|
1
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
2
|
Peto J, Hodgson JT, Matthews FE and Jones
JR: Continuing increase in mesothelioma mortality in Britain.
Lancet. 345:535–539. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Driscoll T, Nelson DI, Steenland K, Leigh
J, Concha-Barrientos M, Fingerhut M and Prüss-Ustün A: The global
burden of disease due to occupational carcinogens. Am J Ind Med.
48:419–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park EK, Takahashi K, Hoshuyama T, Cheng
TJ, Delgermaa V, Le GV and Sorahan T: Global magnitude of reported
and unreported mesothelioma. Environ Health Perspect. 119:514–518.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baas P, Fennell D, Kerr KM, Van Schil PE,
Haas RL and Peters S: Malignant pleural mesothelioma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 26(Suppl 5): v31–v39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dawson MA and Kouzarides T: Cancer
epigenetics: From mechanism to therapy. Cell. 150:12–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baird A, Richard D, O'Byrne KJ and Gray
SG: Epigenetic therapy in lung cancer and mesothelioma. Epigenetic
Cancer Therapy. 1st edition. Gray SG: Academic Press; pp. 189–213.
2015, View Article : Google Scholar
|
|
10
|
Jenuwein T and Allis CD: Translating the
histone code. Science. 293:1074–1080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rothbart SB and Strahl BD: Interpreting
the language of histone and DNA modifications. Biochim Biophys
Acta. 1839:627–643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allis CD, Berger SL, Cote J, Dent S,
Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar
R, et al: New nomenclature for chromatin-modifying enzymes. Cell.
131:633–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krug LM, Kindler HL, Calvert H, Manegold
C, Tsao AS, Fennell D, Öhman R, Plummer R, Eberhardt WE, Fukuoka K,
et al: Vorinostat in patients with advanced malignant pleural
mesothelioma who have progressed on previous chemotherapy
(VANTAGE-014): A phase 3, double-blind, randomised,
placebo-controlled trial. Lancet Oncol. 16:447–456. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farria A, Li W and Dent SY: KATs in
cancer: Functions and therapies. Oncogene. 34:4901–4913. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furdas SD, Kannan S, Sippl W and Jung M:
Small molecule inhibitors of histone acetyltransferases as
epigenetic tools and drug candidates. Arch Pharm (Weinheim).
345:7–21. 2012. View Article : Google Scholar
|
|
17
|
Yang XJ: The diverse superfamily of lysine
acetyltransferases and their roles in leukemia and other diseases.
Nucleic Acids Res. 32:959–976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sapountzi V and Côté J: MYST-family
histone acetyltransferases: Beyond chromatin. Cell Mol Life Sci.
68:1147–1156. 2011. View Article : Google Scholar
|
|
19
|
Doyon Y and Côté J: The highly conserved
and multifunctional NuA4 HAT complex. Curr Opin Genet Dev.
14:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bird AW, Yu DY, Pray-Grant MG, Qiu Q,
Harmon KE, Megee PC, Grant PA, Smith MM and Christman MF:
Acetylation of histone H4 by Esa1 is required for DNA double-strand
break repair. Nature. 419:411–415. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Den Broeck A, Nissou D, Brambilla E,
Eymin B and Gazzeri S: Activation of a Tip60/E2F1/ERCC1 network in
human lung adenocarcinoma cells exposed to cisplatin.
Carcinogenesis. 33:320–325. 2012. View Article : Google Scholar
|
|
22
|
Miyamoto N, Izumi H, Noguchi T, Nakajima
Y, Ohmiya Y, Shiota M, Kidani A, Tawara A and Kohno K: Tip60 is
regulated by circadian transcription factor clock and is involved
in cisplatin resistance. J Biol Chem. 283:18218–18226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghizzoni M, Wu J, Gao T, Haisma HJ, Dekker
FJ and George Zheng Y: 6-alkylsalicylates are selective Tip60
inhibitors and target the acetyl-CoA binding site. Eur J Med Chem.
