Introduction
Despite decades of research and robust cancer
preventive strategies, lung cancer remained the most common cancer
since 1985 with a recorded 1.8 million new cases in 2012 (1). It has a poor 5-year survival rate and
is the commonest cause of cancer related mortality worldwide
accounting for 1.6 million deaths annually, which is an estimate of
one-in-five deaths from all cancers (2). In the United Kingdom (UK), 43,463
individuals (13% of all cancer cases) were diagnosed with lung
cancer in 2011 and 35,371 lung cancer deaths (22% of all cancer
deaths) were reported in 2012 (3).
Five percent of adult lung cancer patients (4% of men and 7% of
women) diagnosed in 2010–2011 in England and Wales are predicted to
survive ten or more years (3).
Although the risk factors for lung cancer are
multifactorial, tobacco smoking is still primarily responsible for
the development of the disease (4,5).
Throughout history, socioeconomic status has been identified to
contribute to health disparities. Health behaviors such as smoking,
lack of exercise and poor diet partly explain socioeconomic
disparity in health (6).
Socioeconomic gradient in lung cancer reflects differences in
health behavior and exposure to occupational and environmental
carcinogens, and air pollution among the various socioeconomic
groups (7). The patterns in lung
cancer incidence are influenced by exposure many years before
diagnosis, which is unfortunately higher in socioeconomic deprived
geographical areas (8).
Rising trends in lung cancer incidence have been
observed internationally and also within the UK (9). Conducting periodic trend analysis has
been found to be invaluable because studying historical data
provides insights into the disease pattern, which is expedient for
projection and future resource planning. The aim of this study is
to present an analysis of incidence of lung cancer trends in
England over the period 2002–2011, as well as project rates up to
year 2020. In addition, we compared trends in comparable countries
in the world.
Methods and methods
Lung cancer cases were defined, according to the
tenth revision of the International Classification of Diseases
(ICD-10), by code C33-34. In the UK, it is obligatory for the
National Health Service (NHS) to provide agreed core cancer
registration dataset to regional cancer registries. New cancer
registrations from the National Cancer Registration Service are
submitted to the Office of National Statistics (ONS) for validation
and data processing (for England and Wales), and are later compiled
into the National Cancer Data Repository (10).
Information on new lung cancer registration and
age-specific incidence data for this study were obtained from the
cancer registration series published yearly by ONS. The ONS
database records incidence by age band, region, gender, and ICD
codes. Lung cancer incidences are reported as age-standardised per
100,000 population, allowing for better comparisons between groups
in the study. Mid-year population estimates were used to calculate
annual incidence rates for each calendar year of study. All cases
with ICD C33-34 in England between 2002 and 2011 were included.
We also obtained age-standardised incidence data for
10 other countries from Europe, North America, Australia and Asia
(Japan) from their respective cancer repositories. The selection of
European countries to this study was purely based on population
size and accessibility to validated data. Lung cancer incidence
data for Russia, Italy, Spain, Poland and France were obtained from
EUREG registry data from European Cancer Observatory (ECO) and for
Germany, from the German Centre for Cancer Registry Data (ZfKD).
Incidence data outside Europe were obtained for Australia, from the
Australian Institute of Health and Welfare; for Canada, from Public
Health Agency of Canada (Canada); for Japan, from National Cancer
Centre (Japan); and for USA, from National Cancer Institute
(USA).
Statistical methods
Trends between groups were compared using
age-standardised incidence rates. Cases were categorised into four
age groups (0–59, 60–69, 70–79, and 80+) and nine English regions
including London. Poisson regression models were used to examine
time trends in the overall incidence of lung cancer and by age
category, region of residence and gender in England between 2002
and 2011. The average annual percentage change (AAPC) was used to
estimate the rate of change of incidence during the study period.
AAPC is a useful tool in epidemiology because of its ability to
summarise trend transitions within each grouped data and allow the
use of a single value to describe the trend (11). Overall AAPC by gender was
calculated for England, which was ranked against AAPC from the 10
countries mentioned above, in order to compare lung cancer trend in
England with that seen elsewhere.
Age-standardised rates over the ten-year period were
fitted into a mathematical model and the year variable extended by
nine years to 2020. Model choice was largely based on whether the
overall trend is decreasing or increasing (12) and the best fitting and biologically
plausible models were used for both male and female incidence
projection. Three models for lung cancer incidence were developed
using three separate datasets: incidence (new registrations) data
covering a forty-year period (1972–2011), twenty-year (1992–2011)
age-standardised incidence rate data and, England projected
population structure data (2012–2020). The first model (model 1)
was developed using data from ONS new lung cancer registration
collected over a forty-year period (1971–2011). The second model
(model 2) highlighted the impact of the impending ageing population
structure on future lung cancer incidence, since 9 out of 10 lung
cancer cases are in 60+ age group and 4 in 10 cases in 75+
(13). Model 3 was developed to
demonstrate the prospects of implementing a comprehensive tobacco
control programme similar to the widely lauded California Tobacco
Programme across the country. Such control programmes have the
potential of reducing lung cancer incidence by ~14% over ten-year
period (14). Furthermore,
stratified analyses were conducted for men and women in order to
explore gender differences in lung cancer trends. Analyses were
considered to be statistically significant at p-value <0.05.
