Introduction
Paclitaxel (Taxol) is a potent anticancer agent
derived from the Pacific yew tree; it acts through the
overstabilization of cellular microtubules. This natural product
causes disruption of the mitotic machinery and inhibition of cell
growth (1–3). Paclitaxel also exhibits
anti-angiogenic properties, expanding the application of this
family of drugs, known as taxanes, to various tumor types in breast
(4), prostate (5), ovarian (6) and lung cancers (7). The effects of paclitaxel are believed
to be mediated by the stabilization of microtubule polymerization,
which leads to cell cycle arrest at the mitotic phase of the cell
cycle, induction of detectable DNA fragmentation, and apoptosis.
Taken together, these processes result in tumor regression
(8). However, the use of
paclitaxel is limited because the precise mechanisms underlying its
antitumor effects are not completely elucidated.
Adenosine monophosphate (AMP)-activated protein
kinase (AMPK) is a cellular fuel sensor that monitors the AMP/ATP
ratio and maintains cellular homeostasis (9). Metabolic stresses, including hypoxia,
exercise, and starvation, lead to the activation of AMPK (10,11).
AMPK induces apoptosis in several cell types (12–15).
These findings suggest that AMPK signaling is a potential
therapeutic target in cancer. However, the molecular mechanisms
underlying AMPK-dependent apoptosis of cancer cells remain
unclear.
Elongation factor 1 α (EF1α) is a ubiquitously
expressed protein that plays a key role in the elongation cycle
during translation. EF1α is also involved in GTP-binding protein
activity during signal transduction and oncogenesis (16–22).
It is the most abundant protein in normal cells, accounting for
1–2% of the total protein content, and is highly expressed in the
brain, heart and skeletal muscle, i.e., tissues consisting largely
of long-lived, terminally differentiated cells (23–25).
Regulation of EF1α levels may be important for normal cell
function. Rapidly growing cells usually exhibit a large increase in
EF1α mRNA levels (26), whereas
overexpression of EF1α correlates with the emergence of metastases
(27).
Forkhead box O3 (FOXO3; FKHRL1) is a member of the
FOXO family of forkhead transcription factors that promote
resistance to oxidative stress, tumor suppression and longevity
(28–33). FOXO proteins primarily act as
potent transcriptional activators by binding to the conserved
consensus core recognition motif TTGTTTAC (34,35).
By upregulating specific gene expression programs, FOXO
transcription factors promote cell cycle arrest, repair of damaged
DNA, de-toxification of reactive oxygen species, apoptosis and
autophagy (36–45).
Based on earlier reports suggesting that AMPK
mediates antitumor effects, considerable attention has been focused
on the role of AMPK in tumor cells. In the present study, we
investigated the effects of paclitaxel on the activity of AMPK in
breast tumor MCF7 cells. We revealed that paclitaxel activated AMPK
in MCF7 cells, whereas EF1α and forkhead box O3a (FOXO3a) affected
AMPK-induced growth inhibition. Our results provide new therapeutic
possibilities for the use of AMPK modulators as antitumor
agents.
Materials and methods
Experimental agents
EF1α, caspase-3, GAPDH, AMPKα2, and GAPDH antibodies
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA) and New England BioLabs, Inc. (Ipswich, MA, USA).
Phospho-specific AMPK (Thr172) and anti-AMPK antibodies
were purchased from Upstate Biotechnology (Temecula, CA, USA). A
FOXO3a/FKHRL1 (Ser253) antibody was purchased from
Sigma-Aldrich (St. Louis, MO, USA). A phospho-specific
FOXO3a/FKHRL1 (Ser253) antibody was purchased from
Millipore Corp. (Billerica, MA, USA). An anti-p21Waf1
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). HRP-conjugated secondary antibodies were obtained
from the Kirkegaard and Perry Laboratory (Gaithersburg, MD, USA).
Paclitaxel was purchased from Sigma-Aldrich.
5-Amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) and
6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine
(compound C) were obtained from Calbiochem (San Diego, CA,
USA).
Cell cultures
The MCF7 cell line was grown in a 1:1 mixture of
RPMI-1640 (Gibco, Auckland, New Zealand) containing 0.584 g/l of
L-glutamate, 4.5 g/l of glucose, 100 g/ml of gentamicin, 2.5 g/l of
sodium carbonate and 10% heat-inactivated fetal bovine serum
(FBS).
