1. Introduction
The pancreas is a retroperitoneal organ. The
peripancreatic nerves form a structure of network arranged in a
crisscross pattern like clouds in the retroperitoneum, spreading
along the abdominal aorta and its main branches, around the celiac
artery and at the root of the superior mesenteric artery within the
retroperitoneal soft tissues. In addition to the common biological
behavior of cancers that come with direct invasion, hematogenous
spread and lymphatic metastasis, pancreatic cancers have unique
neurotropic growth characteristics which result in early perineural
invasion. Some scholars (1)
believe in neural invasion (NI), tumor cells grow along a nerve in
any layer including the endoneurium, perineurium or epineurium.
However, the exact mechanism of how NI occurs is still unclear
(2). One theory proposes that NI
occurs in a low resistance fissure between the pancreas and the
nervous system. When tumor cells infiltrate the epineurium, immune
injury by invasive tumor cells significantly alter the
microenvironment making it further conducive to tumor invasion and
metastasis along the neurons, eventually causing pain (3). Other studies show NI occurs because
of secretion of neurotransmitters between the tumor cells and the
nerves which attract tumor cells. Many signal molecules, including
neurotrophic factors, cytokines, and cell surface ligands/receptors
are involved in NI. Intraoperative nerve dissection methods, scope,
and postoperative treatment have been the main foci in pancreatic
cancer research to study the basis of pancreatic neural invasion
and the mechanisms of neural invasion.
2. Anatomy and factors related to neural
invasion of pancreatic carcinomas
The pancreas has a rich nerve supply coming from the
internal pancreatic nerve, extrapancreatic nerve and peripancreatic
nerve. The internal pancreatic nerve is branched and it travels
interlobularly, being accompanied by both the pancreatic vessels
and pancreatic duct. Its nerve endings are distributed among the
acinar pancreatic cells, creating the necessary infrastructure to
meet the requirements of neurotropic growth. The extrapancreatic
nerve originates from the right celiac ganglion, goes through the
hepatic plexus and the right side of the celiac plexus to form
specifically the pancreatic head nerve. A thorough understanding of
the classification and distribution of the peripancreatic nerve is
critical in pancreatic resection and nerve dissection. During
treatment, innervation of the pancreas should be viewed from the
perspective of perineural invasion by the pancreatic cancer. A
study using post-mortem dissection to observe the nerve fiber
distribution in the pancreas and its relationship to tumor invasion
was carried out on 9 patients who succumbed to pancreatic cancer
(4). The present study found the
pancreatic nerve to originate in the region of the superior
mesenteric artery. The nerve then runs along the inferior
pancreaticoduodenal artery, but not forming large nerve bundles to
innervate the pancreatic head. For the pancreatic body and tail,
nerve fibers originate from the celiac plexus and go straight into
the pancreatic tissues after leaving the celiac plexus, finally
branching along the pancreatic duct to form the basis of the
anatomical structure of the neural pancreatic plexus (Fig. 1).
3. Factors associated with neural invasion
of pancreatic carcinoma
Lymph nodes, blood vessels and lymphatic
invasion
The relationship between peripancreatic nerve
invasion and lymph node metastasis is controversial, but the
current thinking is that nerve infiltration is associated with the
peripheral lymphatic network structure and nerve distribution
(3). Studies have found carcinomas
presenting with lymph node metastases are closely related to the
incidence of pancreatic nerve invasion. Pancreatic cancer can
easily invade the lymphatic system to violate the peripheral nerve.
In the superior mesenteric artery (SMA) with peripheral nerve
plexus within the reticular lymphatic capillary distribution,
pancreatic cancer neural invasion may have a close relationship
with lymphangiogenesis (5).
The nature of the tumor
At present, the majority of researchers believe that
perineural invasion of pancreatic carcinomas has nothing to do with
tumor size and degree of lymphatic invasion, but instead is related
to a certain extent to tumor location and its embryologic
differentiation (6). In addition,
the number of tumor interstitial tissues may also influence the
incidence of neural invasion. The interstitial tissues may play an
important role in pancreatic cancer neuropathology.
Hyperglycemia
Diabetes is present in 34–40% of patients with
pancreatic cancer, and it has often been diagnosed only in these
patients (7). Long-term diabetes
is considered to be one of the pathogenic factors of pancreatic
cancer, and a recent onset of diabetes may be a manifestation of
the cancer (8). With fasting
glucose levels, for each additional 0.56 mmol/l (10 mg/dl), the
corresponding risk of pancreatic cancer increases by roughly 14%
(9). A significant increase in
diagnostic frequency of pancreatic cancer has subsequently been
associated with a recent diagnosis of diabetes (10). Research has also confirmed that
hyperglycemia may be related to neural invasion of pancreatic
cancer. In general, cancer cells, high blood sugar, and presence of
nerves are three factors that exist in the pancreatic cancer
microenvironment; and cancer is the result of interactions among
these three factors (11,12).
4. Molecular biological mechanism of neural
invasion in pancreatic carcinomas
With recent in-depth study of tumor neurobiology, a
series of studies have shown that many biological molecules and
signaling pathways are closely associated with NI in pancreatic
cancer, pointing to a molecular mechanism for NI (3). Relevant pathways include GDNF-RET
(13,14), NGF-TrkA, Hedgehog (Hh) (15,16),
CX3CR1 stimulation, ATDC (17),
vitamin D receptor (18) and
matrix metalloproteinases (MMP). The combined effects of NI,
secondary tumor masses, destruction of and abnormal regeneration of
neurons, and angiogenesis are important factors that signal a poor
prognosis in pancreatic cancer.
5. Composition of pancreatic tumor
microenvironment
The tumor microenvironment is an integrated system
composing of tumor cells, stromal cells (e.g. endothelial cells),
infiltrating cells (e.g. macrophages and lymphocytes) and the
products that they release (19).
The tumor microenvironment directly affects the characteristics of
NI, and NI changes the microenvironment and the interactions
between the tumor and the microenvironment. Therefore, studying the
relationship between these factors has important clinical
significance (Fig. 2).
Cancer-associated fibroblasts
Pancreatic stellate cells (PSCs) are a type of
fibroblast cells that surround the pancreatic lobule and pancreatic
acini (20–22). Previous studies have shown that
during development of a pancreatic carcinoma, PSCs promote growth
and invasion of pancreatic cancer cells which in turn activate
PSCs. This constitutes a positive feedback loop that plays an
important role in the development of pancreatic carcinomas
(23).
The latest study found pancreatic tumor-associated
stromal cells to limit tumor development, rather than to promote
its development (24). In fact,
stromal cells appear to limit further damage through a protective
rebuilding mechanism. Even in the end-stages of the disease, the
body continues to take measures to try and stall disease
progression. However, for pancreatic cancer patients that present
with high levels of fibrosis, a combination of gemcitabine and
hedgehog inhibitor drugs used to deplete myofibroblasts improves
prognosis (25).
Previous treatments have called for complete removal
of fibrous tissue, which in all likelihood is not an ideal
treatment strategy. New findings may subvert traditional cancer
treatment strategies. Our current assumptions about cancer must be
re-examined, and only when we can distinguish cells that are
directly influenced by growing cancer cells, can we formulate
therapeutic strategies to remove cancerous cells with minimal harm.
At the same time, we need to make every effort to preserve the
beneficial surrounding cells not impacted by tumor to reduce total
damage.
