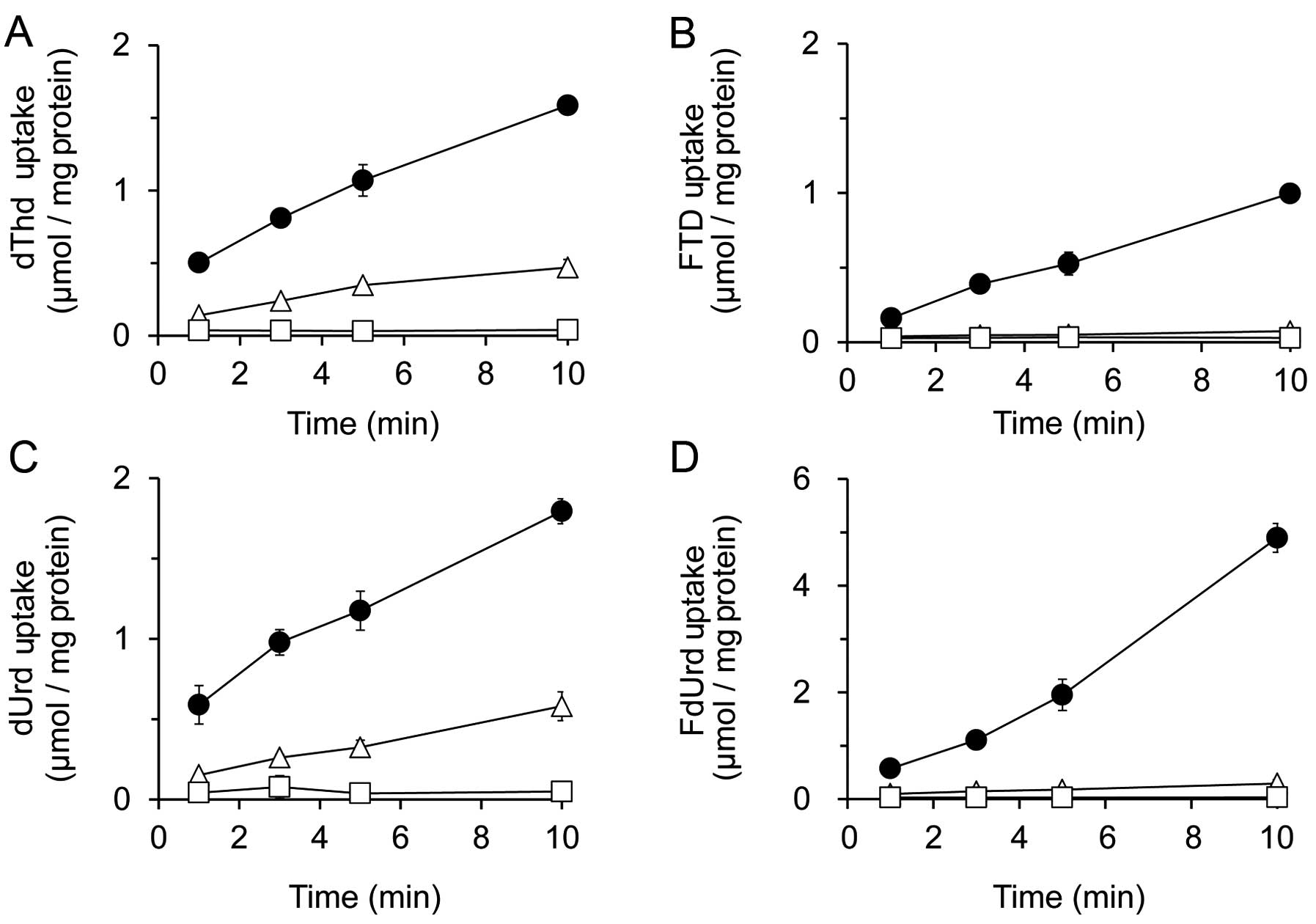
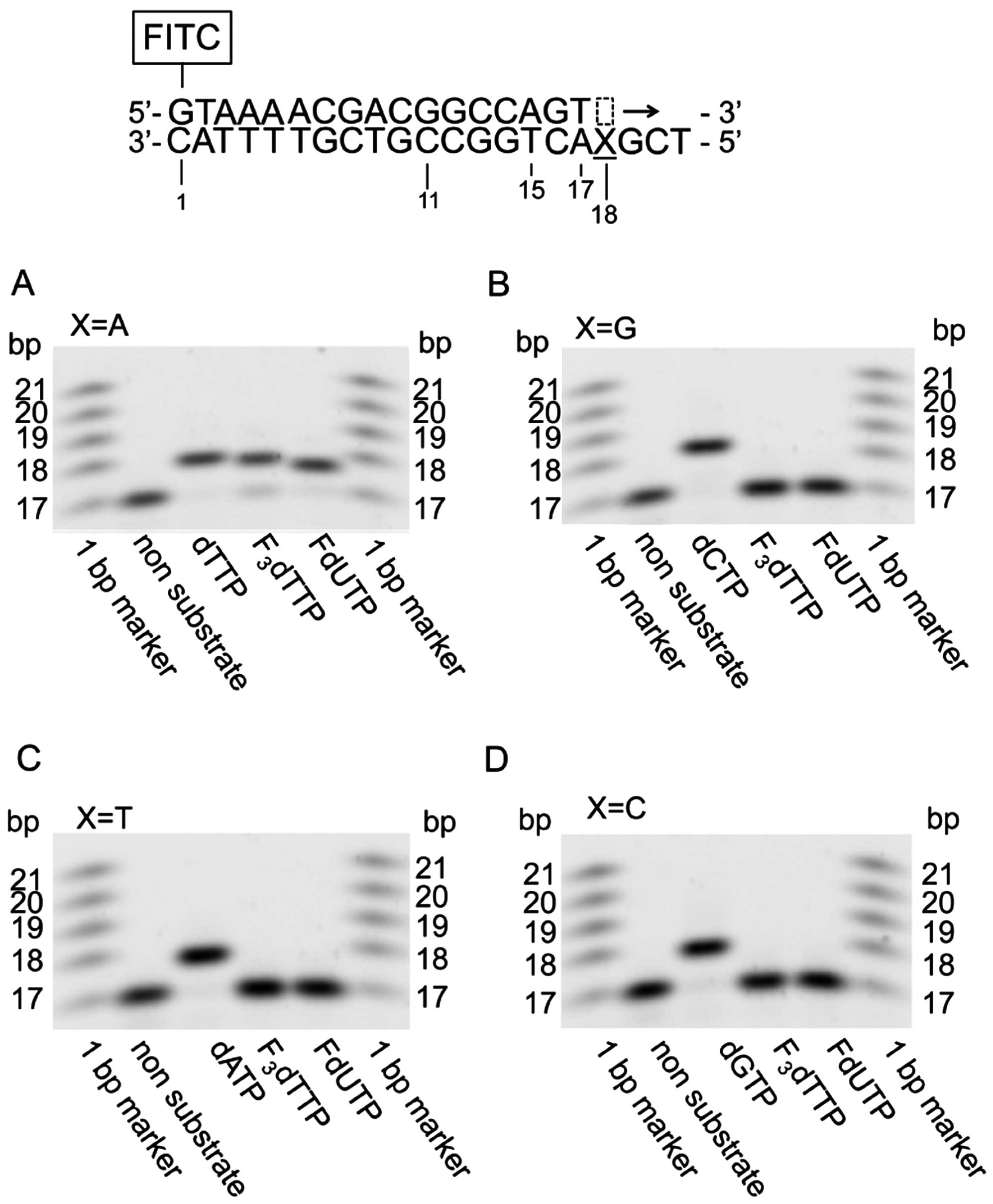
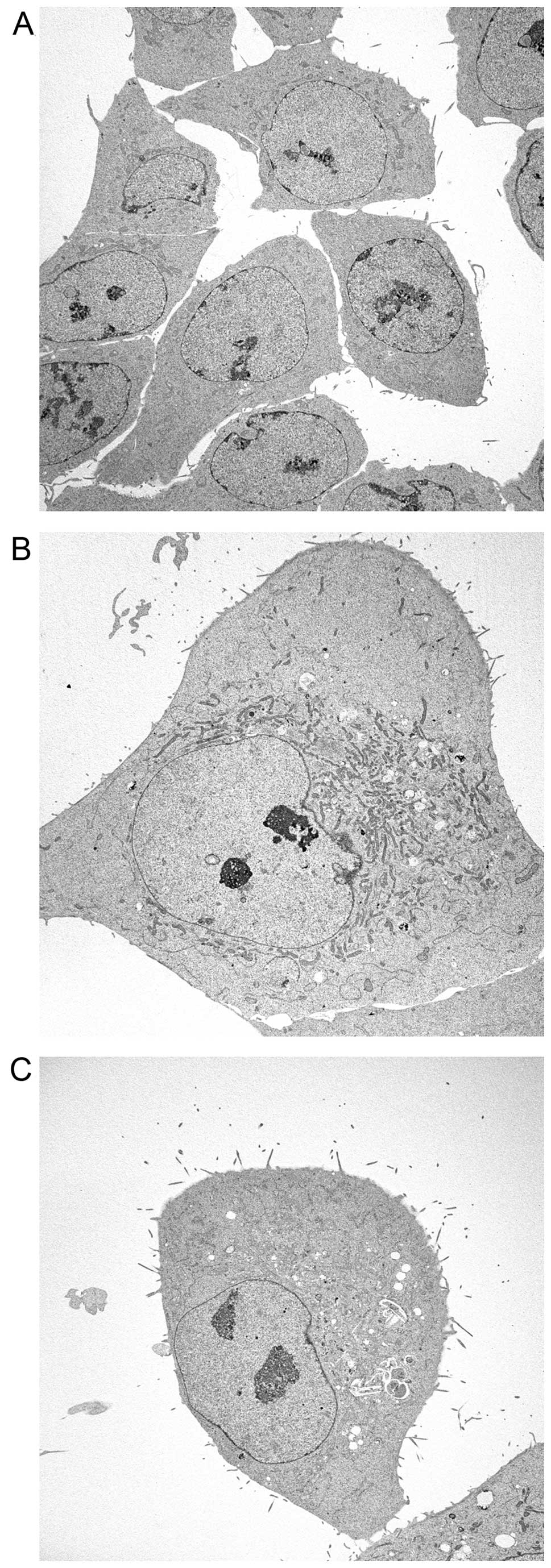
|
1
|
Hu J, Locasale JW, Bielas JH, O’Sullivan
J, Sheahan K, Cantley LC, Vander Heiden MG and Vitkup D:
Heterogeneity of tumor-induced gene expression changes in the human
metabolic network. Nat Biotechnol. 31:522–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber G: Biochemical strategy of cancer
cells and the design of chemotherapy: G. H. A. Clowes Memorial
Lecture. Cancer Res. 43:3466–3492. 1983.PubMed/NCBI
|
|
3
|
Heidelberger C, Chaudhuri NK, Danneberg P,
Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E and
Scheiner J: Fluorinated pyrimidines, a new class of
tumour-inhibitory compounds. Nature. 179:663–666. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muggia FM, Peters GJ and Landolph JR Jr:
XIII International Charles Heidelberger Symposium and 50 Years of
Fluoropyrimidines in Cancer Therapy; September 6 to 8, 2007; New
York University Cancer Institute, Smilow Conference Center. Mol
Cancer Ther. 8. pp. 992–999. 2009
|
|
5
|
Johnston PG, Benson AB III, Catalano P,
Rao MS, O’Dwyer PJ and Allegra CJ: Thymidylate synthase protein
