Introduction
Current cancer medications are costly and often
cause serious side effects. The US National Cancer Institute began
investigating antitumor plant extracts in the 1960s, and the
premise that natural compounds obtained from therapeutic plants
could produce anticancer medications has henceforth been of great
research interest. Traditional Chinese medicines (TCMs) using dried
plants or plant extracts have provided low cost diet and
pharmaceutical therapies for thousands of years and experimental
and clinical studies have proven that >400 plant species used in
TCMs as anticancer herbal medications are significantly effective
in the prevention or treatment of various cancers (1–4).
However, much work remains to be done to determine the
effectiveness of the individual compounds present in the TCMs.
Paeonia suffruticosa, or Paeoniaceae,
is a widely utilized Chinese medicinal plant within the
Paeonia genus. This genus comprises ~35 species that are
classified into three groups: Oneapia, Paeonia, and
Moutan (5). The Cortex
Moutan (root cortex) of Paeonia has been recorded by
China's Pharmacopoeia as a significant source of herbal medicine
(6). Extracts of Paeonia
have been shown to possess cytotoxic, antitumor, anti-inflammatory
and anti-oxidative activities (5).
Previous photochemical research on Paeonia identified
>260 bioactive compounds, including phenols,
monoterpenoidglucosides, paeonols, flavonoids, tannins, steroids,
triterpenoids and stilbenes (7). A
more recent study showed that the seeds of Paeonia contain
considerable quantities of stilbenes compared to the other
compounds (7,8).
Stilbenes are a class of polyphenols widely found in
plants that contain a 1.2-diphenylethylene nucleus in their
structure (9). Stilbenes have
aroused great interest due to their antitumor, anti-steroidal,
anti-mutagenic, anti-oxidative, anti-malarial, and
anti-inflammatory bioactivities (10–16).
One well-known example of the stilbenes is resveratrol, and its
antitumor activity has been intensively studied. Several in
vivo and in vitro studies have shown that resveratrol
inhibits the growth of cancer cells and effects various molecular
targets associated with cancer progression such as the Wnt
signaling pathway, nuclear factor-kappa B (NF-κB), and the MAPK/ERK
pathway in different types of cancer (17,18).
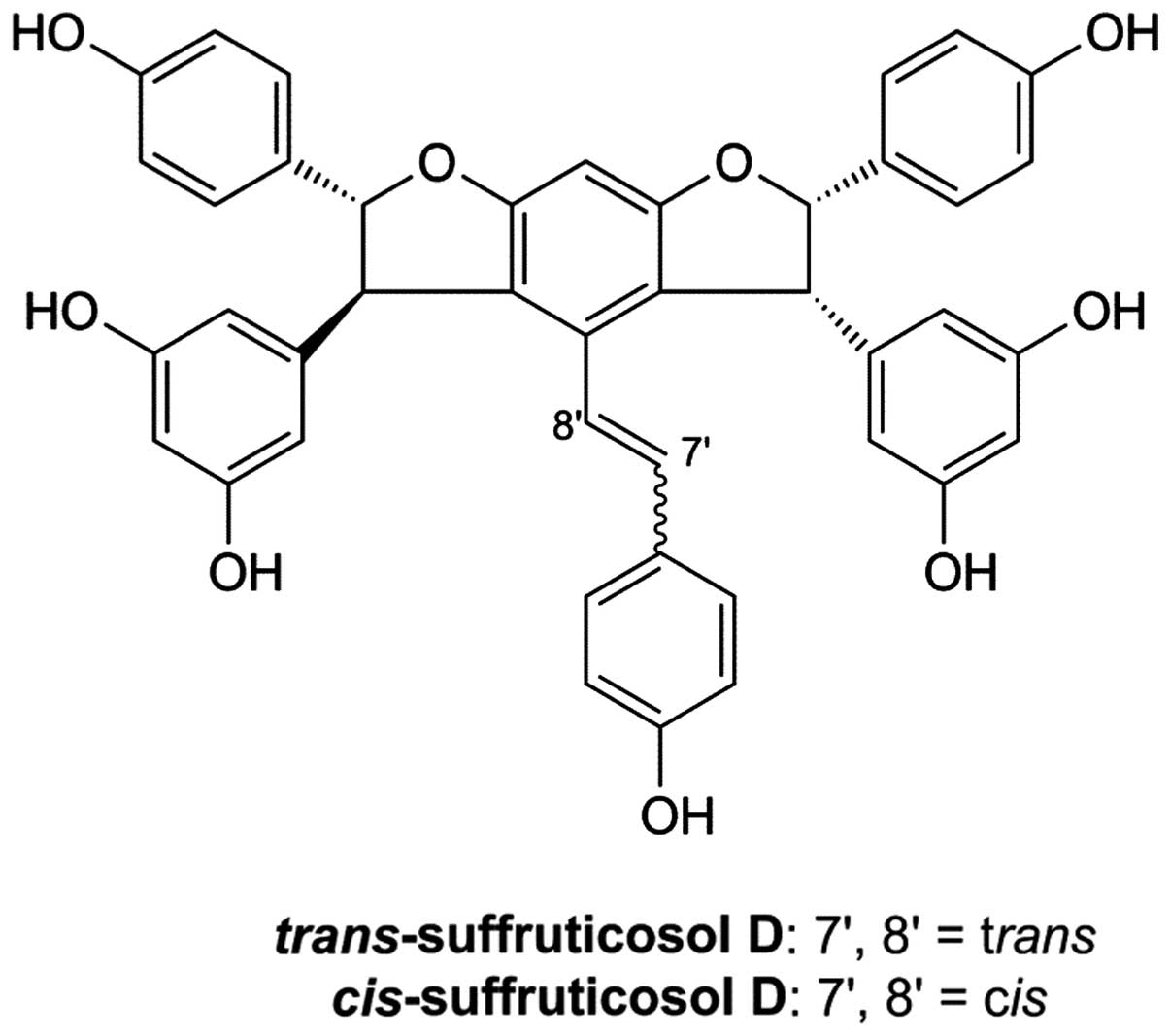
Previously, two novel stilbenes, cis- and
trans-suffruticosol D, were extracted from the seeds of
Paeonia (5). The two
chemicals have similar structures as the mass fragmentation pattern
of trans-suffruticosol D was very similar to
cis-suffruticosol D, with cis-suffruticosol D varying
only from trans-suffruticosol D in its olefinic hydrogen
signal (Fig. 1). In this study, we
investigated the antitumor activities of cis- and
trans-suffruticosol D and examined how these two chemicals
act against cancer cells in vitro.
Materials and methods
Plant material and compound
isolation
The seeds of P. suffruticosa were collected
in Tongling, Anhui, China, and identified in September 2012. A
voucher specimen (2012001) has been deposited in the Seed Resource
Bank at the Institute of Medicinal Plant Development, Chinese
Academy of Medical Sciences and Peking Union Medical College.
cis- and trans-suffruticosol D were extracted and
isolated from the dried seeds of P. suffruticosa as
described previously (5).