Poisson regression was carried out using SAS 9.3 (SAS Institute,
Cary, NC) but all other analyses were performed using Stata 13.1
(Stata Corp, College Station, TX, USA).
Results
A total of 318,417 lung cancer cases were extracted
from ONS during the study period and more males were diagnosed with
lung cancer (57.4%) than females (42.6%). Lung cancer
age-standardised rates were also higher in men than in women in all
age groups and the overall rate ratio between male and female was
3:2, respectively (Table I).
 | Table IDistribution of lung cancer cases by
gender, age group, and region from 2002 to 2011. |
Table I
Distribution of lung cancer cases by
gender, age group, and region from 2002 to 2011.
| Characteristics | Lung cancer cases
(%)
n=318,417 | DASR | AAPC (%) |
|---|
| Gender |
| Male | 182,628 (57.4) | 63.3 | −1.0 |
| Female | 135,789 (42.6) | 41.6 | +1.9 |
| All | 318,417 (100) | 52.5 | +0.5 |
| Male:Female | 1.34:1 (134:100) | 1.5:1 (3:2) | |
| Age group |
| 0–59 | 44,339 (13.9) | 10.6 | −0.5 |
| 60–69 | 81,606 (25.6) | 164.3 | +0.9 |
| 70–79 | 112,493 (35.3) | 322.7 | −0.3 |
| 80+ | 79,979 (25.1) | 384.8 | +2.6 |
| Males |
| 0–59 | 24,296 (13.3) | 11.8 | −1.4 |
| 60–69 | 48,329 (26.5) | 202.2 | −1.5 |
| 70–79 | 66,362 (36.3) | 412.2 | −1.3 |
| 80+ | 43,641 (23.9) | 532.3 | +0.5 |
| Females |
| 0–59 | 20,043 (14.8) | 9.4 | +0.4 |
| 60–69 | 33,277 (24.5) | 126.5 | +3.3 |
| 70–79 | 46,131 (34.0) | 233.2 | +0.6 |
| 80+ | 36,338 (26.8) | 237.2 | +4.8 |
| Average incidence per
year |
| Male (range) | 18,300
(18,056–19,200) | | +0.3 |
| Female (range) | 13,600
(11,992–15,700) | | +3.0 |
As expected, rates increased directly with
increasing age and the greatest percentage change was seen in the
80+ group (+2.6%). Although the bulk of the cases seen in the
10-year study were observed in the 70–79 age-group, a total of
35.3% of all cases, this age group showed a remarkable decrease in
AAPC by -0.3%. Regionally, London was the only region that showed a
decrease in overall trend in the last ten years (Table II), which mirrors England’s
smoking prevalence data showing the region to have the lowest
prevalence of smoking (15), as
well as the largest percentage decrease in smoking prevalence
between 2002 and 2011 (Table
III).
 | Table IITen-year regional statistics by
gender. |
Table II
Ten-year regional statistics by
gender.
| Gender | England | North East | North West | Yorkshire and
Humber | East Midlands | West Midlands | East of England | London | South East | South West |
|---|
| Regional DASR |
| Males | 63.3 | 87.1 | 77.4 | 73.6 | 64.5 | 64.2 | 56.4 | 56.7 | 53.0 | 55.7 |
| Females | 41.6 | 64.2 | 55.1 | 51.1 | 40.4 | 37.9 | 34.8 | 36.2 | 34.1 | 34.7 |
| Overall | 52.5 | 75.7 | 66.3 | 62.4 | 52.5 | 51.1 | 45.6 | 46.5 | 43.6 | 45.2 |
| New cases |
| Males | 182,628 | 12,878 | 30,089 | 21,363 | 16,462 | 19,855 | 18,671 | 20,267 | 25,420 | 17,623 |
| Females | 135,789 | 10,919 | 24,572 | 16,938 | 11,374 | 13,146 | 13,027 | 14,932 | 18,529 | 12,352 |
| Total | 318,417 | 23,797 | 54,661 | 38,301 | 27,836 | 33,001 | 31,698 | 35,199 | 43,949 | 29,975 |
| Percent | 100 | 7.5 | 17.2 | 12.0 | 8.7 | 10.4 | 10.0 | 11.1 | 13.8 | 9.4 |
| Regional AAPC |
| Males | −0.7 | −2.1 | +0.4 | −0.8 | +0.6 | −0.9 | −0.8 | −2.1 | −0.8 | +0.5 |
| Females | +2.3 | +2.3 | +2.3 | +2.5 | +2.8 | +1.6 | +3.3 | +1.1 | +2.0 | +4.1 |
| Overall | +0.8 | +0.1 | +1.4 | +0.9 | +1.7 | +0.4 | +1.3 | −0.5 | +0.6 | +2.3 |
 | Table IIIPrevalence of Smoking in England in
numbers and average annual percentage change (AAPC) in bracket from
2002–2011. |
Table III
Prevalence of Smoking in England in
numbers and average annual percentage change (AAPC) in bracket from
2002–2011.