Immunoblot analysis
Cells were grown in 6-well plates and serum-starved
for 24 h prior to the treatment with indicated agents. The medium
was aspirated and the cells were washed twice in ice-cold
phosphate-buffered saline (PBS) and lysed in 100 μl of lysis
buffer [50 mM Tris-Cl (pH 7.4), 1% Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, 1 mM EDTA and 150 mM NaCl]. The lysed
samples were briefly sonicated, heated for 5 min at 95°C, and
centrifuged for 5 min. The supernatants were electrophoresed on 8%
SDS-PAGE gels and transferred to polyvinylidene difluoride
membranes. The blots were incubated overnight at 4°C with a primary
antibody. They were then washed 6 times in Tris-buffered
saline/0.1% Tween-20 and probed for 1 h with an HRP-conjugated
secondary antibody at room temperature. The blots were visualized
using an ECL detection system (Amersham Biosciences,
Buckinghamshire, UK).
3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay
The MTT assay was performed as a crude measure of
cell viability. MCF7 cells were seeded at a density of
5×104/ml in 96-well plates and allowed to grow for 24 h.
The growth medium was replaced with serum-free medium 24 h prior to
treatment. Subsequently, MTT reagents (10 μl/well; 7.5 mg/ml
in PBS) were added and the cultures were incubated for 30 min at
37°C. The reaction was stopped by addition of acidified Triton
buffer [0.1 M HCl in 10% (v/v) Triton X-100; 50 μl/well],
and tetrazolium crystals were dissolved by mixing on a plate shaker
at room temperature for 20 min. The samples were then measured on a
plate reader (Bio-Rad 450; Bio-Rad Laboratories, Richmond, CA, USA)
at a test wavelength of 595 nm and a reference wavelength of 650
nm. The results are representative of experiments repeated at least
in triplicate.
Silencing EF1α and AMPK
MCF7 cells were seeded in 6-well plates and allowed
to grow to 70% confluence for 24 h. Transient transfections were
performed with the transfection reagent (Lipofectamine 2000;
Invitrogen, Paisley, UK) as per the manufacturer's instructions. In
brief, EF1α (NM_001013367; Dharmacon, Lafayette, CO, USA), AMPKα1,
and non-targeted control siRNAs were designed. siRNA (5 μl)
and transfection reagent (5 μl; Lipofectamine 2000) were
each diluted in 95 μl of the reduced serum medium (Opti-MEM;
Invitrogen) and then mixed. The mixtures incubated for 30 min at
room temperature and then added in drops to each culture well
containing 800 μl of the reduced serum medium (Opti-MEM;
final siRNA concentration, 100 nM). The medium was replaced with a
fresh medium at 4 h after transfection. The cells were cultivated
for 24 h, lysed, and the expression of AMPKα1 protein was assayed
by western blotting.
Semi-quantitative RT-PCR
First-strand cDNA synthesis was performed using 1
μg of total RNA isolated from frozen tissues at 55°C for 20
min using a ThermoScript II One-Step RT-PCR kit (Invitrogen).
Amplification of cDNA was performed in the same tube using the ABI
GeneAmp PCR System 9700 thermal cycler (Applied Biosystems,
Warrington, UK). The reverse transcriptase was inactivated by
heating to 94°C. The following PCR conditions were used: 27 cycles
for 30 sec at 94°C, 30 sec at 56°C and 30 sec at 72°C, followed by
7 min at 72°C. The number of PCR cycles used was optimized to
ensure amplification in the exponential phase. Samples (10
μl) of each RT-PCR product were analyzed by agarose gel
electrophoresis. The bands were stained with ethidium bromide and
visualized under ultraviolet light. Band intensity quantification
was determined by a gel documentation system (Gene Genius; Syngene,
UK). The following primers were used: EF1α sense,
5′-GATATGGTTCCTGGCAAGCCC-3′ and antisense,
5′-CATTTAGCCTTGTGAGCTTTC-3′; GAPDH sense,
5′-ATTTGGTCGTATTGGGCGCCTGGTCACC-3′ and antisense,
5′-GAAGATGGTGATGGGATTTC-3′.
Data analysis
The data are expressed as mean ± SEM Statistical
analyses were conducted using SigmaStat (SPSS Inc., Chicago, IL,
USA). Differences were considered significant at P-value
<0.05.
