Introduction
Thyroid carcinoma is a common malignant tumor of
endocrine glands and the most common cancer in head and neck; it
exhibits the full range of malignant behaviors from the relatively
indolent occult differentiated type to uniformly aggressive and
lethal anaplastic variety (1).
Thyroid tumor formation is associated with many genetic and
environmental causes. In addition, numerous factors are also
required to promote thyroid cancer progression (2). Although well differentiated thyroid
carcinomas are efficiently cured by surgery and radioiodine,
undifferentiated thyroid carcinomas (10–20% of total) are very
resistant to chemotherapy (3–6).
Therefore, novel and more efficacious therapies are urgently needed
for these tumors.
TP53 is the most commonly mutated gene in human
cancers, thus, >50% of all human cancer cases carry mutations
within the TP53 locus (7). Unlike
other oncosuppressors, p53 gene always carries a single monoallelic
missense mutation that mainly resides in its DNA-binding domain
(DBD) that abolishes its ability to bind to specific DNA sequences
recognized by wild-type (wt) p53 (8). Loss of wtp53 activity leads to
inhibition of its oncosuppressor function promoting resistance to
chemo- and radio-therapies (9).
Mutant p53 (mutp53) proteins frequently exhibit a dominant-negative
activity over the wtp53 allele by interacting with wtp53 and
reducing cellular concentration of functional wtp53 (10). Recent studies have shown that
mutations of the TP53 gene can confer oncogenic functions (gain of
function, GOF) that are exerted in a variety of ways, ranging from
enhanced proliferation in culture, increased tumorigenicity in
vivo, and enhanced resistance to a variety of commonly used
anticancer drugs (11–13). Indeed, tumors emerging from
mutant-p53 knock-in mice display aggressive and metastatic traits
that are never detected in tumors developing in a p53-deficient
mice (14,15). Mutations of p53 are detectable in
15% of malignant thyroid tumors and are associated with the
progression from differentiated to anaplastic carcinoma (16). Thus, p53 mutations have an effect
on infiltration, lymphatic metastasis and prognosis of thyroid
carcinoma (17).
Several efforts are in progress to discover
efficient agents, such as small molecules, that target mutp53 in
cancers, including thyroid cancers (18), both to abolish mutp53 function or
to reactivate the wtp53 counterparts (19). The final aim is to restore p53
oncosuppressor functions and improve tumor response to therapies.
We recently showed that Zn(II) supplementation in several cancer
cells carrying mutp53, including colon, breast, and glioblastoma,
restores a folded conformation from mutp53 misfolding, rescuing
wtp53/DNA-binding and transactivation (20–22).
The rationale to use Zn(II) comes from the fact that p53 protein
includes one zinc ion (Zn2+) as an important cofactor
for the proper protein folding to efficiently bind the DNA of
sequence-specific target genes (23). Interestingly, mutp53 proteins are
prone to the loss of the Zn(II) bound to the DNA binding domain
(DBD) with the consequence of protein unfolding and aggregation
with loss of wild-type activity (24), therefore, zinc supplementation has
been shown to be a useful strategy to target mutp53 proteins
(25,26).
In this study we asked whether zinc supplementation
might affect p53 in two different thyroid cancer cell lines, one
carrying p53-R273H (Arg to His at codon 273) mutation (FTC-133)
that is one of the p53 mutation responsible of resistance to
antitumor drugs (11) and another
with wtp53 (WRO). We used a novel curcumin-based zinc compound
[Zn(II)-curc] that we previously showed to reactivate mutp53
proteins (27) and to induce
mutp53 degradation through autophagy (28). Here, we found that Zn(II)-curc
reduced mutp53 protein levels in FTC-133 cells, reactivating the
wtp53 functions with increased response to therapies. Of note,
Zn(II)-curc also inducted wtp53 transcriptional activity in
wtp53-carrying cell line. These findings suggest that Zn(II)-curc
may be a useful compound in therapeutic regimens of thyroid
cancers, carrying both mutant and wtp53, that will be worth further
testing for translational purpose in clinical practice.
Materials and methods
Cell culture and reagents
Human thyroid FTC-133 cell line was obtained from
European Collection of Cell Cultures (ECACC, Salisbury, UK). It
derives from a lymph node metastasis of a follicular thyroid
carcinoma and carries p53R273H mutation and EGFR overexpression.
FTC-133 and WRO (wtp53) (ECACC) cancer cell lines were routinely
maintained in DMEM-Ham's-F12 (Life Technology-Invitrogen),
supplemented with 10% heat-inactivated fetal bovine serum (FBS),
100 U/ml penicillin, 100 μg/ml streptomycin (Life
Technology-Invitrogen), and 2 mmol/l L-glutamine in a humidified
atmosphere with 5% CO2 and 95% air at 37°C.
