Introduction
High-grade malignant gliomas are among the most
rapidly growing and lethal human tumors. Despite multimodal
therapies, the prognosis of glioblastoma multiforme (GBM) remains
poor and the tumor inevitably recurs. The failure of therapeutic
treatments is mainly due to the diffusion modalities of the tumor
and to several resistance mechanisms of cancer cells, such as
elevated expression of drug efflux transporters (1–3),
reduced sensitivity to apoptotic signals and increased expression
of growth factors. A pivotal role in resistance is played by the
ability of tumor cells to repair DNA damage caused by radiation and
chemotherapeutic agents, through a DNA damage response (DDR)
cascade (4–8).
Multiple complex pathways occur in the eukaryotic
cells for the surveillance and repair of genetic material and cell
cycle control. To DNA damage the cancer cells respond with: i) cell
cycle arrest and lesion repair; ii) entry into apoptosis or other
cell deaths; iii) proliferation without repairing the damage
favouring the effects of more genetic mutations. In response to
genotoxic stress, cells do not progress through the cycle until the
stability of the DNA molecule is ensured: the MRN
(Mre11-Rad50-Nbs1) sensor complex recruits the protein kinases
ataxia telangiectasia mutated (ATM) and ataxia telangiectasia
Rad3-related (ATR), that initiate a transduction cascade activating
downstream effectors, including H2AX histone, 53 binding protein 1
(53BP1) and the checkpoint kinases Checkpoint 1 (Chk1) and
Checkpoint 2 (Chk2). These last two molecules act as a molecular
switch determining, through phosphorylation of tumor suppressor
protein p53, cell cycle arrest in order to allow DNA damage repair
(Fig. 1A). If the damage is too
extensive, apoptosis is triggered (9). Double strand breaks (DSBs), the most
dangerous DNA lesions in mammalian cells, are repaired by two main
pathways: homologous recombination (HR) and non-homologous end
joining (NHEJ) (10,11). The former takes place only in
actively cycling cells, during the S/G2 phases and its key effector
is RAD51 protein. The latter occurs during G0/G1 phases and is
driven by DNA-dependent protein kinase (DNA-PK), which consists of
a regulatory subunit (Ku70/Ku80 heterodimer) and a catalytic
subunit (DNA-PKcs) (Fig. 1A).
Glioma stem cells (GSCs), responsible for GBM growth
and recurrence, show a relative resistance to apoptosis and an
increased DNA repair capacity (12–14).
Thanks to preferential activation of DDR and to an increased
Chk1/Chk2 activity, a delayed cell cycle may be a major resistance
mechanism (7) and Chk1/Chk2
inhibition is reported to sensitize to radio-treatments (13).
Temozolomide (TMZ), doxorubicin (Dox) and paclitaxel
(PTX) are drugs commonly used in the clinical practice of solid
tumors. TMZ, an alkylating agent at present employed in the
standard therapy of GBM, induces methylation in multiple sites on
DNA. The O6-methylguanines (O6-meGs) are the
most cytotoxic adducts and are normally repaired by the
O6-methylguanine-DNA methyltransferase (MGMT). The
hypermethylation of the MGMT promoter determines epigenetic
silencing of the protein and correlates with a better prognosis
(15). If the cell is
MGMT-deficient, a futile mismatch repair (MMR) cycle is triggered
with formation of DNA DSBs and activation of ATM/ATR-Chk1/Chk2
signaling, G2/M-cell cycle arrest and ultimately apoptosis
(16–18) (Fig.
1B). The anthracycline Dox interferes with cell growth by
intercalation between DNA paired bases, finally causing cell death.
PTX acts as antimitotic drug determining apoptosis. Also Dox and
PTX are reported to induce DNA strand breaks and a repair response
in some cell types (19,20); their use on brain tumors is limited
due to the poor blood-brain barrier (BBB) penetration capacity,
even though their in vitro effectiveness on GBM cells and in
glioma animal models is proven (21–23).
The present study explored the in vitro
effects of TMZ, Dox and PTX on primary GBM cell lines, focusing the
attention on TMZ and by investigating DNA damage extent and the
molecular mechanisms leading to a repair response, i.e., a
resistant phenotype, or to cell death.
Materials and methods
Cell lines, culture conditions and tumor
specimens
Primary human GBM cultures were established from
tumors surgically resected at the Department of Neurosurgery of CTO
Hospital (Turin, Italy). Malignant glioma U87-MG and 010627 cell
lines were kindly supplied by Dr Rossella Galli (DIBIT San
Raffaele, Milan, Italy). Ten cell lines were cultured, as
previously described (24), in
Dulbecco’s modified Eagle’s medium (DMEM)/F-12 supplemented with 20
ng/ml epidermal growth factor (EGF) and 10 ng/ml basic fibroblast
growth factor (bFGF) for neurosphere (NS) assay and 6 cell lines in
DMEM supplemented with 10% fetal bovine serum (FBS) for adherent
cell (AC) growth (Table I). Both
cultures were maintained at 37°C in 5% O2 and 5%
CO2. All cell lines were characterized for MGMT gene
promoter and p53 gene status (24,25)
(Table I). Experiments with
primary GBM lines were carried out using cells from passages 10–20
and cultures were checked for Mycoplasma contamination
before use (e-Myco™ Mycoplasma PCR Detection kit, iNtRON
Biotechnology, Korea). Formalin-fixed paraffin-embedded (FFPE)
brain tumor samples were collected from 8 GBMs, one pilocytic
astrocytoma and one oligodendroglioma. The histological diagnosis
was performed according to World Health Organization (WHO)
guidelines (26). The study was in
compliance with the local institutional review board and Committee
on Human Research and with the ethical human-subject standards of
the World Medical Association Declaration of Helsinki Research.