Results
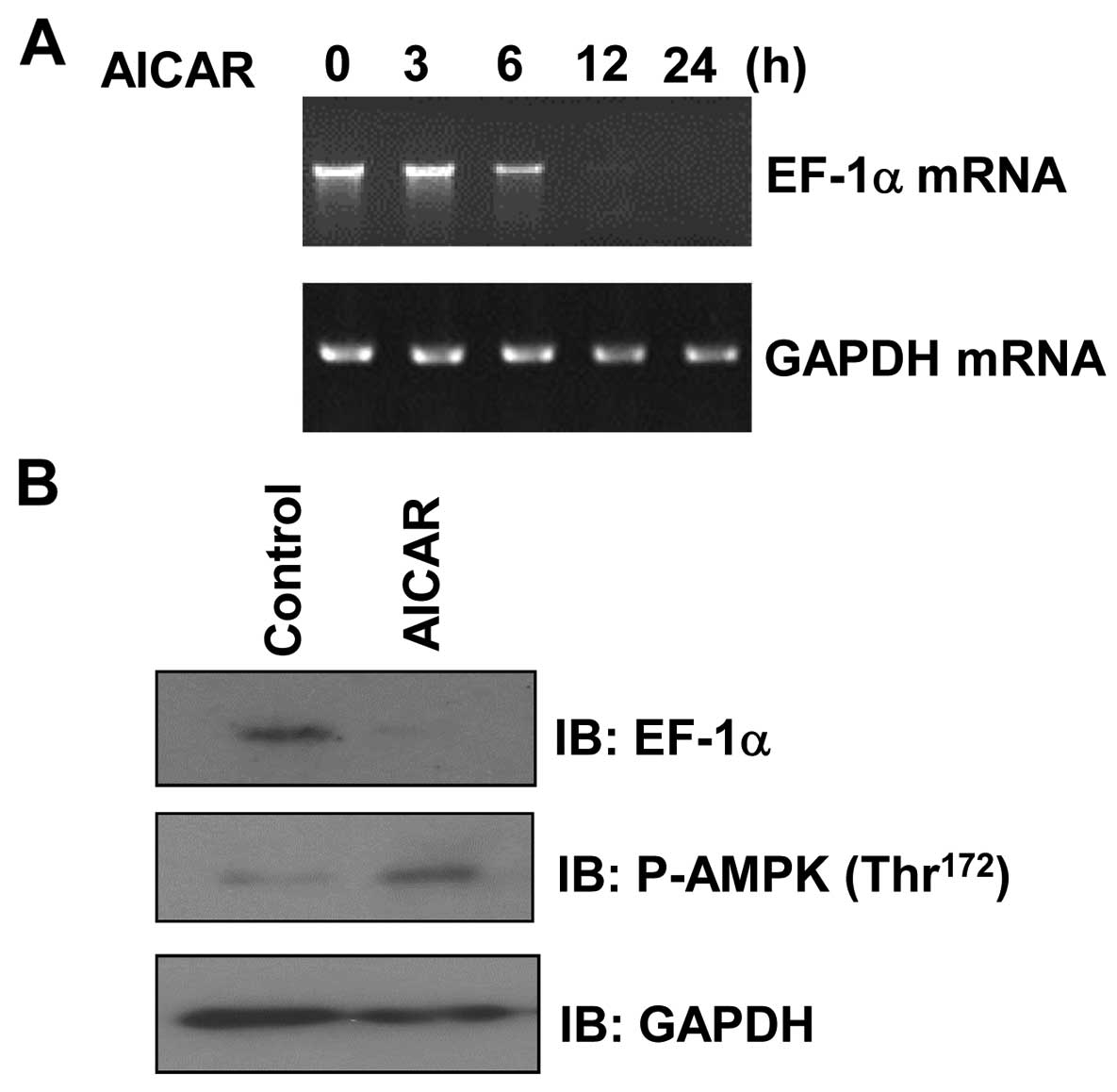
AICAR downregulates EF1α expression in
MCF7 cells
To ascertain whether the activity of AMPK affects
EF1α expression, MCF7 cells were treated with AICAR, a
pharmacologic activator of AMPK, and then analyzed by RT-PCR. We
observed that EF1α mRNA levels were decreased in a
time-dependent manner following AICAR treatment (Fig. 1A). In addition, examination of EF1α
protein levels in AICAR-treated MCF7 cells by western blotting
revealed that EF1α expression was significantly decreased following
incubation with AICAR compared with the expression under control
conditions (Fig. 1B).
Phosphorylation of AMPK was increased by AICAR treatment,
confirming the appropriate conditions of our assay. Taken together,
these results indicate that AMPK can suppress the expression of
EF1α in MCF7 cells.