Compounds were re-suspended in dimethyl sulfoxide (DMSO)
(Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 10 mM and
stored at 4°C.
Cell culture
Four human cancer cell lines including A549 (lung
carcinoma), BT20 (estrogen receptor-negative human breast
adenocarcinoma), MCF-7 (estrogen receptor-positive human breast
adenocarcinoma) and U2OS (human osteosarcoma) were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). An A549
cell line that stably expresses green fluorescent protein (GFP) was
purchased from Cell BioLabs Inc. (San Diego, CA, USA). A549,
A549-GFP and BT20 cells were cultured in RPMI-1640 media
(Sigma-Aldrich), MCF-7 cells were cultured in DMEM medium (ATCC),
and U20S cells were cultured in McCoy's 5A medium (ATCC). As a
control, HPL1A cells (human peripheral lung epithelial cells) were
obtained from Nagoya University and cultured in DMEM/F12 medium
(Sigma-Aldrich); HMEC cells (primary human mammary breast
epithelial cells) were purchased from ATCC and cultured in McCoy's
5A medium. HPL1A cells (primary human peripheral lung epithelial
cells) were obtained from Nagoya University, Japan, and cultured in
DMEM/F-12K medium (Sigma-Aldrich). All medium contained 10% FBS
(Sigma-Aldrich) and 1% streptomycin and penicillin (Sigma-Aldrich).
These cells were incubated in a humid environment with 5%
CO2 at 37°C.
Cell proliferation assay
The resazurin reduction reagent AlamarBlue
(Invitrogen, Frederick, MD, USA) was used to evaluate the
cytotoxicity of the compounds. Cells were plated at a density of
5×103 cells per well in 96-well microplates with 100 μl
culture medium, and were allowed to attach for 16 h prior to
treatment. Next, all the medium was replaced with medium containing
the cis- or trans-suffruticosol D compounds at seven
different concentrations: 320, 100, 32, 10, 3.2, 1.0 and 0.32 μM.
1% DMSO was used as vehicle control. The cells were placed in an
incubator for 48 h at 37°C. Cells that were treated with medium
containing vehicle only serve as negative control. Subsequently,
AlamarBlue solution was added to the medium and the cells were
incubated in the CO2 incubator for 1 h. The fluorescent
intensity change of the dye was measured at Ex 555 nm and Em 590 nm
using a plate reader (Molecular Devices, Sunnyvale, CA, USA). The
cytotoxicity was examined by determining by IC50, the
dose that inhibited 50% of cell growth, using GraphPad Prism
software (GraphPad Software, La Jolla, CA, USA). For the
N-acetylL-cysteine (NAC) attenuation assay, the cells were treated
with different concentrations of cis- or
trans-suffruticosol D followed by incubation with or without
10 mM NAC (Sigma-Aldrich) for 48 h, and then cell viability was
assessed using the AlamarBlue assay.
Apoptosis assay
The FlowCellect Annexin Red kit (EMD Millipore,
Billerica, MA, USA) was used to determine the apoptosis rate in
A549 cells according to the manufacturer's instructions. Briefly,
A549 cells were plated in 96-well plates. After a 24-h treatment
with cis- or trans-suffruticosol D at concentrations
of 100, 32 and 10 μM, the floating and attached cells were
collected for analysis. The cells were centrifuged at 700 × g for 7
min and were resuspended in 100 μl assay buffer (EMD Millipore).
Afterwards, the cells were stained with Annexin V for 15 min and
7-amino-actinomycin D (7-AAD) for 5 min, and examined with a Guava
EasyCyte Flow Cytometer (EMD Millipore). Data were analyzed using
Guava InCyte software.
Apoptosis antibody array
The Human Apoptosis Antibody Array kit (RayBiotech,
Inc., Norcross, GA, USA) was used to evaluate apoptotic protein
expression according to the manufacturer's instructions. A549 cells
were plated at 8,000 cells/well intensity in a 96-well plate and
then treated with cis- or trans-suffruticosol D at a
concentration of 50 μM for 6 h. The cells were lysed in lysis
buffer with protease inhibitors. The cell lysates were concentrated
using a protein concentration column (EMD Millipore) to a total
protein concentration of 2 mg/ml. The samples were then diluted
10-fold with assay buffer and incubated with an array membrane for
2 h at room temperature, and washed with washing buffer for five
times. Subsequently, the cocktail of biotin-conjugated antibody mix
was added to the membrane and incubated overnight at 4°C. The
samples were then incubated with HRP-conjugated streptavidin for 2
h at room temperature and chemiluminescence substrate was used to
detect the signal. Image Studio software (LI-COR Biotechnology,
Lincoln, NE, USA) was used to quantify the intensity of each array
dot and then normalized to the internal control.
Oxidative stress assay
The Hitkit oxidative stress kit (Thermo Scientific,
Waltham, MA, USA) was used to determine the generation of reactive
oxygen species (ROS) according to the manufacturer's instructions.
Briefly, A549 cells were treated with cis- or
trans-suffruticosol D for 24 h, fixed with warm 37%
formaldehyde and stained with Hoechst and dihydroethidium (DHE) dye
for 30 min at 37°C with 5% CO2. Doxorubicin (DOX) at 1
μM concentration was used as a positive control and cells treated
with vehicle only were used as negative control. ROS generation in
the nuclei was indicated by the production of the fluorescent
ethidium, and assessed by measuring the nuclear fluorescent
intensity using an ArrayScan VTI High-content screening (HCS)
reader (Thermo Scientific). Images were acquired and data was
analyzed by vHCS Scan software.
Cell motility assay
A 96-well collagen plate (Corning, Corning, NY, USA)
was coated with blue fluorescent beads (Life Technologies, Eugene,
OR, USA) as follows. The beads were centrifuged for 1 min at 14,000
g and washed twice with PBS, then 75 μl beads were added to each
well of the 96-well collagen plate and incubated for 1 h at 37°C.
The cells were seeded on the lawn of fluorescent beads and the
sizes of the tracks generated by migrating cells were measured.
After the plate was washed 5 times with PBS, A549-GFP cells were
seeded at 500 cells/well in the coated plate and incubated for 1 h
at 37°C. Subsequently the cells were treated with different
concentrations of cis- or trans-suffruticosol D in
medium containing 10% FBS for 18 h. Cells treated with serum-free
medium serve as the negative control and cells treated with medium
containing 10% FBS serve as the positive control. Cell tracks were
imaged using an Arrayscan VTI HCS reader (Thermo Scientific) and
the data were analyzed by vHCS Scan software. The mean of the full
track area per cell for the test compound and the controls was
calculated.
