Introduction
Gallbladder carcinoma (GBC), an aggressive type of
biliary tract cancer, is the fifth most common gastrointestinal
malignancy worldwide (1). In spite
of recent advances in the understanding and treatment of other
gastrointestinal malignancies, GBC has a very poor prognosis, with
a <10% 5-year survival rate (2,3). GBC
often remains undiagnosed until the late disease stage because
patients in the early stage of GBC often exhibit no symptoms. Given
this background, it is important to develop a new therapeutic
approach for GBC.
Galectin-9 (Gal-9) is a β-galactoside-binding lectin
of the galectin family; this protein is involved in various
biological processes, including cell aggregation, adhesion,
chemoattraction, and apoptosis (4). The functions of Gal-9 have been
reported to include the induction of apoptosis in T-cells,
particularly CD4+ Th1 and Th17 cells, and the
stimulation of regulatory T-cell activity (5–7).
Interestingly, Gal-9 has been tested as a potential therapeutic
agent for various autoimmune diseases (7) and allergic diseases (8). Furthermore, it has been reported that
Gal-9 suppresses the cell proliferation and tumor growth of various
human cancer types (9,10), such as melanoma (11) and chronic myelogenous leukemia
(12). We have previously shown
that Gal-9 suppressed the cell proliferation and tumor growth of
human hepatocellular carcinoma (HCC) and cholangiocarcinoma by
inducing apoptosis, and we have identified several microRNAs
(miRNAs) that are associated with the antitumor effect of Gal-9
(13,14). However, the effect of Gal-9 on GBC
remains unknown.
miRNAs, small, endogenous, non-coding RNAs that are
21–30 nucleotides in length, modulate the expression of various
target genes at the post-transcriptional and translational levels
(15,16); 1,881 human miRNAs have been
registered at miRbase in release 21 (http://microrna.sanger.ac.uk/). However, little is
known concerning the association of certain miRNAs with the
antitumor effects of Gal-9 on GBC cells.
Therefore, the purpose of the present study was to
determine whether Gal-9 suppresses the tumor growth of GBC and to
identify miRNAs associated with the antitumor effect of Gal-9.
Materials and methods
Chemicals
Recombinant mutant forms of human Gal-9 lacking
linker peptides were expressed and purified as previously described
(17). This mutant protein is
stable against proteolysis (17).
Cell lines and culture
The human GBC cell lines NOZ and OCUG-1 were
obtained from the Japanese Collection of Research Bioresources
(Osaka, Japan). The GBC cell lines G-415 and TGBC24TKB were
obtained from Riken Cell Bank (Tsukuba, Japan). NOZ cells were
cultured in Williams' medium E (Sigma-Aldrich, St. Louis, MO, USA),
and OCUG-1 cells were cultured in Dulbecco's modified Eagle's
medium (Gibco, Tokyo, Japan) containing 0.5 mM pyruvate. G-415
cells were cultured in RPMI-1640 medium, and TGBC24TKB cells were
cultured in Dulbecco's modified Eagle's medium. All media contained
10% fetal bovine serum (Wako Pure Chemical Industries, Osaka,
Japan) and 100 mg/l penicillin-streptomycin (Invitrogen, Tokyo,
Japan), and the cells were incubated in a humidified atmosphere
containing 5% CO2 at 37ºC.
Cell proliferation assay
Cell proliferation assays were conducted using a
cell counting kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's instructions. Cells
(5.0×103) from each cell line were seeded into the wells
of a 96-well plate and were cultured in 100 μl of the corresponding
medium. After 24 h, the seeded cells were treated with 0, 0.1, 0.3,
or 1 μM Gal-9 diluted in the culture medium. At the indicated
time-points, the medium was exchanged for 100 μl of medium
containing CCK-8 reagent, and the cells were incubated for 3 h. The
absorbance at a wavelength of 450 nm was measured in each well
using an automated microplate reader.
Enzyme-linked immunosorbent assays
(ELISAs) measuring apoptosis
Caspase-cleaved cytokeratin 18 (cCK18) was evaluated
using the M30 Apoptosense ELISA kit obtained from PEVIVA AB
(Bromma, Sweden) (18). The cells
(5×103) were seeded in a 96-well plate and cultured for
6, 24 or 48 h following the addition of 0.3 μM Gal-9. The cells
were lysed in polyoxyethylene octylphenyl ether (NP-40) (Wako Pure
Chemical Industries). The subsequent ELISA procedures were
performed according to the manufacturer's instructions. The
abundance of antigen in the control and unknown samples was
calculated via interpolation from a standard curve.
Analysis of apoptosis-related protein
profiles using an antibody array
The cells were seeded in 100-mm culture dishes.
Subsequently, the cells were treated with 0.3 μM Gal-9 for 24 h.
The cells were lysed in Pro-Prep (iNtRON Biotechnology). A human
apoptosis antibody array kit (R&D Systems, Minneapolis, MN,
USA) was used to measure apoptosis-related proteins according to
the manufacturer's instructions. Briefly, in this method, proteins
were captured by antibodies spotted on a nitrocellulose membrane.
Then, the levels of apoptosis-related proteins were assessed using
an HRP-conjugated antibody, followed by detection via
chemiluminescence. Finally, each array membrane was exposed to
X-ray film using a chemiluminescence detection system (Perkin-Elmer
Co. Waltham, MA, USA).
Gel electrophoresis and western blot
analysis
NOZ cells (1.0×106/dish) were seeded in
100-mm culture dishes and cultured for 24 or 48 h, then 0.3 μM
Gal-9 was added. The cells were lysed using a protease inhibitor
cocktail (Pro-Prep complete protease inhibitor mixture; iNtRON
Biotechnology, Sungnam, Korea). Then, the samples were subjected to
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) on 12% agarose gels, and the proteins were transferred
to nitrocellulose membranes. After blocking, the membranes were
incubated in primary antibodies followed by secondary antibodies.
The immunoreactive proteins were visualized on X-ray film using an
enhanced chemiluminescence detection system (Perkin-Elmer Co.). The
primary antibodies included an anti-β-actin monoclonal antibody
(A5441, used at 1:10,000; Sigma-Aldrich) and antibodies against
cyclin D1 (RB-9041, used at 1:1,000; Thermo Fisher Scientific,
Waltham, MA, USA), cyclin E (used at 1:1,000; Thermo Fisher
Scientific), Cdk6 (sc-177, used at 1:1,000, Santa Cruz
Biotechnology, Santa Cruz, CA, USA), Cdk4 (sc-749, used at 1:1,000;
Santa Cruz Biotechnology), and Cdk2 (sc-163, used at 1:2,000; Santa
Cruz Biotechnology). The secondary antibodies included horseradish
peroxidase (HRP)-linked anti-mouse and anti-rabbit IgG (used at
1:2,000; GE Healthcare, UK).
Flow cytometric analysis
To evaluate the mechanism by which Gal-9 inhibits
tumor growth, the cell cycle profile was analyzed after treatment
with Gal-9. Flow cytometric analysis was conducted using the Cell
Cycle Phase Determination kit (Cayman Chemical Co., MI, USA)
according to the manufacturer's instructions. NOZ or OCUG-1 cells
(1.0×106 cells in a 100-mm dish) were treated with 0 or
0.3 μM Gal-9 for 48 h. Then, the cells were analyzed using a
Cytomics FC 500 flow cytom-eter (Beckman Coulter, Indianapolis, IN,
USA). The results were analyzed using Kaluza software (Beckman
Coulter).