Capillary network
Rapid growth of pancreatic tumor tissue requires a
rich blood supply. A low density of blood vessels places tumor
tissue in a hypoxic and nutrient-poor state, which requires
angiogenesis to meet its growing needs. Clinical studies show that
angiogenesis is related to rapid growth and poor prognosis in
patients with pancreatic cancer (26). Angiogenesis depends on a dynamic
balance between growth factors and inhibitors (27,28).
However, recent studies have found an interesting tumor treatment,
called ‘vascular promotion therapy’ which increases vessel leakage
and reduces tumor hypoxia, therefore, suggesting a potentially
enhanced intratumoural drug delivery and a reduction in
desmoplastic reaction. There is also an interesting approach to
cancer treatment by promoting, rather than inhibiting, vascular
formation. Treatment with a triple combination of
cilengitide-verpamil-gemcitabine reduced tumor burden and number of
metastases considerably, and these effects were sustained after
cessation of treatment (29).
Immune cells
In pancreatic cancer, there is obvious infiltration
by immune cells, but these immune cells do not appear to play a
role in immune surveillance (30–32).
Pancreatic cancer cells evade immune recognition through
modification of their surface antigens and changing the surrounding
microenvironment. Changes made to the pancreatic tumor
microenvironment largely attenuate the immune response to cancerous
cells.
Abundant deposition of extracellular
matrix
In the pancreatic tumor microenvironment, cancer
cells interact with stromal cells (33). During this process, cytokines
(34) and products influencing ECM
development, such as MMP (35),
TGF-β (36), HGF (37) and VEGF (38) also play an important role in the
formation of the tumor.
6. Tumor-neural microenvironment
The existing literature proposes the concept of a
tumor-neural microenvironment (23,39)
in that cancer cells and nerves constitute a microenvironment which
mutually promotes proliferation and inhibits apoptosis. This
microenvironment can promote the occurrence of NI, thus making the
tumor microenvironment a critical factor in the progression of
pancreatic cancer.
In the process of evolution, tumor cells gradually
form a favorable microenvironment to foster development, promote
tumor cells to grow towards nerve tissue and invade. At the same
time, nerve tissue has a specific (favorable) microenvironment to
include neurons, glial cells, and their expressed products to exert
chemotactic effects on cancer cells, thus promoting invasion.
Through NI, the interaction between tumor cells and nerve tissue
can further change the microenvironment, thus enhancing NI
(3).
As the microenvironment plays an important role in
cancer cell invasion and metastasis, changing the microenvironment
to reduce the invasiveness of cancer cells has theoretical
feasibility. However, since the prognosis is influenced by both
tumor and stromal cells, there is not sufficient evidence to show
that the microenvironment alone can cause or impair cancer cell
invasion. Furthermore, cancer cells exhibit great heterogeneity,
such that the ‘fittest’ cells are selected for survival, allowing
the tumor to constantly adapt to changes in the environment. Even
when therapeutic strategies successfully intercept the main
mechanism of pathogenesis, the problem is often not completely
resolved. After cessation of therapy, other (hidden) pathogenesis
mechanisms often surface and eventually lead to recurrence.
Therefore, the tumor microenvironment is a complex, dynamic network
(40), and currently only a few
individual factors related to the microenvironment have undergone
in depth study. Although the tumor microenvironment plays a complex
and important role in cancer pathogenesis, the total influence of
the stromal environment is currently poorly understood and
necessitates further studies.
7. Surgical treatment, adjuvant therapy, and
monitoring technology to follow neural invasion in pancreatic
carcinoma
The traditional Whipple operation focuses only on
excision of the tumor, which often results in inadequate extent of
resection and tumor clearance. Lymph node metastasis and pancreatic
nerve infiltration are important biological features that occur
frequently with pancreatic cancer. The keys to technical
improvements on the Whipple operation lie in extending lymph node
dissection, performing complete peripancreatic retroperitoneal
resection with or without portal vein/mesenteric vascular resection
and reconstruction, emphasizing on negative margins, removing all
metastatic lymph nodes, preventing peritoneal infiltration, and
utilizing microscopic resection techniques to achieve R0 resection
(41). Japanese scholars believe
that to include the tumor and the surrounding connective tissues,
lymphoid tissues, and nervous tissue into a radical resection is
the best treatment for pancreatic tumors at present. However, there
are serious limitations to such approaches: i) the clinical
technology currently available is limited and it is difficult to
definitively diagnose the range of neural invasion in the
preoperative and intraoperative periods. These predictive measures
often rely on lymph node metastasis, tissue grade, tumor size, and
other factors that may not accurately characterize the extent of
tumor progression; ii) complete resection of retroperitoneal nerve
plexus may lead to complications such as severe diarrhea and
malnutrition; iii) complete resection involves operating in a deep
surgical site with a small visual field and poor exposure in a
region surrounded by vital structures. Therefore, there is a
balance between the extent of radical resection with the likelihood
of recurrence, which is the main dilemma for surgeons. Studies
focusing on nerve invasion in pancreatic cancer may help to improve
the complete surgical resection rate in early cases, while reducing
the postoperative recurrence rate. With a better understanding of
the biological characteristics of neurotropic growth in pancreatic
cancer, a better decision can be made on the extent of resection
and a better long-term post-operative survival can be achieved.
8. Treatment of retroperitoneal nerve
dissection and vascular skeletonization
Need
Clearing the retroperitoneal nerve plexus,
especially the SMA peripheral nerve plexus during surgical
treatment of pancreatic cancer has a neuroanatomical basis.
Recurrences of pancreatic cancer after a Whipple
procedure has four patterns: liver metastasis, peritoneal
dissemination, retroperitoneal recurrence and distant metastasis.
The most important reason for retroperitoneal recurrence to occur
is because of perineural invasion. Reports show that >50% of
pancreatic resection specimens display obvious nerve infiltration.
Even after radical pancreaticoduodenectomy (PD), the local
retroperitoneal recurrence rate within one year post-surgery is
>80%. The most common site of recurrence is in soft tissues at
the rear of the pancreatic head. Therefore, pancreatic nerve
invasion has become an independent prognostic marker of long-term
survival for pancreatic cancer. The accuracy in prediction is
greater than using the T stage, involved lymph node counts, and
other indicators (42). In actual
fact, 20–40% of clinically resectable pancreatic cancers have been
pathologically confirmed to be non-radically resected. The main
reason is due to the residual margins caused by invasion of
peripancreatic plexus. To avoid nerve invasion through other
possible means (lymphangiogenesis), the main tumor should be
entirely resected (Fig. 3).
