Introduction
Tumor necrosis factor (TNF)-α-induced protein 8-like
2 (also referred to as TNFAIP8L2, TIPE2) is a newly described
anti-inflammatory factor in an experimental autoimmune
encephalomyelitis (EAE) mouse model, and an important member of the
TIPE family including TNFAIP8, TIPE1, TIPE2 and TIPE3 the four
known members (1,2). TIPE2 as a novel death effector domain
(DED) like domain-containing immune negative molecule is crucial
for homeostasis maintenance in both the adaptive and innate
immunity (1,3). TIPE2-deficient mouse displays
multiple organ inflammation, splenomegaly, leukocytosis and
hyper-responsiveness to septic shock, resulting in premature death
(1). TIPE2-deleted macrophages and
T cells are hyper-responsive to Toll-like receptor (TLR) and T cell
receptor (TCR) activation and drastically secrete inflammatory
cytokines (1). TIPE2 inhibits TLR
and TCR signaling via suppressing activation of activating
protein-1 (AP-1), nuclear factor-κB (NF-κB), c-Jun N-terminal
kinase (JNK) and p38 MAPK while facilitating Fas-triggered T cell
apoptosis (1). TIPE2 also control
macrophage innate immunity to bacterial infection by negatively
regulating phagocytosis and oxidative burst through directly
interacting with and blocking Rac GTPases (4). Moreover, TIPE2 negatively modulates
dendritic cell (DC) innate immunity to RNA viral infection by
inhibiting phosphatidylinositol-3-kinase (PI3K)-Rac pathway
(5). Additionally, TIPE2 restrains
inducible nitric oxide synthase (iNOS) and NO generation by
arginine metabolism switch from nitric oxide synthase to arginase,
leading to inflammation suppression (6). Notably, TIPE2 maintains
immunosuppressive property of CD4+CD25+
regulatory T cells via orchestrating expression of
immunosuppressive molecules such as cytotoxic
T-lymphocyte-associated protein 4 (CTLA4), forkhead box P3 (Foxp3),
TGF-β and IL-10 (7).
The cell-autonomous role of TIPE2 in human cancer is
receiving increasing attention. A seminal study (8) demonstrated that TIPE2 can act as a
novel inhibitor of oncogenic Ras, and dramatically suppresses cell
survival and motility via attenuating activation of downstream
signaling molecules AKT and Ral GTPase by competitively binding to
Ras-interacting domain (RID) of Ras effector RGL and preventing
Ras/RGL active complex formation. Overexpression of TIPE2 initiates
hepatocellular carcinoma (HCC) death and retards Ras-induced
tumorigenesis (8,9). TIPE2 also suppresses HCC metastasis
by inhibiting Rac1 GTPase and subsequently reducing polymerization
of F-actin, matrix metallopeptidase 9 (MMP9) and urokinase
plasminogen activator (uPA) (9).
Furthermore, TIPE2 has been found to be markedly downregulated or
lost in human HCC clinical tissues, which is significantly
associated with tumor metastasis (8,9).
Subsequent studies also showed that TIPE2 is frequently reduced or
absent in human gastric cancer and lung cancer tissues,
contributing to poor prognosis (10–12).
Forced expression of TIPE2 inhibits tumor cell growth and induces
cell cycle alteration and apoptosis by upregulating expression of
cyclin-dependent kinase (CDK) inhibitors and apoptosis-related
molecules (10–12). These reports indicated that TIPE2
is capable of exerting tumor-suppressive effects in a
cell-autonomous manner.
Gastric cancer is one of the most common cancers
world-wide and the leading cause of cancer-related deaths (13). Metastatic gastric cancer is a
life-threatening disease with no effective treatment. Thus, deep
understanding of the mechanism underlying gastric cancer metastasis
and discovery of novel treatment targets is urgently needed.
Accumulating evidence has demonstrated that TIPE2 exhibits
tumor-suppressive effects in several tumor types (8–12).
Clinical data have also shown that TIPE2 is reduced in human
gastric cancer tissues (10).
However, its effects on gastric cancer metastasis are largely
unclear. In the present study, we analyzed TIPE2 expression in a
panel of human gastric cancer cells, examined the effects of TIPE2
on human gastric cancer cell migration and invasion in vitro
by adenovirus-mediated TIPE2 overexpression, and elucidated its
molecular mechanism.
Materials and methods
Vectors, cell lines and antibodies
The marker gene green fluorescent protein
(GFP)-expressing pAdTrack-CMV (14) adenoviral transfer plasmid, BJ5183
bacteria and QBI-293A human embryonic kidney cell line for
adenovirus packaging and amplification were kindly provided by
Professor Jiang Zhong (Department of Microbiology, College of Life
Science, Fudan University, Shanghai, China). The integrin-binding
motif Arg-Gly-Asp (RGD)-modified pAdEasy-1 (Ad5E1/E3-deleted)
adenoviral backbone plasmid (15)
was kindly provided by Professor Jim Xiang (Saskatoon Cancer
Agency, Saskatoon, SK, Canada). The AGS, HGC-27 and SGC-7901 human
gastric cancer cell lines and GES-1 human gastric mucous epithelial
cell line were purchased from the Cell Bank, Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). The
goat anti-TIPE2 (C-18) antibody and secondary antibodies such as
anti-goat IgG-horseradish peroxidase (HRP), anti-rabbit IgG-HRP and
anti-rabbit IgG-phycoerythrin (PE) were purchased from Santa Cruz
Biotechnology (Shanghai, China). The rabbit antibodies specific for
pAKT (244F9), AKT (11E7), pGSK3β (D3A4), GSK3β (27C10), β-catenin
(D10A8), β-actin (13E5) and histone H3 (3H1) were purchased from
Cell Signaling Technology (Danvers, MA, USA).