expression in primary colorectal cancer: Lack of correlation with
outcome and response to fluorouracil in metastatic disease sites. J
Clin Oncol. 21:815–819. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leichman CG: Predictive and prognostic
markers in gastrointestinal cancers. Curr Opin Oncol. 13:291–299.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shintani M, Urano M, Takakuwa Y, Kuroda M
and Kamoshida S: Immunohistochemical characterization of pyrimidine
synthetic enzymes, thymidine kinase-1 and thymidylate synthase, in
various types of cancer. Oncol Rep. 23:1345–1350. 2010.PubMed/NCBI
|
|
8
|
Sherley JL and Kelly TJ: Regulation of
human thymidine kinase during the cell cycle. J Biol Chem.
263:8350–8358. 1988.PubMed/NCBI
|
|
9
|
Arnér ES and Eriksson S: Mammalian
deoxyribonucleoside kinases. Pharmacol Ther. 67:155–186. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heidelberger C, Parsons DG and Remy DC:
Syntheses of 5-trifluoromethyluracil and
5-trifluoromethyl-2′-Deoxyuridine. J Med Chem. 7:1–5. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ansfield FJ and Ramirez G: Phase I and II
studies of 2′-deoxy-5-(trifluoromethyl)-uridine (NSC-75520). Cancer
Chemother Rep. 55:205–208. 1971.PubMed/NCBI
|
|
12
|
Skevaki CL, Galani IE, Pararas MV,
Giannopoulou KP and Tsakris A: Treatment of viral conjunctivitis
with antiviral drugs. Drugs. 71:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Temmink OH, Emura T, de Bruin M, Fukushima
M and Peters GJ: Therapeutic potential of the dual-targeted TAS-102
formulation in the treatment of gastrointestinal malignancies.
Cancer Sci. 98:779–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Emura T, Nakagawa F, Fujioka A, Ohshimo H,
Yokogawa T, Okabe H and Kitazato K: An optimal dosing schedule for
a novel combination antimetabolite, TAS-102, based on its
intracellular metabolism and its incorporation into DNA. Int J Mol
Med. 13:249–255. 2004.PubMed/NCBI
|
|
17
|
Tanaka N, Sakamoto K, Okabe H, Fujioka A,
Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K,
et al: Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI
|
|
18
|
Eckstein JW, Foster PG, Finer-Moore J,
Wataya Y and Santi DV: Mechanism-based inhibition of thymidylate
synthase by 5-(trifluoromethyl)-2′-deoxyuridine 5′-monophosphate.