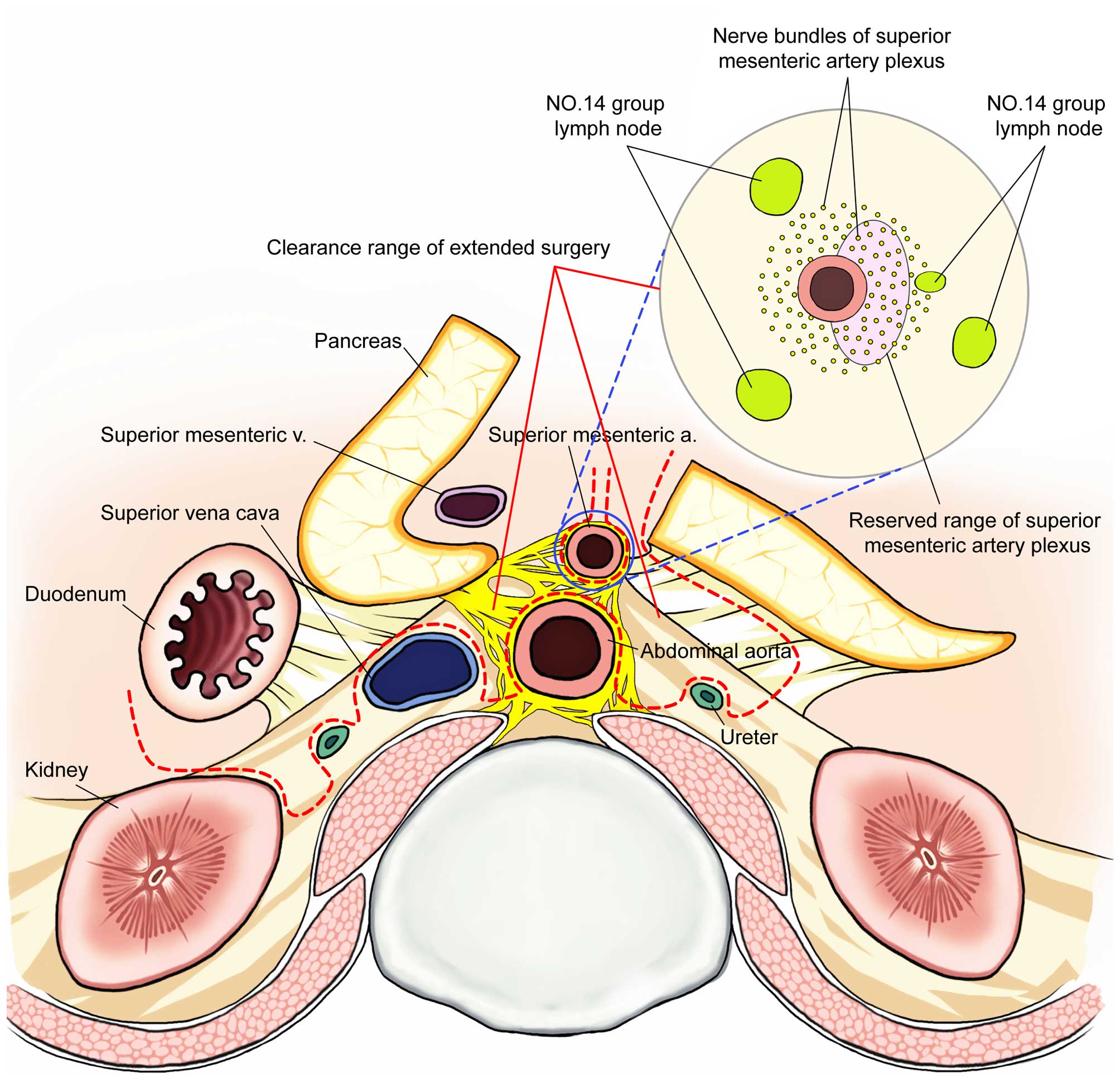 | Figure 3Clearance range of extended surgery
(dotted line represents the cutting line) + SMA clearance range
profile. 1, upper - lower; Plexus, celiac plexus (left, right
celiac ganglion), superior mesenteric artery plexus, plexus around
the abdominal aorta, inferior mesenteric artery plexus; Lymph
nodes, abdominal aortic hiatus - both sides of the abdominal aorta
~2 cm below the root of the inferior mesenteric artery; 2, before -
after; Lymph node, plexus: root of colon artery - superior
mesenteric artery - abdominal aorta: exposure to lumbar artery and
psoas muscle level (reserved sympathetic trunk); 3, right - left;
Right renal hilum - the inside of the left ureter. |
What to do?
i) Improvement of existing surgical
treatments: intraoperative detection, technology-assisted
clearance, left side semicircle clearance around the SMA, or total
mesopancreas excision (TMpE)/modified surgical excision
Auxiliary technology
In 10 patients who died of non-digestive diseases,
their corpses were used to show the extent of the NO. 14 group of
lymph nodes needed to be dissected to retain the SMA peripheral
plexus (43). The results showed
93.7% of the lymph nodes were located 3 mm away from the adventitia
of the SMA. The average distance between all the SMA lymph nodes
from the adventitia was 5.5+2.0 mm. The corresponding distances on
the right side and on the left side of the SMA were 5.8±2.1 and
5.3±1.9 mm, respectively. There was no significant difference
between the distances on the left and right sides. In addition, the
lymph node distributions in the horizontal direction and the
vertical direction of SMA were the same. On the other hand, the
average width of the nerve plexus wrapping around the SMA (left and
right side of the SMA) was 4.2+1.3 mm. Whereas, the positional
relationship between the lymph nodes and the nerve plexus in all
the 142 lymph nodes which completely wrap around the SMA plexus was
>8 (5.6%). The remaining lymph nodes were partially or entirely
located outside of the nerve plexus. For the 8 lymph nodes which
were embedded in the plexus, 7 (87.5%) had a distance of <3 mm
from the SMA arterial adventitia. Combining with the previous data,
nearly 94% of all the NO. 14 group of lymph nodes were outside of
the SMA peripheral nerve plexus. Thus it was shown that by only
retaining the 3-mm thick SMA plexus, almost all of the surrounding
lymph nodes can be cleared away, thus, supporting surgical
clearance of the NO. 14d group of lymph nodes is technically
feasible (43).
Some scholars believe that complete clearance of the
connective tissues surrounding the SMA is ideal for patients with
infiltrating pancreatic cancer. On taking into account the
resulting quality of life, only complete right sided semicircular
clearance of the SMA nerve plexus is recommended as these tissues
are especially vulnerable to cancer infiltration. As the patient
retains the left sided semicircular SMA nerve plexus, even after
completing a circumferential resection of the NO. 14 group of lymph
nodes, additional intraoperative irradiation may be necessary
(43).
There have been reports that show that
intraoperative ultrasound or IPUS (intraductal ultrasound) can be
used to determine whether the tumor has infiltrated the PV (portal
vein) or the pancreatic peripheral nerve plexus intraoperatively.
Using IPUS to evaluate invasion of the pancreatic head nerve
plexus, the sensitivity, specificity, and accuracy were 94, 98 and
97%, respectively (43).
The development of optogenetic technology helps to
identify areas to avoid dissecting into. Using optogenetic neuron
staining techniques to peroperatively identify the neural location
susceptible to injuries, fluorescence can prompt the surgeon to
avoid causing accidental injury intraoperatively. As ganglion
excision may result in postoperative gastrointestinal dysfunctions
and energy absorption disorders, its downstream neurons can be
transfected by photosensitive genes and illuminated to preserve
gastrointestinal motility and to maximize the patient's
post-operative quality of life.
Total mesopancreas excision (TMpE) and
modified surgical excision
Research shows that the long-term survival of
patients is influenced by the tumor biological characteristics,
patient health status, and whether R0 (curative) resection has been
achieved (44). Improvements in
surgical treatment of pancreatic head carcinomas aim to improve
both the surgical resection and the R0 resection rates.