Reverse transcription (RT)-PCR assay
The endogenous TIPE2 expression in human gastric
cancer cells was evaluated by RT-PCR analysis. The total RNAs were
extracted from AGS, HGC-27 and SGC-7901 human gastric cancer cells
(2×106 cells/each) and GES-1 normal human gastric mucous
epithelial cells (2×106 cells) using MiniBEST universal
RNA extraction kit (Takara, Dalian, Liaoning, China) and
first-strand cDNAs were synthesized from RNAs using reverse
transcriptase MuMLV (Thermo Fisher Scientific, Shanghai, China).
The PCR reactions were then performed using cDNAs as templates and
primers (Sangon Biotechnology Inc., Shanghai, China) specific for
human TIPE2 (TIPE2-F1, 5′-gac tga cca cat acc cca ctc-3′ and
TIPE2-R1, 5′-tca cca aag cta agt gcc gt-3′ for amplifying 348 bp;
TIPE2-F2, 5′-cag tga ctg acc aca tac ccc-3′ and TIPE2-R2, 5′-tgg
cca ctt tga tca ggt cc-3′ for amplifying 237 bp) or the
housekeeping gene β-actin (β-actin-F, 5′-tgc gtg aca tta agg aga
ag-3′ and β-actin-R, 5′-ctg cat cct gtc ggc aat g-3′ for amplifying
317 bp) (an internal control). The human TIPE2 cDNA fragment
containing full-length coding sequence (CDS) (GenBank accession no.
NM_024575) was amplified from human peripheral blood mononuclear
cells (PBMCs) (5×106 cells) using TIPE2-specific primers
(TIPE2-F3, 5′-gtg act gac cac ata ccc ca-3′ and TIPE2-R3, 5′-agt
gtt agt gcc agg tga gc-3′ for amplifying 684 bp) by RT-PCR,
subcloned into pMD19-T cloning vector (Takara) and sequenced as
previously described (16). The
adenovirus-mediated exogenous human TIPE2 transcriptional
expression was also analyzed by RT-PCR using TIPE2 CDS-specific
primers (TIPE2-F4, 5′-acc gtc gac gcc acc atg gag tcc ttc agc tca
aag-3′ and TIPE2-R4, 5′-gca ctc gag tca gag ctt ccc ttc gtc tag cag
c-3′ for amplifying 555 bp).
Recombinant adenovirus AdVTIPE2
construction
The human TIPE2 CDS fragment (555 bp) was amplified
by PCR using pMD19-T-TIPE2 plasmids as templates and primers
(TIPE2-F4, 5′-acc gtc gac gcc acc atg gag tcc ttc agc tca aag-3′
and TIPE2-R4, 5′-gca ctc gag tca gag ctt ccc ttc gtc tag cag c-3′),
and inserted into GFP marker gene-expressing pAdTrack-CMV
adenoviral transfer plasmid between SalI and XhoI
sites to form pAdTrack-CMV-TIPE2. To develop a more efficient gene
delivery system, we used Arg-Gly-Asp (RGD)-modified and
Ad5E1/E3-deleted pAdEasy-1 which contains an integrin-binding motif
RGD sequence in the HI loop of adenoviral fiber as an adenoviral
backbone plasmid (15). Based upon
the homologous recombination of pAdTrack-CMV-TIPE2 or pAdTrack-CMV
with RGD-modified pAdEasy-1 in BJ5183 bacteria, the RGD-modified
replication-incompetent AdVTIPE2 and AdV (blank adenovirus control)
adenoviruses expressing GFP were subsequently generated and
amplified in QBI-293A cells as reported previously (14), and further purified by cesium
chloride (CsCl) density-gradient ultracentrifugation.
Fluorescence microscopic analysis
The titer of AdVTIPE2 and AdV adenoviruses was
determined using gene transfer unit (GTU) method by calculating the
number of GFP-expressing QBI-293A cells within 18 h after
adenoviral infection by fluorescence microscopy. Accordingly, the
ratio of infectious adenovirus (GTU) to target cells is called
multiplicity of infection (MOI). To select an optimal MOI for a
maximal adenoviral infection and human TIPE2 transgene expression
in tumor cells, the AGS human gastric cancer cells
(1×104 cells/200 μl culture medium, i.e., RPMI-1640
medium supplemented with 10% fetal bovine serum) (Hyclone, Logan,
UT, USA) were plated into 96-well plates overnight and treated with
AdVTIPE2, AdV or PBS without adenovirus at various MOIs (0, 10, 25,
50, 100 or 200) in culture medium. Twenty-four hours after
infection, the GFP expression was observed and photographed at ×200
higher-power field by fluorescence microscopy and the adenoviral
infection efficiency was analyzed.