Antibody arrays of phosphorylated
receptor tyrosine kinases (p-RTKs)
Human p-RTK array kits (R&D Systems) were used
to measure protein phosphorylation according to the manufacturer's
instructions. The cells were seeded in 100-mm culture dishes and
cultured for 24 h, after 0.3 μM Gal-9 was added. Briefly, in this
method, proteins were captured by antibodies spotted on a
nitrocellulose membrane. Then, the levels of the phosphoproteins
were assessed using an HRP-conjugated antibody, followed by
detection via chemiluminescence. Finally, each array membrane was
exposed to X-ray film using a chemiluminescence detection
system.
Xenograft model analysis
Animal experiments were performed according to the
guidelines of the Committee on Experimental Animals of Kagawa
University and the guidelines regarding the use of animal tissue by
the UK National Cancer Research Institute (19). We purchased 20 female athymic mice
(BALB/c-nu/nu; 6-week-old; 18–23 g) from Japan SLC (Shizuoka,
Japan). The mice were housed in a temperature-controlled
environment under a 12-h light/dark photoperiod. Each mouse was
subcutaneously inoculated with NOZ cells (3×106 cells
per animal) in the flank region of the mouse. Then, when their
xenografts were palpable as a mass of >3 mm in diameter, all
recipient mice were randomly assigned to the control group or the
treated group. The treated mice (n=10) were intraperitone-ally
injected with 90 μg of Gal-9 three times a week. PBS alone was
administered to the control group at the same time-points. The
tumor volume (mm3) was calculated as [tumor length (mm)
× tumor width (mm)2]/2 (20). The body weight of the mice was also
recorded. All animals were sacrificed on day 24 after treatment,
and all survived this treatment period.
Antibody arrays of phosphorylated
receptor tyrosine kinases (p-RTKs)
Human p-RTK array kits (R&D Systems,
Minneapolis, MN, USA) were used to measure protein phosphorylation
according to the manufacturer's instructions. The cells were seeded
in 100-mm culture dishes and cultured for 24 hours, after 0.3 μM
Gal-9 was added. Briefly, in this method, proteins were captured by
antibodies spotted on a nitrocellulose membrane. Then, the levels
of the phosphoproteins were assessed using an HRP-conjugated
antibody, followed by detection via chemiluminescence. Finally,
each array membrane was exposed to X-ray film using a
chemiluminescence detection system.
Analysis of miRNA arrays
The NOZ cells were treated with 0.3 μM Gal-9 for 24
h and were stored in RNAprotect reagent (Qiagen, Venlo, The
Netherlands). The samples for each cell line were processed for
total RNA extraction using the miRNeasy Mini kit (Qiagen) according
to the manufacturer's instructions. After measurement of RNA
quantity and quality using an RNA 6000 Nano kit (Agilent
Technologies, Santa Clara, CA, USA), the samples were labeled using
a miRCURY Hy3 Power Labeling kit (Exiqon, Vedbaek, Denmark) and
were hybridized to a human miRNA Oligo chip (v.20; Toray
Industries, Tokyo, Japan). Scanning was conducted using the 3D-Gene
Scanner 3000 (Toray Industries). 3D-Gene extraction version 1.2
software (Toray Industries) was used to calculate the raw intensity
of the images. To determine the difference in miRNA expression
between the Gal-9-treated and control samples, the raw data were
analyzed using GeneSpring GX 10.0 software (Agilent Technologies).
On the raw data that were above the background level, quantile
normalization was performed. Differentially expressed miRNAs were
determined by Mann-Whitney U test. Hierarchical clustering was
performed using the farthest neighbor method with the absolute
uncentered Pearson's correlation coefficient as a metric. A heat
map was produced with the relative expression intensity for each
miRNA, in which the base-2 logarithm of the intensity was
median-centered for each row.
Statistical analysis
All analyses were conducted using GraphPad Prism
software version 6.0 (GraphPad Software, San Diego, CA, USA). The
unpaired t-test was conducted for comparison between the groups. A
P-value of 0.05 was considered to indicate a significant difference
between the groups.
Results
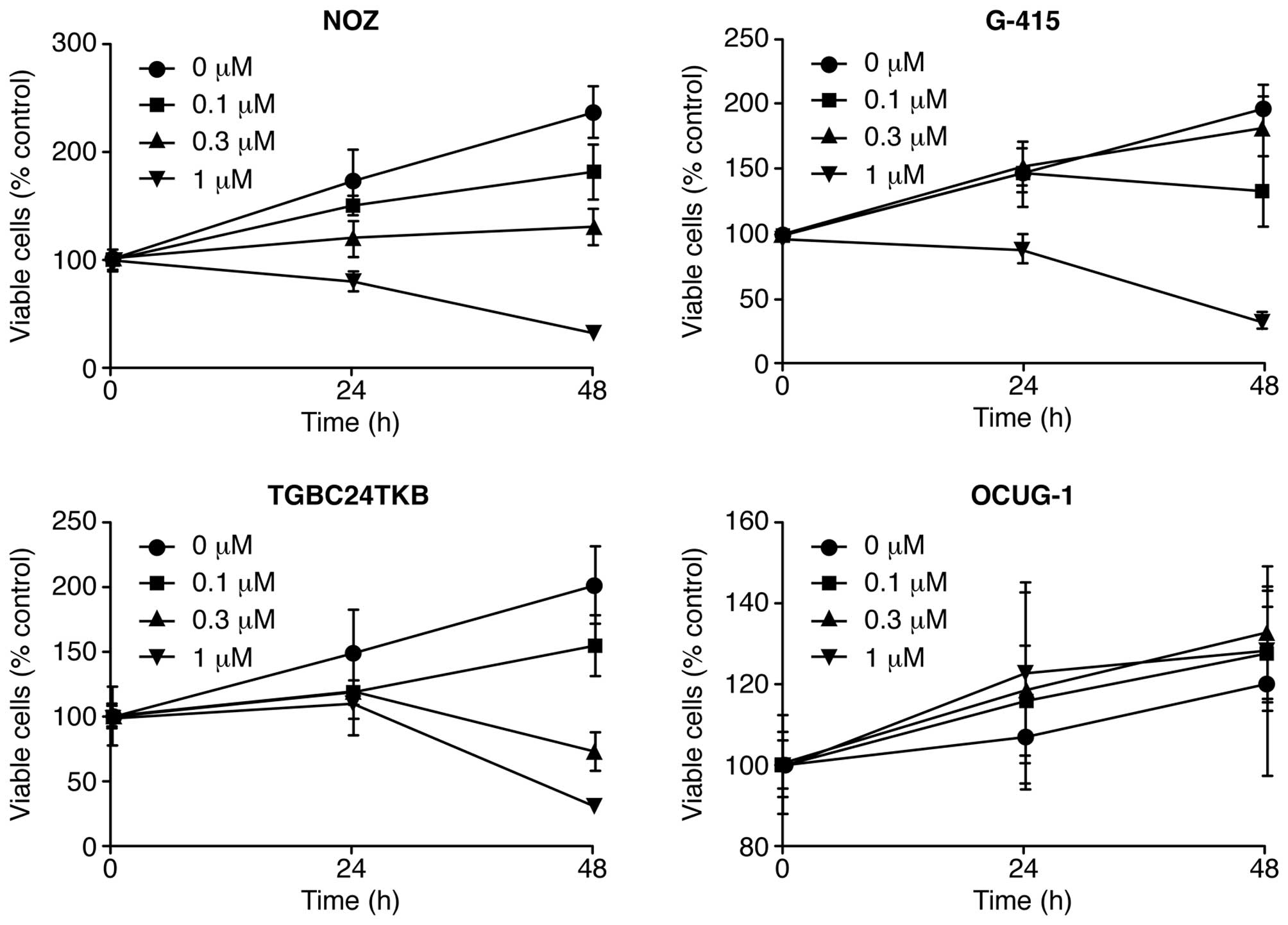
Gal-9 suppresses the proliferation of
human GBC cells (NOZ, G-415, and TGBC24TKB cells) except for OCUG-1
cells
To evaluate the effect of Gal-9 on the growth
activity of human GBC cells in vitro, we examined the effect
of Gal-9 on cell proliferation in four GBC cell lines (NOZ, G-415,
TGBC24TKB, and OCUG-1) by culturing the cells for 48 h in the
presence (0.1, 0.3 or 1 μM) or absence of Gal-9 in the
corresponding medium. We found that Gal-9 suppressed cell
proliferation in three GBC cell lines (NOZ, G-415, and TGBC24TKB)
in a dose-dependent manner (Fig.