Biochemistry. 33:15086–15094. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Temmink OH, Comijn EM, Fukushima M and
Peters GJ: Intracellular thymidylate synthase inhibition by
trifluorothymidine in FM3A cells. Nucleosides Nucleotides Nucleic
Acids. 23:1491–1494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reyes P and Heidelberger C: Fluorinated
pyrimidines. XXVI. Mammalian thymidylate synthetase: Its mechanism
of action and inhibition by fluorinated nucleotides. Mol Pharmacol.
1:14–30. 1965.PubMed/NCBI
|
|
21
|
Belt JA and Noel LD: Isolation and
characterization of a mutant of L1210 murine leukemia deficient in
nitrobenzylthioinosine-insensitive nucleoside transport. J Biol
Chem. 263:13819–13822. 1988.PubMed/NCBI
|
|
22
|
Heidelberger C: Fluorinated pyrimidines.
Prog Nucleic Acid Res Mol Biol. 4:1–50. 1965.PubMed/NCBI
|
|
23
|
Temmink OH, Bijnsdorp IV, Prins HJ,
Losekoot N, Adema AD, Smid K, Honeywell RJ, Ylstra B, Eijk PP,
Fukushima M, et al: Trifluorothymidine resistance is associated
with decreased thymidine kinase and equilibrative nucleoside
transporter expression or increased secretory phospholipase A2. Mol
Cancer Ther. 9:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ladner RD, Lynch FJ, Groshen S, Xiong YP,
Sherrod A, Caradonna SJ, Stoehlmacher J and Lenz HJ: dUTP
nucleotido-hydrolase isoform expression in normal and neoplastic
tissues: Association with survival and response to 5-fluorouracil
in colorectal cancer. Cancer Res. 60:3493–3503. 2000.PubMed/NCBI
|
|
25
|
Temmink OH, de Bruin M, Comijn EM,
Fukushima M and Peters GJ: Determinants of trifluorothymidine
sensitivity and metabolism in colon and lung cancer cells.
Anticancer Drugs. 16:285–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi D, Nozawa T, Imai K, Nezu J,
Tsuji A and Tamai I: Involvement of human organic anion
transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport
across intestinal apical membrane. J Pharmacol Exp Ther.
306:703–708. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams MV and Cheng Y: Human
deoxyuridine triphosphate nucleotidohydrolase. Purification and
characterization of the deoxyuridine triphosphate
nucleotidohydrolase from acute lymphocytic leukemia. J Biol Chem.
254:2897–2901. 1979.PubMed/NCBI
|
|
28
|
Ward JL, Sherali A, Mo ZP and Tse CM:
Kinetic and pharmacological properties of cloned human
equilibrative nucleoside transporters, ENT1 and ENT2, stably
expressed in nucleoside transporter-deficient PK15 cells. Ent2
exhibits a low affinity for guanosine and cytidine but a high
affinity for inosine. J Biol Chem. 275:8375–8381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parsels LA, Parsels JD, Wagner LM, Loney
TL, Radany EH and Maybaum J: Mechanism and pharmacological
specificity of dUTPase-mediated protection from DNA damage and
cytotoxicity in human tumor cells. Cancer Chemother Pharmacol.
42:357–362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilson PM, LaBonte MJ, Lenz HJ, Mack PC
and Ladner RD: Inhibition of dUTPase induces synthetic lethality
with thymidylate synthase-targeted therapies in non-small cell lung
cancer. Mol Cancer Ther. 11:616–628. 2012. View Article : Google Scholar
|
|
31
|
Nobili S, Napoli C, Landini I, Morganti M,
Cianchi F, Valanzano R, Tonelli F, Cortesini C, Mazzei T and Mini
E: Identification of potential pharmacogenomic markers of clinical
efficacy of 5-fluorouracil in colorectal cancer. Int J Cancer.
128:1935–1945. 2011. View Article : Google Scholar
|
|
32
|
Aufderklamm S, Todenhöfer T, Gakis G,
Kruck S, Hennenlotter J, Stenzl A and Schwentner C: Thymidine
kinase and cancer monitoring. Cancer Lett. 316:6–10. 2012.
View Article : Google Scholar
|
|
33
|
Meyers M, Wagner MW, Hwang HS, Kinsella TJ
and Boothman DA: Role of the hMLH1 DNA mismatch repair protein in
fluoropyrimidine-mediated cell death and cell cycle responses.
Cancer Res. 61:5193–5201. 2001.PubMed/NCBI
|