Wound healing assay
The effect of adenovirus-mediated TIPE2
overexpression on gastric cancer cell migration in vitro was
analyzed by wound healing assay. Briefly, the outer bottom of
6-well culture plates was marked to be used as a reference point
during image acquisition. The AGS human gastric cancer cells were
plated to 70% confluence as a monolayer in marked 6-well plates and
treated with AdVTIPE2 or AdV at the optimal MOI of 10. The
AdVTIPE2- or AdV-infected AGS tumor cells were termed AGS-TIPE2 and
AGS-Mock, respectively. Twenty-four hours after infection,
scratches were created in the monolayer with a pipette tip and the
wells were gently washed with fresh medium to remove the detached
cells. Progression of tumor cell migration was observed and
photographed by microscopy (x100 low-power field) at the beginning
and at 24 h after wounding. The gap distance was then
quantitatively calculated using ImageJ software (National
Institutes of Health, Bethesda, MD, USA) and the migratory ability
of tumor cells was analyzed.
Transwell invasion assay
The effect of adenovirus-mediated TIPE2
overexpression on gastric cancer cell invasion in vitro was
assessed by Transwell invasion assay. Briefly, the 12.5 μl Matrigel
(50 mg/l) (BD Biosciences, Shanghai, China) was diluted in 87.5 μl
serum-free RPMI-1640 medium. The 100 μl Matrigel diluted solution
was added to 24-well Transwell chamber (Corning Inc., Corning, NY,
USA) that incorporated a polycarbonate filter membrane with a
diameter of 6.5 mm and a pore size of 8 μm, dried in a laminar hood
overnight, and reconstituted in 100 μl serum-free RPMI-1640 medium
for 2 h. The AGS-TIPE2 and AGS-Mock tumor cells (2×105
cells/100 μl serum-free RPMI-1640 medium) were added to the upper
chamber of the Transwell. The lower chamber was filled with 600 μl
of culture medium. After 24-h incubation, tumor cells on the upper
surface of the filter were removed using a cottons wab and cells
invading into the bottom side of the insert were fixed by 4%
paraformaldehyde, stained with crystal violet, photographed and
quantified by counting them in 5 random ×200 high-power fields. The
invasive ability of tumor cells was then analyzed.
Western blot analysis
The AGS human gastric cancer cells (1×107
cells) were treated with AdVTIPE2 (10 MOI) or AdV (10 MOI). After
24-h treatment, the AGS-TIPE2 and AGS-Mock tumor cells were
collected, washed with cold PBS and lysed in lysis buffer
(1×107 cells/1 ml lysis buffer) for preparation of total
cellular proteins using mammalian cell lysis kit (Sigma-Aldrich,
Shanghai, China). The nuclear proteins derived from AGS-TIPE2 and
AGS-Mock tumor cells were also isolated using nuclear extraction
kit (Sigma-Aldrich, Shanghai, China). The total and nuclear
proteins (100 μg/lane) were resolved by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred
onto polyvinylidene difluoride (PVDF) membranes (Merck Millipore,
Shanghai, China) and then subjected to western blot analysis of
TIPE2, pAKT, AKT, pGSK3β, GSK3β, β-catenin, β-actin (a total
protein loading control); β-catenin, histone H3 (a nuclear protein
loading control), respectively. The membranes were developed using
SuperEnhanced chemiluminescence detection kit (Applygen Technology
Inc., Beijing, China) and the protein bands were visualized after
their exposure to Kodak X-ray film.
Confocal microscopic analysis
The AGS human gastric cancer cells were cultured in
chamber slides (Sigma-Aldrich, Shanghai, China) and treated with
AdVTIPE2 (10 MOI) or AdV (10 MOI) for 24 h. The AGS-TIPE2 and
AGS-Mock tumor cells were fixed in 4% paraformaldehyde for 20 min,
washed 3 times with PBS, permeabilized in 0.2% Triton X-100,
blocked with 5% normal goat serum and incubated with rabbit
anti-β-catenin primary antibody for 1 h. The slides were washed and
stained with PE-conjugated anti-rabbit IgG secondary antibody. The
slides were then washed and prevented from fading using Prolong
diamond antifade with DAPI (Life Technology, Shanghai, China). The
β-catenin expression in AGS tumor cells was observed by confocal
microscopy.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD) and statistically processed by Student's t-test for
comparison of differences between two groups using SPSS 10.0
software (SPSS, Chicago, IL, USA). A value of p<0.05 was
considered statistically significant.
Results
TIPE2 expression is absent in gastric
cancer
Previous studies showed that TIPE2 is not expressed
or poorly expressed in most human cancer cells (17). To further examine TIPE2 expression
in human gastric cancer cells, we assessed TIPE2 mRNA in a panel of
human gastric cancer cell lines including AGS, HGC-27 and SGC-7901
by RT-PCR analysis. In contrast to GES-1 normal human gastric
mucous epithelial cells, TIPE2 expression was almost undetectable
in all the three human gastric cancer cells using two
TIPE2-specific primer pairs for amplifying either 348 or 237 bp
human TIPE2 cDNA (Fig. 1A).
Consistent with our results, TIPE2 is also absent in U-87 MG and
U251 brain tumor cells (17). A
recent study (10) revealed that
TIPE2 is markedly decreased or undetectable in gastric cancer
tissues compared with adjacent control tissues. Our cellular model
data together with the clinical data strongly indicated that TIPE2
is almost completely lost in human gastric cancer, which may be
connected to gastric cancer progression.