1). In contrast, the OCUG-1 cell line, which represents a
poorly differentiated adenocarcinoma exhibiting transition from
adenocarcinoma to squamous cell carcinoma, were less susceptible to
Gal-9 than the other GBC cell lines (Fig. 1). Therefore, the differences in
sensitivity to Gal-9 between NOZ cells (Gal-9-sensitive cells) and
OCUG-1 cells (Gal-9-resistant cells) were examined in human
GBC.
Gal-9 induces apoptosis of
Gal-9-sensitive GBC cells
To determine whether Gal-9 induced apoptosis, NOZ
and OCUG-1 cells were treated with or without 0.3 μM Gal-9, and the
levels of cCK18 following treatment were determined using the M30
ELISA kit. We found that Gal-9 significantly increased the levels
of cCK-18 in NOZ cells, but not in OCUG-1 cells (Fig. 2). These results highlight the
difference in sensitivity to Gal-9 between NOZ cells and OCUG-1
cells. Consequently, Gal-9 suppressed the proliferation of
Gal-9-sensitive GBC cells, which represent a well-to-moderately
differentiated cancer type, by inducing apoptosis. In contrast,
Gal-9-resistant cells, which represent a poorly differentiated
cancer type, were less sensitive to the anti-proliferative effect
of Gal-9.
Effects of Gal-9 on the levels of
apoptosis-associated proteins in Gal-9-sensitive versus
Gal-9-resistant GBC cells
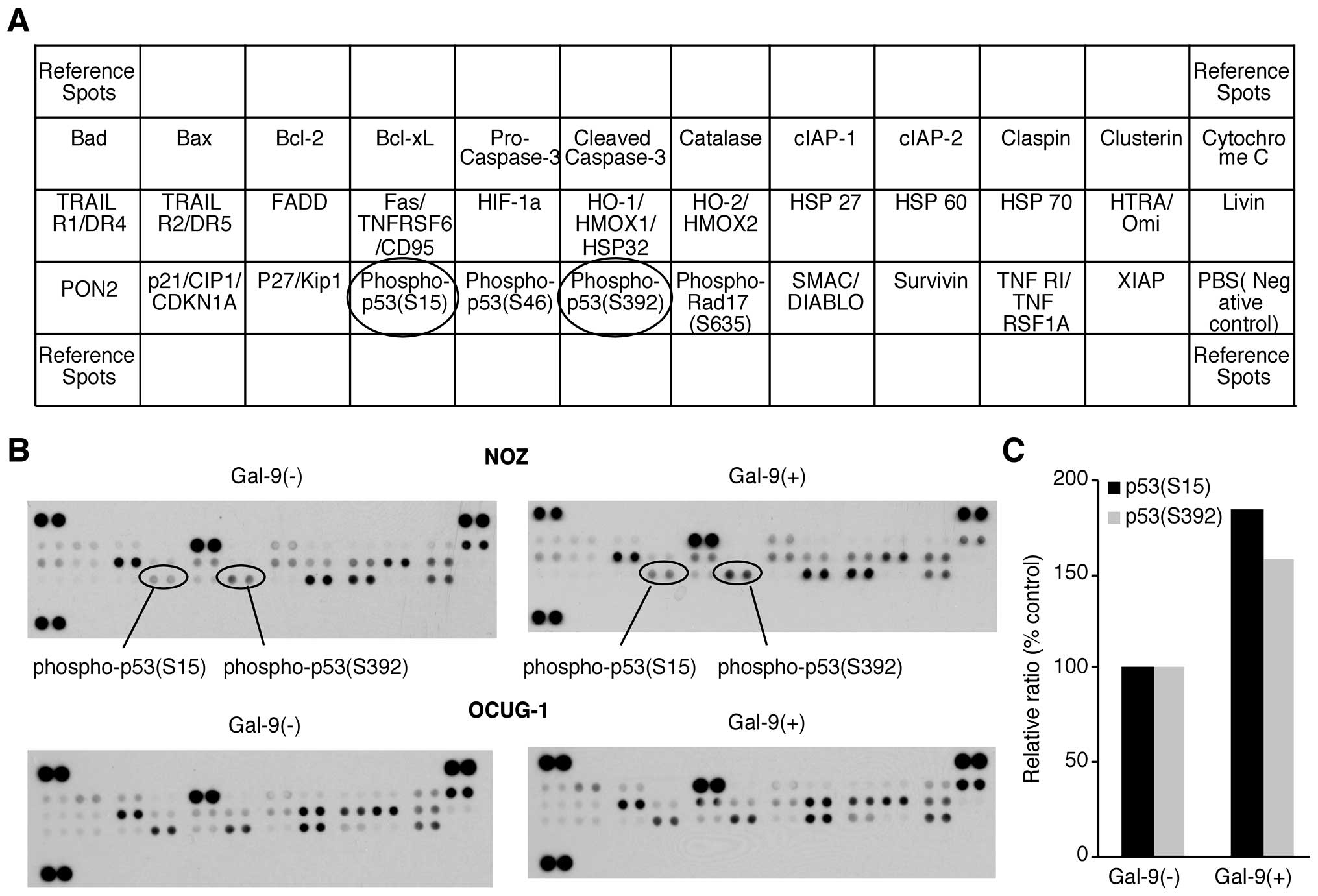
We used an apoptosis array system to identify which
apoptosis-associated proteins are involved in the antitumor effects
of Gal-9. Using an antibody array enabled the screening of the
expression of 35 apoptosis-associated proteins in NOZ and OCUG-1
cells in the presence or absence of Gal-9. Gal-9 increased the
expression of phosphorylated p53 (phospho-p53), especially in NOZ
cells. Densitometry showed that the intensity of the phospho-p53
spot (S15) for the Gal-9-treated NOZ cells was 185.5% of that for
untreated NOZ cells (Fig. 3).