For treatments the following reagents were used:
Zn(II)-curc (29,30) dissolved in DMSO was added to the
culture medium to a final concentration ranging between 40 and 120
μM; chemotherapeutic agent adriamycin (ADR) (Sigma), dissolved in
PBS was added to the culture medium to a final concentration
ranging between 0.2 and 2 μg/ml and cisplatin (Teva Pharma-Italia,
Italy) was added to the culture medium to a final concentration
ranging between 0.5 and 5 μg/ml; PRIMA-1 (p53 reactivation and
induction of massive apoptosis-1) (Sigma) was added to the culture
medium to a final concentration 10 μM for 24 h. PBS and DMSO
solvents were used as control.
Viability assay
Exponentially proliferating cells were plated at
subconfluence in triplicate in 60-mm Petri dishes and 24 h later
treated with Zn(II)-curc alone or combination with chemotherapeutic
agents for 24 h. Both floating and adherent cells were collected
and cell viability was determined by trypan blue exclusion by
direct counting with a hemocytometer, as reported (31). The percentage of cell viability, as
blue/total cells, was assayed by scoring 200 cells per well three
times.
Western blot analysis
Total cell extracts were prepared by incubation at
4°C for 30 min in lysis buffer [50 mmol/l Tris-HCl, pH 7.5, 150
mmol/l NaCl, 150 mmol/l KCl, 1 mmol/l dithiothreitol, 5 mmol/l
EDTA, pH 8.0, 1% Nonidet P-40] plus a mix of protease and
phosphatase inhibitors (Roche Diagnostic). Cell lysates were spun
at 15,000 g for 15 min to remove debris and collect the supernatant
(total cell extracts). Protein concentration was then determined
using BCA Protein Assay kit (Bio-Rad). Equal amount of total cell
extracts were resolved according to molecular weight on 10–18%
SDS-polyacrylamide gel electrophoresis. Proteins were transferred
to a polyvinylidene difluoride membrane (PVDF, Millipore).
Unspecific binding sites were blocked by incubating membranes for 1
h in 0.05% Tween-20 (v/v in TBS) supplemented with 5% non-fat
powdered milk or bovine serum albumin, followed by overnight
incubation with the following primary antibodies: LC3B
(Sigma-Aldrich), rabbit polyclonal anti-p53 (FL393), rabbit
polyclonal anti-p21 and anti-p62 (Santa Cruz Biotechnology, Dallas,
TX, USA), (p)-Akt (Ser473), tot-Akt (Cell Signaling Technologies,
Danvers, MA, USA), polyclonal anti-EGFR antibodies (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and purified mouse
anti-phospho-histone H2AX (Ser139, γH2AX) (Millipore, clone
JBW301). Equal lane loading was monitored by probing membranes with
antibodies specific for β-actin (Calbiochem, San Diego, CA, USA) or
tubulin (Immunological Sciences, Rome, Italy). Primary antibodies
were detected with appropriate horseradish peroxidase-labeled
secondary antibodies (Bio-Rad, Hercules, CA, USA). Enzymatic
signals were visualized using chemiluminescence (ECL Detection
system, GE Healthcare, Milan, Italy).
RNA extraction and semi-quantitative
RT-PCR
Cells were harvested in TRIzol reagent (Invitrogen)
and total RNA was isolated following the manufacturer's
instructions. The first strand cDNA was synthesized from 2 μg of
total RNA with MuLV reverse transcriptase kit (Applied Biosystems,
Foster City, CA, USA). Semi-quantitative RT-PCR was performed
essentially as previously described (20). cDNA was synthesized from 2 μg of
total RNA with MuLV reverse transcriptase kit (Applied Biosystems).
Semi-quantitative reverse-transcribed (RT)-PCR was carried out by
using Hot-Master Taq polymerase (Eppendorf S.r.l., Milan, Italy)
with 2 μl cDNA reaction and gene-specific oligonucleotides under
conditions of linear amplification. PCR products were resolved on
2% agarose gel and visualized with ethidium bromide staining using
UV light. The 28S mRNA sequence was used as control for efficiency
of RNA extraction and transcription. Densitometric analysis was
applied to quantify mRNA levels compared to control gene
expression.
Chromatin immunoprecipitation (ChIP)
assay
ChIP analysis was carried out essentially as
previously described (20).
Briefly, cells were crosslinked with 1% formaldehyde for 10 min at
room temperature and then inactivated by the addition of 125 mM
glycine. Chromatin extracts containing DNA fragments with an
average size of 500 bp were incubated overnight at 4°C with milk,
shaking using polyclonal anti-p53 antibody (FL393, Santa Cruz
Biotechnology). Before use, protein G (Pierce) was blocked with 1
μg/μl sheared herring sperm DNA and 1 μg/μl BSA for 3 h at 4°C and
then incubated with chromatin and antibody for 2 h at 4°C. PCR was
performed with Hot-Master Taq (Eppendorf) using 2 μl of
immunoprecipitated DNA and promoter-specific primers spanning p53
binding sites. Immunoprecipitation with non-specific
immunoglobulins (IgG, Santa Cruz Biotechnology) was performed as
negative controls. PCR products were resolved on 2% agarose gel and
visualized by ethidium bromide staining using UV light.