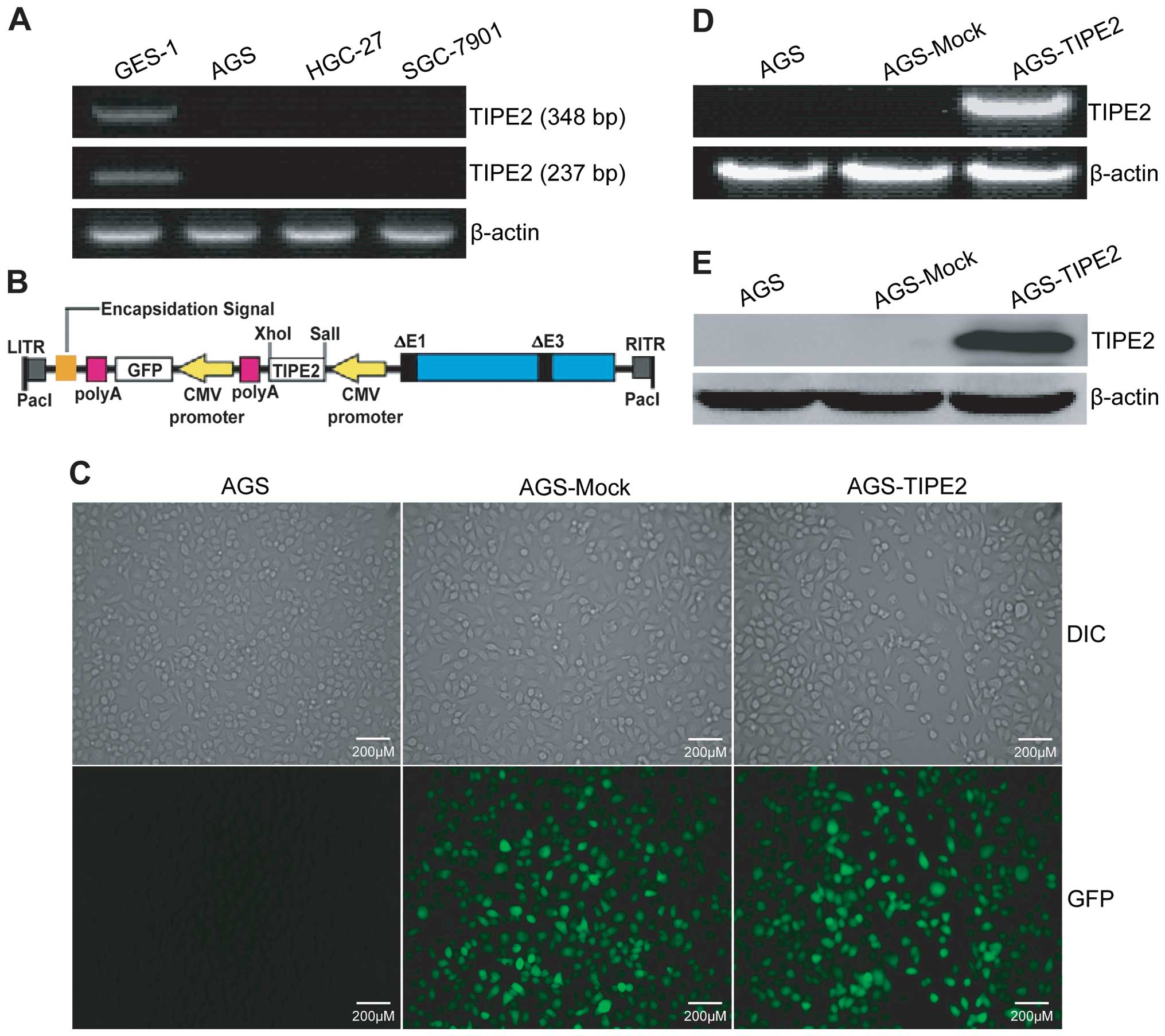 | Figure 1Adenovirus-mediated TIPE2 gene
transfer in gastric cancer cells. (A) RT-PCR analysis of endogenous
human TIPE2 expression in gastric cancer cells. Total RNAs were
extracted from AGS, HGC-27 and SGC-7901 human gastric cancer cells
and GES-1 human gastric mucous epithelial cells. The first-strand
cDNAs were synthesized from RNAs using reverse transcriptase; PCRs
were conducted using primer sets specific for human TIPE2 (348 or
237 bp cDNA) and housekeep gene β-actin, respectively. (B) AdVTIPE2
construction strategy. The human TIPE2 555 bp CDS fragment was
amplified by PCR using pMD19-T-TIPE2 plasmids as templates and
subcloned into pAdTrack-CMV adenoviral transfer plasmid between
SalI and XhoI sites, where TIPE2 transcription is
under control of CMV (1stCMV) promoter, while GFP
transcription is under control of CMV (2ndCMV) promoter.