However, the expression levels of apoptosis-associated proteins
other than phospho-p53 were not affected by Gal-9.
Effects of Gal-9 on the cell cycle in
Gal-9-sensitive versus Gal-9-resistant GBC cells
The possible effect of Gal-9 on cell cycle
progression in GBC cells was examined via flow cytometry. Our data
suggested that the proportion of Gal-9-treated NOZ cells in the S
phase and the G2/M phase was slightly decreased and increased,
respectively (Fig. 4A).
Conversely, OCUG-1 cells did not display any difference in cell
cycle progression in response to Gal-9 treatment (Fig. 4A).
The effects of Gal-9 on the protein expression of
various cell cycle-related molecules in NOZ cells were evaluated by
western blotting. The cells were treated with 0 or 0.3 μM Gal-9 for
24–48 h. Then, we examined the levels of various cell cycle-related
molecules, such as cyclin D1, Cdk4, Cdk6, cyclin E, and Cdk2.
However, no significant differences in the expression of these cell
cycle-related proteins were detected (Fig. 4B).
These results suggested that Gal-9 suppresses the
proliferation of Gal-9-sensitive NOZ cells, predominantly by
inducing apoptosis, but not by promoting cell cycle arrest.
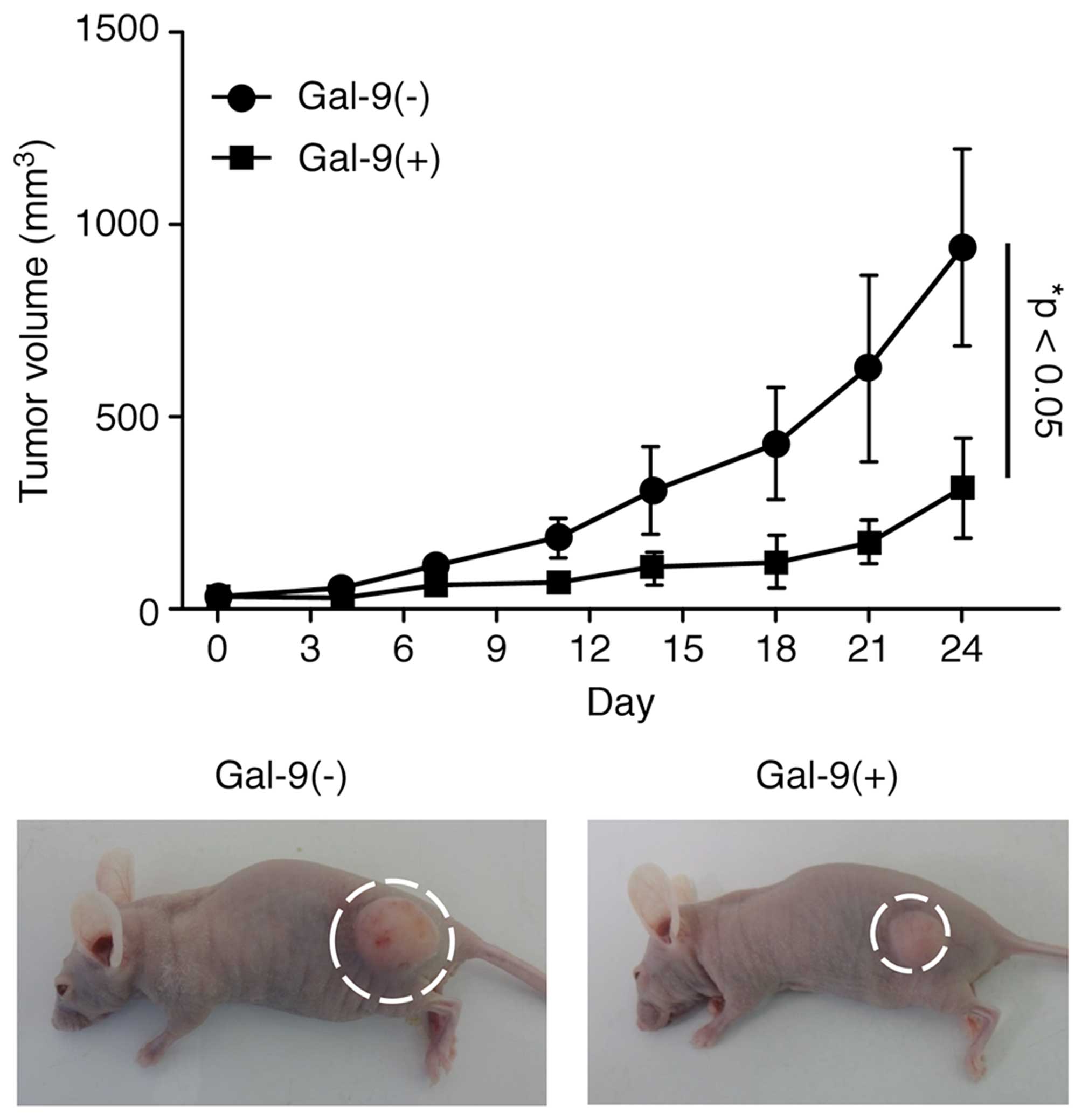
Gal-9 suppresses tumor cell growth in
vivo
Then experiments were performed to clarify whether
Gal-9 exhibits tumor-suppressive activity in vivo. Nude mice
were subcutaneously injected with NOZ cells, followed by
intraperitoneal injection of Gal-9. The Gal-9-treated mice
exhibited significantly suppressed NOZ cell-based tumor growth
compared to the untreated mice (Fig.
5). However, Gal-9 had no further apparent effects on these
mice, as Gal-9 treatment did not affect their body weight and all
animals survived the entire experimental period.