Statistical analysis
Each experiment, unless otherwise specified, was
performed at least three times, and data are presented as the mean
± SD. Statistical significance was determined using Student's
t-test and a P-value of ≤0.05 was considered statistically
significant.
Results
Zn(II)-curc induces autophagy and mutp53
downregulation in FTC-133 cells
We previously reported that Zn(II)-curc induces
mutp53 degradation through autophagy in breast cancer cells
(28). As defects in autophagy may
be found in cancer, we evaluated whether Zn-curc could induce
autophagy in FTC-133 thyroid cancer cells. We evaluated the
expression of microtubule-associated protein light chain 3 (LC3)
protein that, after conversion from LC3-I to its autophagosome
membrane-associated lipidated form LC3-II, is considered a cellular
readout of autophagy, and the expression levels of p62, a bona
fide autophagic substrate (32). As shown in Fig. 1, Zn(II)-curc increased LC3-II
expression and reduced p62 levels; parallel to induction of
autophagy, Zn(II)-curc triggered mutp53 downregulation in FTC-133
cells (Fig. 1). The use of
chemical inhibitors of autophagic/lysosomal degradation cloroquine
(CQ) (32) prevented
Zn(II)-curc-induced mutp53 degradation (Fig. 1). These findings indicate that
Zn(II)-curc induced mutp53 degradation in FTC-133 cells, carrying
p52R273H mutation, likely through autophagy.
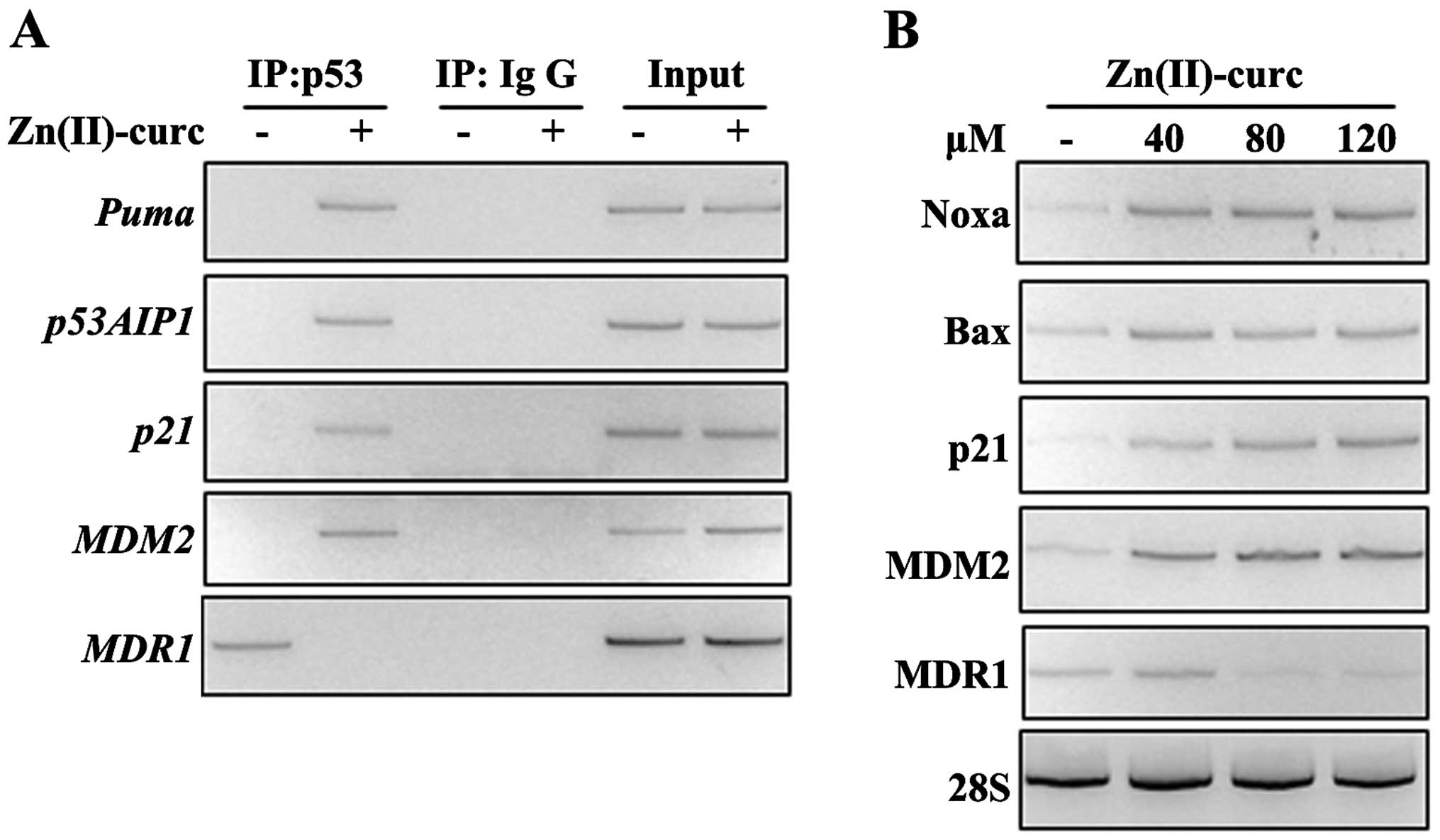
Zn(II)-curc restores wtp53/DNA binding
and target gene transcription in FTC-133 thyroid cancer cells
We then evaluated whether Zn(II)-curc was promoting
reactivation of wtp53 function in FTC-133 cells. We first performed
p53/DNA binding by ChIP analyses and found that Zn(II)-curc
treatment indeed restored the wtp53 binding to several specific
target promoters, including Puma, p53AIP1, MDM2, and
p21, while abolished the p53 binding to the mutant p53
target promoter MDR1 (multi-drug resistance 1) (Fig. 2A). As a consequence, the expression
of the canonical wtp53 target genes was evaluated by reverse
transcription polymerase chain reaction (RT-PCR) analyses. FTC-133
cells were exposed to increasing doses of Zn-curc (40, 80 and 120
μM for 24 h) that induced expression of the typical p53 target
genes already at the lowest dose used, while reduced the expression
of mutp53 target MDR1 expression starting from 80 μM dose (Fig. 2B). These findings indicate that
Zn(II)-curc produced an imbalance between misfolded/folded p53
proteins in FTC-133 cells that favoured wild-type over mutant p53
functions.
Comparison between Zn(II)-curc and
PRIMA-1 on p53 activity
PRIMA-1 is a low molecular weight compound with
antitumor activity that has been shown to be more effective in