| Region | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Overall AAPC |
|---|
| North East | 27 (−6.9) | 28 (3.7) | 29 (3.6) | 29 (0.0) | 25 (13.8) | 22 (12.0) | 21 (−4.5) | 22 (4.8) | 21 (−4.5) | 21 (−4.8) | −2.8 |
| North West | 28 (−3.4) | 30 (7.1) | 28 (−6.7) | 24 (14.3) | 25 (4.2) | 23 (8.0) | 23 (0.0) | 23 (0.0) | 22 (−4.3) | 22 (−4.5) | −3.0 |
| Yorkshire and
Humber | 27 (−6.9) | 25 (7.4) | 28 (12.0) | 25 (10.7) | 23 (−8.0) | 22 (−4.3) | 25 (13.6) | 22 (12.0) | 23 (4.5) | 23 (−8.7) | −2.3 |
| East Midlands | 24 (14.3) | 27 (12.) | 27 (0.0) | 25 (−7.4) | 20 (20.0) | 19 (−5.0) | 20 (5.3) | 19 (−5.0) | 16 (15.8) | 16 (18.0) | −2.2 |
| West Midlands | 23 (−4.2) | 25 (8.7) | 23 (−8.0) | 22 (−4.3) | 22 (0.0) | 23 (4.5) | 20 (13.0) | 22 (10.0) | 21 (4.5) | 21 (−4.8) | −2.8 |
| East of
England | 25 (−3.8) | 24 (0.0) | 24 (−4.0) | 23 (−4.2) | 19 (17.4) | 18 (−5.3) | 19 (5.6) | 19 (0.0) | 19 (0.0) | 19 (0.0) | −2.1 |
| London | 24 (11.1) | 24 (0.0) | 22 (−8.3) | 22 (0.0) | 21 (−4.5) | 19 (−9.5) | 19 (0.0) | 22 (15.8) | 17 (22.7) | 17 (−5.9) | −4.9 |
| South East | 26 (8.3) | 24 (7.7) | 22 (−8.3) | 22 (0.0) | 20 (−9.1) | 19 (−5.0) | 20 (5.3) | 19 (−5.0) | 19 (0.0) | 19 (0.0) | −1.8 |
| South West | 25 (4.2) | 25 (4.0) | 23 (−4.2) | 25 (8.7) | 23 (−8.0) | 21 (−8.7) | 21 (0.0) | 18 (−14.3) | 17 (−5.6) | 17 (5.9) | −2.4 |
Analysis showed significant decline in
age-standardised rates of lung cancer in males in all age groups,
from 2002 to 2011 with an AAPC of −1.0%. In contrast, the
age-standardised rates of lung cancer in females increased steadily
in all age groups over the same period with an overall AAPC of
+1.9% (Table I and Fig. 1). The greatest change seen in women
was an increase of +4.8% in the 80+ age group followed by those in
60–69 age group with an AAPC of +3.3% (Table I). Overall, lung cancer trend
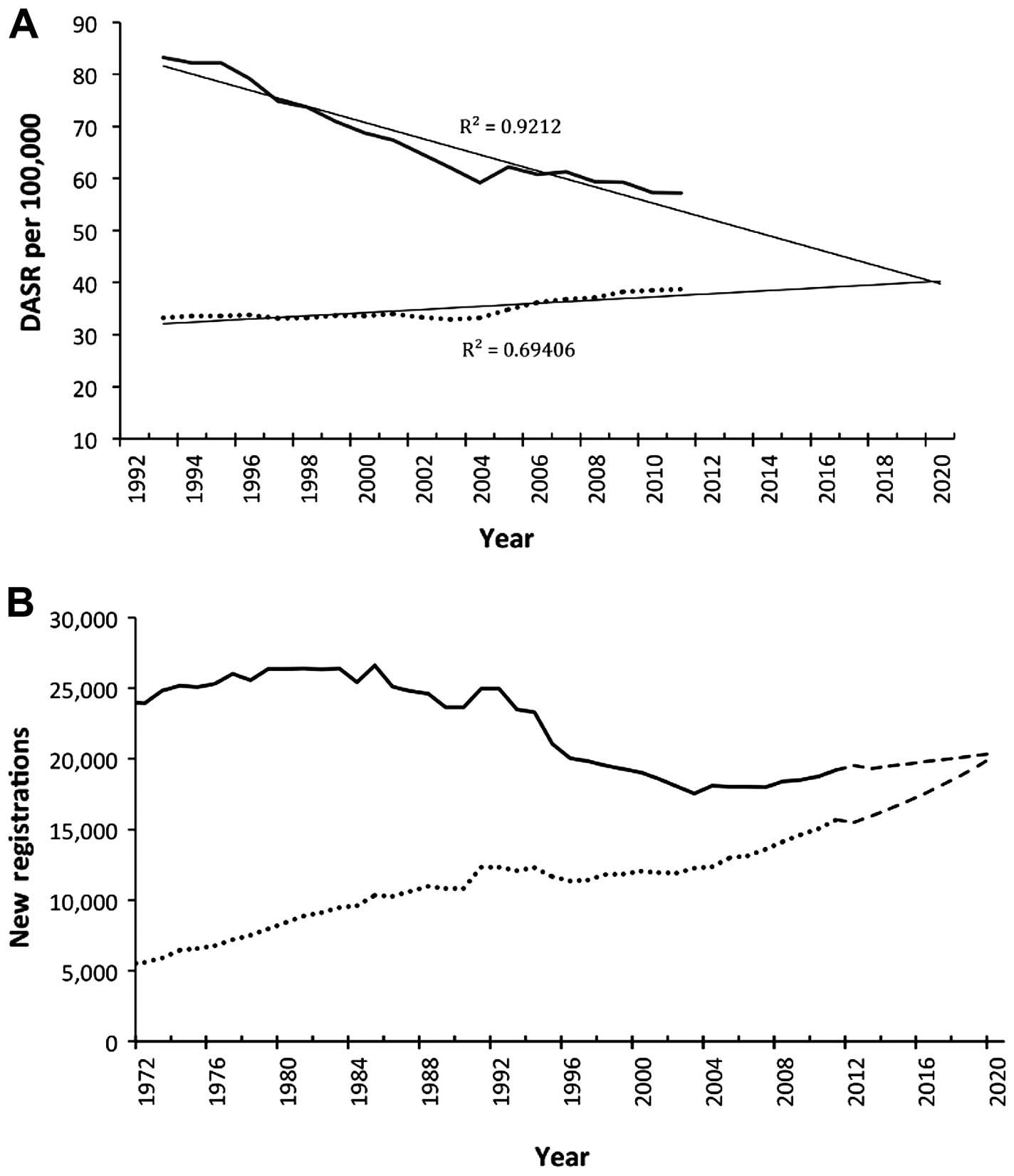
increased by +0.5% in England and our projections to 2020 revealed
that incidence rates in men would continue to fall from the current
57.2 per 100,000 to 39.8 per 100,000 in 2020 whilst rates in women
will increase from the current value of 38.7 to 40.3 per 100,000
using a very modest model (R2=0.69) (Fig. 2). Actually, the best fitting model
(R2=0.91) predicted age-standardised rate of 50.5 per
100,000 in women by 2020.
While the decrease in lung cancer rates in men is