Unfortunately, 20–86% of patients fail to achieve a real R0
resection (45–49). In clinical case studies when most
operations were considered to be ‘radical’/R0
pancreaticoduodenectomy, the positive specimen margin (R1
resection) rate with microscopic residual tumor visible under the
optical microscope was 30–40% (47). Although the specific definitions
and standards of R1 resection have not yet been internationally
standardized, residual cancer 1 mm from the cutting edge (as
visible under a light microscope) is recognized as one of the
criteria (50). This explains why
many patients with ‘R0 resection’ do not have long-term survival.
For patients with pancreatic head cancer who underwent
pancreaticoduodenectomy, the most common site of R1 residual cancer
is in the ‘mesopancreas’ (51,52).
Recently, total mesopancreas excision (TMpE) has been implemented
and this may help to increase the R0 resection rate and to improve
patient's prognosis (53). Verbeke
(50) pointed out that the
mesopancreas is the most important part of the retroperitoneal
resection margin, and according to this standard, the pancreatic
carcinoma R1 resection rate reached 85% through improved
pathological examination. Therefore, the retroperitoneal margin is
the most critical margin related to R0 resection, and the fact that
R1 resection has been misjudged as R0 resection in pancreatic head
cancers is the main reason why ‘R0 resections’ have poor results
(53).
First described in 2007, the mesopancreas (54) includes the dorsal pancreatic nerve
and the lymph tissue layer. Total mesopancreas excision (TMpE) is
based on the principle of total mesorectal excision (TME) and
includes the peripancreatic lymphatic and adipose tissue layers in
an en bloc resection (55). In
order to achieve a negative retroperitoneal margin, unlike TME,
TMpE does not follow a fixed tissue clearance using complete
resection of a predetermined interstitial structure or organ
range.
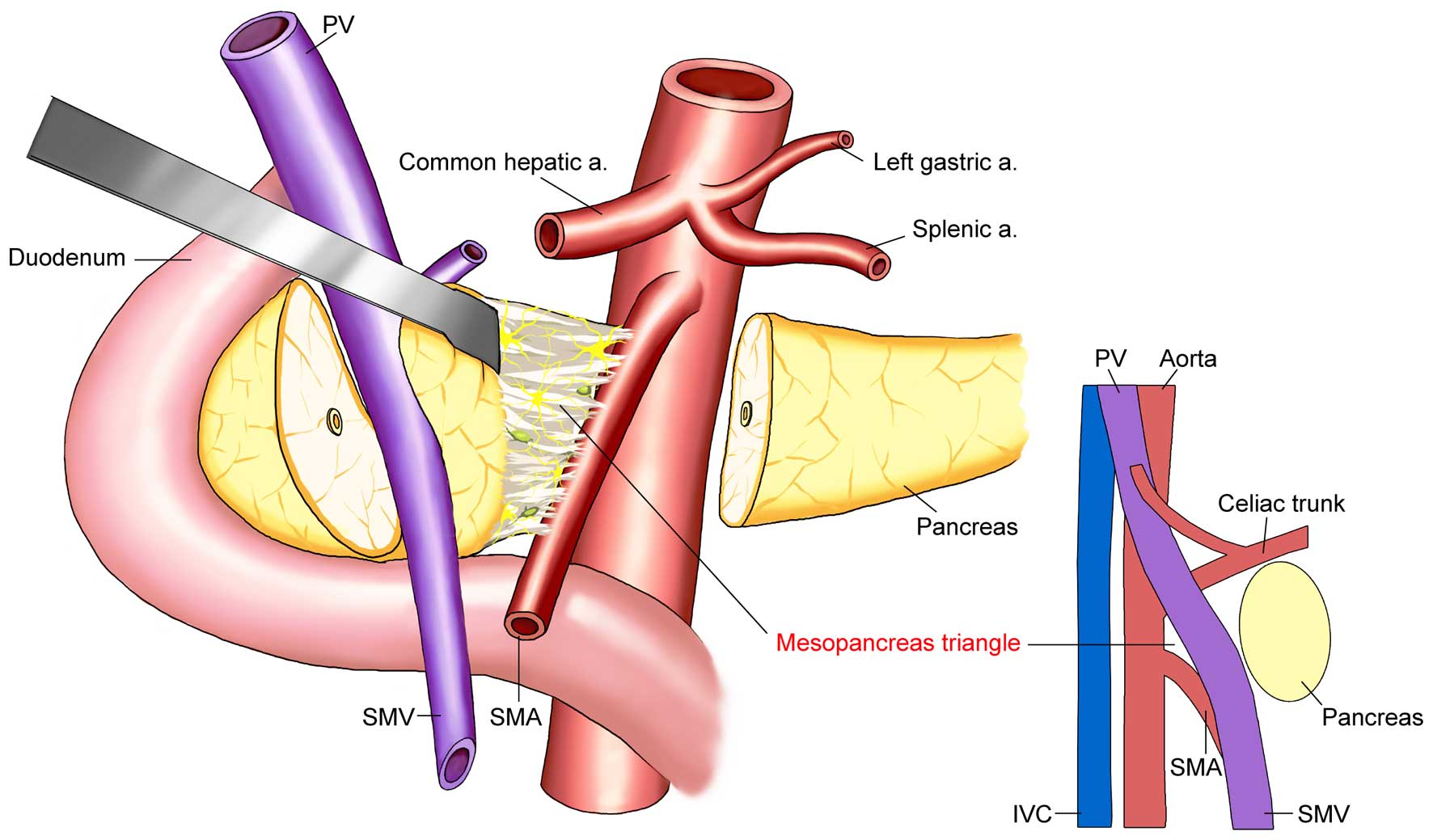
The concept of the mesopancreas triangle was
proposed to define the scope of mesopancreas excision (53). The base of the triangle is the
superior mesenteric vein and the rear of the portal vein, while the
vertex is in the front of the abdominal aorta, between the celiac
axis and the starting point of the superior mesenteric artery, to
include the superior mesenteric artery and the right side of the
semicircle of the celiac plexus. These structures form an inverted
triangle (Fig. 4). The range of
resection required by TMpE is partially covered by regional lymph
node dissection, but TMpE emphasizes the en bloc resection with
soft tissues behind the head of the pancreas to include the lymph
nodes and plexus. This is done to complete the surgical field with
a three-dimensional (3D) negative edge to achieve a real R0
resection. According to the pathological examination of
intraoperatively-obtained and labeled specimens, the number of
cancer positive pancreatic mesopancreas samples in patients with
pancreatic head carcinomas was 23%, while the median number of
lymph nodes dissected was 24, and the overall R0 resection rate was
as high as 80% after TMpE (53).
However, the notion that the pancreatic mesopancreas
exists and is involved is not universally accepted at present. Many
surgeons believe that there is no fascia surrounding the pancreas
and/or that it is not involved in the general or fascia tissue
pathology. Therefore, there is no true concept of the pancreatic
mesopancreas anatomically (52).
However, clinical studies have shown that this structure does exist
as a ‘category mesopancreas organization’ (56). The posterior pancreatic head,
superior mesenteric artery, celiac trunk, and the soft tissue
around the abdominal aorta margin lack uniform definitions, yet,
they comprise the region that is the most common site of invasion
and metastasis in pancreatic head carcinomas. The objective facts
that residual tumors and local recurrence often occur in this area
after surgical resection help to define this area as the pancreatic
mesopancreas, even though there may not be any enveloping fascia.