Written informed consent was obtained from all patients.
 | Table ICell lines with the IC50
values for each drug and the MGMT and p53 gene status. |
Table I
Cell lines with the IC50
values for each drug and the MGMT and p53 gene status.
| Cell line | IC50
(μM)a for TMZ | IC50
(μM)a for Dox | IC50
(μM)a for PTX | Methylation status
of MGMT promoter | Status of p53
gene |
|---|
| U87-MG NS | 170 | 0.92 | - | Methylated | Wild-type |
| 010627 NS | 135 | - | - | Methylated | Mutated |
| CV10 NS | 8.5 | - | - | Methylated | Wild-type |
| CV21 NS | 44 | 0.68 | 0.03 | Methylated | Wild-type |
| NO3 NS | 120 | 0.85 | 0.044 | Unmethylated | Wild-type |
| NO4 NS | 100 | 0.98 | 0.1 | Methylated | Mutated |
| NO6 NS | 170 | - | 0.0094 | Unmethylated | Wild-type |
| CTO3 NS | 97 | 1.85 | 0.066 | Methylated | Mutated |
| CTO5 NS | 130 | 1.18 | 0.08 | Unmethylated | Wild-type |
| CTO15 NS | 130 | 0.65 | 0.035 | Unmethylated | Mutated |
| U87-MG AC | 42 | 0.72 | - | Methylated | Wild-type |
| 010627 AC | 130 | - | - | Methylated | Mutated |
| CV10 AC | 5 | - | - | Methylated | Wild-type |
| NO3 AC | 72 | 0.78 | 0.05 | Unmethylated | Wild-type |
| CTO3 AC | 50 | 0.83 | 0.077 | Methylated | Mutated |
| CTO15 AC | 46 | 0.67 | 0.027 | Unmethylated | Mutated |
Drug treatment and cytotoxicity
assay
TMZ, Dox and PTX (all from Sigma-Aldrich Co., St.
Louis, MO, USA) were dissolved in 100% dimethylsulfoxide (DMSO) for
stock solutions. The final concentration of DMSO never exceeded
0.3% (v/v). All cell lines (10 NS and 6 AC) were treated with TMZ
at increasing doses (5, 50, 100, 200 and 500 μM) and times [6, 24,
48, 72 and 120 hours (h)]; eleven cell lines (7 NS and 4 AC) were
treated with 1, 2 and 5 μM Dox at 2, 24, 48 and 72 h; ten cell
lines (7 NS and 3 AC) were treated with 10, 100, 1,000 and 5,000 nM
PTX at 24, 48, 72 and 120 h. The drugs were added to dissociated NS
cultures. After exposure, the cytotoxicity of the drugs was
evaluated assessing the number of viable cells by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay (Roche, Diagnostic Corp., Indianapolis, IN, USA), measuring
optical density at 570 nm (test wavelength) and 660 nm (reference
wavelength) by a microplate spectrophotometer (Synergy HT, BioTek
Instruments Inc., Winooski, VT, USA). For NS, cell counts were also
performed by trypan blue using a TC20 automated cell counter
(Bio-Rad, Berkeley, CA, USA). Cytotoxicity was expressed as number
of surviving cells as percentage of control (untreated cells). The
concentration of the drugs which caused a 50% inhibition of the
cell growth, defined IC50, was calculated for each cell
line at 72 h by non-linear regression from the survival curves of
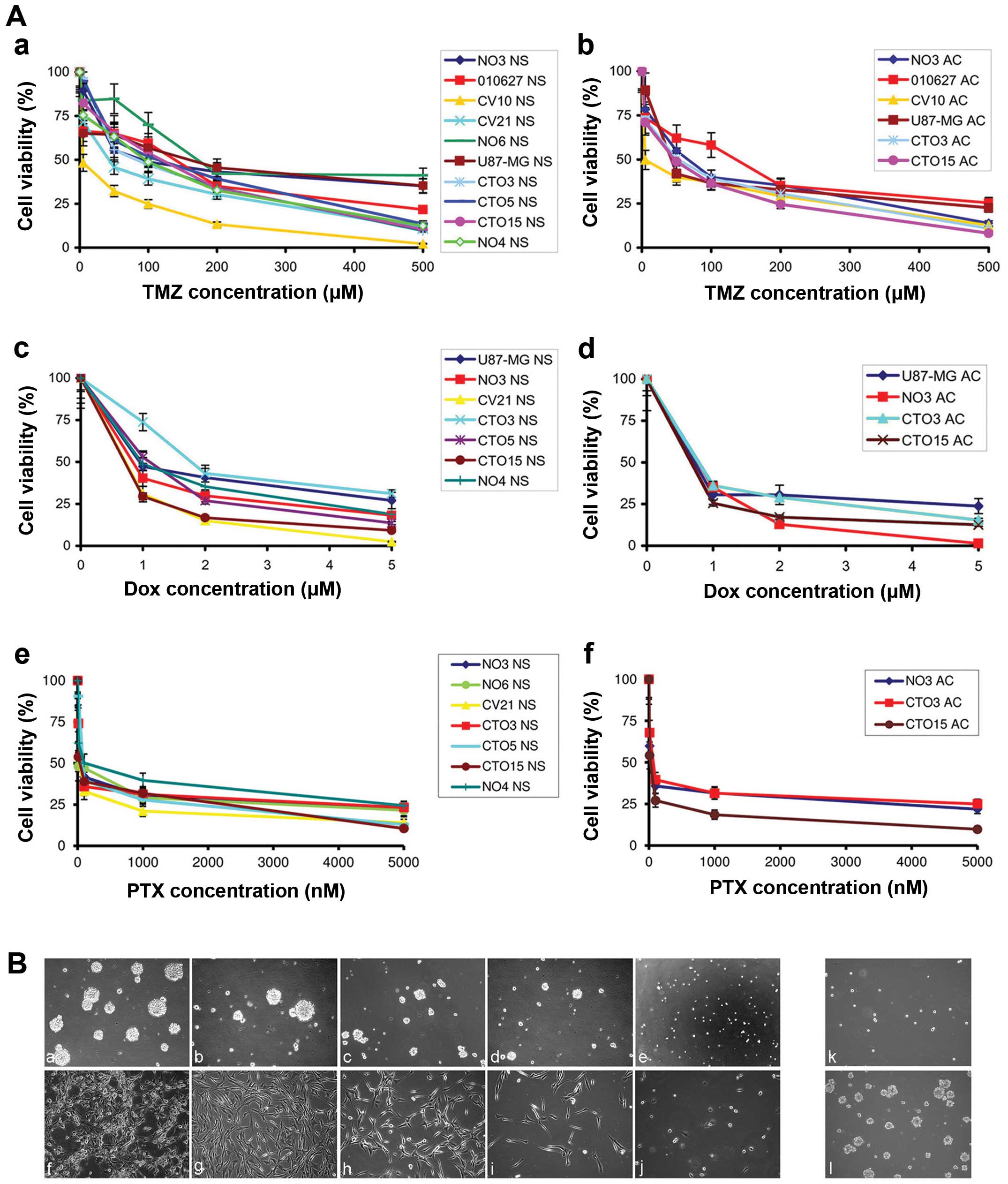
Fig. 2A.
