Introduction
Acute myeloid leukemias are a heterogeneous group of
clonal neoplastic diseases due to genetic and epigenetic
alterations of hematopoietic stem cells (HSCs) or committed
progenitors, causing an aberrant growth of myeloid precursor
cells.
Acute promyelocytic leukemia (APL), a subtype of
AML, is characterized by an abnormal accumulation of promyelocytes
following specific translocation t(15;17) (q21;12) and formation of
promyelocytic leukemia (PML)/retinoic acid receptor α (RARα) fusion
gene. It has been demonstrated that the PML-RARα fusion protein is
involved in the pathogenesis of APL through the recruitment of a
complex that, composed of nuclear co-repressor molecule (NCOR) and
histone deacetylase (HDAC), inhibits the expression of myeloid
differentiation genes (1). Under
treatment with all-trans-retinoic acid (ATRA), the NCOR/HDAC
complex is dissociated from RAR and the maturation of promyelocytes
is restored. Standard protocols combining ATRA with
anthracycline-based chemotherapy are demonstrated to guarantee ~70%
cure rate (2).
Originally isolated from human pheochromocytoma,
adrenomedullin is a 52 aa peptide belonging to the calcitonin
gene-related peptide family (3).
Its maturation involves the proteolytic cleavage of
preproadrenomedullin, a precursor of 185 amino acid residues that
includes at amino terminal end the so-called proadrenomedullin
N-terminal 20 peptide (PAMP), a peptide with known transient
hypotensive activity (4,5).
The biological effects of ADM are mainly mediated by
its interaction with two cell-surface receptors, multimeric
complexes of calcitonin receptor-like receptor (CRLR) and
receptor-activity-modifying proteins (RAMPs). It is known that the
RAMP family includes three members (RAMP1, RAMP2 and RAMP3) and
regulates both transport and ligand specificity of CRLR. When RAMP2
or RAMP3 is associated with CRLR, adrenomedullin receptors 1 (AMR1)
and 2 (AMR2) are generated, respectively, while RAMP1 is included
in calcitonin gene-related peptide (CGRP) receptors (6,7).
Although ADM was first described as a potent
vasodilator and hypotensive factor, several studies reported that
it exerts various biological activities acting in an
autocrine/paracrine manner (8).
ADM is produced and secreted by several cell types, such as
neurons, epithelial and endothelial cells supporting their survival
and/or proliferation (9).
Compelling evidence has shown that adrenomedullin can contribute to
the pathogenesis of solid tumors in several ways (10). Firstly, the hypoxic conditions
induce an upregulation of the peptide in the tumor cells leading to
stimulation of cell growth and inhibition of apoptosis (11). Secondly, ADM exerts proangiogenic
effects thus providing nutrients and oxygen to the tumor and
allowing the spreading of tumor cells (12). Thirdly, it decreases the expression
of proinflammatory cytokines, thus, inhibiting the immune system
(13).
ADM is involved in the regulation of hematopoietic
compartment as demonstrated by its expression in peripheral blood
granulocytes, lymphocytes, monocytes and monocyte-derived
macrophages under homeostatic and lipopolysaccharide-induced
inflammation (14–16). Notably, ADM stimulates the
proliferation of human cord blood hematopoietic stem cells through
autocrine mechanism (17,18).
Based on the above evidence and considering that ADM
plays a critical role in cancer cell proliferation, the aim of the
present study was to evaluate whether ADM signaling is involved in
the maintenance of impaired differentiation of APL cells to mature
granulocytes or monocytes. Thus, the proliferative and
differentiative effects induced by ADM and its inhibitor,
ADM22-52, were evaluated in vitro at different
time-points in HL60 cells, a human promyelocytic leukaemia cell
line widely used to study granulocyte differentiation (19).
Materials and methods
Cell culture
Human promyelocytic leukaemia cell line HL60 (kindly
provided by CRO, Aviano, Italy) was grown in Iscove's medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; Sigma-Aldrich) and 1% antibiotic antimycotic
solution (Sigma-Aldrich) at 37°C in a humidified atmosphere
containing 5% CO2.