Paclitaxel activates AMPK
EF1α is known to be critical for breast tumor cell
maintenance. Thus, we hypothesized that AMPK-mediated
downregulation of EF1α has an anticancer effect. To test this
hypothesis, we determined whether EF1α is involved in
paclitaxel-stimulated breast cancer MCF7 cell apoptosis. The
phosphorylation status of AMPK in pacli-taxel-treated MCF7 cells
was examined to determine whether the effects of paclitaxel are
mediated by AMPK activation. We performed western blotting using a
phosphorylation-specific (Thr172) AMPK antibody and
found that the level of AMPK phosphorylation in paclitaxel-treated
MCF7 cells increased in a time- and dose-dependent manner compared
the level in the controls (Fig. 2A and
B). This observation provided further indirect evidence of
increased AMPK phosphorylation following the treatment of MCF7
cells with paclitaxel.
Paclitaxel downregulates EF1α and
suppresses the viability of MCF7 cells
EF1α not only functions as a translation factor but
also binds to and severs microtubules (17,18).
To better understand the role of EF1α in MCF7 cells, we determined
EF1α levels in paclitaxel-treated MCF7 cells by western blotting.
We found that EF1α expression was decreased in a dose-dependent
manner by paclitaxel (Fig. 3A).
Paclitaxel also downregulated the expression of EF1α in a
time-dependent manner (Fig. 3B).
Then, the effect of paclitaxel on cell viability was assessed.
Treatment with paclitaxel resulted in a reduction of cell viability
in a dose-dependent manner (Fig.
3C). Paclitaxel also activated caspase-3, a key mediator of
apoptosis (Fig. 3D). These results
suggest that paclitaxel suppresses MCF7 cell viability through the
downregulation of EF1α expression.
Paclitaxel induces FOXO3a expression and
increases FOXO3a phosphorylation in an AMPK-dependent manner
FOXO3a belongs to the forkhead family of
transcription factors, which are characterized by a distinct
forkhead DNA-binding domain. This protein functions as a trigger
for apoptosis and is known to be a tumor suppressor. To evaluate
the activation of FOXO3a by paclitaxel, we analyzed the changes in
FOXO3a levels in paclitaxel-treated MCF7 cells. The expression of
FOXO3a and p21Waf1, its downstream effector molecule,
was induced by paclitaxel treatment (Fig. 4A). A similar pattern of FOXO3a
expression was also observed after AICAR treatment (Fig. 4B). Furthermore, FOXO3a
phosphorylation was transiently increased by paclitaxel treatment
(Fig. 4C). To determine whether
AMPK activity was involved in the effect of paclitaxel, we
investigated FOXO3a phosphorylation following treatment with 10
μM compound C, an AMPK inhibitor. Paclitaxel-induced
phosphorylation of FOXO3a was inhibited in cells pretreated with
compound C (Fig. 4D). These
results indicate that FOXO3a is involved in the
paclitaxel-stimulated signaling pathway in an AMPK-dependent
manner.
AMPK silencing blocks paclitaxel-induced
EF1α downregulation and FOXO3a induction
To explore the role of AMPK in the
paclitaxel-activated biochemical pathway, we investigated the
expression of EF1α and FOXO3 in AMPK-silenced cells. The expression
of AMPKα1 was downregulated by AMPKα1 siRNA (Fig. 5A). The expression of EF1α and
FOXO3a were suppressed in AMPK knockdown cells (Fig. 5B), suggesting the involvement of
these molecules in AMPK-mediated signaling. To confirm the role of
AMPK in the regulation of FOXO3a and EF1α, we examined the effect
of AMPK silencing on FOXO3a and EF1α levels in paclitaxel-treated
cells. We found that paclitaxel failed to upregulate FOXO3a in
AMPKα knockdown cells (Fig. 5B).
Similarly, paclitaxel did not decrease EF1α levels in AMPKα
knockdown cells. These results indicated that AMPK plays an
important role in the regulation of EF1α and FOXO3a levels via
paclitaxel-activated signaling.