inducing apoptosis in mutp53 cells than in wtp53 cells (33). We then compared the p53
reactivating effect of Zn(II)-curc with that of PRIMA-1 in both
FTC-133 (mutp53) and WRO (wtp53) cells. In vivo
transcription of endogenous p53 target genes was examined by
employing RT-PCR analysis. The results show that the induction of
wtp53 target genes such as Bax, Noxa, Puma and
downregulation of the mutp53 target gene MDR1 was comparably
and efficiently achieved by both treatments, that is, Zn(II)-curc
and PRIMA-1 (Fig. 3A); of note,
Zn(II)-curc induced also the wtp53 target, cell cycle-related
p21 gene, unlike PRIMA-1 (Fig.
3A). This latter result is in agreement with PRIMA-1
pro-apoptotic specific function (33). Interestingly, Zn(II)-curc,
differently from PRIMA-1, induced p53 target genes in WRO cells,
carrying wtp53 (Fig. 3B), likely
due to the DNA intercalating ability of Zn(II)-curc compound
sensing the DNA damage response (29). Thus, Zn-curc induced H2AX
phosphorylation (γH2AX) (Fig. 3C)
that is a marker to detect the genotoxic effect of different
anti-cancer agents (34).
Zn(II)-curc modifies prosurvival
molecular pathways in FTC-133 cells
P53H273 mutation has been shown to increase
epidermal growth factor receptor (EGFR) levels as well as
EGFR/PI3K/Akt signaling pathway, facilitating cell proliferation,
tumor growth and migration (35).
EGFR overexpression is described in several thyroid carcinomas,
including follicular thyroid carcinomas (36), and recent evidence implicates EGFR
overexpression in advanced thyroid carcinoma progression (37). Therefore, we evaluated the effect
of Zn(II)-curc on EGFR and Akt expression levels in FTC-133 cells.
Western blot analyses show that the strong expression of EGFR was
markedly reduced by Zn(II)-curc (Fig.
4A); similarly, Akt phosphorylation was noticeably inhibited by
Zn(II)-curc treatment (Fig. 4B).
Moreover, p53 mutants have been shown to regulate E-cadherin
expression (38). Downregulation
of the E-cadherin cell-cell adhesion molecule is a key event for
epithelial to mesenchymal transition (EMT) in tumor progression and
it is associated with the development of invasive carcinoma,
metastatic dissemination, and poor clinical prognosis (39). We found that Zn(II)-curc induced
re-expression of E-cadherin mRNA levels in FTC-133 cells, that
strongly correlated with downregulation of N-cadherin expression, a
mesenchymal marker (Fig. 4C), as
evidenced by densitometric analysis (Fig. 4C, lower panel) indicative of a
reversion of EMT phenotype. Altogether, these findings indicate
that Zn(II)-curc could modify prosurvival pathways and counteract
mutp53-regulated pathways responsible of GOF.