The AdVTIPE2 adenovirus was then generated by homologous
recombination with RGD-modified and Ad5E1/E3-deleted pAdEasy-1 in
BJ5183 bacteria followed by packaging/amplification in QBI-293A
cells. CMV, cytomegalovirus promoter; GFP, green fluorescent
protein; polyA, SV40 polyadenylation signal; LITR, left-hand
inverted terminal repeat; RITR, right-hand inverted terminal
repeat. (C) Representative images of differential interference
contrast (DIC) and GFP. The AGS tumor cells were infected with
AdVTIPE2 or AdV at the MOI of 10 for 24 h, and then observed under
GFP fluorescence and DIC images by fluorescence microscopy. (D)
RT-PCR analysis of adenovirus-mediated TIPE2 transcriptional
expression. Total RNAs were extracted from AdVTIPE2- or
AdV-infected (AGS-TIPE2 or AGS-Mock) and uninfected AGS tumor
cells. The first-strand cDNAs were synthesized from RNAs using
reverse transcriptase; PCRs were conducted using primer sets
specific for human TIPE2 (555 bp CDS) and housekeeping gene
β-actin, respectively. (E) Western blot analysis of
adenovirus-mediated TIPE2 translational expression. Total cellular
proteins derived from AdVTIPE2- or AdV-infected (AGS-TIPE2 or
AGS-Mock) and uninfected AGS tumor cells were immunoblotted with
anti-TIPE2 and anti-β-actin antibody, respectively. Data shown are
representative of three independent experiments. |
Adenovirus-directed TIPE2 overexpression
in gastric cancer
To decipher the pathological role of TIPE2
dysregulation in human gastric cancer, we cloned the human TIPE2
CDS by RT-PCR from PBMCs and constructed the RGD-modified
replication-deficient recombinant adenovirus expressing human TIPE2
and marker gene GFP (AdVTIPE2) (Fig.
1B). To select an optimal MOI for a maximal adenovirus-mediated
TIPE2 expression, but a minimal adenovirus-induced cytotoxic
effect, the AGS human gastric cancer cells were infected with
GFP-expressing AdVTIPE2 or AdV at different MOIs for 24 h and then
examined by fluorescence microscopy. More than 90% of GFP
expression was found in AdVTIPE2- or AdV-infected AGS tumor cells
at a MOI of 10 (Fig. 1C) or above
(data not shown). In addition, there was almost no
adenovirus-elicited cytotoxicity in 10 MOI blank adenovirus
AdV-infected AGS tumor cells compared to uninfected control AGS
tumor cells (Fig. 1C). RT-PCR
(Fig. 1D) and western blot
(Fig. 1E) analysis further showed
that adenovirus-mediated TIPE2 exogenous gene was abundantly
expressed at both the transcriptional and translational levels in
10 MOI AdVTIPE2-infected AGS tumor cells, but not in AdV-infected
or uninfected control cells. These data suggested that 10 MOI can
be employed as an optimal infection dose for adenovirus-mediated
TIPE2 gene transfer and overexpression in AGS tumor cells.
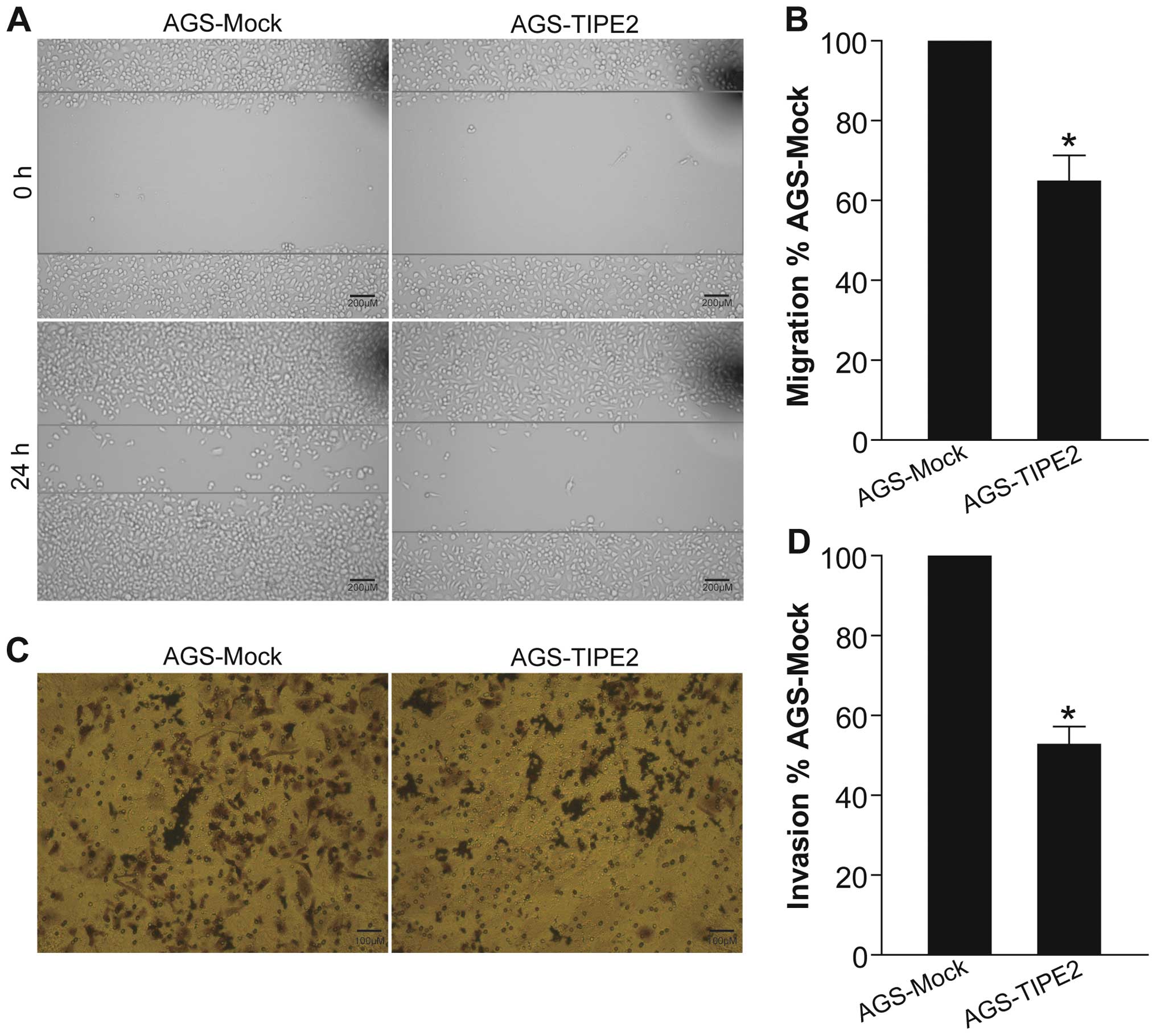
TIPE2 suppresses gastric cancer migration
and invasion
Tumor metastasis is a key hallmark of cancer,
causing as much as 90% of cancer-associated deaths (18,19).