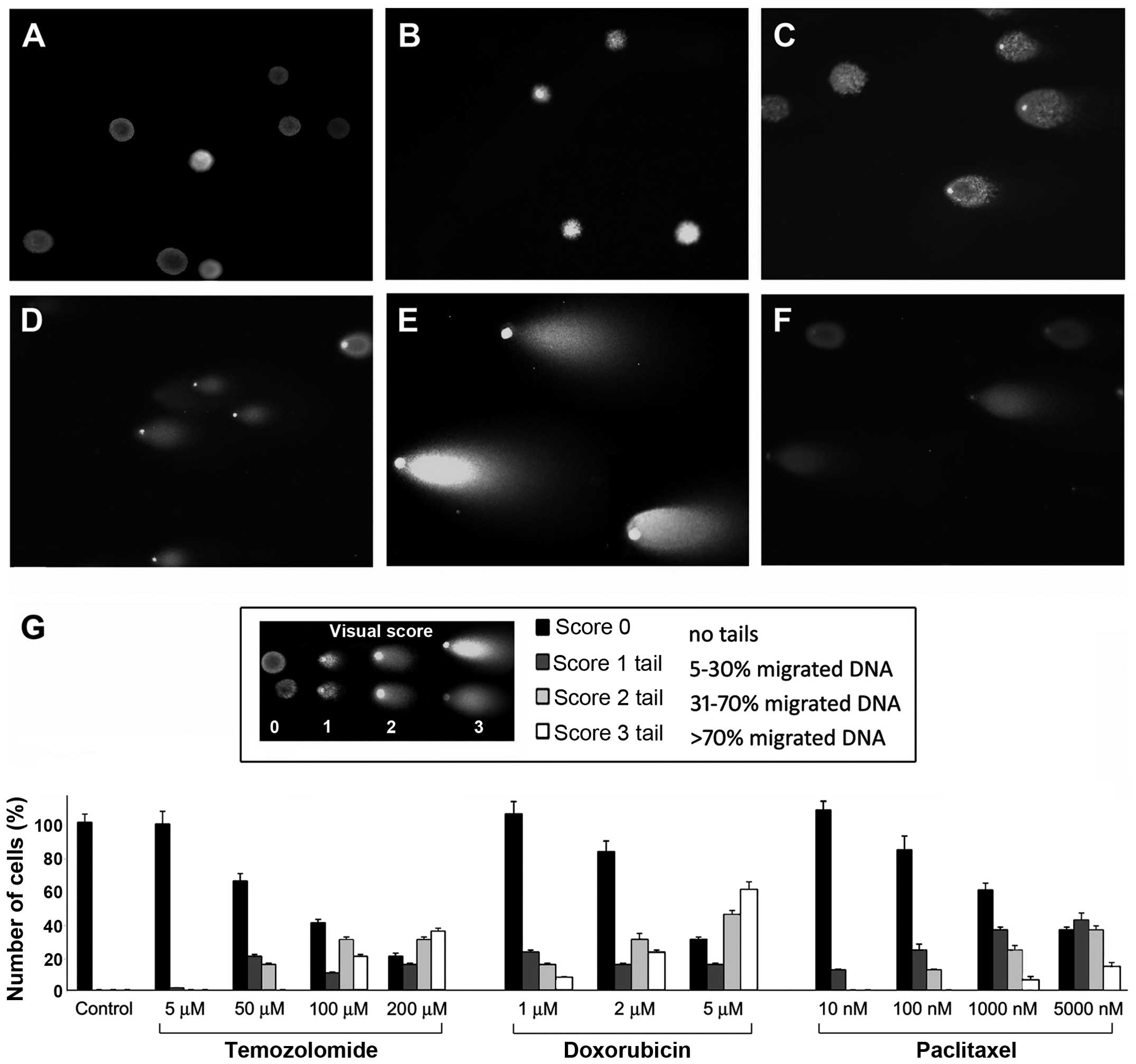
Comet assay
Cells were treated with different drug doses at
various time-points and the occurrence of DNA damage induced by the
drugs was investigated using the Comet (or single cell gel
electrophoresis, SCGE) assay kit (Trevigen Inc., Gaithersburg, MD,
USA) at neutral pH. Nuclei were labeled with SYBR Green I dye.
Observations were made on a Zeiss Axioskop fluorescence microscope
(Carl Zeiss, Oberkochen, Germany) equipped with an AxioCam5 MR5c
and coupled to an Imaging system (AxioVision Release 4.5; Carl
Zeiss). Cleaved DNA fragments caused by DSBs are detected as a tail
(comet), the length of which is a measure of the DNA damage degree
(27). Cells with comet tails were
quantified as percentage over the total cell number and a visual
score was assigned to the comets (28): score 0 representing undamaged cells
(comets with no or barely detectable tails); score 1, 5–30% of
migrated DNA; score 2, 31–70% of migrated DNA; score 3, >70% of
migrated DNA (panel in Fig.