Zn(II)-curc increases the cytotoxic
effect of drugs
In order to study the biological effect of
Zn(II)-curc we treated FTC-133 and WRO cells with Zn(II)-curc alone
or combination with cisplatin (cis) or adriamycin (ADR) and
assessed viability using trypan blue staining. We found that the
weak antitumor effect achieved by two different doses (0.2 and 2
μg/ml) of ADR in FTC-133 cells was significantly increased by
Zn(II)-curc co-treatment (Fig.
5A). Similarly, cell death induced by both low (0.5 μg/ml) or
high (5 μg/ml) dose of cisplatin was increased by Zn(II)-curc
co-treatment, although FTC-133 resulted more sensitive to high dose
of cisplatin (5 μg/ml) (Fig. 5A);
in agreement with the activation of wtp53 target genes, seen in
Fig. 3B, Zn(II)-curc increased the
cytotoxic effect of cisplatin and ADR also in this wtp53-carrying
cell line (Fig. 5B). In
comparison, PRIMA-1 increased chemosensitivity of mutp53 FTC133
cells, while did not affect the response to drugs of wtp53 WRO
cells (Fig. 5C). Altogether, these
results show that Zn(II)-curc enhanced the cytotoxic effect of
chemotherapeutic drugs in both mutant and wild-type-carrying cancer
cells, unlike PRIMA-1 that was effective only in mutp53 cells.
Discussion
In this study we found that Zn(II)-curc, a mutant
p53 reactivator, was able to downregulate mutp53 in FTC-133
(p53H273) thyroid cancer cells restoring the wtp53 functions and
improving cancer cell response to antitumor drugs. In addition,
Zn(II)-curc was also able to activate endogenous wtp53 in wtp53
cells WRO.
Several studies have shown that restoration of
wild-type p53 activity in vitro (40) and in mice (41–43)
induces rapid tumor regression, demonstrating that reconstitution
of p53 is indeed sufficient for elimination of tumors even in the
presence of other tumor-associated genetic alterations (41–43).
Over the past years several strategies have been approached to
develop p53-targeted drugs, and in particular to develop small
molecule compounds that activate wtp53 and restore wtp53 function
of mutant p53 proteins (44), as
abrogation of mutp53 has been shown to reduce tumor malignancy
(45). Thus, some mutp53 proteins
such as p53H273 have been shown to acquire gain of function (GOF)
that actively contributes to cancer development and progression and
chemoresistance (11). At the
molecular levels GOF effects were shown to be linked to the ability
of mutp53 to increase the expression of several genes including
MDR1 (multi-drug resistance gp180 protein). Moreover,
p53H273 mutation has been shown to increase epidermal growth factor
receptor (EGFR) levels as well as EGFR/PI3K/Akt signaling pathway,
facilitating cell proliferation and tumor growth (35). Here, we found that Zn(II)-curc was
able to down-regulate mutp53 protein levels. In agreement with the
loss of mutant p53 protein, Zn(II)-curc reduced EGFR expression and
Akt phosphorylation as well as induced reversion of EMT, suggesting
modulation of mutp53-dependent gain of function pathways. The
reversion of EMT by Zn(II)-curc is particularly intriguing because
EMT induction has been shown to promote cancer stem cell (CSC)
traits in the thyroid cancer cells, with the potential to induce
cancer stem cell generation and tumor progression in thyroid
cancers (46). The effect of
Zn(II)-curc on mutp53 downregulation depended in part on induction
of autophagy, as previously reported (28). Autophagy plays a dual role during
cancer development, progression and treatment, mainly depending on
the type and stage of the tumor. The induction of autophagy by
Zn(II)-curc is interesting in light of studies showing that, in
thyroid cancer, autophagy activation increases chemosensitivity,
enhances the iodine uptake ability, and reverses the
dedifferentiation phenotype (47).
Parallel to mutp53 downregulation, Zn(II)-curc reactivated the
wild-type p53 functions, likely through the imbalance of
misfolded/folded protein conformation. These molecular changes
correlated with improvement, at biological level, of the cytotoxic
effect of chemotherapeutic drugs, increasing thyroid cancer cell
death.
The presence of p53 mutations is a late event in
thyroid cancer progression. The mutations of p53 or increased
expression of p53 protein is associated with the progression from
differentiated to anaplastic carcinoma and is implicated in
chemoresistance (17). Recently,
some attention has been focused on Zinc compounds for p53
reactivation (48). In this
regard, compounds that can both promote mutp53 degradation and
reactivation of wtp53 functions may have therapeutic advantages, as
is the case with Zn(II)-curc. To strengthen the role of Zn(II)-curc
as mutp53 reactivator, the effect of Zn(II)-curc was compared to
that of PRIMA-1, a low molecular weight compound that reactivates
mutp53 and that has been shown to be more effective in inducing
apoptosis in mutp53 cells than in wtp53 cells (33). Such compound has been shown to
induce cell death in thyroid cancer cells carrying p53 mutations
and particularly p53H273 mutation (18). Of note, Zn(II)-curc restored wtp53
activity similarly to PRIMA-1 in FTC-133 cells and increased the
cytotoxic effect of drugs. In addition, Zn(II)-curc was able to
activate endogenous wtp53 in WRO cells, unlike PRIMA-1 that did not
have such effect on wtp53 protein.