Flow cytometry (FCM)
The expression level of endogenous ADM
(ADMendo), RAMP1, RAMP2, RAMP3 and CRLR was studied in
HL60 cells cultured under resting or primed conditions with
exogenous ADM (ADMexo) and ADM22-52. After
washing with 0.5% BSA in PBS, the samples were incubated with
primary antibodies (Table I) for
15 min at room temperature (RT). For indirect staining, the
detection of specific binding sites was carried out by staining for
15 min at RT with Alexa Fluor® 488- or PE-conjugated
secondary antibodies. For intracellular staining, HL60 cells were
pre-fixed with BD Cytofix/Cytoperm solution (BD Biosciences, San
Josè, CA, USA) for 20 min at 4°C. Data were analyzed by FACSCanto
II flow cytometer (BD Biosciences) and expressed as percentage (%)
± standard deviation (SD) of positive cells compared with II
antibody (Ab)- or isotype-matched controls. Statistical
significance was calculated by Student's t-test.
 | Table IAntibodies used for flow cytometric
analysis. |
Table I
Antibodies used for flow cytometric
analysis.
| Antibodies | Manufacturing
company |
|---|
| Primary
antibodies |
| FITC rat
anti-human CD11b | BD Biosciences |
| FITC mouse
anti-human CD11c | BD Biosciences |
| PE mouse
anti-human CD14 | Santa Cruz
Biotecnology |
| PE mouse
anti-human CD31 | Dako |
| FITC mouse
anti-human CD33 | BD Biosciences |
| FITC mouse
anti-human CD34 | BD Biosciences |
| Mouse anti-human
CD38 | Santa Cruz
Biotecnology |
| PE mouse
anti-human CD45 | Santa Cruz
Biotecnology |
| PE mouse
anti-human cKIT | Santa Cruz
Biotecnology |
| PE mouse
anti-human HLA DR | Santa Cruz
Biotecnology |
| FITC mouse
anti-human Lineage cocktail 1 (CD3, CD14, CD16, CD19, CD20,
CD56) | BD Biosciences |
| Goat anti-human
ADM | Santa Cruz
Biotecnology |
| Rabbit anti-human
RAMP1 | Santa Cruz
Biotecnology |
| Rabbit anti-human
RAMP2 | Santa Cruz
Biotecnology |
| Rabbit anti-human
RAMP3 | Santa Cruz
Biotecnology |
| Goat anti-human
CRLR | Santa Cruz
Biotecnology |
| Rabbit anti-human
Akt | Santa Cruz
Biotecnology |
| Rabbit anti-human
p(Ser473)-Akt | Santa Cruz
Biotecnology |
| Rabbit anti-human
p44/42 MAPK | Cell Signaling
Technology |
| Rabbit anti-human
phosho (Thr202/Tyr204)-p44/42 MAPK | Cell Signaling
Technology |
| Secondary
antibodies |
| PE goat
anti-mouse | Santa Cruz
Biotecnology |
| PE donkey
anti-goat | Santa Cruz
Biotecnology |
| Alexa Fluor 488
goat anti-rabbit | Invitrogen-Life
Technologies |
| Isotype
controls |
| PE isotype
control | Santa Cruz
Biotecnology |
| FITC isotype
control | BD Biosciences |
Gene expression study of PAM
Total mRNA of HL60 cells was isolated using
TRIzol® reagent (Sigma-Aldrich), according to the
manufacturer's protocol. From each sample, 1 μg total RNA was
reverse transcribed into cDNA by using M-MLV reverse transcriptase
(Sigma-Aldrich), and oligo (dT)23 primers
(Invitrogen-Life Technologies, Paisley, UK). The amplification
reaction was performed by PTC-100 thermal cycler (Bio-Rad
Laboratories, Hercules, CA, USA) using ReadyMix™ Taq PCR Reaction
Mix with MgCl2 (Sigma-Aldrich), primer pairs designed to
detect peptidylglycine α-amidating monooxygenase (PAM) and
housekeeping gene GAPDH (Table
II). PCR products were electrophoresed on 2% agarose gel
(Sigma-Aldrich) pre-stained with GelRed™, and then visualized by UV
transilluminator Gel Doc 2000 Gel Documentation system (Bio-Rad
Laboratories).