Multi-parameter cytotoxicity assay
HCS analysis was used to measure nuclear morphology,
cell membrane permeability, and mitochondrial membrane potential
changes, the three parameters associated with cytotoxicity. A549
cells were treated with different concentrations of cis- or
trans-suffruticosol D for 24 h. The cells were then fixed
and stained with a warm solution containing Hoechst dye, membrane
permeability dye, and mitochondrial membrane potential dye (Thermo
Scientific). Cells were imaged using an Arrayscan VTI HCS reader
(Thermo Scientific). Data on nuclear size, cell permeability, and
mitochondria membrane potential were collected and analyzed using
vHCS Scan software.
Western blot analysis
A549 cells were treated with 50 μM of cis- or
trans-suffruticosol D for 3 h then incubated with 10 ng/ml
of TNF-α for 30 min. Cells treated with the NF-κB inhibitor
Bay11-7082 (10 μM) (Sigma-Aldrich) were used as a positive control,
and cells treated with vehicle only were used as a negative
control. After treatment, the cells were lysed using M-PER
mammalian protein extraction reagent (Thermo Scientific) containing
proteinase and phosphatase inhibitors (Sigma-Aldrich) and
centrifuged at 13,000 rpm for 5 min at 4°C. A Pierce BCA protein
assay kit (Thermo Scientific) was used to determined protein
concentrations. Proteins were separated on a 4–20% Tris-glycine gel
(Thermo Scientific), and electrophoretically transferred to a PVDF
membrane. The following primary antibodies were used:
phosphorylated-NF-κB p65, NF-κB p65 (Cell Signaling Technology,
Danver, MA, USA) and actin (Santa Cruz Biotechnology, Dallas, TX,
USA). The membrane was incubated with the primary antibodies at a
1:1,000 concentration at 4°C overnight. After washing with 1X PBS 5
times, the membrane was incubated for 2 h at room temperature with
HRP linked anti-rabbit IgG secondary antibodies. Membranes were
developed with chemiluminescent substrates (Thermo Scientific) and
scanned with a chemiDoc MP imaging system (Bio-Rad, Hercules, CA,
USA).
NF-κB nuclear translocation assay
The Multiplexed NF-κB activation HCS kit (Thermo
Scientific) was used to assess NF-κB nuclear translocation. A549
cells were pre-treated with different concentrations of cis-
or trans-suffruticosol D for 4 h, then 10 ng/ml of TNF-α
(Sigma-Aldrich) was added to the cells for an additional 30 min.
After treatment, cells were fixed and permeabilized prior to
detection. NF-κB distribution was detected by adding NF-κB p65
primary antibodies and then staining with a secondary antibody
conjugated with DyLight 549 and Hoechst dye (Thermo Scientific).
Cells treated with medium containing only the vehicle were used as
negative control, and cells treated with 25 ng/ml TNF-α were used
as a positive control. Cells were imaged using an Arrayscan VTI HCS
reader. Data on the mean difference of NF-κB fluorescent intensity
between the nuclear and cytoplasmic areas were collected and
analyzed by vHCS Scan software.
Results
Cytotoxicity of cis- and
trans-suffruticosol D in lung, breast, and bone cancer cells
After 48-h treatment, both cis- and
trans-suffruticosol D showed significant cytotoxic effects
against A549 (lung), BT20 (breast), MCF-7 (breast) and U2OS
(osteosarcoma) cancer cell lines. IC50 values for
cis- and trans-suffruticosol D against these cancer
cells ranged from 9.93 to 46.79 μM as shown in Table I. Interestingly, we observed that
trans-suffruticosol D had lower IC50 values
(9.93–15.84 μM) than cis-suffruticosol D (13.42–46.79 μM) in
all four cancer cell lines. In addition, both cis- and
trans-suffruticosol D showed notably weaker cytotoxicity
against normal breast epithelial cells HMEC (IC50 values