3G).
Determination of apoptosis
Apoptosis was evaluated on cells after treatment
with TMZ and PTX and on tissue sections by in situ terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling
(TUNEL) assay, using the in situ cell death detection kit,
fluorescein (Roche) according to the manufacturer’s protocols.
Apoptotic indexes were calculated scoring 4 randomly selected
fields and counting number of apoptotic cells over the total of
viable cells which represented a quota in comparison with untreated
cells.
Immunofluorescence (IF)
IF was performed on NS and AC following TMZ and PTX
treatments to monitor the activation of the damage/repair molecules
p-ATM, p-Chk2, p-53BP1, γ-H2AX histone, HR effector RAD51, NHEJ
effectors Ku70/Ku80 and DNA-PKcs. Cells were fixed for 20 min with
4% paraformaldehyde at room temperature, rinsed three times with
PBS, blocked/permeabilized for 30 min with PBS containing 2% of the
appropriate serum and 0.1% Triton X-100 and finally stained with
the following primary antibodies: monoclonal mouse anti-human
γ-H2AX (Ser139) (05-636; Millipore, Bedford, MA, USA; dilution
1:200), monoclonal mouse anti-human p-ATM (Ser1981) (05-740;
Millipore; dilution 1:100), polyclonal rabbit anti-human p-Chk2
(Thr68) (BK2197S; Cell Signaling Technology, Beverly, MA, USA;
dilution 1:100), polyclonal rabbit anti-human p-53BP1 (Ser25)
(IHC-00053; Bethyl Laboratories, Inc., Montgomery, TX, USA;
dilution 1:200), monoclonal mouse anti-human RAD51 (MS-988;
NeoMarkers, Fremont, CA, USA; dilution 1:100), monoclonal mouse
anti-human DNA-PKcs (MS-423; NeoMarkers; dilution 1:100) and
monoclonal mouse anti-human Ku70/Ku80 (MS-286; NeoMarkers; dilution
1:200). Negative controls were obtained by omitting the primary
antibody. Alexa Fluor® 488-AffiniPure goat anti-rabbit
IgG and Alexa Fluor® 594-AffiniPure rabbit anti-mouse
IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) were used as secondary antibodies. Cell nuclei were stained
with 4′,6-diamidino-2-phenylindole (DAPI) and examined under a
Zeiss Axioskop fluorescence microscope.
Immunocytochemistry (ICC) and
ımmunohistochemistry (IHC)
Due to the interfering spontaneous fluorescence of
Dox, ICC, instead of IF, was carried out on cells treated with Dox.
IHC was performed on the 8 GBM and 2 low-grade tissues. The
analyses were made using a Ventana Full BenchMark® XT
automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ,
USA) and UltraView™ Universal DAB Detection kit (Ventana Medical
Systems Inc.) as detection system. Heat-induced epitope retrieval
(HIER) was performed in Tris-EDTA, pH 8.0. Primary antibodies were
the same used for IF with the same dilutions. Negative controls
were obtained by omitting the primary antibody.
Western blotting (WB)
WB was performed as previously described (24) using the following primary
antibodies: monoclonal mouse anti-human γ-H2AX (Ser139) (05-636;
Millipore; dilution 1:1,000), monoclonal mouse anti-human p-ATM
(Ser1981) (05-740; Millipore; dilution 1:2,000), polyclonal rabbit
anti-human p-Chk2 (Thr68) (BK2197S; Cell Signaling Technology;
dilution 1:1,000), monoclonal mouse anti-human RAD51 (MS-988;
NeoMarkers; dilution 1:500), and monoclonal mouse anti-human
Ku70/Ku80 (MS-286; NeoMarkers; dilution 1:1,000). A polyclonal
rabbit anti-human α-tubulin (LF-PA0146; LabFrontier, Seoul, Korea;
dilution 1:5,000) was used to normalize sample loading and
transfer.
Statistical analysis
The level of significance was determined by a
two-tailed Student’s t-test. All quantitative data presented are
the average value ± standard error (SE) from at least three
independent determinations. Statistical significance was defined as
p<0.05 or p<0.01.
Results
Cytotoxicity studies on GBM cell lines
after TMZ, Dox and PTX treatments
NS lines were self-renewing, clonogenic, multipotent
and expressing undifferentiation antigens; AC displayed a
differentiation antigen profile (24). TMZ, Dox and PTX inhibited cell
proliferation, both in NS and in AC by MTT assay, controlled by
trypan blue method. The cytotoxic effect of the three drugs were
dose- and time-dependent and more evident on AC than on NS
(Fig. 2A-a-f). NS were resistant
to dosages of TMZ <10 μM, with the exception of CV10 and CV21
lines, that presented hypermethylation of MGMT promoter (Table I). Even high TMZ concentrations
resulted in the survival of ≤30% of the cells in some NS lines. On
the contrary, on most AC, TMZ concentrations <50 μM reduced cell
growth to 50% after 72-h exposure. Compared with growth of
untreated NS and AC (Fig. 2B-a and
-f, respectively, from NO3 cell line, taken as a representative
line), cell proliferation in NS (Fig.
2B-b-e) and AC adhesion (Fig.
2B-g-j) was hindered by TMZ in a dose and time-dependent
manner. However, the growth inhibition in NS was not irreversible
and the depletion of cells was not complete, even at high drug
dosages. Even after 5-day treatment with high TMZ dosage (200 μM),
a few single cells survived re-acquiring growth capacity within one
month after treatment suspension (Fig.
2B-k and -l).
Dox uptake by NS and AC was evident in most cells
already after 2 h with 2 μM drug and, after 24-h exposure, the
number of viable cells showed 20% decrease with inhibition of
clonogenicity in NS and of cell adhesion in AC. After 72-h
treatment, 80% cells were dead. Also 100 nM PTX at 48 h inhibited
evidently cell proliferation both in NS and in AC. After 5-day
treatment a few single cells of NS lines survived re-acquiring
growth capacity within one month after treatment suspension.