consistent throughout this study, the lung cancer burden, defined
as the total number of new registration per year, is nevertheless
on the increase by an average of +0.3% per year in males; and by
3.0% per year in females. This higher rate of increase in females
is responsible for narrowing the existing incidence gap between
males and females, from a difference of 6,000 new registrations
between men and women in 2002 to about 3,500 in 2011 (Fig. 1). Historically this gap was wider
by about 18,000 back in 1972 (Fig.
2). Our projections, which incorporated the impact of expected
population structure in England (model 2), showed that this
existing gap between men and women would cease to exist by 2020 and
women will then begin to have higher number of new lung cancer
registrations per year subsequently (Fig. 3).
We also observed that although the North West
recorded the largest number of new cases over the 10 years in both
males and females (30,089 and 24,572 new cases, respectively), the
North East region had the highest incidence rates in both males and
females (87.1 and 64.2, respectively) (Table II). Rates varied differently in
all the regions over the last 10 years, with a decrease in AAPC
seen in men in all the regions except in East Midlands (+0.6%),
South West (+0.5%) and North West (+0.4%). North East and London
regions both have AAPC that is less than the England average (AAPC)
for men. Regional trends in females varied between +1.1 and +4.1%,
indicating an increase in female lung cancer incidence trend in all
regions in England from 2002 to 2011. South West region had the
highest rate of growth in female lung cancer incidence with an AAPC
of +4.1 and London had the least (+1.1). The overall DASR (Directly
Age-Standarised Rate) ratio between England worst (North East) and
England best (South East) region is 1.7.
Further regional analyses showed London to have
significantly lower than England average trend in both men and
women (Fig. 4), which could be
attributed to the low smoking prevalence in that region. London
currently has the lowest prevalence of smoking (15) and their smoking data also showed
the largest regional percentage decrease (−4.9%) in smoking
prevalence between 2002 and 2011. London was the only region that
demonstrated an overall trend (AAPC) decrease in lung cancer in the
last ten years (Table II).