Clearer definitions and recognition of this area can help to
standardize the rate of radical cure of pancreatic head cancer and
improve efficacy. The principles of TMpE emphasize that pancreatic
head carcinoma resection should be combined with en bloc of this
area to achieve R0 resection. When compared with other current
surgical methods through existing clinical studies, TMpE shows no
significant differences in operation time, intraoperative blood
loss, incidence of complications, postoperative hospital stay or
perioperative mortality. Therefore, despite all the controversies,
TMpE is a comparatively safe and viable option for treatment of
pancreatic head tumors (53).
Viewed from the perspective of embryology, due to a
duodenal loop, the ‘meso’ disappears during embryonic development
(57). The embryonic mesentery
facilitates the pancreatic germ attachment to the abdominal wall,
thus, forming the pancreatic mesopancreas. This is why the
pancreatic mesopancreas must be completely resected up to the SMA.
The concept of ‘meso-pancreatoduodenum excision’ (tMPDe) was put
forward to address this (58). The
meso-pancreatoduodenum is defined as a mesangial mesentery supplied
by the inferior pancreaticoduodenal artery (IPDA), which runs
towards the third and fourth portions of the duodenum and the
proximal jejunal mesentery supplied by the first jejunal artery
(FJA) at the back of the SMA. During tMPDe, the scope of the TmpE
(Fig. 5) is extended to the left
side of the SMA from the coronal plane to ensure thorough cleaning
of the retroperitoneal margin. As a result the R0 resection rate
significantly increases when compared with the standard
pancreaticoduodenectomy procedure (93 vs. 60%) (58).
 | Figure 5The key extent of
meso-pancreatoduodenum excision (tMPDe): i) The left side
semicircle lymphadenectomy around the SMA from the origin of the
MCA up to the origin of the SMA in a longitudinal direction on the
ventral side of the SMA. The origin of the common trunk of the
inferior pancreaticoduodenal artery (IPDA) and first jejunal artery
(FJA) need to be secured at the left posterior side of the SMA. A
right-side semicircle lymphadenectomy is then performed, soft
connective tissue around the SMA containing lymph nodes,
mesopancreas, and PL around the SMV is dissected up to the origin
of the SMA in a longitudinal direction. After completion of the
circumferential lymphadenectomy, the IPDA and FJA are ligated and
divided. ii) If the portal venous system was involved in tumor
invasion, a quick resection of the vein followed by an end-to-end
anastomosis was conducted. The resected specimens demonstrated that
the mesoduodenum, which is fed by the IPDA, and the jejunal
mesentery, which is dominated by the FJA, form a common mesentery,
and thus named this common mesentery the
‘meso-pancreatoduodenum’. |
However, current TMpE studies are retrospective
studies. Larger cohort studies and randomized controlled trials are
needed to evaluate the clinical effects. Also, the long-term
prognosis of these patients remains unclear. Some clinical studies
using extended retroperitoneal clearance failed to show that
extended resection improves patient prognosis. Although the
concepts of mesopancreas and TMpE are controversial, there are some
other reports which show TMpE has definite advantages. We believe
TMpE is an entirely new concept that requires further evaluation.
Its clinical application may improve the prognosis of patients with
pancreatic carcinomas, but large randomized clinical studies are
necessary to demonstrate its true role in the treatment of
pancreatic head cancer.
ii) Breakthroughs in the existing
operation procedures using a circumferential resection plus
adjuvant therapy?
Extensive resection of the retroperitoneal nerve
plexus will cause the bowel to lose its dual innervation, thus,
resulting in serious complications such as severe diarrhea and
malabsorption of nutrients. These can be difficult to control even
with medication (59) and can
significantly impair the quality of life after operation. In some
situations, these complications can be life-threatening. However,
in clinical practice, the incidence of postoperative intractable
diarrhea does not occur more frequently than after extensive
resection, suggesting that the incidence may be low and/or readily
controlled (58). However, if the
tumor is circumferentially wrapping around or is invading the SMA,
radical operations should not be attempted. In this case,
palliative operation or implantation of I125 are better
options. If preoperative imaging and intraoperative exploration
only detect suspicious lymph node metastasis on the left edge of
the SMA, the SMA can theoretically be completely skeletonized,
which is then combined with optogenetic adjuvant therapy.
Based on the concept of TMpE (en bloc resection and
R0 resection), the ‘no touch’ tumor principle and integrated with a
large number of clinical reports, the SMA should be dissected first
to determine whether it is feasible to perform radical resection.
Total mesopancreas excision should be carried out to remove the
lymphatics and blood vessels in the mesopancreas, which falls in
line with the principle of ‘no contact’ tumor resection before the
pancreaticoduodenectomy (58).
What do we need?
i) Randomized controlled studies
worldwide at this stage do not support extended resection and
extended lymph node clearance
The latest clinical study on 200 patients with
pancreatic head carcinomas (59)
showed no improvement in long-term survival by using extended
resection, including extended lymph node dissection and plexus
dissection when compared to the traditional standard
pancreaticoduodenectomy (Whipple procedure). This large prospective
study showed that extended resection did not improve long-term
outcomes.
However, the present review has many debatable
points. For example, in the cases of radical operation, it is not
clear what criteria were used to define the extent of clearance or
how the SMA peripheral nerve plexus was distinguished from the No.
14d group of lymph node during dissection. The review also
mentioned on several occasions that complete circumferential
clearance of the SMA plexus would inevitably lead to intractable
diarrhea, poor nutrient intake, and immune dysfunction, which would
affect the postoperative quality of life and survival of the
patients. Therefore, in the extended resection group there was no
clearance of the left half of the SMA. However, there were no clear
data in the medical literature to indicate the severity and
intractability of the diarrhea. References used were at least 10
years old. The lengths of hospital stay mainly caused by the
national health care system were also different between the two
groups, which affected the accuracy of the results. Notably, the
report also indicated that in the extended resection group there
were significantly more patients with peritoneal metastasis.
The report also noted that in the standard group and
the extended group, operation time and blood loss were
significantly different. As dissection was performed near to major
blood vessels and nerves in the extended group, balancing the risk
of prolonged operation times, increased bleeding volumes, and
surgical complications with the potential extension of
postoperative survival warrants further investigations.
Retrospective clinical data from Japan indicated
that extended resection could result in significantly improved
survival (60,61). However, randomized controlled
trials in Europe and the United States do not support the use of
extended resection in pancreatic cancer (61). Prospective randomized studies in
Europe and the United States showed that the survival rate was not
significantly different between extended radical and standard
operations, while the extended radical operation had a higher rate
of surgical complications (62).
In summary, each center has its own view on the
scope and extent of retroperitoneal dissection. Most European and
American scholars tend to advocate for a standard Whipple
procedure. For extended retroperitoneal dissection, information
from the Japanese scholars cannot lead to a conclusion as to
whether an extended resection is superior to a standard Whipple in
long-term survival outcomes.
ii) Costs related to pancreatic cancer
management
The majority of patients with pancreatic cancer are
diagnosed late. The patients are old, and the survival rate is very
low because of rapid tumor progression (63). In 2014, the median age of patients
diagnosed with pancreatic cancer was 71-years old in the United
States. The incidence has increased each year, and the numbers of
new cases and fatal cases are expected to reach 46,420 and 39,590,
respectively. Pancreatic cancer is ranked number four in malignant
tumor mortality, and the 5-year survival rate is <6% (64,65).