Evaluation of DNA damage by Comet
assay
By Comet assay no damage was detectable on untreated
NS (Fig. 3A) and AC (data not
shown) and on NS treated with 50 μM TMZ for 24 h (Fig. 3B); a comet tail was evident only
after 72-h exposure with 50 μM drug (Fig. 3C): >10% of cells showed a
score-2-length tail. Comets with score-3 length were visible in
>50% of cells treated with 200 μM TMZ (Fig. 3D). The damage resulted more
elevated in AC and in NS lines with methylated MGMT promoter. DNA
lesions were revealed in NS (Fig.
3E) and even more in AC (data not shown) with 2 μM Dox at 72 h
and the same effect but weaker was observed after 72-h treatment
with 100 nM PTX both in NS (Fig.
3F) and in AC (data not shown) with score-2 length tails.
Quantification of cell damage by Comet assay in untreated and
treated NO3 NS is reported in Fig.
3G.
Determination of cell apoptosis induced
by drug treatment
Apoptosis was a late phenomenon, evident only after
72-h drug exposure, both in NS and AC (Fig. 4A and B). The frequency of apoptotic
cells following treatment with increasing concentrations of TMZ for
6, 24 and 72 h is shown in Fig. 4C and
D for NS and AC, respectively. In treated NS, apoptosis did not
exceed 10–15% and it was ≤30% in AC. PTX-induced apoptosis was
evidenced at 72 h already at concentration of 10 nM. At the highest
drug dosages, the percentage of apoptotic cells was ≤20% in NS
(Fig. 4E) and ≤30% in AC (Fig. 4F).
Studies of checkpoint/repair pathways in
treated cells
The antigens of repair cascade resulted negative in
untreated NS, with the exception of p-ATM, γ-H2AX, Ku70/Ku80,
DNA-PKcs, moderately expressed in some lines (Fig. 5A-a-g). No expression was shown in
untreated AC (Fig. 5B-a-g). The
expression of all the sensors and effectors, except RAD51, was
evident after 48-h exposure to 100 μM TMZ, both in NS and, at a
minor extent, in AC (Fig. 5A and
B-h-n). Expression of p-ATM, γ-H2AX, p-Chk2, Ku70/80 and RAD51
in 4 NS lines treated with TMZ was confirmed by WB analysis
(Fig. 5D). Dox effects were the
same as TMZ but earlier. γ-H2AX, indicator of DSBs, was evident
after 48-h treatment with 50 μM TMZ or 5 μM Dox, both in NS and in
AC. Concurrent administration of the two drugs increased the effect
with γ-H2AX expressed in ~90% of cells and a significant decrease
in cell number (Fig. 5E-a-d for NS and
-e-h for AC). All repair markers became negative after 72 h
with high drug concentrations (500 μM TMZ or 5 μM Dox): cells were
dying or no longer able to activate the repair cascade. After 72-h
treatment with 100 nM PTX, a moderate activation of
checkpoint/repair response with a mild expression of all markers
except RAD51 was found in NS, whereas, interestingly, in treated
AC, several cells appeared arrested in metaphase and with a strong
expression of p-ATM, p-Chk2, p-53BP1, γ-H2AX, DNA-PKcs, Ku70/Ku80
(Fig. 5C-a-g). After 24 h from the
beginning of treatments, the presence of activated damage sensors
(p-ATM, p-53BP1, γ-H2AX, p-Chk2) was more evident, whereas at
longer times (72 h), expression level of effectors (DNA-PKcs,
Ku70/80) increased and that of sensor molecules decreased as repair
proceeded (Fig. 5F).
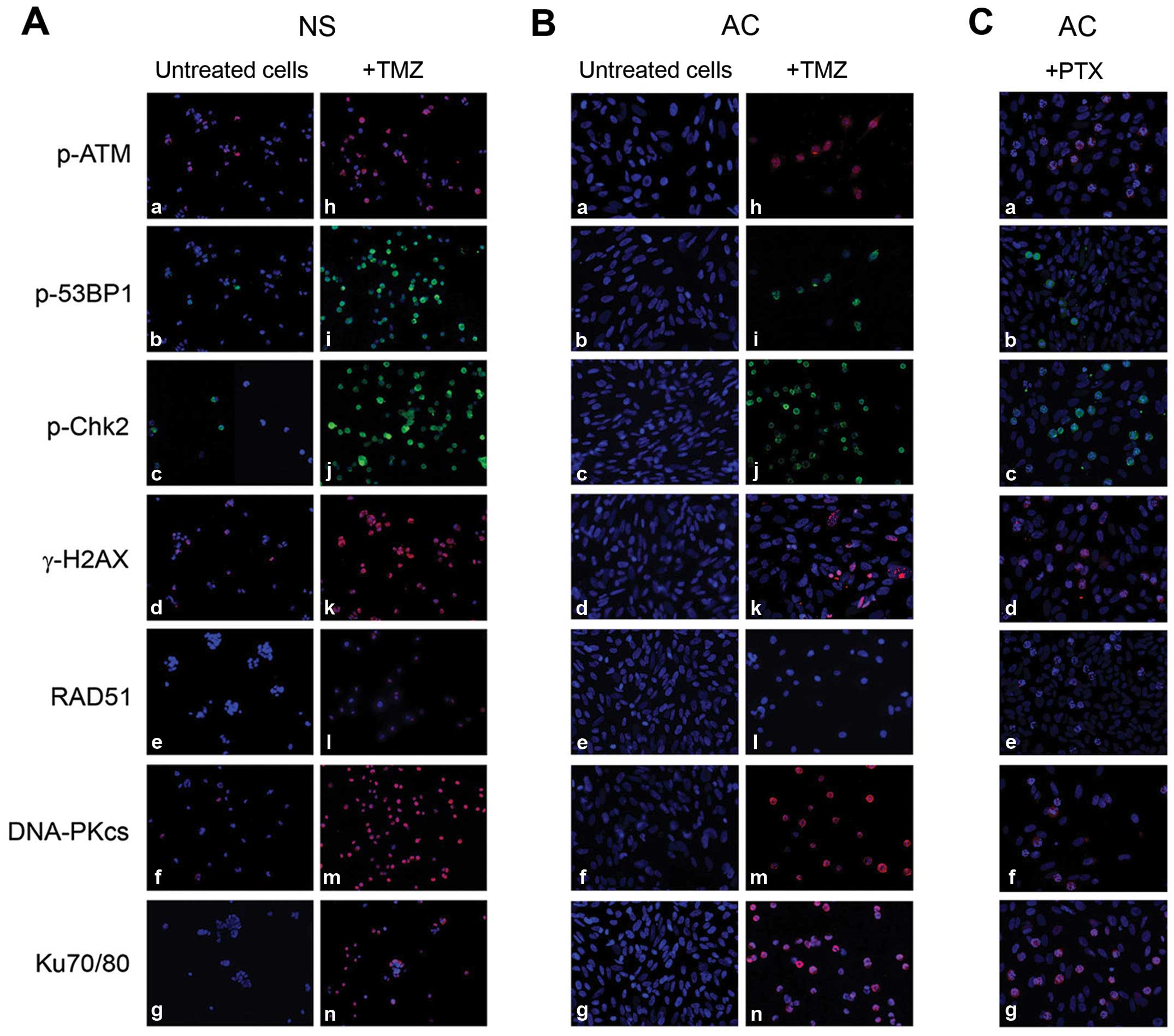 | Figure 5Study of checkpoint/repair response
in NO3 NS and AC. (A) Expression by immunofluorescence of
checkpoint/repair proteins in untreated NO3 NS (a-g) and in cells