TIPE2 has been found to inhibit hepatocellular carcinoma metastasis
(8,9). To investigate the effect of TIPE2 on
gastric cancer migration and invasion in vitro, we carried
out a gain-of-function study by adenovirus-mediated TIPE2 gene
transfer into AGS human gastric cancer cells and performed wound
healing and Transwell chamber invasion assays. As shown in Fig. 2A and B, adenovirus-mediated TIPE2
overexpression obviously inhibited the migratory speed of AGS
gastric cancer cells compared with that of AdV-infected AGS-Mock
tumor cells (p<0.05). Furthermore, TIPE2 overexpression also
remarkably suppressed the invasive ability of AGS tumor cells
(Fig. 2C and D) (p<0.05). Our
data for the first time demonstrated that TIPE2 negatively
modulated gastric cancer cell motility and invasion.
TIPE2 downregulates β-catenin signaling
via inhibiting AKT and activating GSK3β
To further address the molecular mechanism
responsible for TIPE2-mediated inhibition of gastric cancer cell
migration and invasion, the expression of pAKT, AKT, pGSK3β, GSK3β
and β-catenin in AdVTIPE2- and AdV-infected AGS human gastric
cancer cells was determined by western blot analysis. As shown in
Fig. 3A and B, the total levels of
pAKT, pGSK3β and β-catenin in AGS-TIPE2 tumor cells was markedly
reduced compared with that in AGS-Mock tumor cells. Moreover, the
nuclear level of β-catenin in AGS-TIPE2 tumor cells was also
clearly decreased. Confocal microscopic analysis (Fig. 3C) further showed that AdVTIPE2
promoted β-catenin degradation and decreased its translocation into
nucleus in AGS tumor cells. The data indicated that TIPE2
restoration suppresses gastric cancer metastasis very possibly via
downregulating β-catenin signaling through inhibiting AKT and
activating GSK3β (Fig. 4).
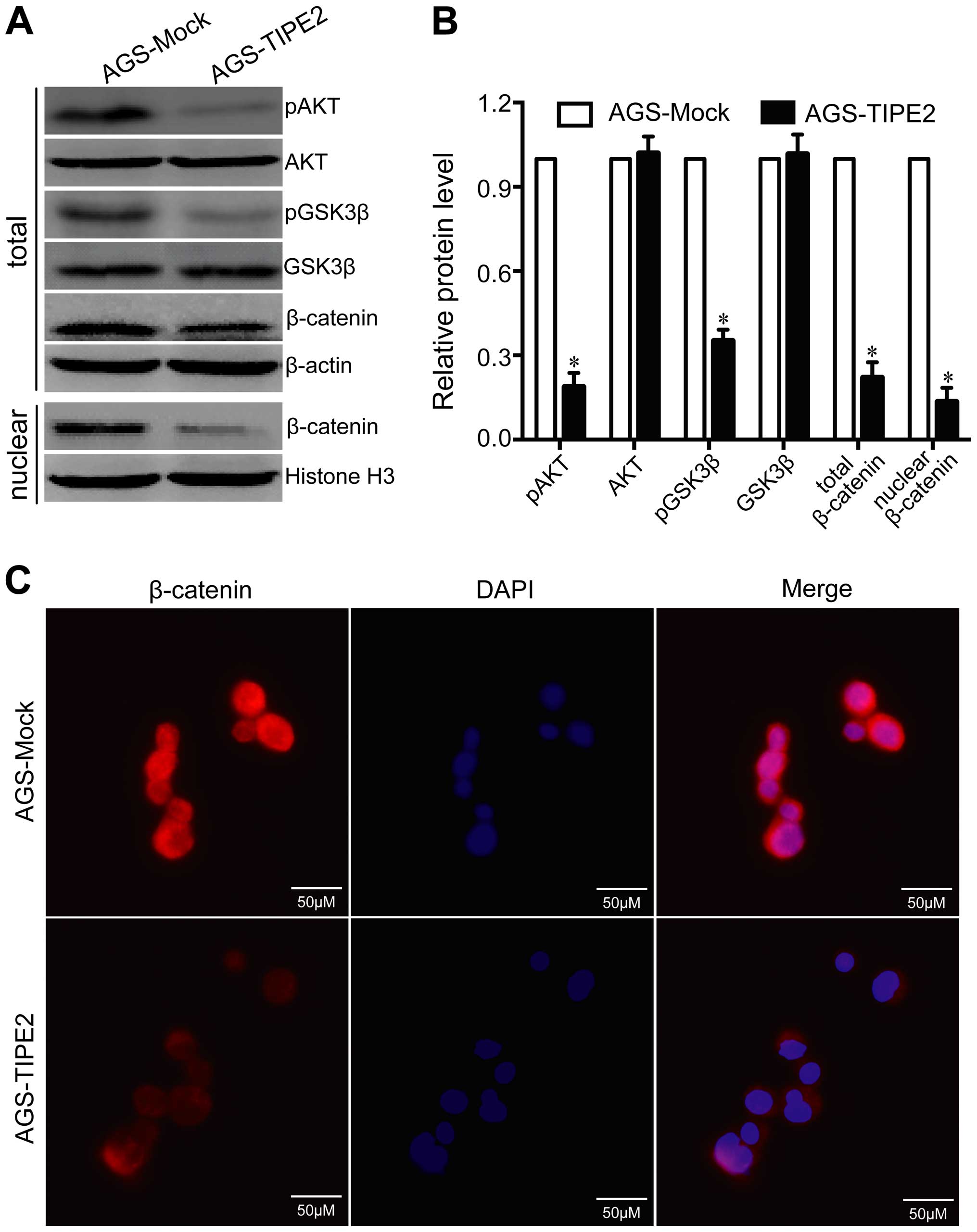 | Figure 3AdVTIPE2 suppresses gastric cancer
metastasis via downregulating β-catenin through inhibiting AKT and
activating GSK3β. (A and B) Western blot analysis of AKT, GSK3β and
β-catenin. The total cellular proteins derived from AdVTIPE2- and
AdV-infected AGS tumor cells (AGS-TIPE2 or AGS-Mock) were
immunoblotted with a panel of antibodies specific for pAKT, AKT,
pGSK3β, GSK3β, β-catenin and β-actin; the nuclear proteins were
immunoblotted with anti-β-catenin and anti-histone H3 antibody,
respectively. The representative images of western blot analysis
are shown (A). The expression of each index was normalized to
expression level of β-actin (total cellular proteins) or histone H3
(nuclear proteins), and the relative change was expressed as a
ratio or fold, with 1 being the value for AGS-Mock group (B).
*p<0.05, Student's t-test, n=3 replicates per
condition, n=3 replicates per sample. (C) Confocal microscopic
analysis of β-catenin immunofluorescence. The AGS-TIPE2 and
AGS-Mock tumor cells were fixed in 4% paraformaldehyde,