The main cost of diagnosis and treatment for these patients include
costs for hospitalization, postoperative drug treatment, nursing
and other costs (66,67). Pancreatic resection is arguably the
most complex and difficult surgery in the medical profession. Even
eliminating all objective conditions, the technical level of the
surgeons and other human factors, the risk of surgical
complications remains high. Surgical complications (aside from the
cancer) can be life-threatening. At the same time, with increasing
costs for long-term postoperative care after reconstruction of
digestive tract and the risk of resulting malabsorption (68), the costs to the patient's family,
patient and society are a heavy burden that will increase with
disease incidence.
9. Optogenetic technology with pancreatic
cancer for detection and adjuvant therapy
To study the basis of pancreatic neural invasion
which includes the mechanisms of neural invasion, optogenetic
technology can be used to control the optics of a cell after making
it to express a light-sensitive protein with genetic techniques
which integrates optics, electrophysiology, genetic engineering and
other disciplines. By using light regulation of specific neuronal
cell types, the function of neural circuits and the specific
control of biological behavior can be studied (69,70).
Optogenetic technology is being applied more widely because of its
ease in handling, intact stimulus, high temporal and spatial
precision, quantifiability and repeatability (71,72).
The core of optogenetic technology includes real-time imaging
technology of specific neurons and light-sensitive control
technology. As a possible way to go beyond traditional neuroscience
research methods and to regulate neuronal activity,
channelrhodopsin-2 (ChR2), a light-sensitive ion channel protein
that can cause changes in membrane potential in response to light,
represents a useful tool (73,74).
Optogenetic technology can target ChR2 to specific neural circuits
and control the function effectively. From the neural networks of
the nematode to brain research in rhesus monkeys, ChR2 has been
systemically tested in higher level animals (75). From basic research studies on
neural circuits to clinical diseases of the nervous system, ChR2
has become a new method to explore neural system diseases. With a
more practical significance, optogenetic technology can be applied
in vivo (76). Some
scholars use this technique (with good results) in conjunction with
lentivirus application to alter the pathogenesis of sleep disorders
(77).
During pancreatic resection, injured nerves can
cause postoperative gastrointestinal disorders. However, injury is
common due to the limited resolution of the naked eye, making it
difficult to visualize tiny nerve endings. Real-time neuronal
staining using optogenetic technology in vivo allows for the
visualization of such nerves and their distribution to avoid
accidental injury. Unilateral or bilateral celiac ganglia that have
been violated by pancreatic cancer cells must be removed.
Considering that gastrointestinal disorders can significantly
reduce the quality of life, downstream neurons can be transfected
with light-sensitive proteins and illuminated to preserve the
nerves and thus gastrointestinal motility.
ChR2 can also be used to regulate other cellular
calcium signaling regulatory pathways (78), such as the calcium signaling
pathway to control pancreatic stellate cells, which is helpful to
further understand how they affect neurons with regards to support,
nourish, protect and communicate. Such techniques can be used to
further explore the molecular mechanisms of neural invasion in
pancreatic carcinomas (79,80).
With development of new technology and
identification of light-sensitive channel proteins with long-term
stability which can then be expressed in specific neurons and
monitored using fast 3D beam scanning methods, we have reasons to
believe that pancreatic cancers will soon be treatable using
optogenetic technology (81).
10. Conclusions
In conclusion, a better understanding of the
mechanisms which are involved in NI and the role of NI in
pancreatic cancer progression is essential. Promoting advanced
preoperative evaluation systems, strengthening the collaboration of
multi-disciplinary teams (MDT), making a reasonable choice of
treatment, and continuing to standardize pancreatic surgical
procedures are the keys to achieving higher R0 resection rates and
improving prognoses of patients with pancreatic cancer.
Acknowledgements
The present study was supported by the Funds of the
National Natural Science Foundation of China (61371066), the Key
Medical Talents of Jiangsu Province (RC2011090), and the 333
Program for High Level Talents of Jiangsu Province (grant no.
2011III-2640).
References
|
1
|
Liebig C, Ayala G, Wilks JA, Berger DH and
Albo D: Perineural invasion in cancer: A review of the literature.
Cancer. 115:3379–3391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alderton GK: Microenvironment: An exercise
in restraint. Nat Rev Cancer. 14:4492014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bapat AA, Hostetter G, Von Hoff DD and Han
H: Perineural invasion and associated pain in pancreatic cancer.
Nat Rev Cancer. 11:695–707. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi SQ, Miwa K, Ohta T, Kayahara M,
Kitagawa H, Tanaka A, Shimokawa T, Akita K and Tanaka S:
Innervation of the pancreas from the perspective of perineural
invasion of pancreatic cancer. Pancreas. 27:225–229. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu
R, Zhou Y, Shao C, Zheng J and Zhu M: Analysis of tumor-induced
lymphangiogenesis and lymphatic vessel invasion of pancreatic
carcinoma in the peripheral nerve plexus. Cancer Sci.
103:1756–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Makino I, Kitagawa H, Ohta T, Nakagawara
H, Tajima H, Ohnishi I, Takamura H, Tani T and Kayahara M: Nerve
plexus invasion in pancreatic cancer: Spread patterns on
histopathologic and embryological analyses. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chari ST, Leibson CL, Rabe KG, Timmons LJ,
Ransom J, de Andrade M and Petersen GM: Pancreatic
cancer-associated diabetes mellitus: Prevalence and temporal
association with diagnosis of cancer. Gastroenterology. 134:95–101.
2008. View Article : Google Scholar
|
|
8
|
Satija A, Spiegelman D, Giovannucci E and
Hu FB: Type 2 diabetes and risk of cancer. BMJ. 350:g77072015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao WC, Tu YK, Wu MS, Lin JT, Wang HP and
Chien KL: Blood glucose concentration and risk of pancreatic
cancer: Systematic review and dose-response meta-analysis. BMJ.
349:g73712015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pannala R, Basu A, Petersen GM and Chari
ST: New-onset diabetes: A potential clue to the early diagnosis of
pancreatic cancer. Lancet Oncol. 10:88–95. 2009. View Article : Google Scholar :
|
|
11
|
Giovannucci E and Michaud D: The role of
obesity and related metabolic disturbances in cancers of the colon,
prostate, and pancreas. Gastroenterology. 132:2208–2225. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J and Ma Q: Hyperglycemia promotes the
perineural invasion in pancreatic cancer. Med Hypotheses.
71:386–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He S, Chen CH, Chernichenko N, He S, Bakst
RL, Barajas F, Deborde S, Allen PJ, Vakiani E, Yu Z, et al: GFRα1
released by nerves enhances cancer cell perineural invasion through
GDNF-RET signaling. Proc Natl Acad Sci USA. 111:E2008–E2017. 2014.
View Article : Google Scholar
|
|
14
|
Gao L, Bo H, Wang Y, Zhang J and Zhu M:
Neurotrophic factor artemin promotes invasiveness and neurotrophic
function of pancreatic adenocarcinoma in vivo and in vitro.