treated with 100 μM TMZ for 48 h (h-n). (B) The same as above in
untreated (a-g) and treated NO3 AC (h-n). (C) The same as above in
NO3 AC treated with 100 nM PTX for 72 h (a-g): positivity of the
antigens is evident at metaphasis level. Nuclei counterstained with
DAPI. All ×200 magnification. (D) Expression by western blotting of
p-ATM, p-Chk2, γ-H2AX and Ku70/Ku80 in 4 NS lines (CV10, 010627,
CV21 and NO3), untreated and treated with 100 μM TMZ for 48 h. No
band for RAD51 was detectable in any lines, not even after TMZ
treatment. (E) Expression by immunocytochemistry of γ-H2AX, as
indicator of DNA damage, in untreated NO3 NS (a, ×400) and AC (e,
×400) and after 50 μM TMZ for 48 h [(b) and (f) for NS and AC,
respectively, ×400], after 5 μM Dox for 48 h [(c) and (g) for NS
and AC, respectively, ×400] and after combined treatment with 50 μM
TMZ and 5 μM Dox for 48 h [(d) and (h) for NS and AC, ×400 and
×630, respectively]. (F) Levels of checkpoint/repair proteins at 24
(a) and 72 h (b) in NO3 NS, untreated (control) and treated with
100 μM TMZ, 2 μM Dox or 100 nM PTX. |
Studies of checkpoint/repair and
apoptosis in glioma tissues
p-ATM, γ-H2AX and the key proteins of NHEJ system,
DNA-PKcs and Ku70/Ku80, were constitutively expressed in the GBM
specimens studied, particularly in proliferation areas and in
perinecrotic pseudopalisades (Fig.
6A-a, -b, -e and -f). p-ATM and γ-H2AX were sometimes positive
in macrophages and reactive astrocytes. p-Chk2 and RAD51 expression
was rather scarce and heterogeneous (Fig. 6A-c and -d) and p-53BP1 was not
detectable. The two low-grade glioma tissues analyzed were almost
negative for the markers, with the exception of p-ATM and γ-H2AX,
poorly positive at level of mitoses (Fig. 6B). Apoptosis occurred in the
palisades of circumscribed necroses and was scattered in the
proliferating areas of GBM (29)
(Fig. 6C).
 | Figure 6(A) Immunohistochemical expression in
GBM tissues of p-ATM, DAB, ×200 (a); γ-H2AX, DAB, ×200 (b); p-Chk2,
DAB, ×400 (c); RAD51, positive in scattered cells, DAB, ×400 (d);
DNA-PKcs, DAB, ×200 (e); Ku70/Ku80, DAB, ×200 (f). (B)
Immunohistochemical expression of p-ATM in a pilocytic astrocytoma,
DAB, ×400 (a); γ-H2AX in an oligodendroglioma, DAB, ×200 (b);
p-Chk2 in an oligodendroglioma, DAB, ×400 (c); RAD51 in an
oligodendroglioma, DAB, ×400 (d); DNA-PKcs in an oligodendroglioma,
DAB, ×200 (e); Ku70/Ku80 in an oligodendroglioma, DAB, ×200 (f).
(C) Evaluation of apoptosis in GBM tissue sections. Scattered
apoptotic nuclei in palisades of circumscribed necroses (a) and in
proliferating areas (b) of a GBM sample, TUNEL labeling, ×400. |
Discussion
In this study we demonstrated that three commonly
used anticancer drugs, TMZ, Dox and PTX, were able, under in
vitro conditions, to reduce significantly the number of viable
cells in GBM NS lines and even more in AC lines, inhibiting
clonogenic growth in the former and hindering cell adhesion in the
latter. The finding, as for TMZ, is in agreement with recent
reports (30,31). Dox and PTX caused a more evident
reduction of cell viability in comparison with TMZ, both in NS and
in AC. IC50 values for TMZ and Dox at 72 h were, on
average, higher for NS than for AC, proving that the former are
more resistant than the latter, characterized by a more
differentiated state. No significant differences in IC50
values for PTX was observed between NS and AC.