EF1α silencing potentiates the induction
of FOXO3a by paclitaxel
To confirm the role of EF1α in the regulation of
cell viability and to exclude unrelated effects due to EF1α, we
transfected MCF7 cells with double-stranded EF1α siRNA
oligonucleotides. The cells were harvested 24 h after transfection,
and EF1α expression was analyzed by western blotting. EF1α-specific
siRNA completely suppressed EF1α expression, whereas scrambled
siRNA (negative control) had no effect on EF1α levels (Fig. 6A). In addition, EF1α silencing
increased the expression of caspase-3 and FOXO3a (Fig. 6A), key molecules for apoptosis,
suggesting that EF1α may regulate apoptotic cell death. Moreover,
knockdown of EF1α potently enhanced the induction of FOXO3a by
paclitaxel (Fig. 6B). These
results indicate that EF1α is essential for activation of the
FOXO3a pathway by paclitaxel.
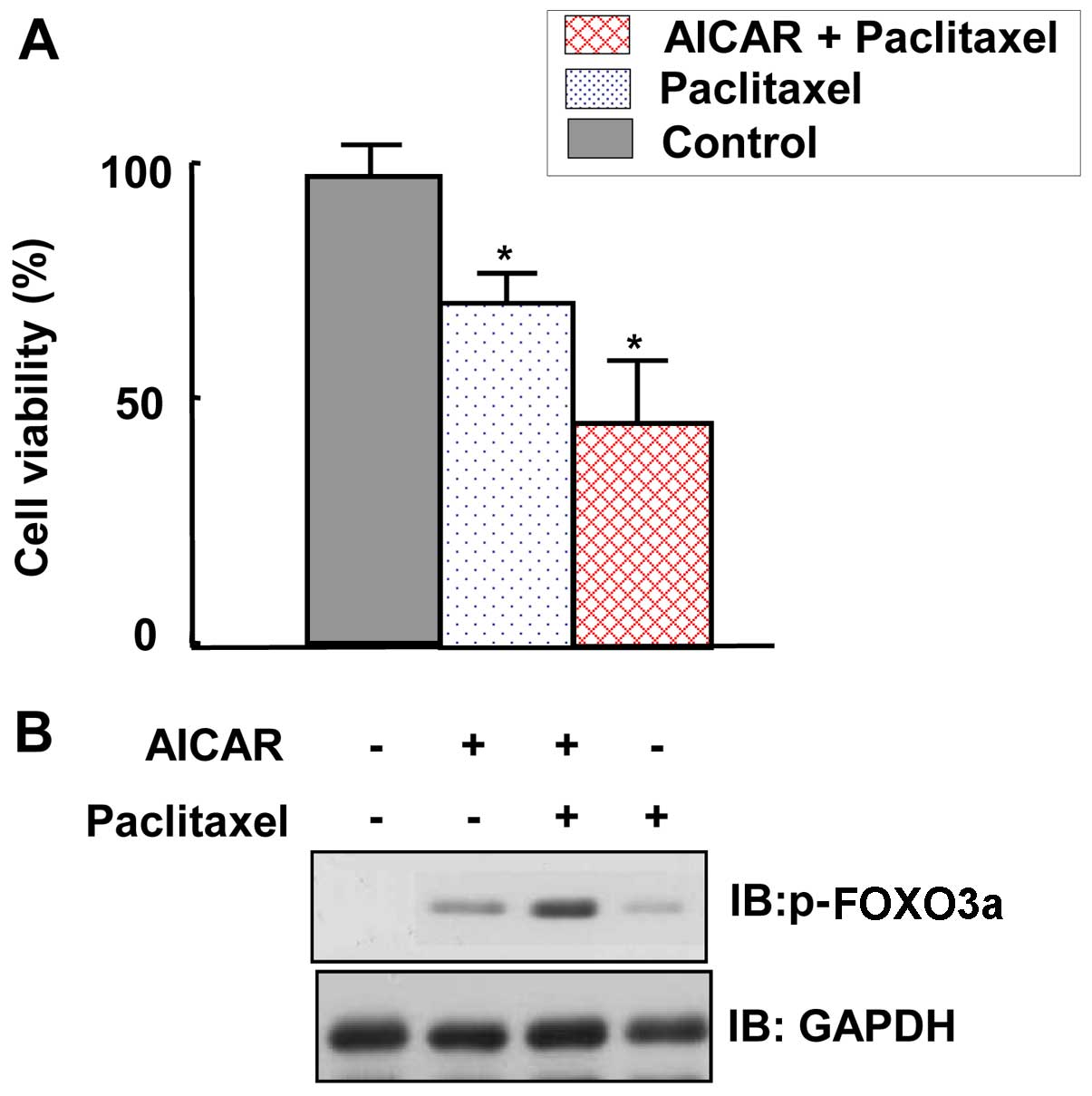
AMPK activation sensitizes MCF7 cells to
the suppression of their viability and induction of FOXO3a by
paclitaxel
To ascertain the role of AMPK in maintaining the
viability of cancer cells, MTT assays were performed. The viability
of MCF7 cells was inhibited by paclitaxel treatment (Fig. 7A). Moreover, co-treatment with
AICAR further inhibited the viability of MCF7 cells (Fig. 7A). To gain insight into the
mechanism by which paclitaxel suppresses MCF7 viability, FOXO3a
expression levels were examined. We observed that FOXO3a expression
was further increased by co-application of AICAR and paclitaxel
compared with the expression following treatment with paclitaxel
alone (Fig. 7B). In conclusion,
these results indicate that AMPK activation increased the
susceptibility of MCF7 cancer cells to the adverse effects of
paclitaxel on cell viability.