Pancreas. 44:134–143. 2015. View Article : Google Scholar
|
|
15
|
Martínez-Bosch N, Fernández-Barrena MG,
Moreno M, Ortiz-Zapater E, Munné-Collado J, Iglesias M, André S,
Gabius HJ, Hwang RF, Poirier F, et al: Galectin-1 drives pancreatic
carcinogenesis through stroma remodeling and Hedgehog signaling
activation. Cancer Res. 74:3512–3524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W,
Lei J, Ma J, Wang X, Lv S, et al: Sonic hedgehog paracrine
signaling activates stromal cells to promote perineural invasion in
pancreatic cancer. Clin Cancer Res. 20:4326–4338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Yang H, Abel EV, Ney GM, Palmbos
PL, Bednar F, Zhang Y, Leflein J, Waghray M, Owens S, et al: ATDC
induces an invasive switch in KRAS-induced pancreatic
tumorigenesis. Genes Dev. 29:171–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sherman MH, Yu RT, Engle DD, Ding N,
Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S,
et al: Vitamin D receptor-mediated stromal reprogramming suppresses
pancreatitis and enhances pancreatic cancer therapy. Cell.
159:80–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witz IP and Levy-Nissenbaum O: The tumor
microenvironment in the post-PAGET era. Cancer Lett. 242:1–10.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Helm O, Mennrich R, Petrick D, Goebel L,
Freitag-Wolf S, Röder C, Kalthoff H, Röcken C, Sipos B, Kabelitz D,
et al: Comparative characterization of stroma cells and ductal
epithelium in chronic pancreatitis and pancreatic ductal
adenocarcinoma. PLoS One. 9:e943572014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gore J and Korc M: Pancreatic cancer
stroma: Friend or foe? Cancer Cell. 25:711–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinemann V, Reni M, Ychou M, Richel DJ,
Macarulla T and Ducreux M: Tumour-stroma interactions in pancreatic
ductal adenocarcinoma: Rationale and current evidence for new
therapeutic strategies. Cancer Treat Rev. 40:118–128. 2014.
View Article : Google Scholar
|
|
24
|
Rhim AD, Oberstein PE, Thomas DH, Mirek
ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP,
Tattersall IW, et al: Stromal elements act to restrain, rather than
support, pancreatic ductal adenocarcinoma. Cancer Cell. 25:735–747.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C,
Novitskiy SV, et al: Depletion of carcinoma-associated fibroblasts
and fibrosis induces immunosuppression and accelerates pancreas
cancer with reduced survival. Cancer Cell. 25:719–734. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karademir S, Sökmen S, Terzi C, Sağol O,
Özer E, Astarcioğlu H, Coker A and Astarcioğlu I: Tumor
angiogenesis as a prognostic predictor in pancreatic cancer. J
Hepatobiliary Pancreat Surg. 7:489–495. 2000. View Article : Google Scholar
|
|
27
|
Khorana AA, Ahrendt SA, Ryan CK, Francis
CW, Hruban RH, Hu YC, Hostetter G, Harvey J and Taubman MB: Tissue
factor expression, angiogenesis, and thrombosis in pancreatic
cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wente MN, Keane MP, Burdick MD, Friess H,
Büchler MW, Ceyhan GO, Reber HA, Strieter RM and Hines OJ: Blockade
of the chemokine receptor CXCR2 inhibits pancreatic cancer
cell-induced angiogenesis. Cancer Lett. 241:221–227. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong PP, Demircioglu F, Ghazaly E,
Alrawashdeh W, Stratford MR, Scudamore CL, Cereser B,
Crnogorac-Jurcevic T, McDonald S, Elia G, et al: Dual-action
combination therapy enhances angiogenesis while reducing tumor
growth and spread. Cancer Cell. 27:123–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark CE, Hingorani SR, Mick R, Combs C,
Tuveson DA and Vonderheide RH: Dynamics of the immune reaction to
pancreatic cancer from inception to invasion. Cancer Res.
67:9518–9527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayne LJ, Beatty GL, Jhala N, Clark CE,
Rhim AD, Stanger BZ and Vonderheide RH: Tumor-derived
granulocyte-macrophage colony-stimulating factor regulates myeloid
inflammation and T cell immunity in pancreatic cancer. Cancer Cell.
21:822–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greten TF: Myeloid-derived suppressor
cells in pancreatic cancer: more than a hidden barrier for
antitumour immunity? Gut. 63:1690–1691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neesse A, Michl P, Frese KK, Feig C, Cook
N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al:
Stromal biology and therapy in pancreatic cancer. Gut. 60:861–868.
2011. View Article : Google Scholar
|
|
34
|
Schlomann U, Koller G, Conrad C, Ferdous
T, Golfi P, Garcia AM, Höfling S, Parsons M, Costa P, Soper R, et
al: ADAM8 as a drug target in pancreatic cancer. Nat Commun.
6:61752015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kahlert C, Fiala M, Musso G, Halama N,
Keim S, Mazzone M, Lasitschka F, Pecqueux M, Klupp F, Schmidt T, et
al: Prognostic impact of a compartment-specific angiogenic marker
profile in patients with pancreatic cancer. Oncotarget.
5:12978–12989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacobs EJ, Newton CC, Silverman DT,
Nogueira LM, Albanes D, Männistö S, Pollak M and
Stolzenberg-Solomon RZ: Serum transforming growth factor-β1 and
risk of pancreatic cancer in three prospective cohort studies.
Cancer Causes Control. 25:1083–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar
|
|
38
|
Bai X, Zhi X, Zhang Q, Liang F, Chen W,
Liang C, Hu Q, Sun X, Zhuang Z and Liang T: Inhibition of protein
phosphatase 2A sensitizes pancreatic cancer to chemotherapy by
increasing drug perfusion via HIF-1α-VEGF mediated angiogenesis.
Cancer Lett. 355:281–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Farrow B, Albo D and Berger DH: The role
of the tumor microenvironment in the progression of pancreatic
cancer. J Surg Res. 149:319–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bockhorn M, Uzunoglu FG, Adham M, Imrie C,
Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley
RM, et al; International Study Group of Pancreatic Surgery.
Borderline resectable pancreatic cancer: A consensus statement by
the International Study Group of Pancreatic Surgery (ISGPS).
Surgery. 155:977–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fouquet T, Germain A, Brunaud L, Bresler L
and Ayav A: Is perineural invasion more accurate than other factors
to predict early recurrence after pancreatoduodenectomy for
pancreatic head adenocarcinoma? World J Surg. 38:2132–2137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kimura W and Makuuchi M: Suihi geka no
youten to mouten. Bunkodo; Tokyo: 2009, (In Japanese).
|
|
44
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Büchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg. 91:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weitz J, Rahbari N, Koch M and Büchler MW:
The ‘artery first’ approach for resection of pancreatic head
cancer. J Am Coll Surg. 210:e1–e4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Verbeke CS, Leitch D, Menon KV, McMahon
MJ, Guillou PJ and Anthoney A: Redefining the R1 resection in
pancreatic cancer. Br J Surg. 93:1232–1237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Esposito I, Kleeff J, Bergmann F, Reiser
C, Herpel E, Friess H, Schirmacher P and Büchler MW: Most
pancreatic cancer resections are R1 resections. Ann Surg Oncol.
15:1651–1660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cameron JL, Riall TS, Coleman J and
Belcher KA: One thousand consecutive pancreaticoduodenectomies. Ann
Surg. 244:10–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schmidt CM, Powell ES, Yiannoutsos CT,
Howard TJ, Wiebke EA, Wiesenauer CA, Baumgardner JA, Cummings OW,
Jacobson LE, Broadie TA, et al: Pancreaticoduodenectomy: A 20-year
experience in 516 patients. Arch Surg. 139:718–725; discussion
725–727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Verbeke CS: Resection margins and R1 rates
in pancreatic cancer - are we there yet? Histopathology.