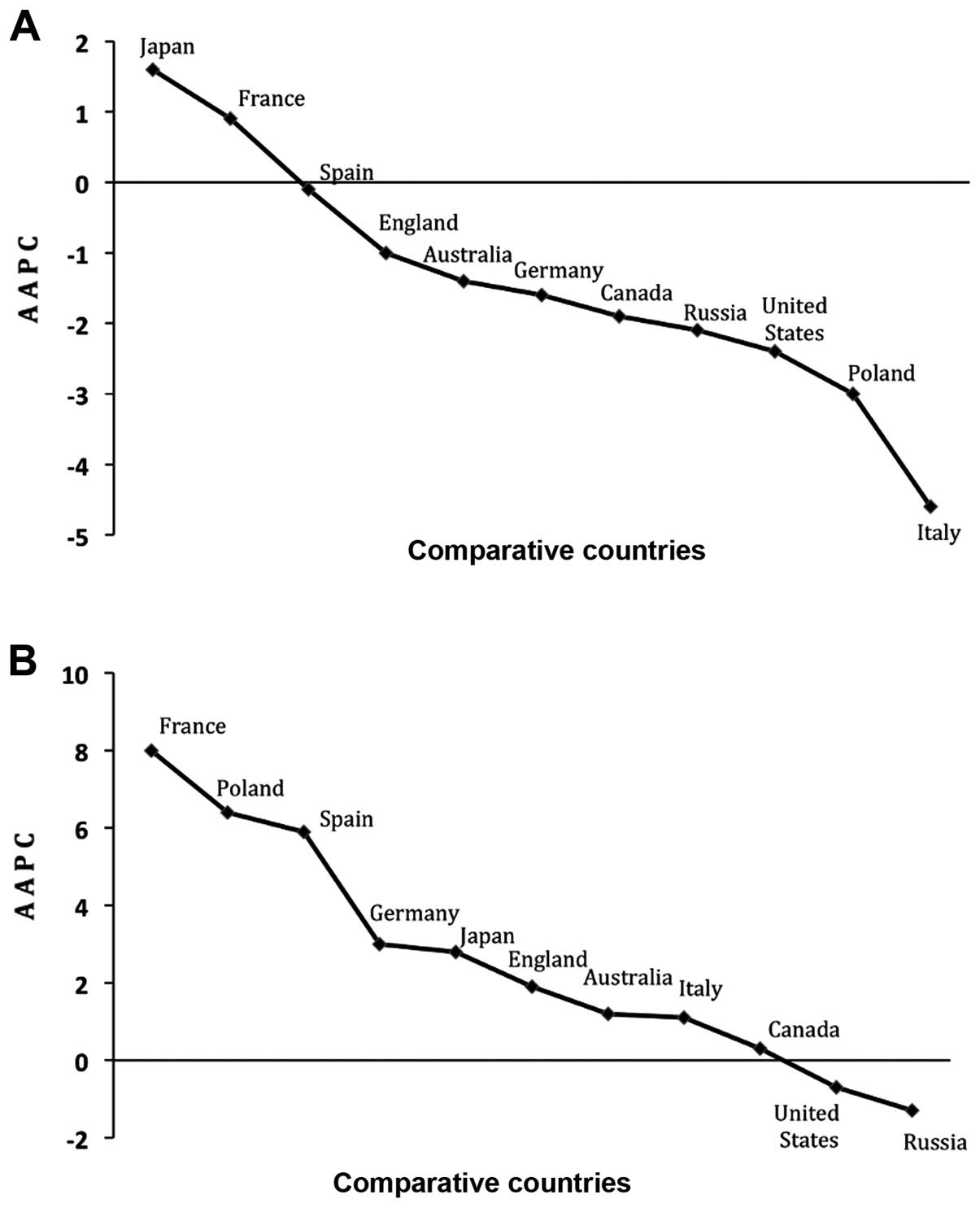
Finally, analysis of lung cancer data from other countries revealed
that Japan had the largest increase over the 10-year study in males
(+1.6%) and France (+8.0%) in females. The largest decrease in men
was seen in Italy (−4.6%) and Russian Federation in women (−1.3%)
(Fig. 5).
Discussion
This study showed that overall lung cancer incidence
in England increased per year by an average of +0.4% between 2002
and 2011. Historically, men have always had higher lung cancer
incidence rates than women across the country, with an incidence
ratio as high as 6:1 documented between males and female,
respectively in 1950s (16).
However, the gender gap is closing due to decreasing rates in men
and increasing rates in women, in spite of the decreasing
prevalence of smoking. The current overall male to female incidence
ratio demonstrated in this study was 3:2, but our projections
showed the rate ratio will be 1:1 by 2020.
The overall trend in women increased by an average
of +1.9, which seemed rather modest, but subgroup analysis revealed
that some age-groups had AAPC values as high as +4.7 (80+) and +3.3
(60–69) during the period of study (Table I). If 9 out of 10 lung cancer cases
occur at age of 60+ (13),
understanding the increasing rate in women over 60 becomes very
vital because it would lead to a significant lung cancer burden
with an increasing ageing population in England. The projected
models in Fig. 3 showed an
impending lung cancer burden in England especially in women. For
instance, if the current incidence rate is not halted, as describes
by model 2, there would be 5,848 excess lung cancer cases in 2020
(when compared with the 2011 figures), with the female population
accounting for 85.4% (4,996) of the excess cases. We also
demonstrated that in spite of the projected decrease in incidence
rates in men, there would still be an excess of 852 lung cancer
cases by 2020, mainly caused by the projected increase in male
population over 60+.
Implementing a comprehensive tobacco control that is
based on the six policies identified by the World Bank has the
potential of reducing incidence. An example is the California
Tobacco Control programme which utilised these policies and saw a
61% decrease per capita cigarette consumption compared to just 41%
across USA, during the same time period (1989–90 and 2006–07).