Discussion
In the present study, we demonstrated that
paclitaxel activates AMPK and that this phenomenon may contribute
to the suppression of breast tumor viability. The relationship
between AMPK and paclitaxel has raised several questions regarding
the mechanism by which paclitaxel can suppress tumor growth. The
results of the present study suggest that the EF1α/FOXO3 pathway
stimulated by paclitaxel may play a critical role in the
suppression of tumor cells.
The aim of this study was to determine whether the
activity of AMPK is directly regulated by paclitaxel, and if so, to
uncover the molecules and signaling pathways involved in this
regulation. The principal finding of this study is that EF1α is
involved in the suppression of breast tumor MCF7 cell viability
caused by paclitaxel. In addition, the results showed that EF1α is
highly overexpressed in several tumor cell lines, including MCF7.
The expression of EF1α is sensitive to the knockdown of AMPK and
combined application of AICAR and paclitaxel. Considering our
observations and the findings of previous studies that the AMPK
pathway regulates transcription factors including EF1α (16,17),
these results implicate the interaction between AMPK and EF1α in
the suppression of tumor viability by paclitaxel. EF1α is known to
be associated with microtubules. Given that paclitaxel is a
microtubule stabilizer, our results suggest that EF1α plays a
critical role in the downstream cascades of AMPK by regulating
cellular phenomena involving microtubules.
AMPK affects FOXO3a through a complex mechanism.
FOXO3a is known to be a sensor of energy levels. AMPK, also a key
cellular energy sensor, may phosphorylate FOXO3a in response to
alterations in energy demands, thereby controlling protein
stability. In the present study, we observed that paclitaxel
increases the expression and phosphorylation of FOXO3a. Given that
one of the AMPK phosphorylation sites on FOXO3a (Thr179)
is located in the DNA-binding domain, FOXO3a phosphorylation may
affect the ability of this protein to bind DNA. Because the
adenosine analog AICAR, an AMPK activator, augments FOXO3 levels,
it is possible that AMPK may also transcriptionally regulate
FOXO3.
We have demonstrated that paclitaxel treatment
activates AMPK in breast tumor cells and that the EF1α/FOXO3
pathway is regulated by AMPK. These results suggest that the
AMPK/EF1α/FOXO3 pathway may exert a profound influence on
paclitaxel-induced inhibition of tumor viability. Future studies
will be necessary to fully elucidate the role of AMPK in
paclitaxel-activated signaling.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea, funded by the Korean Government (no.
NRF-2013R1A2A2A05004796).
Abbreviations:
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
FOXO3a
|
forkhead box O3a
|
|
EF1α
|
elongation factor 1 α
|
|
AICAR
|
5-aminoimidazole-4-carboxy-amide-1-D-ribofuranoside
|
References
|
1
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horwitz SB, Lothstein L, Manfredi JJ,
Mellado W, Parness J, Roy SN, Schiff PB, Sorbara L and Zeheb R:
Taxol: Mechanisms of action and resistance. Ann N Y Acad Sci. 466(1
Dynamic Aspec): 733–744. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmes FA: Combination chemotherapy with
Taxol (paclitaxel) in metastatic breast cancer. Ann Oncol. 5(Suppl
6): S23–S27. 1994.PubMed/NCBI
|
|
5
|
Beer TM, El-Geneidi M and Eilers KM:
Docetaxel (taxotere) in the treatment of prostate cancer. Expert
Rev Anticancer Ther. 3:261–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markman M: Taxol: An important new drug in
the management of epithelial ovarian cancer. Yale J Biol Med.