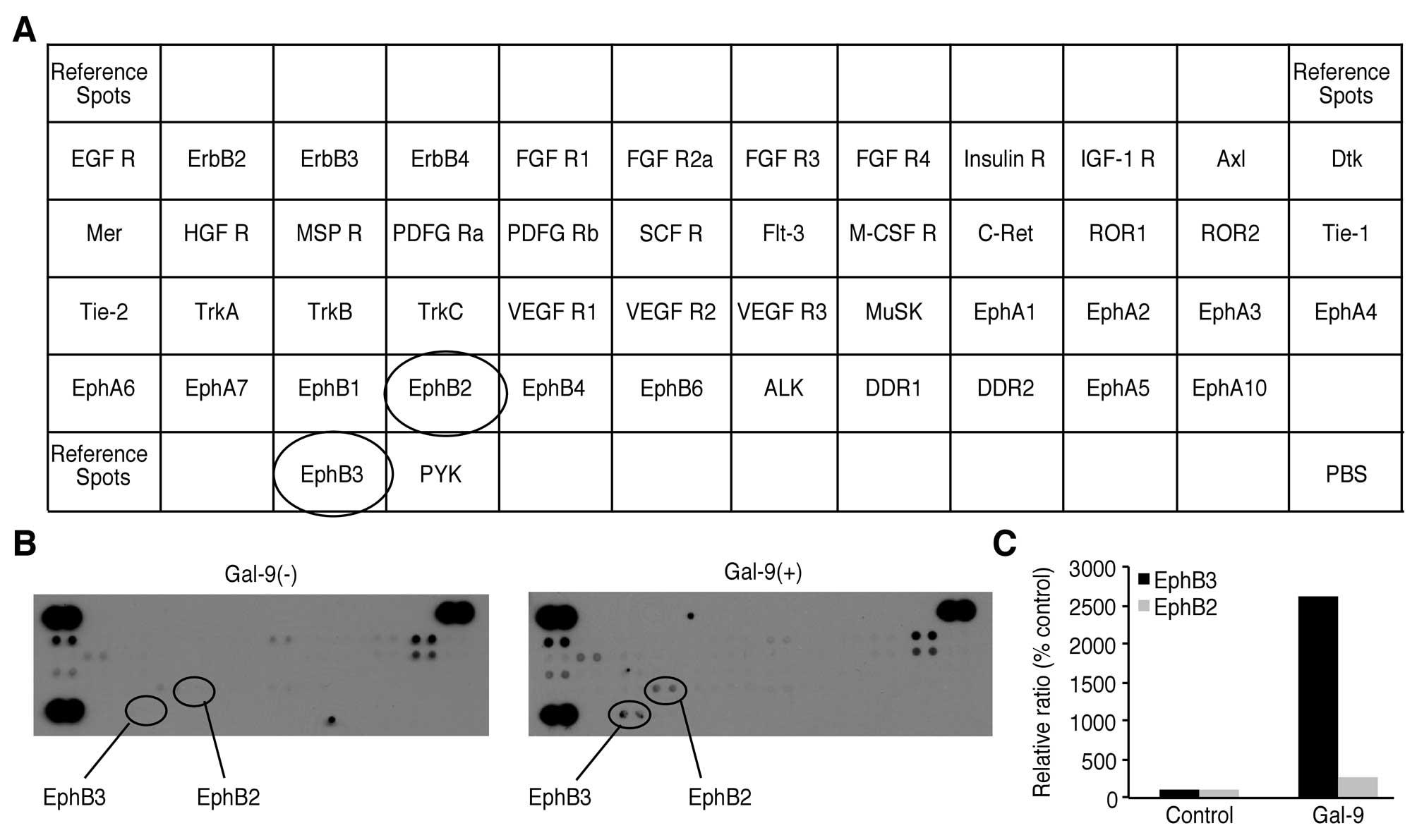
Effects of Gal-9 on the levels of p-RTKs
in NOZ cells
We used a p-RTK array system to identify the key
RTKs associated with the antitumor effect of Gal-9. Using an
antibody array enabled the screening of the expression of 49
activated RTKs in NOZ cells. Gal-9 increased the expression of
phosphorylated Ephrin type-B receptor (EphB) 3 and EphB2.
Densitometric analysis revealed that the EphB3 and EphB2 spots for
the Gal-9-treated cells were 26.15 times and 2.6 times more intense
than those for the untreated cells, respectively (Fig. 6).
Effects of Gal-9 on miRNA expression in
NOZ cells
To further examine the antitumor effect of Gal-9, we
screened the expression levels of miRNAs in NOZ cells and compared
the miRNA profiles obtained with or without Gal-9 treatment. NOZ
cells were treated with 0 or 0.3 μM Gal-9 for 24 h. Unsupervised
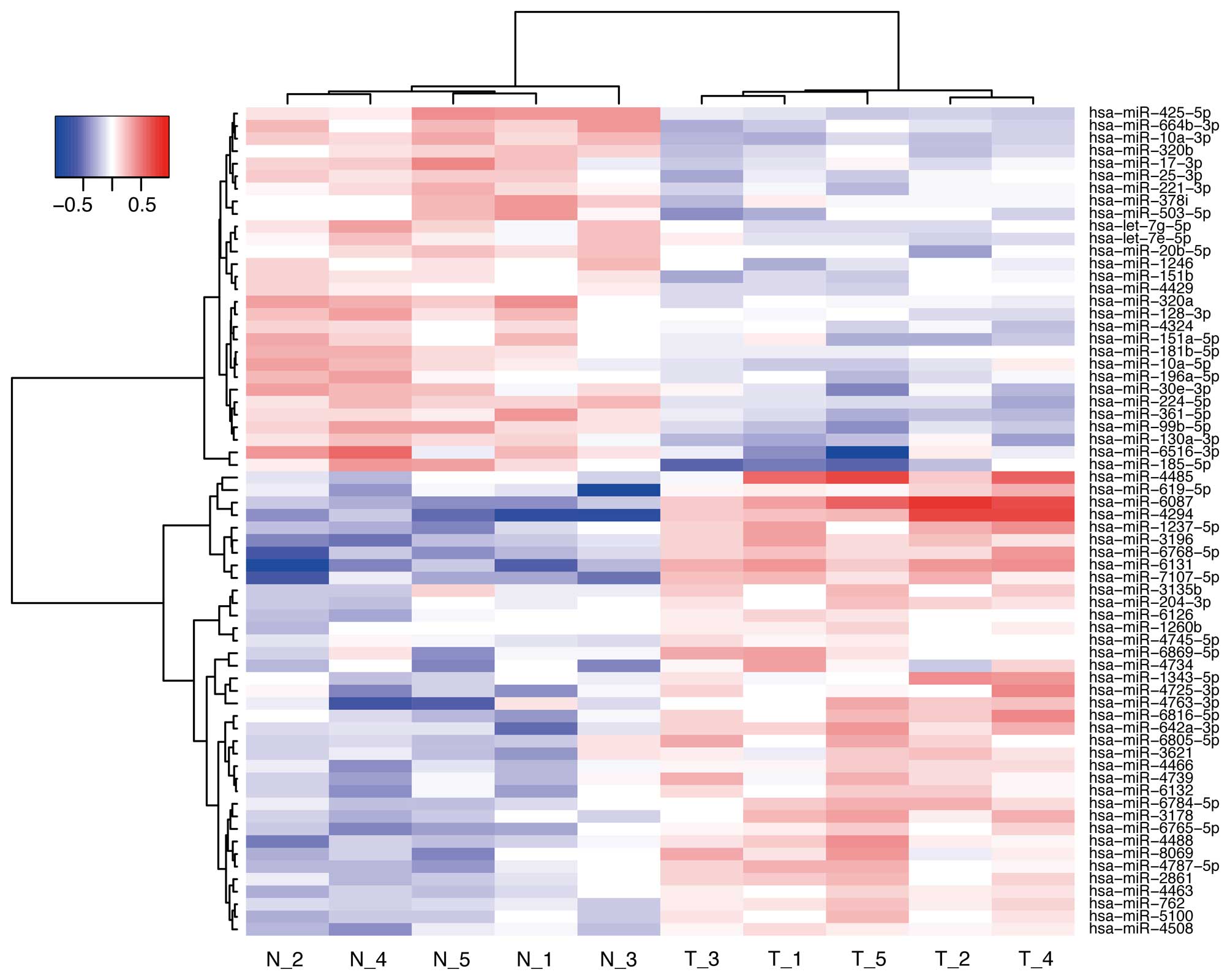
hierarchical clustering analysis showed that the treated group
clustered separately from the control group (Fig. 7). We identified 66 miRNAs that were
differentially expressed between the two groups of NOZ cells (37
upregulated miRNA and 29 downregulated miRNAs) (Table I).
 | Table IStatistical results of miRNAs in NOZ
cells treated with Gal-9 compared with untreated NOZ cells. |
Table I
Statistical results of miRNAs in NOZ
cells treated with Gal-9 compared with untreated NOZ cells.