Within 10 years (1988–1997), California recorded a decrease in lung
cancer incidence by 1.9% per year (p<0.01). The overall
incidence rate decreased by 14.0% in California and by 2.7% in
non-California regions during the 10-year period (14). Our model 3 provides picture of lung
cancer if a similar tobacco control programme was implemented
across England, with the potential of reducing lung cancer
incidence (new registrations) in males by 371 cases per year and in
females by 340 cases per year. Effective tobacco control will
reduce incidence by an overall 6,400 cases (3,340 in men and 3,060
in women) over the projected 9-year period.
Throughout this study, we saw a statistically
significant trend increase in women partly explained by the steady
rise in female smoking prevalence after the Second World War
through to the early 1980s when smoking prevalence realistically
started to decline (17,18). Smoking is the most significant risk
factor in the development of lung cancer (19,20)
and the presence of lag time between exposure to smoking and onset
of lung cancer of ~20–35 years is why we are still experience a
steady rate increase in women (21).
However, recent studies showed that smoking alone
cannot account for the relatively high AAPC currently seen in
females (22). These studies
identified genetic factors that make women more susceptible to
developing lung cancer, smoking patterns in women involving deeper
inhalation and effects of oestrogen on tumor metabolism (23,24),
all contribute to the increasing trend in women. Zang and Wynder
described the relative risk of developing lung cancer in women to
be ~1.5-fold higher than in men, despite men having greater number
of cigarette pack-years (25).
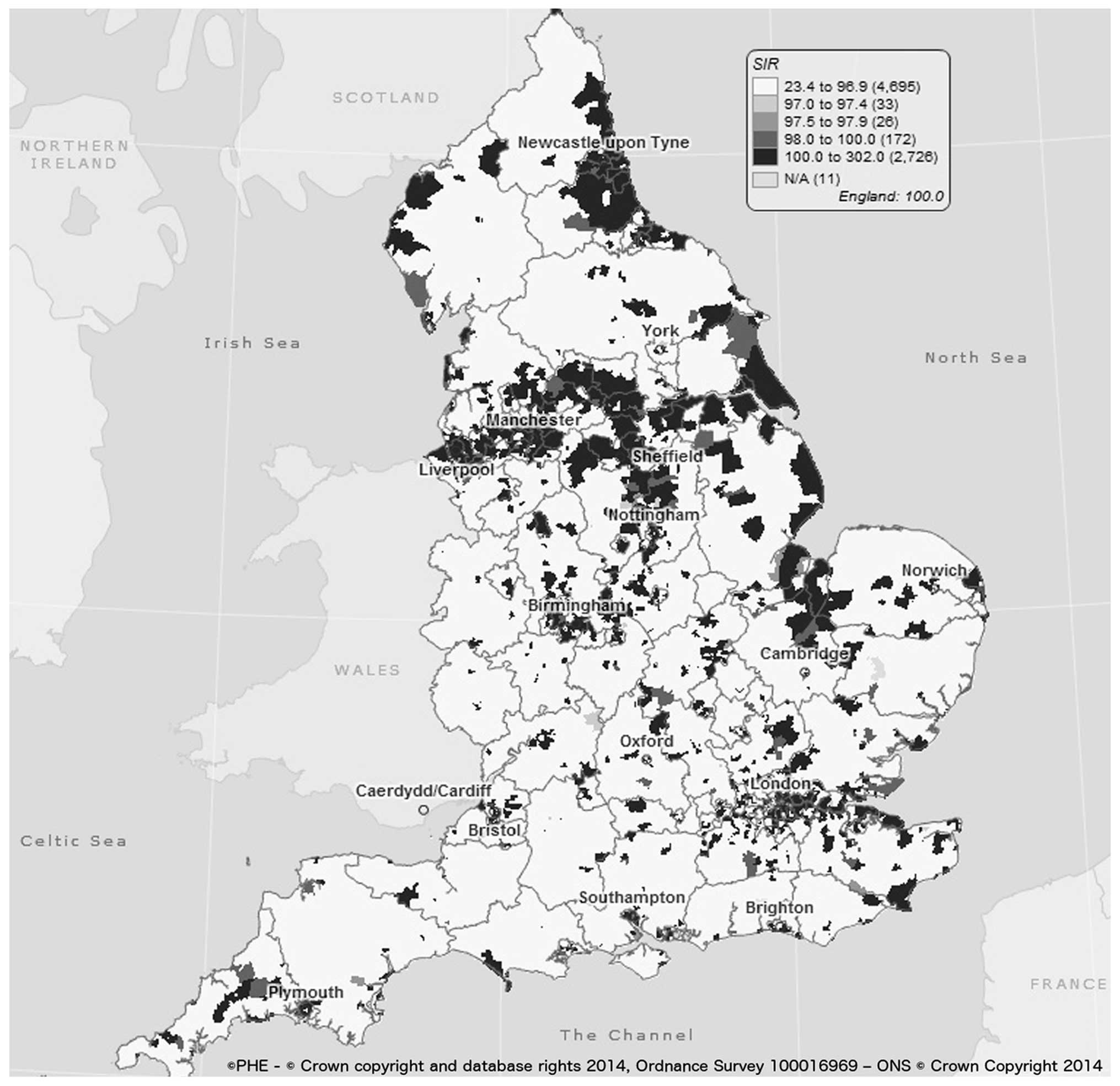
We also established that regional inequalities still
exists across England with higher incidence rates seen in the
northern parts of the country and lower rates in the south creating
a North-South divide (Table II
and Fig. 6). For more than half a
century, the northern part of England is known for its high
deprivation, unemployment and higher smoking prevalence (26,27)
and Quinn et al showed that in the most deprived region in
England, lung cancer incidence was 2.5 and 3.0 times those in
affluent areas in both males and females, respectively (20). Trends in males decreased across all
the regions except in East-Midlands (+0.6), South-West (+0.5) and
North-West (+0.4) regions. While trend increased in females in all
the regions, South-West (+4.1), East of England (+3.3) and
East-Midlands (+2.8) were the top 3 hotspots for female trend in
England. The ratio between England worst region (North East) and
England best region (South East) is 1.6 and 1.9 in both males and
females, respectively (Table
II).