47:337–344. 2012. View Article : Google Scholar :
|
|
24
|
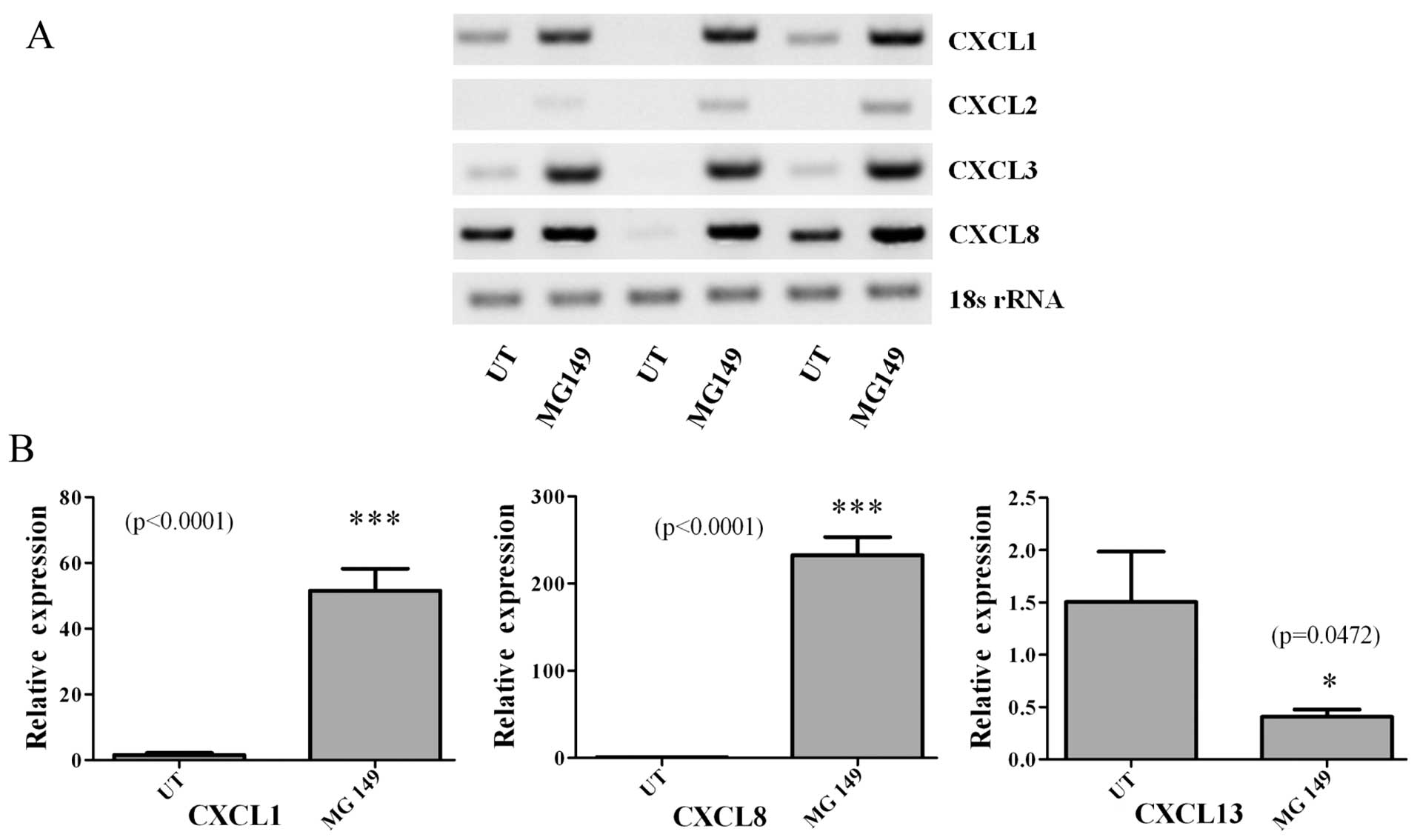
Baird AM, Gray SG and O'Byrne KJ:
Epigenetics underpinning the regulation of the CXC
(ELR+) chemokines in non-small cell lung cancer. PLoS
One. 6:e145932011. View Article : Google Scholar
|
|
25
|
Dragon J, Thompson J, MacPherson M and
Shukla A: Differential susceptibility of human pleural and
peritoneal mesothelial cells to asbestos exposure. J Cell Biochem.