| Upregulated
microRNAs | Fold-change (Gal-9
treated/non-treated) | P-value | Chromosomal
localization |
|---|
| hsa-miR-4294 | 1.97 | 0.0079 | 10 |
| hsa-miR-6087 | 1.80 | 0.0117 | X |
| hsa-miR-6131 | 1.74 | 0.0079 | 5 |
|
hsa-miR-7107-5p | 1.47 | 0.0079 | 12 |
|
hsa-miR-6768-5p | 1.45 | 0.0079 | 16 |
| hsa-miR-4485 | 1.44 | 0.0317 | 11 |
| hsa-miR-3196 | 1.41 | 0.0079 | 20 |
|
hsa-miR-1237-5p | 1.38 | 0.0079 | 11 |
|
hsa-miR-642a-3p | 1.34 | 0.0079 | 19q13.32 |
|
hsa-miR-4763-3p | 1.32 | 0.0317 | 22 |
| hsa-miR-619-5p | 1.31 | 0.0079 | 12q24.11 |
| hsa-miR-4488 | 1.30 | 0.0119 | 11 |
|
hsa-miR-6816-5p | 1.30 | 0.0159 | 22 |
| hsa-miR-3178 | 1.30 | 0.0119 | 16 |
|
hsa-miR-6765-5p | 1.28 | 0.0079 | 14 |
| hsa-miR-8069 | 1.27 | 0.0361 | 21 |
|
hsa-miR-4787-5p | 1.27 | 0.0153 | 3 |
|
hsa-miR-4725-3p | 1.26 | 0.0317 | 17 |
|
hsa-miR-6784-5p | 1.25 | 0.0079 | 17 |
| hsa-miR-4734 | 1.24 | 0.0317 | 17 |
| hsa-miR-4739 | 1.23 | 0.0273 | 17 |
| hsa-miR-6132 | 1.22 | 0.0159 | 7 |
| hsa-miR-4466 | 1.22 | 0.0119 | 6 |
|
hsa-miR-6805-5p | 1.22 | 0.0317 | 19 |
|
hsa-miR-1343-5p | 1.21 | 0.0465 | 11 |
| hsa-miR-2861 | 1.21 | 0.0157 | 9 |
| hsa-miR-4508 | 1.21 | 0.0119 | 15 |
|
hsa-miR-6869-5p | 1.21 | 0.0459 | 20 |
| hsa-miR-5100 | 1.20 | 0.0255 | |
| hsa-miR-762 | 1.20 | 0.0117 | 16 |
| hsa-miR-3621 | 1.20 | 0.0317 | 9 |
| hsa-miR-4463 | 1.19 | 0.0119 | 6 |
| hsa-miR-204-3p | 1.16 | 0.0196 | 9q21.12 |
| hsa-miR-3135b | 1.15 | 0.0356 | 3 |
| hsa-miR-6126 | 1.14 | 0.0465 | 16 |
| hsa-miR-1260b | 1.09 | 0.0238 | 14 |
|
hsa-miR-4745-5p | 1.09 | 0.0317 | 19 |
|
| Downregulated
microRNAs localization | Fold-change (Gal-9
treated/non-treated) | P-value | Chromosomal |
|
| hsa-miR-185-5p | 0.69 | 0.0212 | 22q11.21 |
|
hsa-miR-6516-3p | 0.70 | 0.0159 | 17 |
| hsa-miR-99b-5p | 0.74 | 0.0119 | 19q13.41 |
| hsa-miR-425-5p | 0.74 | 0.0117 | 3p21.31 |
| hsa-miR-10a-3p | 0.75 | 0.0119 | 17q21.32 |
|
hsa-miR-664b-3p | 0.77 | 0.0119 | 1 |
| hsa-miR-361-5p | 0.77 | 0.0119 | Xq21.2 |
| hsa-miR-224-5p | 0.78 | 0.0119 | Xq28 |
| hsa-miR-30e-3p | 0.79 | 0.0317 | 1p34.2 |
|
hsa-miR-130a-3p | 0.79 | 0.0159 | 11q12.1 |
| hsa-miR-320a | 0.80 | 0.0119 | 8p21.3 |
| hsa-miR-503-5p | 0.80 | 0.0278 | Xq26.3 |
|
hsa-miR-151a-5p | 0.81 | 0.0356 | 8q24.3 |
| hsa-miR-17-3p | 0.82 | 0.0317 | 13q31.3 |
| hsa-miR-25-3p | 0.83 | 0.0147 | 7q22.1 |
| hsa-miR-320b | 0.83 | 0.0117 | |
| hsa-miR-10a-5p | 0.83 | 0.0269 | 17q21.32 |
|
hsa-miR-196a-5p | 0.84 | 0.0079 | 17q21.32 |
| hsa-miR-128-3p | 0.84 | 0.0159 | |
| hsa-miR-221-3p | 0.84 | 0.0107 | Xp11.3 |
| hsa-let-7g-5p | 0.84 | 0.0345 | 3p21.1 |
| hsa-miR-151b | 0.85 | 0.0147 | 14 |
| hsa-miR-378i | 0.85 | 0.0361 | 22 |
|
hsa-miR-181b-5p | 0.86 | 0.0119 | |
| hsa-miR-20b-5p | 0.86 | 0.0248 | Xq26.2 |
| hsa-miR-1246 | 0.87 | 0.0200 | 2q31.1 |
| hsa-let-7e-5p | 0.87 | 0.0452 | 19q13.41 |
| hsa-miR-4324 | 0.87 | 0.0079 | 19 |
| hsa-miR-4429 | 0.88 | 0.0114 | 2 |
Discussion
GBC is the most common malignant tumor found in the
biliary tract and the fifth most common digestive malignancy
(1,21). Previously, we demonstrated that
Gal-9 suppressed cell proliferation and tumor growth in
hepatobiliary malignancies (13,14).
The present study revealed that Gal-9 led to dose-dependent
inhibition of cell proliferation in three GBC cell lines (NOZ,
TGBC24TKB and G-415) but not in the OCUG-1 cell line. The OCUG-1
cell line represents a poorly differentiated adenocarcinoma and is
regarded as a unique cell line because the corresponding cancer is
a transitional form from adenocarcinoma to squamous cell carcinoma
(22). Generally, GBC is
classified into three histological types: adenocarcinoma, which
accounts for 90% of all primary gallbladder cancer cases, followed
by squamous carcinoma and adenosquamous carcinoma, both of which
display an incidence of 1.4–10.4% (23,24).
Although the clinical features of squamous carcinoma and
adenosquamous carcinoma are similar to adenocarcinoma of the
gallbladder, squamous and adenosquamous carcinoma patients
frequently presented with an advanced stage of cancer.
Additionally, these two forms of GBC are traditionally considered
as more aggressive and to be associated with a poorer prognosis
than adenocarcinoma (25,26). Accordingly, the data from the
present study indicated that the squamous and adenosquamous forms
of GBC were more resistant to Gal-9 treatment due to their poorly
differentiated phenotype compared to well-to-moderately
differentiated GBC types.
Gal-9, a tandem-repeat-type galectin family member
that consists of two carbohydrate recognition domains connected by
a linker peptide (27), was first
described as an eosinophil chemoattractant (4,28).
Further research showed that Gal-9 induces apoptosis in T-cells and
stimulates regulatory T-cell activity (5–7).