Finally, benchmarking England data against 10
different countries was necessary to evaluate the impact of
existing lung cancer preventive measures in England. Overall, trend
seen in these countries is similar to that observed in England with
only a few exceptions. For instance, all countries demonstrated a
decreasing trend in men except in Japan where the trend in on the
increase (+1.6). Similarly, the trend in women is on the increase
in all the countries except in Russia and the USA where incidence
decreased by −1.3 and −0.7, respectively. Although lung cancer
incidence trend in males is decreasing in England but when compared
with other countries, we concluded that there is room for
improvement. Seven countries achieved better 10-year AAPC than
England, whilst 5 countries had better AAPC in females (Fig. 5). Only 2 countries (USA and Russia)
showed decreasing trend in both males and females and while the
success seen in USA could be attributable to the wide-ranging
smoking cessation programmes and lobbying in that country (28,29),
that seen in Russia remains unclear. A possible explanation for the
decreasing rates in Russia could be the low average life expectancy
of 66 years recorded during our study period, whereas the average
age of diagnosis for lung cancer is 70 years (30,31).
The strengths of this study include the large number
of lung cancer cases and the use of ONS data, which minimises the
chances of missing information on lung cancer incidence. However,
the result of this study must be considered in the light of a
number of limitations. First, it was practically impossible to
stratify and depict trend by race, smoking and other important risk
factors (as the data are not available). Secondly, we did not have
access to stage or grade specific incident data, which would have
allowed us to explore trend according to histological subtypes.
In conclusion, as the UK is already laden with the
challenges of a growing and ageing population, it is necessary to
re-strategise and develop high-quality preventive interventions
that will complement existing national tobacco control measures,
especially in women living in regions that have demonstrated very
high AAPC values (32). Utilising
this targeted approach will achieve a considerable decline in
age-adjusted lung cancer incidence rate similar to those seen in
comparable countries such as USA (33). Our models showed that, unless a
targeted and comprehensive preventive strategy is implemented, lung
cancer rates in women will be on par or higher than that expected
in their male counterparts in England by the year 2020.
Acknowledgements
Dr Michael W. Marcus and Dr Michael P. Davies were
funded by the European Community’s Framework Programme
(FP7/2007–2013) under grant agreement no. HEALTH-F2-2010-258677
(CURELUNG project) and grant agreement no. 258868 (LCAOS
project).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik MDR, Eser
S, Mathers C, Rebelo M, et al: Lung Cancer: Estimated Incidence,
Mortality and Prevalence Worldwide in 2012 GLOBOCAN 2012 v1.0.
International Agency for Research on Cancer (IARC); 2013,
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung.
Accessed July 14, 2014
|
|
2
|
International Agency for Research on
Cancer (IARC). GLOBOCAN Cancer Fact Sheets: Lung Cancer.
International Agency for Research on Cancer (IARC); 2012,
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung.
Accessed April 1, 2014
|
|
3
|
Cancer Research UK. Lung Cancer Mortality
Statistics. CRUK; 2014, http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/mortality/uk-lung-cancer-mortality-statistics.
Accessed March 23, 2015
|
|
4
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of Lung Cancer: Diagnosis and Management
of Lung Cancer, 3rd edition: American College of Chest Physicians
Evidence-Based Clinical Practice Guidelines. Chest. 143:e1S–e29S.
2013. View Article : Google Scholar
|
|
5
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pampel FC, Krueger PM and Denney JT:
Socioeconomic disparities in health behaviors. Annu Rev Sociol.
36:349–370. 2010. View Article : Google Scholar
|
|
7
|
Sidorchuk A, Agardh EE, Aremu O, Hallqvist
J, Allebeck P and Moradi T: Socioeconomic differences in lung
cancer incidence: A systematic review and meta-analysis. Cancer
Causes Control. 20:459–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riaz SP, Horton M, Kang J, Mak V,
Lüchtenborg M and Møller H: Lung cancer incidence and survival in
England: An analysis by socioeconomic deprivation and urbanization.