of 146.3 and 269.5 μM, respectively) and normal lung epithelial
cells HPL1A (IC50 values of 78.3 and 177.5 μM,
respectively) (Table I).
 | Table IIC50 values of cis-
and trans-suffruticosol D in selected cancer and normal cell
lines. |
Table I
IC50 values of cis-
and trans-suffruticosol D in selected cancer and normal cell
lines.
| IC50
(μM) |
trans-SD | cis-SD |
|---|
| A549 | 11.9±1.2 | 17.1±1.0 |
| BT20 | 9.9±3.8 | 13.4±2.5 |
| MCF-7 | 15.8±1.6 | 46.8±3.3 |
| U2OS | 11.3±2.3 | 24.6±4.4 |
| HPL1A | 78.3±6.1 | 177.5±9.3 |
| HMEC | 146.3±2.7 | 269.5±2.2 |
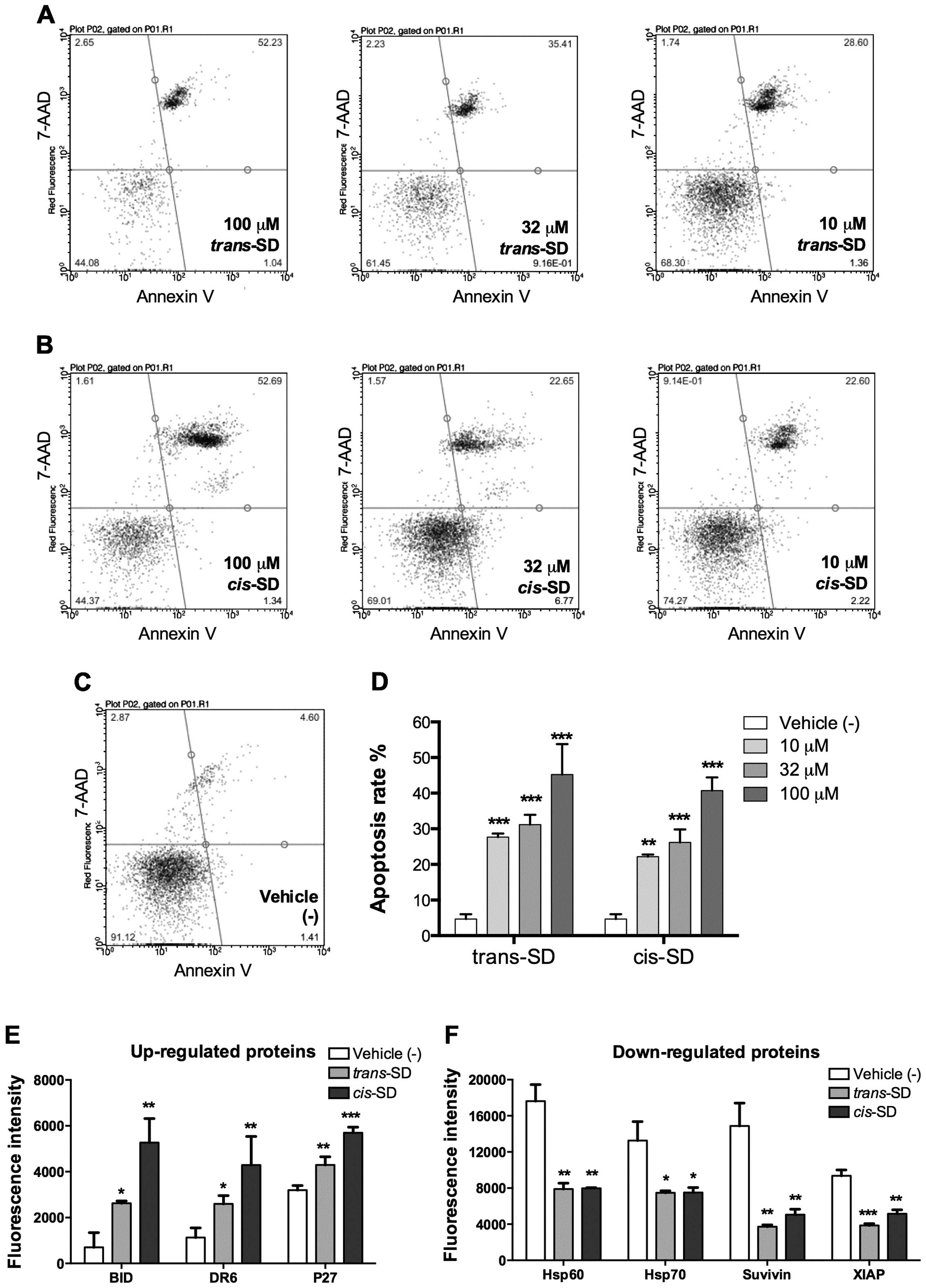
cis- and trans-suffruticosol D induce
apoptosis in A549 lung cancer cells
To find out whether these cytotoxic properties were
due to apoptosis, we conducted an apoptosis assay using A549 cells
treated with cis- or trans-suffruticosol D. Following
a 24-h treatment, both compounds showed significant apoptosis
induction at a wide range of concentrations compared with the
non-treated cells (*P<0.05, **P<0.01 or
***P<0.001) and the apoptotic effects were
concentration-dependent (Fig.
2A–D). trans-suffruticosol D induced 30.1, 39.8 and
41.9% of A549 cells into apoptosis at concentrations of 10, 32 and
100 μM, respectively. cis-suffruticosol D induced 22.2, 27.1
and 45.3% of A549 cells into apoptosis at concentrations of 10, 32
and 100 μM, respectively.
Next, we performed an apoptotic protein array
analysis to investigate the effect of cis- and
trans-suffruticosol D on apoptotic proteins. Two proteins from the
inhibitor of apoptosis proteins family (IAPs), X-linked inhibitor
of apoptosis protein (XIAP) and survivin, as well as the heat shock
proteins Hsp60 and Hsp70, showed significant downregulation after
treatment by cis- and trans-suffruticosol D (Fig. 2E). Whereas, death receptor 6 (DR6),
also known as tumor necrosis factor receptor superfamily member 21
(TNFRSF21), the cyclin-dependent kinase inhibitor 1B (p27), and the
BH3 interacting-domain death agonist (BID), were upregulated by
both cis- and trans-suffruticosol D (Fig. 2F).
cis- and trans-suffruticosol D induce ROS
generation in A549 lung cancer cells
We examined the cellular ROS levels in A549 cells to
determine whether cis- and trans-suffruticosol D
induced oxidative stress. As shown in Fig. 3A, both cis- and
trans-suffruticosol D converted non-fluorescent DHE to
fluorescent ethidium, which binds to DNA, suggesting they induced
ROS generation in A549 cells. Quantitative data showed both
compounds significantly induced ROS generation in a
concentration-dependent manner (**P<0.01,
***P<0.001 or ****P<0.0001). After
treatment for 24 h, trans-suffruticosol increased the ROS
levels by 32.8, 34.6 and 87.2% at concentrations of 10, 32 and 100
μM, respectively, while cis-suffruticosol increased the ROS
levels by 32.8, 55.6 and 73.1% at concentrations of 10, 32, and 100
μM, respectively, in A549 cells (Fig.
3B). To further investigate whether the cytotoxicity induced by
cis- and trans-suffruticosol D was associated with
ROS levels, we co-treated A549 cells with the anti-oxidant
N-acetyl-L-cysteine (NAC) and different concentrations of
cis- or trans-suffruticosol D for 48 h. We observed
that 10 mM NAC attenuated the cell death induced by cis- or
trans-suffruticosol D in A549 cells at all of the
concentrations that were tested (Fig.
3C).
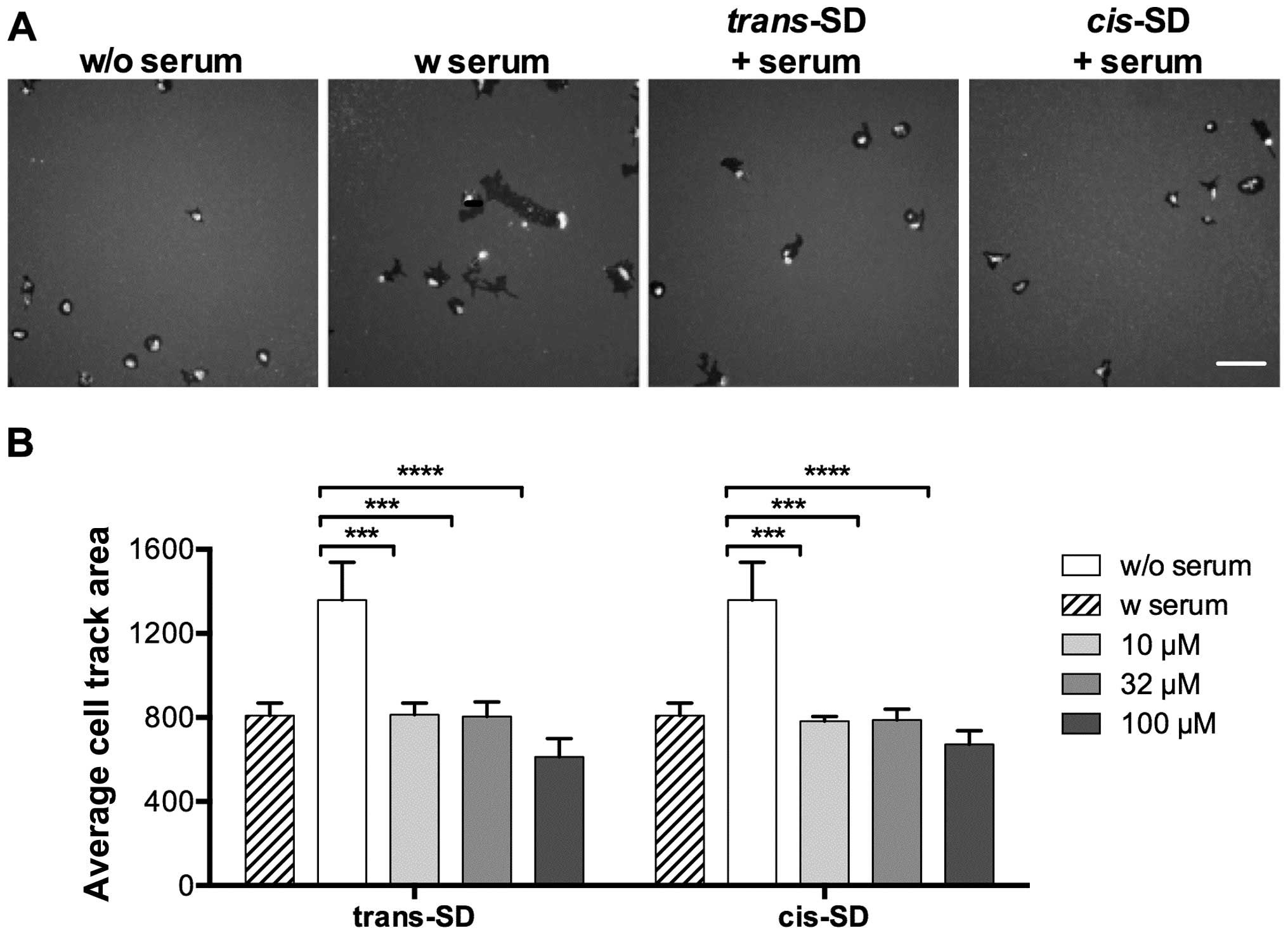
cis- and trans-suffruticosol D inhibit
the motility of A549 lung cancer cells
To test if cis- and
trans-suffruticosol D affected cancer cell motility, we
measured the size of the tracks generated by migrating cells after
treatment, which is proportional to the magnitude of cell movement.