Cell lines with a hypermethylated MGMT promoter were
mostly more sensitive to TMZ, but the data were in some cases
discordant. MGMT expression indeed is not the only factor to be
considered in evaluating TMZ response: p53 wild-type status is
reported as fundamental to determine cell cycle arrest and the
entry in the apoptotic process (32,33).
In our data, among the cell lines with hypermethylated MGMT those
with mutated p53 gene appeared more resistant to the action of TMZ
than the ones with wild-type p53.
In NS cultures treated with TMZ or PTX, the
depletion of cells was never complete even at high drug dosages;
cells could resist the drugs in a non-proliferating state, as
already observed for TMZ (30,31)
and resumed proliferation within 1–2 months after treatment
suspension. The maintenance of a quiescent state and metabolic
inertia may, therefore, represent a mechanism of genome protection
in GSCs; the consequence is that antineoplastic treatments can have
a cytostatic rather than a cytotoxic effect. Dox effect was more
lethal and irreversible.
Apoptosis following treatments was a late phenomenon
demonstrable only after 72 h, as already reported (18,34).
In our observations, it was more evident in AC than in NS, but in
both cases the frequency of apoptosis does not explain the level of
reduction of viable cells, in agreement with the data of Beier
et al (35).
By Comet assay we found that TMZ, Dox and, to lesser
extent, PTX were able to produce in glioma cells a DNA damage, that
increased proportionally with drug doses and times and was higher
in AC than in NS lines, with the consequent activation of the
checkpoint/repair pathways. Moreover, we noted in some untreated NS
lines a basal expression of some checkpoint/repair proteins, which
was significantly elicited following drug exposure. After DNA
injury also AC were able to trigger a repair response, although to
a minor extent. Sensors of damage were the first proteins to be
activated and they decreased with time, as the effectors increased
and repair of lesions took place. From our data, NHEJ repair system
activity seemed higher than the one of HR. It was reported that
high tumor levels of DNA-PK correlate with poor survival in GBM
patients treated with postsurgical radiation (36).
In GBM tissue specimens, we found a constitutive
expression of checkpoint/repair proteins, not present in the
low-grade tumors. At the beginning of tumorigenesis, DDR machinery
acts as a protective mechanism against glioma progression, limiting
the expansion of malignant clones with unstable genome (37). In glioma pathogenesis an aberrant
constitutive activation of repair mechanisms was reported in
response to DNA replication stress produced by oncogenes (38).
In conclusion, cell fate after treatments depends on
the preferential activation of repair or apoptotic pathways. The
intrinsic resistance to genotoxic therapies of malignant glioma
cells could therefore be explained on one hand, by their ability to
stop growth and survive in a quiescent state and, on the other
hand, by the involvement of an enhanced DNA damage signaling.
Moreover, we demonstrated that also Dox and PTX would be effective
cytotoxic/cytostatic agents, similarly to TMZ, on glioma cells and,
once the way to cross the BBB is found (39,40),
they could be potentially useful in the GBM treatment. Targeted
inhibition of the DNA repair factors could, therefore, be useful to
sensitize malignant gliomas to genotoxic treatments and to improve
therapeutic strategies.
Acknowledgements
This study was supported by Grant ID ORTO11WNST and
by Grant no. 4011 SD/cv 2011-0438, both from Compagnia di San
Paolo, Turin, Italy.
References
|
1
|
Caldera V, Mellai M, Annovazzi L,
Monzeglio O, Piazzi A and Schiffer D: MGMT hypermethylation and MDR
system in glioblastoma cancer stem cells. Cancer Genomics
Proteomics. 9:171–178. 2012.PubMed/NCBI
|
|
2
|
Salmaggi A, Boiardi A, Gelati M, Russo A,
Calatozzolo C, Ciusani E, Sciacca FL, Ottolina A, Parati EA, La
Porta C, Alessandri G, Marras C, Croci D and De Rossi M:
Glioblastoma-derived tumorospheres identify a population of tumor
stem-like cells with angiogenic potential and enhanced multidrug
resistance phenotype. Glia. 54:850–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johannessen TC and Bjerkvig R: Molecular
mechanisms of temozolomide resistance in glioblastoma multiforme.
Expert Rev Anticancer Ther. 12:635–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexander BM, Pinnell N, Wen PY and
D’Andrea A: Targeting DNA repair and the cell cycle in
glioblastoma. J Neurooncol. 107:463–477. 2012. View Article : Google Scholar
|
|
5
|
Schmalz PG, Shen MJ and Park JK: Treatment
resistance mechanisms of malignant glioma tumor stem cells. Cancers
(Basel). 3:621–635. 2011. View Article : Google Scholar
|
|
6
|
Frosina G: The bright and the dark sides
of DNA repair in stem cells. J Biomed Biotechnol. 2010:8453962010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frosina G: DNA repair and resistance of
gliomas to chemotherapy and radiotherapy. Mol Cancer Res.
7:989–999. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolderson E, Richard DJ, Zhou BB and
Khanna KK: Recent advances in cancer therapy targeting proteins
involved in DNA double-strand break repair. Clin Cancer Res.
15:6314–6320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pardo B, Gómez-González B and Aguilera A:
DNA repair in mammalian cells: DNA double-strand break repair: how
to fix a broken relationship. Cell Mol Life Sci. 66:1039–1056.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johannessen TC, Bjerkvig R and Tysnes BB:
DNA repair and cancer stem-like cells--potential partners in glioma
drug resistance? Cancer Treat Rev. 34:558–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar
|
|
15
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirose Y, Berger MS and Pieper RO: p53
effects both the duration of G2/M arrest and the fate of
temozolomide-treated human glioblastoma cells. Cancer Res.
61:1957–1963. 2001.PubMed/NCBI
|
|
17
|
Günther W, Pawlak E, Damasceno R, Arnold H
and Terzis AJ: Temozolomide induces apoptosis and senescence in
glioma cells cultured as multicellular spheroids. Br J Cancer.