116:1540–1552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan E, Warren N, Darnton AJ and Hodgson
JT: Projection of mesothelioma mortality in Britain using Bayesian
methods. Br J Cancer. 103:430–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao C, Bourke E, Scobie M, Famme MA,
Koolmeister T, Helleday T, Eriksson LA, Lowndes NF and Brown JA:
Rational design and validation of a Tip60 histone acetyltransferase
inhibitor. Sci Rep. 4:53722014.PubMed/NCBI
|
|
28
|
Coffey K, Blackburn TJ, Cook S, Golding
BT, Griffin RJ, Hardcastle IR, Hewitt L, Huberman K, McNeill HV,
Newell DR, et al: Characterisation of a Tip60 specific inhibitor,
NU9056, in prostate cancer. PLoS One. 7:e455392012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang C, Ngo L and Zheng YG: Rational
design of substrate-based multivalent inhibitors of the histone
acetyltransferase Tip60. ChemMedChem. 9:537–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gryder BE, Sodji QH and Oyelere AK:
Targeted cancer therapy: Giving histone deacetylase inhibitors all
they need to succeed. Future Med Chem. 4:505–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yardley DA, Ismail-Khan RR, Melichar B,
Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee
MJ and Trepel JB: Randomized phase II, double-blind,
placebo-controlled study of exemestane with or without entinostat
in postmenopausal women with locally recurrent or metastatic
estrogen receptor-positive breast cancer progressing on treatment
with a nonsteroidal aromatase inhibitor. J Clin Oncol.
31:2128–2135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fenichel MP: FDA approves new agent for
multiple myeloma. J Natl Cancer Inst. 107:djv1652015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lansley SM, Varano Della Vergiliana JF,
Cleaver AL, Ren SH, Segal A, Xu MY and Lee YC: A commercially
available preparation of Staphylococcus aureus bio-products
potently inhibits tumour growth in a murine model of mesothelioma.
Respirology. 19:1025–1033. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yom SS, Busch TM, Friedberg JS, Wileyto
EP, Smith D, Glatstein E and Hahn SM: Elevated serum cytokine
levels in mesothelioma patients who have undergone pleurectomy or
extrapleural pneumonectomy and adjuvant intraoperative photodynamic
therapy. Photochem Photobiol. 78:75–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stam TC, Swaak AJ, Kruit WH, Stoter G and
Eggermont AM: Intrapleural administration of tumour necrosis
factor-alpha (TNFalpha) in patients with mesothelioma: Cytokine
patterns and acute-phase protein response. Eur J Clin Invest.
30:336–343. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nowak AK, Millward MJ, Creaney J, Francis
RJ, Dick IM, Hasani A, van der Schaaf A, Segal A, Musk AW and Byrne
MJ: A phase II study of intermittent sunitinib malate as
second-line therapy in progressive malignant pleural mesothelioma.
J Thorac Oncol. 7:1449–1456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nowak AK, Cook AM, McDonnell AM, Millward
MJ, Creaney J, Francis RJ, Hasani A, Segal A, Musk AW, Turlach BA,
et al: A phase 1b clinical trial of the CD40-activating antibody
CP-870,893 in combination with cisplatin and pemetrexed in
malignant pleural mesothelioma. Ann Oncol. 26:2483–2490.
2015.PubMed/NCBI
|
|
40
|
Antony VB, Hott JW, Godbey SW and Holm K:
Angiogenesis in mesotheliomas. Role of mesothelial cell derived
IL-8. Chest. 109(Suppl): 21S–22S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Galffy G, Mohammed KA, Dowling PA, Nasreen
N, Ward MJ and Antony VB: Interleukin 8: An autocrine growth factor
for malignant mesothelioma. Cancer Res. 59:367–371. 1999.PubMed/NCBI
|
|
42
|
Cioce M, Canino C, Pulito C, Muti P,
Strano S and Blandino G: Butein impairs the protumorigenic activity
of malignant pleural mesothelioma cells. Cell Cycle. 11:132–140.
2012. View Article : Google Scholar
|
|
43
|
Galffy G, Mohammed KA, Nasreen N, Ward MJ
and Antony VB: Inhibition of interleukin-8 reduces human malignant
pleural mesothelioma propagation in nude mouse model. Oncol Res.
11:187–194. 1999.PubMed/NCBI
|