Previous findings suggested that Gal-9 suppresses the proliferation
of melanoma (11) and chromic
myeloid leukemia (12). Regarding
the mechanism underlying Gal-9-induced cell growth inhibition in
various cancers, Gal-9 induces cancer cell death via an apoptotic
signaling pathway (11–13). This apoptotic signaling in multiple
myeloma was caspase-dependent and was induced by the activation of
the MAP kinases JNK and p38 (22,29).
Alternatively, Gal-9 induced apoptosis in chronic myelogenous
leukemia by inducing Noxa (12).
In the present study, Gal-9 increased the levels of cCK18 in NOZ
cells, which are sensitive to Gal-9. A neo-epitope in cytokeratin
18 becomes available upon an early caspase cleavage event during
apoptosis, and the monoclonal antibody M30, which is specific for
this site, can be utilized to specifically detect apoptotic cells
(30). Additionally, Gal-9
increased the phosphorylation of p53, especially in NOZ cells. The
p53 protein is activated by phosphorylation to induce tumor cell
apoptosis (31). Thus, we regarded
phospho-p53 as a key apoptosis-related protein responsible for the
antitumor effect of Gal-9.
In contrast, Gal-9 treatment for 24 or 48 h did not
affect the G0-G1 transition or the expression levels of cell
cycle-related proteins. These data suggest that Gal-9 suppresses
cell proliferation in Gal-9-sensitive GBC cell lines by inducing
apoptosis but not by promoting cell cycle arrest. In contrast,
Gal-9 did not induce apoptosis of OCUG-1 cells, which are more
resistant to Gal-9.
Moreover, Gal-9 treatment leads to changes in the
phosphorylation status of various proteins. We detected increases
in EphB3 and EphB2 phosphorylation in GBC cells in response to
Gal-9 treatment based on p-RTK array analysis. EphB receptors are
implicated in regulating the malignant progression of cancer. EphB2
and EphB3 show similar changes in expression during colorectal
cancer progression, and EphB receptor activity suppresses cancer
progression by controlling cellular positioning and restricting
tumor cell motility (32,33). A more recent study suggested that
the expression of EphB1 and Ephrin-B is an independent prognostic
factor of not only adenocarcinoma but also squamous adenocarcinoma
and adenosquamous carcinoma of the gallbladder (34). These results raise the possibility
that EphB signaling pathway may be relevant for antitumor effect of
galectin-9 in GBC cells.
The miRNAs associated with the antitumor effects of
Gal-9 were analyzed using miRNA expression arrays. It has become
apparent that miRNA expression is associated with various cancers
(16). Our previous studies
reported that miRNAs lead to apoptosis as a consequence of the
antitumor effect of Gal-9 in HCC and cholangiocarcinoma (11,12).
Hierarchical cluster analyses were performed to clarify the
alteration in the expression of miRNAs by Gal-9 treatment. We
identified 66 differentially expressed miRNAs (37 upregulated and
29 downregulated) in NOZ cells with or without Gal-9 treatment.
Several miRNAs that were downregulated by Gal-9 treatment have been
reported to be associated with cancer cell apoptosis. For instance,
miR-10a has been reported to be upregulated in several solid tumors
including pancreatic cancer (35)
and lung cancer (36). miR-10a
targets the phosphatase and tensin homolog (PTEN), and miR-10a
promotes the migration, invasion, and growth of non-small cell lung
cancer by regulating the PTEN/AKT/ERK signaling pathway (36). In addition, miR-224 promotes lung
cancer cells proliferation and migration by direct targeting of
caspase-3 and caspase-7 (37), and
downregulation of miR-224 might be associated with inhibition of
cancer progression by inducing apoptosis. Furthermore, these
findings suggest that Gal-9 suppresses cell proliferation by
altering the expression of several miRNAs.
Gemcitabine-based chemotherapies are still the main
therapeutic regimens for patients with unresectable advanced or
metastatic GBC (38). Gemcitabine
acts by targeting ribo-nucleotide reductase M1 (RRM1) to elongate
DNA (39) and by targeting cyclin
D1 to induce cell cycle arrest (40). However, the use of gemcitabine
alone is inadequate, and combination therapy of gemcitabine with
other antitumor drug has been attempted. These results suggest that
a combination of Gal-9 with other antitumor drugs that induce cell
cycle arrest would be even more effective for patients with
GBC.
In conclusion, Gal-9 suppresses the cell
proliferation and tumor growth of human GBC in vitro and
in vivo. The anti-tumor effect of Gal-9 appears to depend on
several pathways, such as the induction of apoptosis in cancer
cells via the phosphorylation of p53, the activation of the EphB
receptor and the alteration of expression miRNAs; however, Gal-9
did not affect the cell cycle. Thus, Gal-9 may represent a novel
therapeutic agent as an adjunct to conventional chemotherapy for
the treatment of GBC.
Acknowledgements
Toshiro Niki and Mitsuomi Hirashima are board
members of GalPharma Co., Ltd. These two authors have the following
patent related to material pertinent to this study: ‘Novel modified
galectin 9 proteins and use thereof’, which was applied for by
GalPharma and was issued in Japan (4792390), the USA (8,268,324),
the EPC (1736541), Canada (2,561,696), India (239130), and Korea
[(10-1222281) as of 2013.12.2]. These two authors have the
following products related to material pertinent to this study:
stable-form Gal-9. We thank Ms. Kayo Endo, Ms. Fuyuko Kokado, Ms.
Keiko Fujikawa, Ms. Kayo Hirose, Ms. Miwako Watanabe, Ms. Noriko
Murao, and Ms. Kayo Ogawa for providing technical assistance.
Abbreviations:
|
GBC
|
gallbladder carcinoma
|
|
Gal-9
|
galectin-9
|
|
CCK-8
|
Cell counting kit-8
|
|
p-RTK
|
phosphorylated receptor tyrosine
kinase
|
|
cCK18
|
caspase-cleaved cytokeratin 18
|
|
EphB
|
Ephrin type-B receptor
|
|
miRNAs
|
microRNAs
|
References
|
1
|
Bal MM, Ramadwar M, Deodhar K and
Shrikhande S: Pathology of gallbladder carcinoma: Current
understanding and new perspectives. Pathol Oncol Res. 21:509–525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Randi G, Franceschi S and La Vecchia C:
Gallbladder cancer worldwide: Geographical distribution and risk
factors. Int J Cancer. 118:1591–1602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dwivedi AN, Jain S and Dixit R: Gall
bladder carcinoma: Aggressive malignancy with protean loco-regional
and distant spread. World J Clin Cases. 3:231–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirashima M, Kashio Y, Nishi N, Yamauchi
A, Imaizumi TA, Kageshita T, Saita N and Nakamura T: Galectin-9 in
physiological and pathological conditions. Glycoconj J. 19:593–600.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oomizu S, Arikawa T, Niki T, Kadowaki T,
Ueno M, Nishi N, Yamauchi A and Hirashima M: Galectin-9 suppresses
Th17 cell development in an IL-2-dependent but Tim-3-independent
manner. Clin Immunol. 143:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seki M, Oomizu S, Sakata KM, Sakata A,
Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, et al:
Galectin-9 suppresses the generation of Th17, promotes the
induction of regulatory T cells, and regulates experimental
autoimmune arthritis. Clin Immunol. 127:78–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niki T, Tsutsui S, Hirose S, Aradono S,
Sugimoto Y, Takeshita K, Nishi N and Hirashima M: Galectin-9 is a
high affinity IgE-binding lectin with anti-allergic effect by
blocking IgE-antigen complex formation. J Biol Chem.