88:463–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roos WP, Batista LF, Naumann SC, Wick W,
Weller M, Menck CF and Kaina B: Apoptosis in malignant glioma cells
triggered by the temozolomide-induced DNA lesion O6-methylguanine.
Oncogene. 26:186–197. 2007. View Article : Google Scholar
|
|
19
|
Kurz EU, Douglas P and Lees-Miller SP:
Doxorubicin activates ATM-dependent phosphorylation of multiple
downstream targets in part through the generation of reactive
oxygen species. J Biol Chem. 279:53272–53281. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Branham MT, Nadin SB, Vargas-Roig LM and
Ciocca DR: DNA damage induced by paclitaxel and DNA repair
capability of peripheral blood lymphocytes as evaluated by the
alkaline comet assay. Mutat Res. 560:11–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stan AC, Casares S, Radu D, Walter GF and
Brumeanu TD: Doxorubicin-induced cell death in highly invasive
human gliomas. Anticancer Res. 19(2A): 941–950. 1999.PubMed/NCBI
|
|
22
|
Lesniak MS, Upadhyay U, Goodwin R, Tyler B
and Brem H: Local delivery of doxorubicin for the treatment of
malignant brain tumors in rats. Anticancer Res. 25(6B): 3825–3831.
2005.Erratum in: Anticancer Res 26: 445, 2006. PubMed/NCBI
|
|
23
|
Chang SM, Kuhn JG, Robins HI, Schold SC
Jr, Spence AM, Berger MS, Mehta M, Pollack IF, Rankin C and Prados
MD: A Phase II study of paclitaxel in patients with recurrent
malignant glioma using different doses depending upon the
concomitant use of anticonvulsants: A North American Brain Tumor
Consortium report. Cancer. 91:417–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caldera V, Mellai M, Annovazzi L, Piazzi
A, Lanotte M, Cassoni P and Schiffer D: Antigenic and genotypic
similarity between primary glioblastomas and their derived
neurospheres. J Oncol. 2011:3149622011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mellai M, Monzeglio O, Piazzi A, Caldera
V, Annovazzi L, Cassoni P, Valente G, Cordera S, Mocellini C and
Schiffer D: MGMT promoter hypermethylation and its associations
with genetic alterations in a series of 350 brain tumors. J
Neurooncol. 107:617–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Louis DN, Ohgaki H, Wiestler OD and
Cavanee WK: WHO Classification of Tumors of the Central Nervous
Systems. 4th edition. International Agency for Research on Cancer
(IARC); Lyon: 2007
|
|
27
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Collins AR, Oscoz AA, Brunborg G, Gaivão
I, Giovannelli L, Kruszewski M, Smith CC and Stetina R: The comet
assay: Topical issues. Mutagenesis. 23:143–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mellai M and Schiffer D: Apoptosis in
brain tumors: Prognostic and therapeutic considerations. Anticancer
Res. 27(1A): 437–448. 2007.PubMed/NCBI
|
|
30
|
Beier D, Röhrl S, Pillai DR, Schwarz S,
Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U,
Trampe-Kieslich A, et al: Temozolomide preferentially depletes
cancer stem cells in glioblastoma. Cancer Res. 68:5706–5715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mihaliak AM, Gilbert CA, Li L, Daou MC,
Moser RP, Reeves A, Cochran BH and Ross AH: Clinically relevant
doses of chemotherapy agents reversibly block formation of
glioblastoma neurospheres. Cancer Lett. 296:168–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hermisson M, Klumpp A, Wick W, Wischhusen
J, Nagel G, Roos W, Kaina B and Weller M: O6-methylguanine DNA
methyltransferase and p53 status predict temozolomide sensitivity
in human malignant glioma cells. J Neurochem. 96:766–776. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bocangel DB, Finkelstein S, Schold SC,
Bhakat KK, Mitra S and Kokkinakis DM: Multifaceted resistance of
gliomas to temozolomide. Clin Cancer Res. 8:2725–2734.
2002.PubMed/NCBI
|
|
34
|
Knizhnik AV, Roos WP, Nikolova T, Quiros
S, Tomaszowski KH, Christmann M and Kaina B: Survival and death
strategies in glioma cells: Autophagy, senescence and apoptosis
triggered by a single type of temozolomide-induced DNA damage. PLoS
One. 8:e556652013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beier D, Schriefer B, Brawanski K, Hau P,
Weis J, Schulz JB and Beier CP: Efficacy of clinically relevant
temozolomide dosing schemes in glioblastoma cancer stem cell lines.
J Neurooncol. 109:45–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kase M, Vardja M, Lipping A, Asser T and
Jaal J: Impact of PARP-1 and DNA-PK expression on survival in
patients with glioblastoma multiforme. Radiother Oncol.
101:127–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartek J, Bartkova J and Lukas J: DNA
damage signalling guards against activated oncogenes and tumour
progression. Oncogene. 26:7773–7779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bartkova J, Hamerlik P, Stockhausen MT,
Ehrmann J, Hlobilkova A, Laursen H, Kalita O, Kolar Z, Poulsen HS,
Broholm H, et al: Replication stress and oxidative damage
contribute to aberrant constitutive activation of DNA damage
signalling in human gliomas. Oncogene. 29:5095–5102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Battaglia L, Gallarate M, Peira E, Chirio
D, Muntoni E, Biasibetti E, Capucchio MT, Valazza A, Panciani PP,
Lanotte M, et al: Solid lipid nanoparticles for potential
doxorubicin delivery in glioblastoma treatment: Preliminary in
vitro studies. J Pharm Sci. 103:2157–2165. 2014. View Article : Google Scholar
|
|
40
|
Chirio D, Gallarate M, Peira E, Battaglia
L, Muntoni E, Riganti C, Biasibetti E, Capucchio MT, Valazza A,
Panciani P, et al: Positive-charged solid lipid nanoparticles as
paclitaxel drug delivery system in glioblastoma treatment. Eur J
Pharm Biopharm. 88:746–758. 2014. View Article : Google Scholar : PubMed/NCBI
|