caused by its active
compound FK-3000 through NF-κB deactivation. NF-κB activation is a
double-edged sword and its downstream effects depend on the cell’s
phenotype and context.
NF-κB activation inhibits apoptosis (40,41)
by altering the apoptosis related protein 3 (APR3) levels that
normally change during development and inflammation (42). It also suppresses TNF-α-induced
apoptosis by promoting transcription of apoptotic inhibitors such
as Bcl-2, inhibitor of apoptosis proteins (IAPs), and
TNFR-associated factors (TRAF) 1 and 2 (43–45).
Conversely, NF-κB activation can also promote apoptosis, as
evidenced by doxorubicin-mediated cell death induction through IκB
degradation in N-type neuroblastoma cells (46) and p53-induced apoptosis, which
depends on NF-κB activation (47).
In the case of breast carcinomas, constitutive NF-κB activation is
detrimental to the patient prognosis, and treatment with a compound
like FK-3000 could potentially improve outcomes.
Furthermore, we tested the safety and efficacy of
FK-3000 in a mouse xenograft model. We identified several
advantages to developing FK-3000 as a novel anticancer drug. First,
FK-3000 seems to be very safe with low toxicity (Tables II and III) compared to other small molecule
inhibitors. For example, the COX-2 inhibitor celecoxib has numerous
side effects including gastric bleeding (48). Second, FK-3000 specifically targets
the NF-κB and COX-2 pathway. Third, since Taxol is a major
anticancer drug approved to treat several types of cancer;
combination of FK-3000 and Taxol may improve the outcome of
anticancer chemotherapy. Overall, FK-3000 is a promising candidate
to inhibit cancer proliferation.
 | Table IIIComplete blood chemistry from
MDA-MB-231 xenografted mice in the treatment and control
groups. |
Table III
Complete blood chemistry from
MDA-MB-231 xenografted mice in the treatment and control
groups.
| Control | Taxol | FK-3000 | FK-3000+Taxol |
|---|
| GOT (U/I) | 72.2±10.63a | 63.60±3.71 | 65.20±3.77 | 64.40±4.50 |
| GPT (U/I) | 25.00±2.55a | 30.20±5.20 | 25.80±2.17 | 24.60±2.61 |
| BUN (mg/dl) | 42.84±9.47a | 36.50±4.45 | 52.00±6.32 | 59.30±11.58 |
| NH3
(μg/dl) | 95.4±3.51a | 97.80±5.07 | 92.00±1.41 | 97.40±3.78 |
| TBIL (mg/dl) | 0.36±0.15a | 0.38±0.13 | 0.44±0.09 | 0.43±0.18 |
| ALB (g/dl) | 2.34±0.15a | 2.32±0.22 | 2.46±0.15 | 2.42±0.13 |
References
|
1
|
The World Health Organization. Cancer,
Fact Sheet No. 297. February. 2014
|
|
2
|
International Agency for Research on
Cancer. Estimated cancer incidence, mortality and prevalence
worldwide in 2012. Globocan 2012. 2012.
|
|
3
|
Gupta SC, Sundaram C, Reuter S and
Aggarwal BB: Inhibiting NF-κB activation by small molecules as a
therapeutic strategy. Biochim Biophys Acta. 1799:775–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo JL, Kamata H and Karin M:
IKK/NF-kappaB signaling: balancing life and death - a new approach
to cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozakai N, Kikuchi E, Hasegawa M, Suzuki
E, Ide H, Miyajima A, Horiguchi Y, Nakashima J, Umezawa K,
Shigematsu N and Oya M: Enhancement of radiosensitivity by a unique
novel NF-κB inhibitor, DHMEQ, in prostate cancer. Br J Cancer.
107:652–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun XF and Zhang H: NFκB and NFκBI
polymorphisms in relation to susceptibility of tumour and other
diseases. Histol Histopathol. 22:1387–1398. 2007.PubMed/NCBI
|
|
7
|
St-Germain ME, Gagnon V, Parent S and
Asselin E: Regulation of COX-2 protein expression by Akt in
endometrial cancer cells is mediated through NF-kappaB/IkappaB
pathway. Mol Cancer. 3:1–11. 2004. View Article : Google Scholar
|
|
8
|
Monks NR and Pardee AB: Targeting the
NF-kappa B pathway in estrogen receptor negative MDA-MB-231 breast
cancer cells using small inhibitory RNAs. J Cell Biochem.