The classic treatment of thyroid cancer is total
thyroid-ectomy, followed by, in some cases, radioiodine treatment.
Surgically inoperable and radioiodine-refractory differentiated
thyroid cancers, poorly differentiated thyroid cancer, and
anaplastic thyroid cancer are currently the major causes of deaths
related to thyroid cancer and do not have effective treatments
(3–6). In this regard, molecular-based
management strategies for thyroid nodules and thyroid cancer are a
novel and intense area of development in molecular thyroid-cancer
medicine (49). In line with this
strategy, the present findings, although preliminary due to the
small number of cell lines analyzed, suggest that Zn(II)-curc may
be a useful adjuvant in the treatment of thyroid cancer cells
carrying both both mutant and wild-type p53 proteins. Thus,
although well differentiated thyroid carcinomas are efficiently
cured by surgery and radioiodine, undifferentiated thyroid
carcinomas (10–20% of total) are very resistant to chemotherapy and
the restoration of chemosensitivity, through p53 reactivation for
instance, may be an efficient antitumor strategy. The use of
Zn(II)-curc in clinical practice is strengthen also by our previous
studies showing other biological effects of zinc supplementation
such as restoration of immune response (50) and inhibition of hypoxia-induced
molecular pathways (51,52). This is particularly relevant
because hypoxia-inducible factor is often overexpressed in thyroid
cancer contributing to chemoresistance and tumor progression
(53). In earlier observations
Zn(II) has been found to have cytotoxic effect on thyroid cancer
cell lines (55), although the molecular mechanisms were not fully
elucidated. Here we propose the use of Zn(II)-curc as p53 (re)
activator in therapeutic regimens of thyroid cancers, carrying both
mutant and wtp53, that will be worth further testing for
translational purpose in clinical practice.
Acknowledgements
This study was supported by Grant from the Italian
Association for Cancer Research (AIRC, IG11377 to G.D.). The
authors wish to thank Dr Fabiola Moretti and Dr Oreste Segatto for
sharing reagents. We also thank Dr Maria Pia Gentileschi for
technical assistance.
References
|
1
|
Sipos JA and Mazzaferri EL: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol (R Coll Radiol).
22:395–404. 2010. View Article : Google Scholar
|
|
2
|
Kim DS, McCabe CJ, Buchanan MA and
Watkinson JC: Oncogenes in thyroid cancer. Clin Otolaryngol Allied
Sci. 28:386–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Urciuoli P, Ghinassi S, Iavarone C,
Peparini N, D’Orazi V, Gabatel R, Pichelli D, Iachetta RP and
Custureri F: Thyroid anaplastic tumor: Our experience. Chir Ital.
55:835–840. 2003.(In Italian).
|
|
4
|
Pulcrano M, Boukheris H, Talbot M, Caillou
B, Dupuy C, Virion A, De Vathaire F and Schlumberger M: Poorly
differentiated follicular thyroid carcinoma: Prognostic factors and
relevance of histological classification. Thyroid. 17:639–646.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajhbeharrysingh U, Taylor M and Milas M:
Medical therapy for advanced forms of thyroid cancer. Surg Clin
North Am. 94:541–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen QT, Lee EJ, Huang MG, Park YI,
Khullar A and Plodkowski RA: Diagnosis and treatment of patients
with thyroid cancer. Am Health Drug Benefits. 8:30–40.
2015.PubMed/NCBI
|
|
7
|
Hainaut P and Hollstein M: p53 and human
cancer: The first ten thousand mutations. Adv Cancer Res.
77:81–137. 2000. View Article : Google Scholar
|
|
8
|
Beckerman R and Prives C: Transcriptional
regulation by p53. Cold Spring Harb Perspect Biol. 2:a0009352010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tchelebi L, Ashamalla H and Graves PR:
Mutant p53 and the response to chemotherapy and radiation. Subcell
Biochem. 85:133–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milner J and Medcalf EA: Cotranslation of
activated mutant p53 with wild type drives the wild-type p53
protein into the mutant conformation. Cell. 65:765–774. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blandino G, Levine AJ and Oren M: Mutant
p53 gain of function: Differential effects of different p53 mutants
on resistance of cultured cells to chemotherapy. Oncogene.