As shown in Fig. 4A, A549 cells
treated with cis- or trans-suffruticosol D in
serum-containing medium showed less motility activity evidenced by
a smaller track area per cell than the untreated cells. Both
cis- and trans-suffruticosol D significantly
inhibited cell movement at all the concentrations that were tested
in A549 cells (***P<0.001 or
****P<0.0001) (Fig.
4B). trans-suffruticosol D decreased the A549 cell
motility by 40.7, 40.7 and 54.9% at concentrations of 10, 32 and
100 μM, respectively, while cis-suffruticosol D decreased
the A549 cell motility by 42.3%, 42.0 and 50.4% at concentrations
of 10, 32 and 100 μM, respectively.
cis- and trans-suffruticosol D decreased
mitochondrial membrane potential in A549 cells
To determine the cytotoxic effect of cis- and
trans-suffruticosol D in human lung cancer cells, we
measured three cell health parameters, nuclear morphology, cell
membrane permeability and mitochondrial membrane potential changes,
using an HCS reader. As shown in Fig.
5, in the mitochondrial potential channel, untreated A549 cells
exhibited bright fluorescent intensity, indicating intact
mitochondrial membranes. In comparison, in cells treated with
cis- or trans-suffruticosol D the fluorescent
intensity of the dye was significantly decreased at all tested
concentrations, indicating that cis- and
trans-suffruticosol D induced a significant decrease of the
mitochondrial membrane potential in A549 cells
(***P<0.001). We also observed nuclei shrinkage and
increased cell membrane permeability in cells treated with a
high-concentration (100 μM) of trans-suffruticosol D
(*P<0.05 or **P<0.01). However, no
significant change was detected in nuclear size and cell membrane
permeability in cells treated with cis-suffruticosol D.
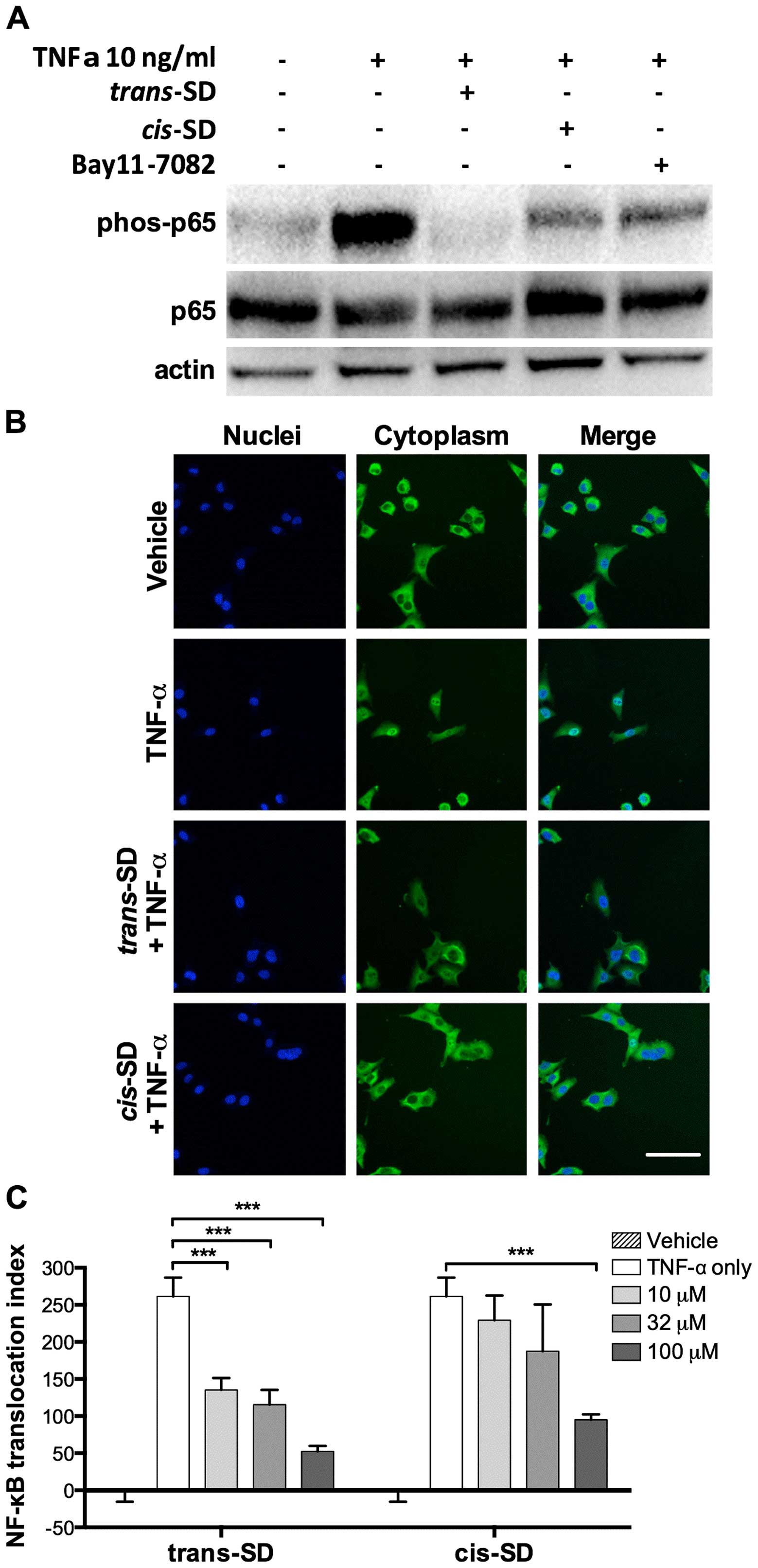
cis- and trans-suffruticosol D inhibit
TNF-α-induced NF-κB activation
We performed western blot analysis to examine the
effects of cis- and trans-suffruticosol D on the
expression of NF-κB in A549 cells. As shown in Fig. 6A, upon TNF-α stimulation,
overexpression of phosphorylated NF-κB p65 was detected, and the
overexpression was significantly inhibited by cis- and
trans-suffruticosol D. In trans-suffruticosol
D-treated cells, the expression of phosphorylated NF-κB p65 was
almost completely blocked, and in cis-suffruticosol
D-treated cells, the expression of phosphorylated NF-κB p65 was
blocked as effectively as the blockage caused by the Bay11-7082
inhibitor control.