98:221–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang BC, Sanchez T, Schaefers HJ, Trifan
OC, Liu CH, Creminon C, Huang CK and Hla T: Serum
withdrawal-induced post-transcriptional stabilization of
cyclooxygenase-2 mRNA in MDA-MB-231 mammary carcinoma cells
requires the activity of the p38 stress-activated protein kinase. J
Biol Chem. 275:39507–39515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karin M, Yamamoto Y and Wang QM: The IKK
NF-kappa B system: a treasure trove for drug development. Nat Rev
Drug Discov. 3:17–26. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orlowski RZ and Baldwin AS Jr: NF-kappaB
as a therapeutic target in cancer. Trends Mol Med. 8:385–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guttridge DC, Albanese C, Reuther JY,
Pestell RG and Baldwin AS Jr: NF-kappaB controls cell growth and
differentiation through transcriptional regulation of cyclin D1.
Mol Cell Biol. 19:5785–5799. 1999.PubMed/NCBI
|
|
13
|
Dannenberg AJ, Altorki NK, Boyle JO, Dang
C, Howe LR, Weksler BB and Subbaramaiah K: Cyclo-oxygenase 2: a
pharmacological target for the prevention of cancer. Lancet Oncol.
2:544–551. 2001. View Article : Google Scholar
|
|
14
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Shi ZL, Feng J and Tao HM:
Celecoxib, a cyclooxygenase-2 inhibitor, induces apoptosis in human
osteosarcoma cell line MG-63 via down-regulation of PI3K/Akt. Cell
Biol Int. 32:494–501. 2008. View Article : Google Scholar
|
|
16
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Half E, Sun Y and Sinicrope FA: Anti-EGFR
and ErbB-2 antibodies attenuate cyclooxygenase-2 expression and
cooperatively inhibit survival of human colon cancer cells. Cancer
Lett. 251:237–246. 2007. View Article : Google Scholar
|
|
18
|
Park MK, Hwang SY, Kim JO, Kwack MH, Kim
JC, Kim MK and Sung YK: NS398 inhibits the growth of Hep3B human
hepatocellular carcinoma cells via caspase-independent apoptosis.
Mol Cells. 17:45–50. 2004.PubMed/NCBI
|
|
19
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: a global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giovino GA: The tobacco epidemic in the
United States. Am J Prev Med. 33:S318–S326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boileau TW, Liao Z, Kim S, Lemeshow S,
Erdman JW Jr and Clinton SK: Prostate carcinogenesis in
N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato
powder, lycopene, or energy-restricted diets. J Natl Cancer Inst.
95:1578–1586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halicka D, Ita M, Tanaka T, Kurose A and
Darzynkiewicz Z: Biscoclaurine alkaloid cepharanthine protects DNA
in TK6 lymphoblastoid cells from constitutive oxidative damage.
Pharmacol Rep. 60:93–100. 2008.PubMed/NCBI
|
|
24
|
Kikukawa Y, Okuno Y, Tatetsu H, Nakamura
M, Harada N, Ueno S, Kamizaki Y, Mitsuya H and Hata H: Induction of
cell cycle arrest and apoptosis in myeloma cells by cepharanthine,
a biscoclaurine alkaloid. Int J Oncol. 33:807–814. 2008.PubMed/NCBI
|
|
25
|
Meng LH, Zhang H, Hayward L, Takemura H,
Shao RG and Pommier Y: Tetrandrine induces early G1 arrest in human
colon carcinoma cells by down-regulating the activity and inducing
the degradation of G1-S-specific cyclin-dependent kinases and by
inducing p53 and p21Cip1. Cancer Res. 64:9086–9092.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park D-H, Xu HD, Shim J, Li Y-C, Lee J-H,
Cho S-C, Han S-S, Lee Y-L, Lee M-J and Kwon S-W: Stephania delavayi
Diels. inhibits breast carcinoma proliferation through the p38MAPK/
NF-κB/COX-2 pathway. Oncol Rep. 26:833–841. 2011.PubMed/NCBI
|
|
27
|
Nawawi A, Nakamura N, Meselhy MR, Hattori
M, Kurokawa M, Shiraki K, Kashiwaba N and Ono M: In vivo antiviral
activity of Stephania cepharantha against herpes simplex virus
type-1. Phytother Res. 15:497–500. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma CM, Nakamura N, Hattori M, Kawahata T
and Otake T: Inhibitory effects of triterpene-azidothymidine
conjugates on proliferation of human immunodeficiency virus type 1
and its protease. Chem Pharm Bull (Tokyo). 50:877–880. 2002.