18:477–485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gurtner A, Starace G, Norelli G, Piaggio
G, Sacchi A and Bossi G: Mutant p53-induced up-regulation of
mitogen-activated protein kinase kinase 3 contributes to gain of
function. J Biol Chem. 285:14160–14169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ubertini V, Norelli G, D’Arcangelo D,
Gurtner A, Cesareo E, Baldari S, Gentileschi MP, Piaggio G, Nisticò
P, Soddu S, et al: Mutant p53 gains new function in promoting
inflammatory signals by repression of the secreted interleukin-1
receptor antagonist. Oncogene. 34:2493–2504. 2015. View Article : Google Scholar
|
|
14
|
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA,
Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et
al: Gain of function of a p53 hot spot mutation in a mouse model of
Li-Fraumeni syndrome. Cell. 119:861–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olive KP, Tuveson DA, Ruhe ZC, Yin B,
Willis NA, Bronson RT, Crowley D and Jacks T: Mutant p53 gain of
function in two mouse models of Li-Fraumeni syndrome. Cell.
119:847–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donghi R, Longoni A, Pilotti S, Michieli
P, Della Porta G and Pierotti MA: Gene p53 mutations are restricted
to poorly differentiated and undifferentiated carcinomas of the
thyroid gland. J Clin Invest. 91:1753–1760. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parameswaran R, Brooks S and Sadler GP:
Molecular pathogenesis of follicular cell derived thyroid cancers.
Int J Surg. 8:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Messina RL, Sanfilippo M, Vella V, Pandini
G, Vigneri P, Nicolosi ML, Gianì F, Vigneri R and Frasca F:
Reactivation of p53 mutants by prima-1 [corrected] in thyroid
cancer cells. Int J Cancer. 130:2259–2270. 2012. View Article : Google Scholar
|
|
19
|
Zawacka-Pankau J and Selivanova G:
Pharmacological reactivation of p53 as a strategy to treat cancer.
J Intern Med. 277:248–259. 2015. View Article : Google Scholar
|
|
20
|
Puca R, Nardinocchi L, Gal H, Rechavi G,
Amariglio N, Domany E, Notterman DA, Scarsella M, Leonetti C,
Sacchi A, et al: Reversible dysfunction of wild-type p53 following
home-odomain-interacting protein kinase-2 knockdown. Cancer Res.
68:3707–3714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puca R, Nardinocchi L, Bossi G, Sacchi A,
Rechavi G, Givol D and D’Orazi G: Restoring wtp53 activity in HIPK2
depleted MCF7 cells by modulating metallothionein and zinc. Exp
Cell Res. 315:67–75. 2009. View Article : Google Scholar
|
|
22
|
Puca R, Nardinocchi L, Porru M, Simon AJ,
Rechavi G, Leonetti C, Givol D and D’Orazi G: Restoring p53 active
conformation by zinc increases the response of mutant p53 tumor
cells to anticancer drugs. Cell Cycle. 10:1679–1689. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho Y, Gorina S, Jeffrey PD and Pavletich
NP: Crystal structure of a p53 tumor suppressor-DNA complex:
Understanding tumorigenic mutations. Science. 265:346–355. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loh SN: The missing zinc: p53 misfolding
and cancer. Metallomics. 2:442–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D’Orazi G and Givol D: p53 reactivation:
The link to zinc. Cell Cycle. 11:2581–2582. 2012. View Article : Google Scholar :
|
|
26
|
Blanden AR, Yu X, Wolfe AJ, Gilleran JA,
Augeri DJ, O’Dell RS, Olson EC, Kimball SD, Emge TJ, Movileanu L,
et al: Synthetic metallochaperone ZMC1 rescues mutant p53
conformation by transporting zinc into cells as an ionophore. Mol
Pharmacol. 87:825–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garufi A, Trisciuoglio D, Porru M,
Leonetti C, Stoppacciaro A, D’Orazi V, Avantaggiati M, Crispini A,
Pucci D and D’Orazi G: A fluorescent curcumin-based Zn(II)-complex
reactivates mutant (R175H and R273H) p53 in cancer cells. J Exp
Clin Cancer Res. 32:722013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garufi A, Pucci D, D’Orazi V, Cirone M,
Bossi G, Avantaggiati ML and D’Orazi G: Degradation of mutant
p53H175 protein by Zn(II) through autophagy. Cell Death Dis.
5:e12712014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pucci D, Bellini T, Crispini A, D’Agnano
I, Liguori PF, Garcia-Orduña P, Pirillo S, Valentini A and
Zanchetta G: DNA binding and cytotoxicity of fluorescent
curcumin-based Zn(II) complexes. Med Chem Comm. 3:462–468. 2012.
View Article : Google Scholar
|
|
30
|
Pucci D, Crispini A, Sanz Mendiguchía B,
Pirillo S, Ghedini M, Morelli S and De Bartolo L: Improving the
bioactivity of Zn(II)-curcumin based complexes. Dalton Trans.