Next, we used HCS analysis to test whether
cis- or trans-suffruticosol D could block NF-κB
nuclear translocation in A549 cells. As shown in Fig. 6B, NF-κB fluorescent staining
remained in the cytoplasmic area and no fluorescence was detected
in the nuclear area in non-treated cells, however, in cells treated
with TNF-α the NF-κB fluorescent staining was detected in the
nuclear area, indicating that NF-κB was translocated from the
cytoplasm to the nucleus. In A549 cells treated with cis- or
trans-suffruticosol D, NF-κB fluorescent staining remained
in the cytoplasm, suggesting that NF-κB translocation to the
nucleus was blocked. Treatment with trans-suffruticosol D at
all the tested concentrations, caused a significant inhibition of
NF-κB activation (***P<0.001) (Fig. 6C). In contrast, treatment with
cis-suffruticosol D only caused a significant inhibition of
NF-κB at 100 μM (***P<0.001).
Discussion
Oligostilbenes have been widely considered to be
valuable resources of antitumor agents. Previously, two novel
oligostilbenes, cis- and trans-suffruticosol D, were
extracted from the seeds of P. suffruticosa, but their
antitumor activities were not determined. In this study, we found
that both of these oligostilbenes exhibited remarkable
anti-proliferation activities against several types of cancer cell
lines, and their cytotoxicity effects and related mechanisms were
investigated.
trans-suffruticosol D exhibited lower
IC50 values (9.93–20.8 μM) than cis-suffruticosol
D (13.42–46.79 μM) in all of the cancer cell lines that were
tested, indicating that trans-suffruticosol D is more
cytotoxic than its cis-isomer. Consistent with this
conclusion, trans-suffruticosol D had stronger effects than
cis-suffruticosol D on three cytotoxicity parameters,
changes in nuclear size, cell membrane permeability and
mitochondrial transmembrane potential. trans-suffruticosol D
also showed higher inhibition activity of NF-κB activation than
cis-suffruticosol D. These observations are consistent with
a previous report, which showed that trans-resveratrol had
stronger cytotoxicity than its cis-isomer (19). In addition, both chemicals showed
selective cytotoxicity against cancer cell lines versus a normal
cell line.
Cancer cells usually develop the ability to escape
apoptosis (programmed cell death), which is a homeostatic mechanism
to maintain cell populations in the body (20). Hence, targeting apoptotic induction
has become an important strategy of anticancer therapies. It is
commonly known that there are two apoptotic pathways, the
extrinsic, or the death receptor pathway, and the intrinsic, or the
mitochondrial pathway. Previous studies have shown that
mitochondria play a critical role in apoptosis, especially in the
intrinsic apoptosis pathways (21,22).
Mitochondria are the main source of ROS inside the cell, and
increases in ROS production can damage the mitochondrial membrane
and subsequently lead to the release of pro-apoptotic proteins and
cytochrome c, thus activating the apoptotic pathway
(23–25). In this study, we found that
cis- and trans-suffruticosol D induced apoptosis in
A549 lung cancer cells after 24-h treatment in a
concentration-dependent manner. Both oligostilbenes significantly
decreased the mitochondrial membrane potential in lung cancer
cells, suggesting they might induce the mitochondrial apoptosis
pathway. Since both chemicals significantly increased cellular ROS
levels in lung cancer cells and their cytotoxicity was associated
with ROS levels as shown by the NAC attenuation assay, it can be
speculated that the excessive ROS induced by cis- and
trans-suffruticosol D act as an apoptosis mediator by
damaging the mitochondrial membrane, causing the release of the
mitochodria's contents, which eventually leads to apoptosis. In
addition, cis- and trans-suffruticosol D affected the
expression of several key regulators involved in apoptosis; XIAP,
survivin, Hsp60 and Hsp70 were downregulated, while BID, DR6 and
p27 were upregulated.
XIAP and survivin are known apoptosis inhibitors
that prevent apoptosis by inhibiting caspase-3, −7, and −9
(26–28). Downregulation of XIAP or survivin
has been demonstrated to inhibit the progression of cancer and
increase the sensitivity of cancer cells to chemo-reagents
(29–32). Heat shock proteins Hsp60 and Hsp70
are chaperones that play essential roles in tumor cell survival and
proliferation due to their ability to block both the intrinsic and
extrinsic apoptosis pathways (33,34).
BID is a pro-apoptotic member of the Bcl-2 protein family, and is a
mediator of mitochondrial damage induced by caspase-8 (35). p27, the cyclin-dependent kinase
inhibitor, controls the cell cycle progression at G1 by preventing
the activation of cyclin E-Cdk2 or cyclin D1-Cdk4 complexes
(36,37). DR6, also known as TNFRSF21, is a
member of the death receptor family, which induces apoptosis in
mammalian cells and its apoptotic function is inhibited by survivin
(38). Downregulation of XIAP,
survivin, Hsp60 and Hsp70, as well as upregulation of BID, DR6 and
p27 by cis- and trans-suffruticosol D at least
partially contribute to the apoptotic effect of cis- and
trans-suffruticosol D.
Tumor cells have the ability to migrate to
surrounding tissues and organs through reorganization of the actin
cytoskeleton (39,40). Most of the fatality from tumors
occurs when cells move from the initial organs where they
originated (41). Therefore,
control of cancer cell motility and migration is an essential issue
in cancer treatment and represents a new opportunity for a
potential tumor therapy (42).
cis- and trans-suffruticosol D significantly
inhibited the mobility of lung cancer cells after treatment for 18
h at all the concentrations that were tested. Therefore, both
chemicals exhibit therapeutic potential as an inhibitor of cancer
cell mobility.