J Thorac Oncol. 6:2005–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai J, Elleray R, Nordin A, Hirschowitz L,
Rous B, Gildea C and Poole J: Vulval cancer incidence, mortality
and survival in England: Age-related trends. BJOG. 121:728–738;
discussion 739. 2014. View Article : Google Scholar
|
|
11
|
Clegg LX, Hankey BF, Tiwari R, Feuer EJ
and Edwards BK: Estimating average annual per cent change in trend
analysis. Stat Med. 28:3670–3682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Godlewski D, Wojtyś P and Antczak A:
Predictions of cancer incidence in Wielkopolska in 2018. Contemp
Oncol (Pozn). 16:38–43. 2012.
|
|
13
|
Cancer Research UK. Lung cancer incidence
statistics. Cancer Research UK. CRUK; 2014, http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/incidence/uk-lung-cancer-incidence-statistics.
Accessed October 12, 2014
|
|
14
|
Centers for Disease Control and Prevention
(CDC). Declines in lung cancer rates - California, 1988–1997. MMWR
Morb Mortal Wkly Rep. 49:1066–1069. 2000.
|
|
15
|
Action on Smoking and Health (ASH).
Smoking Statistics. London: ASH; 2014, http://www.ash.org.uk/files/documents/ASH_93.pdf.
Accessed October 1, 2014
|
|
16
|
Field JK, Oudkerk M, Pedersen JH and Duffy
SW: Prospects for population screening and diagnosis of lung
cancer. Lancet. 382:732–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agudo A, Ahrens W, Benhamou E, Benhamou S,
Boffetta P, Darby SC, Forastiere F, Fortes C, Gaborieau V, González
CA, et al: Lung cancer and cigarette smoking in women: A
multi-center case-control study in Europe. Int J Cancer.
88:820–827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Action on Smoking and Health. Smoking
statistics: who smokes and how much. London: ASH; 2013, http://ash.org.uk/files/documents/ASH_106.pdf.
Accessed July 23, 2014
|
|
19
|
Doll R, Peto R, Wheatley K, Gray R and
Sutherland I: Mortality in relation to smoking: 40 years’
observations on male British doctors. BMJ. 309:901–911. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quinn M, Babb P, Brock A, Kirby L and
Jones J: Cancer trends in England and Wales 1950–1999. Office of
National Statistics, Studies in Medical and Population Subjects No.
66. The Stationery Office; London: pp. 84–89. 2001
|
|
21
|
McCartney G, Mahmood L, Leyland AH, Batty
GD and Hunt K: Contribution of smoking-related and alcohol-related
deaths to the gender gap in mortality: Evidence from 30 European
countries. Tob Control. 20:166–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marcus MW, Chen Y, Liloglou T and Field
JK: New perspectives to respiratory tract cancers. Head Neck Oncol.
5:282013.
|
|
23
|
Lim WY, Tan CS, Loy EY, Omkar Prasad R,
Seow A and Chia KS: Lung cancer incidence in Singapore: Ethnic and
gender differences. Lung Cancer. 84:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Powell HA, Iyen-Omofoman B, Hubbard RB,
Baldwin DR and Tata LJ: The association between smoking quantity
and lung cancer in men and women. Chest. 143:123–129. 2013.
View Article : Google Scholar
|
|
25
|
Zang EA and Wynder EL: Differences in lung
cancer risk between men and women: Examination of the evidence. J
Natl Cancer Inst. 88:183–192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Möller H, Haigh F, Harwood C, Kinsella T
and Pope D: Rising unemployment and increasing spatial health
inequalities in England: Further extension of the North-South
divide. J Public Health (Oxf). 35:313–321. 2013. View Article : Google Scholar
|
|
27
|
Cancer Research UK and National Cancer
Intelligence Network. Cancer by deprivation in England: Incidence,
1996–2010, Mortality, 1997–2011. London: NCIN; 2014
|
|
28
|
World Health Organization. WHO European
Strategy for Smoking Cessation Policy. Europe: 2004, http://www.euro.who.int/__data/assets/pdf_file/0017/68111/E80056.pdf.
|
|
29
|
Mong C, Garon EB, Fuller C, Mahtabifard A,
Mirocha J, Mosenifar Z and McKenna R: High prevalence of lung
cancer in a surgical cohort of lung cancer patients a decade after
smoking cessation. J Cardiothorac Surg. 6:192011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
United Nations. World Population Prospects
(The 2012 Revision): Key Findings and Advance Tables. Department of
Economic and Social Affairs; Population Division. 2013
|
|
31
|
American Cancer Society. Lung Cancer
(Non-Small Cell). American Cancer Society; 2014, http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics.
Accessed October 20, 2014
|
|
32
|
Mistry M, Parkin DM, Ahmad AS and Sasieni
P: Cancer incidence in the United Kingdom: Projections to the year
2030. Br J Cancer. 105:1795–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Møller H, Fairley L, Coupland V, Okello C,
Green M, Forman D, Møller B and Bray F: The future burden of cancer
in England: Incidence and numbers of new patients in 2020. Br J
Cancer. 96:1484–1488. 2007. View Article : Google Scholar : PubMed/NCBI
|