64:583–590. 1991.PubMed/NCBI
|
|
7
|
Ettinger DS: Overview of paclitaxel
(Taxol) in advanced lung cancer. Semin Oncol. 20(Suppl 3): 46–49.
1993.PubMed/NCBI
|
|
8
|
Horwitz SB: Taxol (paclitaxel): Mechanisms
of action. Ann Oncol. 5(Suppl 6): S3–S6. 1994.PubMed/NCBI
|
|
9
|
Hardie DG, Carling D and Carlson M: The
AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of
the eukaryotic cell? Annu Rev Biochem. 67:821–855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hardie DG: Minireview: the AMP-activated
protein kinase cascade: the key sensor of cellular energy status.
Endocrinology. 144:5179–5183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hardie DG and Sakamoto K: AMPK: A key
sensor of fuel and energy status in skeletal muscle. Physiology
(Bethesda). 21:48–60. 2006. View Article : Google Scholar
|
|
12
|
Garcia-Gil M, Pesi R, Perna S, Allegrini
S, Giannecchini M, Camici M and Tozzi MG:
5′-aminoimidazole-4-carboxamide riboside induces apoptosis in human
neuroblastoma cells. Neuroscience. 117:811–820. 2003. View Article : Google Scholar
|
|
13
|
Kefas BA, Cai Y, Kerckhofs K, Ling Z,
Martens G, Heimberg H, Pipeleers D and Van de Casteele M:
Metformin-induced stimulation of AMP-activated protein kinase in
beta-cells impairs their glucose responsiveness and can lead to
apoptosis. Biochem Pharmacol. 68:409–416. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meisse D, Van de Casteele M, Beauloye C,
Hainault I, Kefas BA, Rider MH, Foufelle F and Hue L: Sustained
activation of AMP-activated protein kinase induces c-Jun N-terminal
kinase activation and apoptosis in liver cells. FEBS Lett.
526:38–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saitoh M, Nagai K, Nakagawa K, Yamamura T,
Yamamoto S and Nishizaki T: Adenosine induces apoptosis in the
human gastric cancer cells via an intrinsic pathway relevant to
activation of AMP-activated protein kinase. Biochem Pharmacol.
67:2005–2011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talukder AH, Jorgensen HF, Mandal M,
Mishra SK, Vadlamudi RK, Clark BF, Mendelsohn J and Kumar R:
Regulation of elongation factor-1alpha expression by growth factors
and anti-receptor blocking antibodies. J Biol Chem. 276:5636–5642.
2001. View Article : Google Scholar
|
|
17
|
Bourne HR, Sanders DA and McCormick F: The
GTPase superfamily: Conserved structure and molecular mechanism.
Nature. 349:117–127. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Vos AM, Tong L, Milburn MV, Matias PM,
Jancarik J, Noguchi S, Nishimura S, Miura K, Ohtsuka E and Kim SH:
Three-dimensional structure of an oncogene protein: Catalytic
domain of human c-H-ras p21. Science. 239:888–893. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jurnak F: Structure of the GDP domain of
EF-Tu and location of the amino acids homologous to ras oncogene
proteins. Science. 230:32–36. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
la Cour TF, Nyborg J, Thirup S and Clark
BF: Structural details of the binding of guanosine diphosphate to
elongation factor Tu from E. coli as studied by X-ray
crystallography. EMBO J. 4:2385–2388. 1985.PubMed/NCBI
|
|
21
|
Pai EF, Krengel U, Petsko GA, Goody RS,
Kabsch W and Wittinghofer A: Refined crystal structure of the
triphosphate conformation of H-ras p21 at 1.35 A resolution:
Implications for the mechanism of GTP hydrolysis. EMBO J.
9:2351–2359. 1990.PubMed/NCBI
|
|
22
|
Valencia A, Chardin P, Wittinghofer A and
Sander C: The ras protein family: Evolutionary tree and role of
conserved amino acids. Biochemistry. 30:4637–4648. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ann DK, Moutsatsos IK, Nakamura T, Lin HH,
Mao PL, Lee MJ, Chin S, Liem RK and Wang E: Isolation and
characterization of the rat chromosomal gene for a polypeptide
(pS1) antigenically related to statin. J Biol Chem.