42:9679–9687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garufi A, D’Orazi V, Arbiser JL and
D’Orazi G: Gentian violet induces wtp53 transactivation in cancer
cells. Int J Oncol. 44:1084–1090. 2014.PubMed/NCBI
|
|
32
|
Barth S, Glick D and Macleod KF:
Autophagy: Assays and artifacts. J Pathol. 221:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bykov VJ, Issaeva N, Shilov A, Hultcrantz
M, Pugacheva E, Chumakov P, Bergman J, Wiman KG and Selivanova G:
Restoration of the tumor suppressor function to mutant p53 by a
low-molecular-weight compound. Nat Med. 8:282–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Podhorecka M, Skladanowski A and Bozko P:
H2AX phosphorylation: its role in DNA damage response and cancer
therapy. J Nucleic Acids. 2010:9201612010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Cheng B, Miao L, Mei Y and Wu M:
Mutant p53-R273H gains new function in sustained activation of EGFR
signaling via suppressing miR-27a expression. Cell Death Dis.
4:e5742013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Z, Hou P, Ji M, Guan H, Studeman K,
Jensen K, Vasko V, El-Naggar AK and Xing M: Highly prevalent
genetic alterations in receptor tyrosine kinases and
phosphatidylinositol 3-kinase/akt and mitogen-activated protein
kinase pathways in anaplastic and follicular thyroid cancers. J
Clin Endocrinol Metab. 93:3106–3116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Landriscina M, Pannone G, Piscazzi A, Toti
P, Fabiano A, Tortorella S, Occhini R, Ambrosi A, Bufo P and
Cignarelli M: Epidermal growth factor receptor 1 expression is
upregulated in undifferentiated thyroid carcinomas in humans.
Thyroid. 21:1227–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roger L, Jullien L, Gire V and Roux P:
Gain of oncogenic function of p53 mutants regulates E-cadherin
expression uncoupled from cell invasion in colon cancer cells. J
Cell Sci. 123:1295–1305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
D’Orazi G, Marchetti A, Crescenzi M, Coen
S, Sacchi A and Soddu S: Exogenous wt-p53 protein is active in
transformed cells but not in their non-transformed counterparts:
Implications for cancer gene therapy without tumor targeting. J
Gene Med. 2:11–21. 2000. View Article : Google Scholar
|
|
41
|
Martins CP, Brown-Swigart L and Evan GI:
Modeling the therapeutic efficacy of p53 restoration in tumors.
Cell. 127:1323–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ventura A, Kirsch DG, McLaughlin ME,
Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R
and Jacks T: Restoration of p53 function leads to tumour regression
in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue W, Zender L, Miething C, Dickins RA,
Hernando E, Krizhanovsky V, Cordon-Cardo C and Lowe SW: Senescence
and tumour clearance is triggered by p53 restoration in murine
liver carcinomas. Nature. 445:656–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu X, Narayanan S, Vazquez A and Carpizo
DR: Small molecule compounds targeting the p53 pathway: Are we
finally making progress? Apoptosis. 19:1055–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bossi G, Lapi E, Strano S, Rinaldo C,
Blandino G and Sacchi A: Mutant p53 gain of function: Reduction of
tumor malignancy of human cancer cell lines through abrogation of
mutant p53 expression. Oncogene. 25:304–309. 2006.
|
|
46
|
Lan L, Luo Y, Cui D, Shi B-Y, Deng W, Huo
L-L, Chen H-L, Zhang G-Y and Deng L-L: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013.PubMed/NCBI
|
|
47
|
Yi H, Long B, Ye X, Zhang L, Liu X and
Zhang C: Autophagy: A potential target for thyroid cancer therapy
(Review). Mol Clin Oncol. 2:661–665. 2014.PubMed/NCBI
|
|
48
|
Khoo KH, Verma CS and Lane DP: Drugging
the p53 pathway: Understanding the route to clinical efficacy. Nat
Rev Drug Discov. 13:217–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cirone M, Garufi A, Di Renzo L, Granato M,
Faggioni A and D’Orazi G: Zinc supplementation is required for the
cytotoxic and immunogenic effects of chemotherapy in chemoresistant
p53-functionally deficient cells. OncoImmunology. 2:e261982013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nardinocchi L, Puca R, Sacchi A, Rechavi
G, Givol D and D’Orazi G: Targeting hypoxia in cancer cells by
restoring homeodomain interacting protein-kinase 2 and p53 activity
and suppressing HIF-1alpha. PLoS One. 4:e68192009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nardinocchi L, Pantisano V, Puca R, Porru
M, Aiello A, Grasselli A, Leonetti C, Safran M, Rechavi G, Givol D,
et al: Zinc downregulates HIF-1α and inhibits its activity in tumor
cells in vitro and in vivo. PLoS One. 5:e150482010. View Article : Google Scholar
|
|
53
|
Burrows N, Babur M, Resch J, Williams KJ
and Brabant G: Hypoxia-inducible factor in thyroid carcinoma. J
Thyroid Res. 2011:7629052011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Iitaka M, Kakinuma S, Fujimaki S, Oosuga
I, Fujita T, Yamanaka K, Wada S and Katayama S: Induction of
apoptosis and necrosis by zinc in human thyroid cancer cell lines.
J Endocrinol. 169:417–424. 2001. View Article : Google Scholar : PubMed/NCBI
|