View Article : Google Scholar
|
|
29
|
Ma CM, Nakamura N, Miyashiro H, Hattori M,
Komatsu K, Kawahata T and Otake T: Screening of Chinese and
Mongolian herbal drugs for anti-human immunodeficiency virus type 1
(HIV-1) activity. Phytother Res. 16:186–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baba M: Inhibitors of HIV-1 gene
expression and transcription. Curr Top Med Chem. 4:871–882. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu WK, Wang XK and Che CT: Cytotoxic
effects of sinococuline. Cancer Lett. 99:217–224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carraz M, Jossang A, Rasoanaivo P, Mazier
D and Frappier F: Isolation and antimalarial activity of new
morphinan alkaloids on Plasmodium yoelii liver stage. Bioorg Med
Chem. 16:6186–6192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nawawi A, Ma C, Nakamura N, Hattori M,
Kurokawa M, Shiraki K, Kashiwaba N and Ono M: Anti-herpes simplex
virus activity of alkaloids isolated from Stephania cepharantha.
Biol Pharm Bull. 22:268–274. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Itokawa H, Tsuruoka S, Takeya K, Mori N,
Sonobe T, Kosemura S and Hamanaka T: An antitumor morphinane
alkaloid, sinococuline, from Cocculus trilobus. Chem Pharm Bull.
35:1660–1662. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chinembiri TN, du Plessis LH, Gerber M,
Hamman JH and du Plessis J: Review of natural compounds for
potential skin cancer treatment. Molecules. 19:11679–11721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nesaretnam K and Meganathan P:
Tocotrienols: inflammation and cancer. Ann NY Acad Sci. 1229:18–22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ganguly A, Yang H and Cabral F:
Paclitaxel-dependent cell lines reveal a novel drug activity. Mol
Cancer Ther. 9:2914–2923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghosh R, Garcia GE, Crosby K, Inoue H,
Thompson IM, Troyer DA and Kumar AP: Regulation of Cox-2 by cyclic
AMP response element binding protein in prostate cancer: potential
role for nexrutine. Neoplasia. 9:893–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar AP, Bhaskaran S, Ganapathy M, Crosby
K, Davis MD, Kochunov P, Schoolfield J, Yeh IT, Troyer DA and Ghosh
R: Akt/ cAMP-responsive element binding protein/cyclin D1 network:
a novel target for prostate cancer inhibition in transgenic
adenocarcinoma of mouse prostate model mediated by Nexrutine, a
Phellodendron amurense bark extract. Clin Cancer Res. 13:2784–2794.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
van Hogerlinden M, Rozell BL,
Ahrlund-Richter L and Toftgard R: Squamous cell carcinomas and
increased apoptosis in skin with inhibited Rel/nuclear
factor-kappaB signaling. Cancer Res. 59:3299–3303. 1999.PubMed/NCBI
|
|
41
|
Miyamoto S, Maki M, Schmitt MJ, Hatanaka M
and Verma IM: Tumor necrosis factor alpha-induced phosphorylation
of I kappa B alpha is a signal for its degradation but not
dissociation from NF-kappa B. Proc Natl Acad Sci USA.
91:12740–12744. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang G, Yu F, Fu H, Lu F, Huang B, Bai L,
Zhao Z, Yao L and Lu Z: Identification of the distinct promoters
for the two transcripts of apoptosis related protein 3 and their
transcriptional regulation by NFAT and NFkappaB. Mol Cell Biochem.
302:187–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herrmann JL, Beham AW, Sarkiss M, Chiao
PJ, Rands MT, Bruckheimer EM, Brisbay S and McDonnell TJ: Bcl-2
suppresses apoptosis resulting from disruption of the NF-kappa B
survival pathway. Exp Cell Res. 237:101–109. 1997. View Article : Google Scholar
|
|
44
|
Notarbartolo M, Poma P, Perri D, Dusonchet
L, Cervello M and D’Alessandro N: Antitumor effects of curcumin,
alone or in combination with cisplatin or doxorubicin, on human
hepatic cancer cells. Analysis of their possible relationship to
changes in NF-κB activation levels and in IAP gene expression.
Cancer Lett. 224:53–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bian X, McAllister-Lucas LM, Shao F,
Schumacher KR, Feng Z, Porter AG, Castle VP and Opipari AW Jr:
NF-kappa B activation mediates doxorubicin-induced cell death in
N-type neuroblastoma cells. J Biol Chem. 276:48921–48929. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ryan KM, Ernst MK, Rice NR and Vousden KH:
Role of NF-kappaB in p53-mediated programmed cell death. Nature.
404:892–897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chan FK, Hung LC, Suen BY, Wu JC, Lee KC,
Leung VK, Hui AJ, To KF, Leung WK, Wong VW, Chung SC and Sung JJ:
Celecoxib versus diclofenac and omeprazole in reducing the risk of
recurrent ulcer bleeding in patients with arthritis. N Engl J Med.
347:2104–2110. 2002. View Article : Google Scholar : PubMed/NCBI
|