The NF-κB pathway is known to control cell growth
and survival, and the transcription factor NF-κB has been found to
be permanently activated in various tumors (21). Activation of NF-κB in cancer cells
is often associated with drug resistance as both radio- and
chemo-therapies induce constitutive activation of the NF-κB pathway
(43). Therefore, a compound's
ability to block the NF-κB pathway is important for the efficacy of
cancer therapy (44). In this
study, we evaluated cis- and trans-suffruticosol D
for their abilities to inhibit TNF-α induced NF-κB activation in
lung cancer cells. After a 4-h treatment both chemicals
significantly blocked NF-κB p65 phosphorylation as well as NF-κB
p65 translocation from the nucleus to the cytoplasm, suggesting
they might act as an inhibitor of the NF-κB pathway. Since NF-κB
affects the transcription of a number of anti-apoptotic proteins,
including cellular inhibitor of apoptosis proteins (cIAPs), XIAP,
bcl-2, bcl-XL, and FADD-like IL-1β-converting enzyme-inhibitory
protein (c-FLIP), blocking NF-κB nuclear translocation decreases
the expression of anti-apoptotic proteins and subsequently promotes
apoptosis. In addition, several studies have shown that an increase
of ROS can block the NF-κB pathway by the inhibition of cytokines,
such as TNF and IL-1 (45).
Because cis- and trans-suffruticosol D increased ROS
generation in lung cancer cells, the block in the NF-κB pathway may
be associated with the inhibition of the inducer cytokines by
excessive ROS.
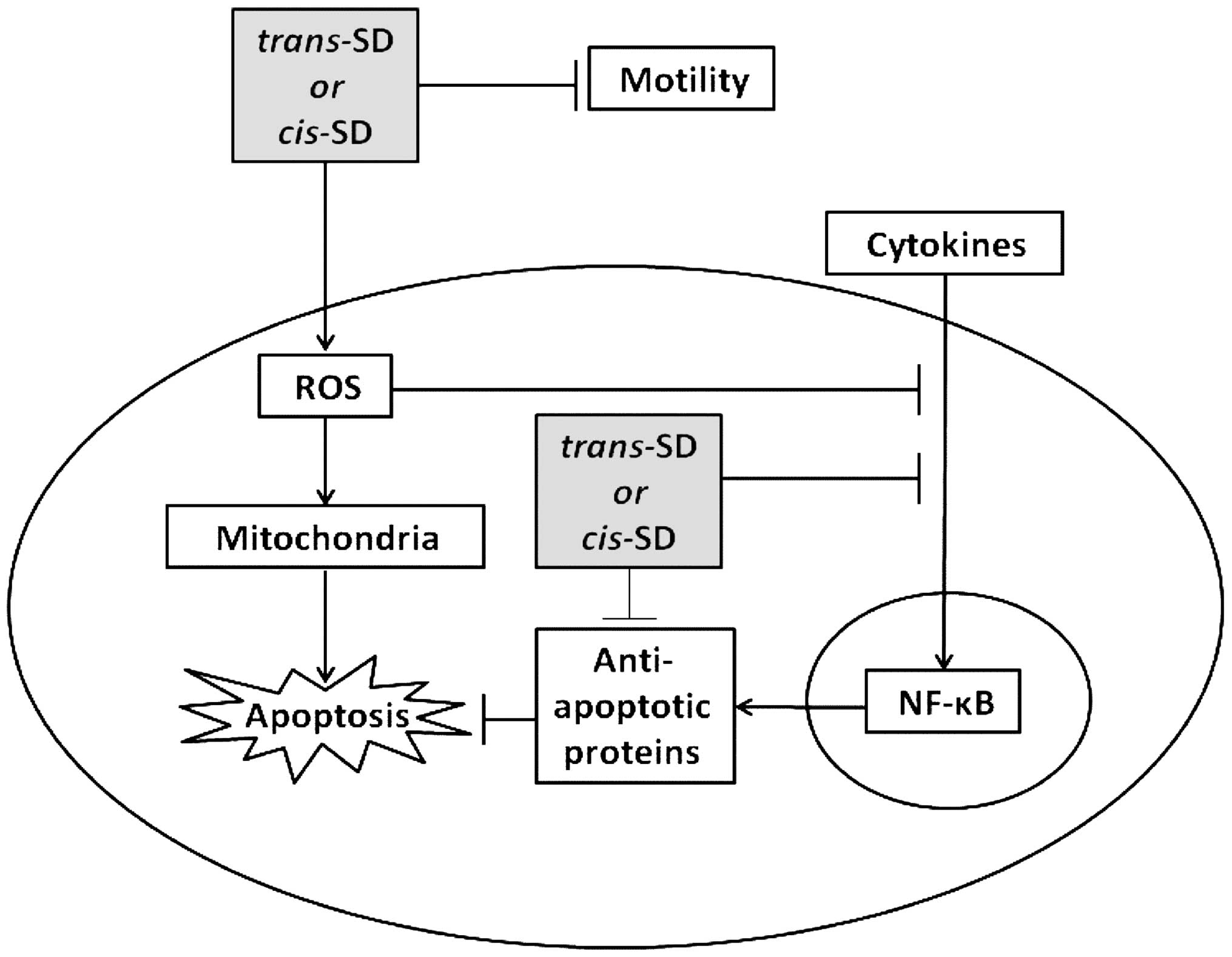
In conclusion, this study provides evidence that
cis- and trans-suffruticosol D have promising
antitumor activities. Both compounds selectively inhibited the
growth of various cancer cells, induced apoptosis in A549 lung
cancer cells, as well as inhibited A549 cell movement. The
induction of apoptosis may be associated with ROS generation and
inhibition of the NF-κB pathway. Collectively, our results suggest
a potential mechanism for the cytotoxicity of cis- and
trans-suffruticosol D. As shown in Fig. 7, in A549 lung cancer cells,
cis- and trans-suffruticosol D trigger oxidative
stress, which in turn leads to mitochondrial damage, blocks NF-κB
activation and ultimately triggers apoptosis. Our findings suggest
that both cis- and trans-suffruticosol D have
promising chemo-therapeutic potential for treating cancer.
Acknowledgements
We thank the Tennessee Center of Botanical Medicine
Research (TCBMR) for providing the funding for this study. We also
thank Dr Takashi Takahashi at Nagoya University for the HPL1A cell
line.