52:787–796. 2008. View Article : Google Scholar
|
|
51
|
Gaedcke J, Gunawan B, Grade M, Szöke R,
Liersch T, Becker H and Ghadimi BM: The mesopancreas is the primary
site for R1 resection in pancreatic head cancer: Relevance for
clinical trials. Langenbecks Arch Surg. 395:451–458. 2010.
View Article : Google Scholar :
|
|
52
|
Agrawal MK, Thakur DS, Somashekar U,
Chandrakar SK and Sharma D: Mesopancreas: Myth or reality? JOP.
11:230–233. 2010.PubMed/NCBI
|
|
53
|
Adham M and Singhirunnusorn J: Surgical
technique and results of total mesopancreas excision (TMpE) in
pancreatic tumors. Eur J Surg Oncol. 38:340–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gockel I, Domeyer M, Wolloscheck T,
Konerding MA and Junginger T: Resection of the mesopancreas (RMP):
A new surgical classification of a known anatomical space. World J
Surg Oncol. 5:442007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bouassida M, Mighri MM, Chtourou MF and
Sassi S, Touinsi H, Hajji H and Sassi S: Retroportal lamina or
mesopancreas? Lessons learned by anatomical and histological study
of thirty three cadaveric dissections. Int J Surg. 11:834–836.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dumitrascu T and Popescu I: Total
mesopancreas excision in pancreatic head adenocarcinoma: The same
impact as total mesorectal excision in rectal carcinoma? Comment on
article “surgical technique and results of total mesopancreas
excision in pancreatic tumours” by Adham M and Singhirunnusorn J,
Eur J Surg Oncol, 2012. Eur J Surg Oncol. 38:725author reply 726.
2012. View Article : Google Scholar
|
|
57
|
Chowdappa R and Challa VR: Mesopancreas in
pancreatic cancer: where do we stand - review of literature. Indian
J Surg Oncol. 6:69–74. 2014. View Article : Google Scholar
|
|
58
|
Kawabata Y, Tanaka T, Nishi T, Monma H,
Yano S and Tajima Y: Appraisal of a total meso-pancreatoduodenum
excision with pancreaticoduodenectomy for pancreatic head
carcinoma. Eur J Surg Oncol. 38:574–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jang JY, Kang MJ, Heo JS, Choi SH, Choi
DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, et al: A prospective
randomized controlled study comparing outcomes of standard
resection and extended resection, including dissection of the nerve
plexus and various lymph nodes, in patients with pancreatic head
cancer. Ann Surg. 259:656–664. 2014. View Article : Google Scholar
|
|
60
|
Nimura Y, Nagino M, Takao S, Takada T,
Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda
Y, et al: Standard versus extended lymphadenectomy in radical
pancreatoduodenectomy for ductal adenocarcinoma of the head of the
pancreas: Long-term results of a Japanese multicenter randomized
controlled trial. J Hepatobiliary Pancreat Sci. 19:230–241. 2012.
View Article : Google Scholar
|
|
61
|
Michalski CW, Kleeff J, Wente MN, Diener
MK, Büchler MW and Friess H: Systematic review and meta-analysis of
standard and extended lymphadenectomy in pancreaticoduodenectomy
for pancreatic cancer. Br J Surg. 94:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Farnell MB, Pearson RK, Sarr MG, DiMagno
EP, Burgart LJ, Dahl TR, Foster N and Sargent DJ; Pancreas Cancer
Working Group. A prospective randomized trial comparing standard
pancreatoduodenectomy with pancreatoduodenectomy with extended
lymphadenectomy in resectable pancreatic head adenocarcinoma.
Surgery. 138:618–628; discussion 628–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee A, Chiu CH, Cho MWA, Gomersall CD, Lee
KF, Cheung YS and Lai PB: Factors associated with failure of
enhanced recovery protocol in patients undergoing major
hepatobiliary and pancreatic surgery: A retrospective cohort study.
BMJ Open. 4:e0053302014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pfister DG: The just price of cancer drugs
and the growing cost of cancer care: Oncologists need to be part of
the solution. J Clin Oncol. 31:3487–3489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Short MN, Aloia TA and Ho V: The influence
of complications on the costs of complex cancer surgery. Cancer.
120:1035–1041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sanford DE, Sanford AM, Fields RC, Hawkins
WG, Strasberg SM and Linehan DC: Severe nutritional risk predicts
decreased long-term survival in geriatric patients undergoing
pancreaticoduodenectomy for benign disease. J Am Coll Surg.
219:1149–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deisseroth K, Feng G, Majewska AK,
Miesenböck G, Ting A and Schnitzer MJ: Next-generation optical
technologies for illuminating genetically targeted brain circuits.
J Neurosci. 26:10380–10386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fenno L, Yizhar O and Deisseroth K: The
development and application of optogenetics. Annu Rev Neurosci.
34:389–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nagel G, Brauner M, Liewald JF, Adeishvili
N, Bamberg E and Gottschalk A: Light activation of
channelrhodopsin-2 in excitable cells of Caenorhabditis elegans
triggers rapid behavioral responses. Curr Biol. 15:2279–2284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li X, Gutierrez DV, Hanson MG, Han J, Mark
MD, Chiel H, Hegemann P, Landmesser LT and Herlitze S: Fast
noninvasive activation and inhibition of neural and network
activity by vertebrate rhodopsin and green algae channelrhodopsin.
Proc Natl Acad Sci USA. 102:17816–17821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang F, Wang LP, Boyden ES and Deisseroth
K: Channelrhodopsin-2 and optical control of excitable cells. Nat
Methods. 3:785–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cardin JA, Carlén M, Meletis K, Knoblich
U, Zhang F, Deisseroth K, Tsai LH and Moore CI: Targeted
optogenetic stimulation and recording of neurons in vivo using
cell-type-specific expression of Channelrhodopsin-2. Nat Protoc.
5:247–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Han X, Qian X, Bernstein JG, Zhou HH,
Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R and
Boyden ES: Millisecond-timescale optical control of neural dynamics
in the nonhuman primate brain. Neuron. 62:191–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yizhar O, Fenno LE, Davidson TJ, Mogri M
and Deisseroth K: Optogenetics in neural systems. Neuron. 71:9–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Adamantidis AR, Zhang F, Aravanis AM,
Deisseroth K and de Lecea L: Neural substrates of awakening probed
with optogenetic control of hypocretin neurons. Nature.
450:420–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hung J and Colicos MA: Astrocytic
Ca2+ waves guide CNS growth cones to remote regions of
neuronal activity. PLoS One. 3:e36922008. View Article : Google Scholar
|
|
79
|
Adelsberger H, Grienberger C, Stroh A and
Konnerth A: In vivo calcium recordings and channelrhodopsin-2
activation through an optical fiber. Cold Spring Harbor Protocols.
2014:pdb. prot084145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang Y, Yue J, Ai M, Ji Z, Liu Z, Cao X
and Li L: Channelrhodopsin-2-expressed dorsal root ganglion neurons
activates calcium channel currents and increases action potential
in spinal cord. Spine. 39:E865–E869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fenno LE and Deisseroth K: Optogenetic
tools for control of neural activity. Optical Imaging of
Neocortical Dynamics. Springer; pp. 73–86. 2014, View Article : Google Scholar
|