266:10429–10437. 1991.PubMed/NCBI
|
|
24
|
Lee S, Francoeur AM, Liu S and Wang E:
Tissue-specific expression in mammalian brain, heart, and muscle of
S1, a member of the elongation factor-1 alpha gene family. J Biol
Chem. 267:24064–24068. 1992.PubMed/NCBI
|
|
25
|
Lee S, LeBlanc A, Duttaroy A and Wang E:
Terminal differentiation-dependent alteration in the expression of
translation elongation factor-1 alpha and its sister gene, S1, in
neurons. Exp Cell Res. 219:589–597. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanders J, Brandsma M, Janssen GM, Dijk J
and Möller W: Immunofluorescence studies of human fibroblasts
demonstrate the presence of the complex of elongation factor-1 beta
gamma delta in the endoplasmic reticulum. J Cell Sci.
109:1113–1117. 1996.PubMed/NCBI
|
|
27
|
Taniguchi S, Miyamoto S, Sadano H and
Kobayashi H: Rat elongation factor 1 alpha: Sequence of cDNA from a
highly metastatic fos-transferred cell line. Nucleic Acids Res.
19:69491991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y,
Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al: FoxOs are
lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakae J, Biggs WH III, Kitamura T, Cavenee
WK, Wright CV, Arden KC and Accili D: Regulation of insulin action
and pancreatic beta-cell function by mutated alleles of the gene
encoding forkhead transcription factor Foxo1. Nat Genet.
32:245–253. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogg S, Paradis S, Gottlieb S, Patterson
GI, Lee L, Tissenbaum HA and Ruvkun G: The Fork head transcription
factor DAF-16 transduces insulin-like metabolic and longevity
signals in C. elegans. Nature. 389:994–999. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin K, Dorman JB, Rodan A and Kenyon C:
daf-16: An HNF-3/forkhead family member that can function to double
the life-span of Caenorhabditis elegans. Science. 278:1319–1322.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwangbo DS, Gershman B, Tu MP, Palmer M
and Tatar M: Drosophila dFOXO controls lifespan and regulates
insulin signalling in brain and fat body. Nature. 429:562–566.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giannakou ME, Goss M, Jünger MA, Hafen E,
Leevers SJ and Partridge L: Long-lived Drosophila with
overexpressed dFOXO in adult fat body. Science. 305:3612004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furuyama T, Yamashita H, Kitayama K,
Higami Y, Shimokawa I and Mori N: Effects of aging and caloric
restriction on the gene expression of Foxo1, 3, and 4 (FKHR,
FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech.
59:331–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xuan Z and Zhang MQ: From worm to human:
Bioinformatics approaches to identify FOXO target genes. Mech
Ageing Dev. 126:209–215. 2005. View Article : Google Scholar
|
|
36
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dijkers PF, Medema RH, Lammers JW,
Koenderman L and Coffer PJ: Expression of the pro-apoptotic Bcl-2
family member Bim is regulated by the forkhead transcription factor
FKHR-L1. Curr Biol. 10:1201–1204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kops GJ, Dansen TB, Polderman PE, Saarloos
I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH and Burgering
BM: Forkhead transcription factor FOXO3a protects quiescent cells
from oxidative stress. Nature. 419:316–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nemoto S, Fergusson MM and Finkel T:
Nutrient availability regulates SIRT1 through a forkhead-dependent
pathway. Science. 306:2105–2108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tran H, Brunet A, Grenier JM, Datta SR,
Fornace AJ Jr, DiStefano PS, Chiang LW and Greenberg ME: DNA repair
pathway stimulated by the forkhead transcription factor FOXO3a
through the Gadd45 protein. Science. 296:530–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee SS, Kennedy S, Tolonen AC and Ruvkun
G: DAF-16 target genes that control C. elegans life-span and
metabolism. Science. 300:644–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murphy CT, McCarroll SA, Bargmann CI,
Fraser A, Kamath RS, Ahringer J, Li H and Kenyon C: Genes that act
downstream of DAF-16 to influence the lifespan of Caenorhabditis
elegans. Nature. 424:277–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mammucari C, Milan G, Romanello V, Masiero
E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J,
et al: FoxO3 controls autophagy in skeletal muscle in vivo. Cell
Metab. 6:458–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao J, Brault JJ, Schild A, Cao P, Sandri
M, Schiaffino S, Lecker SH and Goldberg AL: FoxO3 coordinately
activates protein degradation by the autophagic/lysosomal and
proteasomal pathways in atrophying muscle cells. Cell Metab.
6:472–483. 2007. View Article : Google Scholar : PubMed/NCBI
|