permeabilized in 0.2% Triton X-100, blocked with 5% normal goat
serum and incubated with anti-β-catenin primary antibody followed
by incubation with PE-labeled secondary antibody, and then observed
under confocal microscopy. The representative images of confocal
microscopic analysis are shown. Data shown are representative of
three independent experiments. |
Discussion
TIPE2 was initially found to be a crucial regulator
of homeostasis maintenance (1,3).
Recently, TIPE2 has been shown to be frequently and remarkably
reduced or lost in human hepatocellular, gastric and lung cancer
tissues (8–12) as well as in most human cancer cell
lines (17). Increasing evidence
has revealed that TIPE2 exerts tumor-suppressive activity in
several tumor types (8–12), implying it might be proposed as a
novel candidate tumor suppressor gene. However, its
tumor-suppressive effects and relevant mechanisms of its antitumor
property have not been well characterized. In the present report,
we investigated the significance of the TIPE2 in gastric cancer
progression. We found that TIPE2 was almost absent in a panel of
human gastric cancer cell lines compared with normal human gastric
mucous epithelial cells, which is in accord with the clinical data
(10). Forced expression of TIPE2
by adenovirus-mediated human TIPE2 (AdVTIPE2) gene transfer
markedly suppressed gastric cancer migration and invasion. A
previous study showed that TIPE2-stably transfected gastric cancer
cells using eukaryotic expression plasmid displays similar
migratory ability compared to control cells (10). One plausible explanation for the
discrepancy is the different expression systems used in these
studies. Adenovirus-mediated transient overexpression in our study
may avoid the persistent selection pressure of gastric cancer cells
poisoned by TIPE2 that occurred during establishment of
TIPE2-stably transfected tumor cells, thus providing more authentic
data on tumor-suppressive effects of TIPE2 in gastric cancer.
β-catenin is a critical regulatory molecule of
canonical Wnt signaling pathway (20,21)
and is also crucial for cadherin-based cell-cell adhesion by
linking cadherins to actin cytoskeleton indirectly through
α-catenin (22,23), which plays an important role in
regulation of diverse cellular processes such as cell
proliferation, survival, migration, invasion, polarity,
differentiation, development and stem cell self-renewal. Aberrant
β-catenin signaling underlies a wide range of human diseases
(21). β-catenin is generally
considered as an oncogene that can facilitate cancer progression
and metastasis (20). Upregulation
of β-catenin signaling by its deregulation or mutational activation
has been shown in various human cancers including gastric cancer
(20,24). The aberrant activation of PI3K/AKT
signaling as well as the high inactivation of GSK3β is frequently
found in human cancers (25,26).