284:32344–32352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiersma VR, de Bruyn M, Helfrich W and
Bremer E: Therapeutic potential of Galectin-9 in human disease. Med
Res Rev. 33(Suppl 1): E102–E126. 2013. View Article : Google Scholar
|
|
10
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kageshita T, Kashio Y, Yamauchi A, Seki M,
Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda J, Yamamoto M, Nagoshi H, Kobayashi
T, Sasaki N, Shimura Y, Horiike S, Kimura S, Yamauchi A, Hirashima
M, et al: Targeting activating transcription factor 3 by Galectin-9
induces apoptosis and overcomes various types of treatment
resistance in chronic myelogenous leukemia. Mol Cancer Res.
8:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol. 46:2419–2430.
2015.PubMed/NCBI
|
|
14
|
Kobayashi K, Morishita A, Iwama H, Fujita
K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H,
et al: Galectin-9 suppresses cholangiocarcinoma cell proliferation
by inducing apoptosis but not cell cycle arrest. Oncol Rep.
34:1761–1770. 2015.PubMed/NCBI
|
|
15
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar
|
|
17
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al; Committee of the National Cancer Research
Institute. Guidelines for the welfare and use of animals in cancer
research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Incalci M, Colombo T, Ubezio P,
Nicoletti I, Giavazzi R, Erba E, Ferrarese L, Meco D, Riccardi R,
Sessa C, et al: The combination of yondelis and cisplatin is
synergistic against human tumor xenografts. Eur J Cancer.
39:1920–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapoor VK: Gallbladder cancer: A global
perspective. J Surg Oncol. 93:607–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada N, Chung Y, Ohtani H, Ikeda T,
Onoda N, Sawada T, Nishiguchi Y, Hasuma T and Sowa M: Establishment
and characterization of a new human gallbladder carcinoma cell line
(OCUG-1) producing TA-4. Int J Oncol. 10:1251–1255. 1997.PubMed/NCBI
|
|
23
|
Kim WS, Jang KT, Choi DW, Choi SH, Heo JS,
You DD and Lee HG: Clinicopathologic analysis of
adenosquamous/squamous cell carcinoma of the gallbladder. J Surg
Oncol. 103:239–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamdani NH, Qadri SK, Aggarwalla R,
Bhartia VK, Chaudhuri S, Debakshi S, Baig SJ and Pal NK:
Clinicopathological study of gall bladder carcinoma with special
reference to gallstones: Our 8-year experience from eastern India.
Asian Pac J Cancer Prev. 13:5613–5617. 2012. View Article : Google Scholar
|
|
25
|
Chan KM, Yu MC, Lee WC, Jan YY and Chen
MF: Adeno-squamous/squamous cell carcinoma of the gallbladder. J
Surg Oncol. 95:129–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mingoli A, Brachini G, Petroni R,
Antoniozzi A, Cavaliere F, Simonelli L, Chirletti P and Modini C:
Squamous and adeno-squamous cell carcinomas of the gallbladder. J
Exp Clin Cancer Res. 24:143–150. 2005.PubMed/NCBI
|
|
27
|
Miyanishi N, Nishi N, Abe H, Kashio Y,
Shinonaga R, Nakakita S, Sumiyoshi W, Yamauchi A, Nakamura T,
Hirashima M, et al: Carbohydrate-recognition domains of galectin-9
are involved in intermolecular interaction with galectin-9 itself
and other members of the galectin family. Glycobiology. 17:423–432.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsumoto R, Matsumoto H, Seki M, Hata M,
Asano Y, Kanegasaki S, Stevens RL and Hirashima M: Human ecalectin,
a variant of human galectin-9, is a novel eosinophil
chemoattractant produced by T lymphocytes. J Biol Chem.
273:16976–16984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi T, Kuroda J, Ashihara E, Oomizu
S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N,
et al: Galectin-9 exhibits anti-myeloma activity through JNK and
p38 MAP kinase pathways. Leukemia. 24:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leers MP, Kölgen W, Björklund V, Bergman
T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B,
Nap M, et al: Immunocytochemical detection and mapping of a
cytokeratin 18 neo-epitope exposed during early apoptosis. J
Pathol. 187:567–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
el-Deiry WS: Regulation of p53 downstream
genes. Semin Cancer Biol. 8:345–357. 1998. View Article : Google Scholar
|
|
32
|
Batlle E, Bacani J, Begthel H, Jonkheer S,
Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T,
et al: EphB receptor activity suppresses colorectal cancer
progression. Nature. 435:1126–1130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jägle S, Rönsch K, Timme S, Andrlová H,
Bertrand M, Jäger M, Proske A, Schrempp M, Yousaf A, Michoel T, et
al: Silencing of the EPHB3 tumor-suppressor gene in human
colorectal cancer through decommissioning of a transcriptional
enhancer. Proc Natl Acad Sci USA. 111:4886–4891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan Y, Yang ZL, Miao XY, Liu ZR, Li DQ,
Zou Q, Li JH, Liang LF, Zeng GX and Chen SL: EphB1 and Ephrin-B,
new potential biomarkers for squamous cell/adenosquamous carcinomas
and adenocarcinomas of the gallbladder. Asian Pac J Cancer Prev.
15:1441–1446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohuchida K, Mizumoto K, Lin C, Yamaguchi
H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M, et
al: MicroRNA-10a is overexpressed in human pancreatic cancer and
involved in its invasiveness partially via suppression of the HOXA1
gene. Ann Surg Oncol. 19:2394–2402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu T, Liu L, Li J, Yan M, Lin H, Liu Y,
Chu D, Tu H, Gu A and Yao M: MiRNA-10a is upregulated in NSCLC and
may promote cancer by targeting PTEN. Oncotarget. 6:30239–30250.
2015.PubMed/NCBI
|
|
37
|
Cui R, Kim T, Fassan M, Meng W, Sun HL,
Jeon YJ, Vicentini C, Tili E, Peng Y, Scarpa A, et al: MicroRNA-224
is implicated in lung cancer pathogenesis through targeting
caspase-3 and caspase-7. Oncotarget. 6:21802–21815. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang C, Xu M, Shen HJ, Zhu HY, Li F, He M,
Chen T, Wang J, Shi WJ and Ji F: Potential biomarkers for
sensitivity of gall-bladder cancer cells to gemcitabine. Int J Clin
Exp Pathol. 7:521–528. 2014.
|
|
39
|
Plunkett W, Huang P, Searcy CE and Gandhi
V: Gemcitabine: Preclinical pharmacology and mechanisms of action.
Semin Oncol. 23(Suppl 10): 3–15. 1996.PubMed/NCBI
|
|
40
|
Toyota Y, Iwama H, Kato K, Tani J, Katsura
A, Miyata M, Fujiwara S, Fujita K, Sakamoto T, Fujimori T, et al:
Mechanism of gemcitabine-induced suppression of human
cholangiocellular carcinoma cell growth. Int J Oncol. 47:1293–1302.
2015.PubMed/NCBI
|