References
|
1
|
Cai Y, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Q and Gong H: Clinical Practice of
Anticancer Traditional Chinese Medicines. People's Health
Publishing House; Beijing: 1998
|
|
3
|
Bo QM, Wu ZY, Shun QS, Bao XS, Mao ZS, Ha
SQ, Lu SY and Huang JM: A Selection of the Illustrated Chinese
Anti-Cancer Herbal Medicines. Shanghai Science and Technology
Literature Press; Shanghai: 2002
|
|
4
|
Parekh HS, Liu G and Wei MQ: A new dawn
for the use of traditional Chinese medicine in cancer therapy. Mol
Cancer. 8:212009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He CN, Peng Y, Xu LJ, Liu ZA, Gu J, Zhong
AG and Xiao PG: Three new oligostilbenes from the seeds of Paeonia
suffruticosa. Chem Pharm Bull (Tokyo). 58:843–847. 2010. View Article : Google Scholar
|
|
6
|
Chinese Pharmacopoeia Commission. Chinese
Pharmacopoeia. China Medical Scientific and Technological Press;
Beijing: pp. 160–161. 2010
|
|
7
|
He CN, Peng Y, Wu QL, Xiao W, Peng B, Wang
Z and Xiao PG: Simultaneous determination of ten stilbenes in the
seeds of Paeonia species using HPLC-DAD. J Liquid Chromatogr Relat
Technol. 36:1708–1724. 2013.
|
|
8
|
He CN, Peng Y, Zhang YC, Xu LJ, Gu J and
Xiao PG: Phytochemical and biological studies of Paeoniaceae. Chem
Biodivers. 7:805–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen T, Xie CF, Wang XN and Lou HX:
Stilbenoids. Natural Products. Springer; pp. 1901–1949. 2013,
View Article : Google Scholar
|
|
10
|
Cai T and Cai Y: cis-Ampelopsin E, a
stilbene isolated from the seeds of Paeonia suffruticosa, inhibits
lipopolysaccharide-stimulated nitric oxide production in RAW 264.7
macrophages via blockade of nuclear factor-kappa B signaling
pathway. Biol Pharm Bull. 34:1501–1507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuk HJ, Ryu HW, Jeong SH, Curtis-Long MJ,
Kim HJ, Wang Y, Song YH and Park KH: Profiling of neuraminidase
inhibitory polyphenols from the seeds of Paeonia lactiflora. Food
Chem Toxicol. 55:144–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussain S, Slevin M, Ahmed N, West D,
Choudhary MI, Naz H and Gaffney J: Stilbene glycosides are natural
product inhibitors of FGF-2-induced angiogenesis. BMC Cell Biol.
10:302009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simoni D, Invidiata FP, Eleopra M,
Marchetti P, Rondanin R, Baruchello R, Grisolia G, Tripathi A,
Kellogg GE, Durrant D, et al: Design, synthesis and biological
evaluation of novel stilbene-based antitumor agents. Bioorg Med
Chem. 17:512–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He S, Lu Y, Jiang L, Wu B, Zhang F and Pan
Y: Preparative isolation and purification of antioxidative stilbene
oligomers from Vitis chunganeniss using high-speed counter-current
chromatography in stepwise elution mode. J Sep Sci. 32:2339–2345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung M, Park WH, Jung JC, Lim E, Lee Y, Oh
S and Moon HI: Synthesis, structural characterization and
biological evaluation of novel stilbene derivatives as potential
antimalarial agents. Chem Biol Drug Des. 73:346–354. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee K, Lee JH, Ryu SY, Cho MH and Lee J:
Stilbenes reduce Staphylococcus aureus hemolysis, biofilm
formation, and virulence. Foodborne Pathog Dis. 11:710–717. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukla Y and Singh R: Resveratrol and
cellular mechanisms of cancer prevention. Ann NY Acad Sci.
1215:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whitlock NC and Baek SJ: The anticancer
effects of resveratrol: Modulation of transcription factors. Nutr
Cancer. 64:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pettit GR, Grealish MP, Jung MK, Hamel E,
Pettit RK, Chapuis JC and Schmidt JM: Antineoplastic agents. 465.
Structural modification of resveratrol: Sodium resverastatin
phosphate. J Med Chem. 45:2534–2542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasibhatla S and Tseng B: Why target
apoptosis in cancer treatment? Mol Cancer Ther. 2:573–580.
2003.PubMed/NCBI
|
|
21
|
Cheah SC, Appleton DR, Lee ST, Lam ML,
Hadi AHA and Mustafa MR: Panduratin A inhibits the growth of A549
cells through induction of apoptosis and inhibition of NF-kappaB
translocation. Molecules. 16:2583–2598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (Δψm) in apoptosis; an update.
Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
26
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and −7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schimmer AD, Dalili S, Batey RA and Riedl
SJ: Targeting XIAP for the treatment of malignancy. Cell Death
Differ. 13:179–188. 2006. View Article : Google Scholar
|
|
28
|
Ryan BM, O'Donovan N and Duffy MJ:
Survivin: A new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Cherton-Horvat G, Dragowska V, Baird
S, Korneluk RG, Durkin JP, Mayer LD and LaCasse EC: Antisense
oligonucleotides targeting XIAP induce apoptosis and enhance
chemotherapeutic activity against human lung cancer cells in vitro
and in vivo. Clin Cancer Res. 9:2826–2836. 2003.PubMed/NCBI
|
|
30
|
He X, Khurana A, Maguire JL, Chien J and
Shridhar V: HtrA1 sensitizes ovarian cancer cells to
cisplatin-induced cytotoxicity by targeting XIAP for degradation.
Int J Cancer. 130:1029–1035. 2012. View Article : Google Scholar
|
|
31
|
Oost TK, Sun C, Armstrong RC, Al-Assaad
AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP,
Olejniczak ET, et al: Discovery of potent antagonists of the
antiapoptotic protein XIAP for the treatment of cancer. J Med Chem.
47:4417–4426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cappello F, Conway de Macario E, Marasà L,
Zummo G and Macario AJ: Hsp60 expression, new locations, functions
and perspectives for cancer diagnosis and therapy. Cancer Biol
Ther. 7:801–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto H, Soh JW, Shirin H, Xing WQ, Lim
JT, Yao Y, Slosberg E, Tomita N, Schieren I and Weinstein IB:
Comparative effects of overexpression of p27Kip1 and p21Cip1/Waf1
on growth and differentiation in human colon carcinoma cells.
Oncogene. 18:103–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nickeleit I, Zender S, Kossatz U and Malek
NP: p27kip1: A target for tumor therapies? Cell Div. 2:132007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasof GM, Lu JJ, Liu D, Speer B, Mongan
KN, Gomes BC and Lorenzi MV: Tumor necrosis factor-alpha induces
the expression of DR6, a member of the TNF receptor family, through
activation of NF-kappaB. Oncogene. 20:7965–7975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar
|
|
41
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levin EG: Cancer therapy through control
of cell migration. Curr Cancer Drug Targets. 5:505–518. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Monika SA, Sharma A, Suthar SK, Aggarwal
V, Lee HB and Sharma M: Synthesis of lantadene analogs with marked
in vitro inhibition of lung adenocarcinoma and TNF-α induced
nuclear factor-kappa B (NF-κB) activation. Bioorg Med Chem Lett.
24:3814–3818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|