It has been reported that activated AKT promotes the accumulation
of cytosolic/nuclear β-catenin and enhances its transcriptional
activity via directly phosphorylating β-catenin (27). GSK3β has been recognized as a
primary kinase in the β-catenin multi-protein destruction complex
containing axis inhibition protein (Axin), adenomatous polyposis
coli (APC), GSK3β and casein kinase 1α (CK1α), which can
phosphorylate β-catenin on conserved serine and threonine residues
in its amino terminus (20). The
phosphorylated β-catenin by GSK3β was then recognized by an E3
ubiquitin ligase β-transducin repeat-containing protein (βTrCP),
resulting in proteasomal degradation of β-catenin and low
cytosolic/nuclear β-catenin levels (20). It has also been shown that AKT
indirectly stabilizes β-catenin and consequently increases
β-catenin signaling by phosphorylating GSK3β at Ser9 residue and
inactivating GSK3β kinase activity (28). Thus, in addition to the
Wnt-dependent pathway involved in modulation of β-catenin, the
Wnt-independent pathway such as PI3K/AKT/GSK3β signaling pathway
also critically activates transcriptional activity of β-catenin in
many types of human cancer. Previous studies (5,8)
demonstrated that TIPE2 can inhibit AKT signaling via targeting
RGL-PDK1-AKT and PI3K-Rac pathways. These results made us assume
that TIPE2 suppressed gastric cancer cell migration and invasion
possibly via downregulating β-catenin through activating GSK3β by
attenuating AKT signaling. As expected, western blot and confocal
fluorescence microscopic analysis showed that adenovirus-mediated
TIPE2 overexpression remarkably downregulated pAKT, pGSK3β and
total or nuclear β-catenin levels in human gastric cancer cells.
Our present study indicated that TIPE2 restoration-mediated
inhibition of AKT activation might result in the decreased
phosphorylation of GSK3β and increased active GSK3β, leading to the
phosphorylation and degradation of β-catenin through a
ubiquitin-dependent proteasome pathway. The
TIPE2/AKT/GSK3β/β-catenin axis may constitute a major pathway to
regulate gastric cancer metastasis.
Collectively, we demonstrated that TIPE2 was absent
in human gastric cancer cells. Adenovirus-mediated TIPE2
restoration markedly suppressed gastric cancer cell migration and
invasion possibly via inhibiting β-catenin signaling through
suppressing AKT as well as activating GSK3β. Our data provided the
first evidence that TIPE2 may be a potential therapeutic target for
human gastric cancer metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (NNSFC) (nos. 81372443,
81001016, 81272542 and 81572992) and the Science and Technology
Department of Jiangsu Province (nos. BL2014039 and BY2015039).
References
|
1
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Fayngerts S, Wang P, Sun H,
Johnson DS, Ruan Q, Guo W and Chen YH: TIPE2 protein serves as a
negative regulator of phagocytosis and oxidative burst during
infection. Proc Natl Acad Sci USA. 109:15413–15418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun H, Zhuang G, Chai L, Wang Z, Johnson
D, Ma Y and Chen YH: TIPE2 controls innate immunity to RNA by
targeting the phosphatidylinositol 3-kinase-Rac pathway. J Immunol.
189:2768–2773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lou Y, Zhang G, Geng M, Zhang W, Cui J and
Liu S: TIPE2 negatively regulates inflammation by switching
arginine metabolism from nitric oxide synthase to arginase. PLoS
One. 9:e965082014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luan YY, Yao YM, Zhang L, Dong N, Zhang
QH, Yu Y and Sheng ZY: Expression of tumor necrosis factor-α
induced protein 8 like-2 contributes to the immunosuppressive
property of CD4(+)CD25(+) regulatory T cells in mice. Mol Immunol.
49:219–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Q, Zhao M, Dong T, Zhou C, Peng Y,
Zhou X, Fan B, Ma W, Han M and Liu S: Tumor necrosis
factor-α-induced protein-8 like-2 (TIPE2) upregulates p27 to
decrease gastic cancer cell proliferation. J Cell Biochem.
116:1121–1129. 2015. View Article : Google Scholar
|
|
11
|
Liu QQ, Zhang FF, Wang F, Qiu JH, Luo CH,
Zhu GY and Liu YF: TIPE2 inhibits lung cancer growth attributing to
promotion of apoptosis by regulating some apoptotic molecules
expression. PLoS One. 10:e01261762015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Li X, Liu G, Sun R, Wang L, Wang J
and Wang H: Downregulated TIPE2 is associated with poor prognosis
and promotes cell proliferation in non-small cell lung cancer.
Biochem Biophys Res Commun. 457:43–49. 2015. View Article : Google Scholar
|
|
13
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Ye T, Sun D, Maynard J and
Deisseroth A: Conditionally replication-competent adenoviral
vectors with enhanced infectivity for use in gene therapy of
melanoma. Hum Gene Ther. 15:637–647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Sheng W, Xiang J, Ye Z and Yang J:
Interleukin-17F suppresses hepatocarcinoma cell growth via
inhibition of tumor angiogenesis. Cancer Invest. 28:598–607. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y and Zhang Y: Tissue-specific expression of
TIPE2 provides insights into its function. Mol Immunol.
47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
21
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conacci-Sorrell M, Zhurinsky J and
Ben-Ze'ev A: The cadherin-catenin adhesion system in signaling and
cancer. J Clin Invest. 109:987–991. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang T, Wei Y, Honaker Y, Yamaguchi H,
Appella E, Hung MC and Piwnica-Worms H: GSK-3 beta targets Cdc25A
for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation
correlates with Cdc25A overproduction in human cancers. Cancer
Cell. 13:36–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang D, Hawke D, Zheng Y, Xia Y,
Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T and Lu Z:
Phosphorylation of beta-catenin by AKT promotes beta-catenin
transcriptional activity. J Biol Chem. 282:11221–11229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen P and Frame S: The renaissance of
GSK3. Nat Rev Mol Cell Biol. 2:769–776. 2001. View Article : Google Scholar : PubMed/NCBI
|