 | Table IIPrimer sequences used for RT-PCR and
qPCR analysis. |
Table II
Primer sequences used for RT-PCR and
qPCR analysis.
| Target | Sequence
(5′-3′) | Accession | Length (bp) |
|---|
| Peptidylglycine
α-amidating monooxygenase (PAM) |
F-GCGCAAGCACTTTGATATGCCTCA
R-TCTGCAATTCTGAGGAGGTGGGTT | NM_000919.3 | 220 |
| Cullin 5
(Cul5) |
F-GAACCAAAGACCCAGAGAGAAA
R-GTCCTCCTAAGTTCAGCATCAG | NM_003478.3 | 81 |
| Glyceraldehydes
3-phosphate dehydrogenase (GAPDH) |
F-AGGTCGGAGTCAACGGATTTGGT
R-ACAAAGTGGTCGTTGAGGGCAATG | NM_002046.3 | 910 |
|
Hypoxanthine-guanine
phosphoribosyltransferase (HPRT) |
F-ATGGACAGGACTGAACGTCTTGCT
R-TGAGCACACAGAGGGCTACAATG | NM_000194.2 | 79 |
ADM secretion assay
The constant release of endogenous ADM into culture
medium was studied in HL60 cells using protein transport
inhibition. Cells were seeded at density of 1×105
cells/ml and then cultured for 12, 36 and 60 h in proliferation
medium before incubation for 12 h at 37°C with GolgiPlug™ (BD
Biosciences). At 24, 48 and 72 h after plating, the samples were
collected and then submitted to permeabilization with BD
Cytofix/Cytoperm solution. The analysis was performed by
intracellular ADM staining followed by flow cytometric analysis, as
above reported. In the present study, cultures untreated with
GolgiPlug™ were used as positive control of ADM secretion. The
acquired data were analyzed by the overton substraction tool of
Summit 4.3 software (Beckman Coulter, Inc., Brea, CA, USA) and were
reported as percentage (%) ± SD of ADM positive cells compared with
relative II Ab-matched control.
Treatment of HL60 cells with exogenous
ADM and ADM22-52
Cells were seeded in 25-cm2 flasks at a
density of 1×105 cells/ml and then cultured for 72 h
with different concentrations of ADM (ranging from
0.625×10−8 M to 5×10−8 M) (Sigma-Aldrich) or
5×10−7 M ADM22-52 (Sigma-Aldrich) (13,18,20).
In parallel, untreated cultures were used as reference. At the end
of the treatment, growth assay and FCM analysis of endogenous ADM
system were carried out. In parallel, cytospin preparations for
May-Grunwald Giemsa staining were made to detect morphological
changes of the nuclei.
Growth assay
At 72 h, cell suspensions were collected and then
centrifuged at 1,200 rpm for 5 min at 4°C. After resuspension of
pellets in 1 ml of Iscove's medium, HL60 cells were counted by
Nexcelom Bioscience-Auto T4 Cellometer (Invitrogen-Life
Technologies). Data are expressed as mean of total cell number ± SD
of three independent experiments performed in triplicate.
Statistical analysis was performed using the Student's t-test.
Investigation of PI3K/Akt and MAPK/ERK
signaling pathways
At different time-points (5, 15 and 30 min) after
stimulation, the activation of PI3K/Akt and MAPK/ERK signaling
pathways was evaluated in HL60 cells treated with 5×10−8
M ADM and 5×10−7 M ADM22-52. The analysis was
performed by FCM as previously described, using antibodies specific
for phosphorylated and unphosphorylated forms of Akt and MAPK
enzymes (Table I). In parallel,
untreated samples were used as reference. For each marker and its
corresponding II Ab-matched control, data were reported as
geometric mean fluorescence intensity (MFI) ± SD. Statistical
significance was calculated using the Student's t-test comparing
primed cells with resting samples.
Differentiative response of HL60 cells to
ADMexo and ADM22-52 Quantitative real-time
polymerase chain reaction (qPCR)
The quantitative analysis of Cullin 5 (Cul5) gene
expression was performed using Platinum®
SYBR® Green qPCR SuperMix-UDG (Invitrogen-Life
Technologies) and oligo primers (Invitrogen-Life Technologies)
designed for the detection of Cul5 and hypoxanthine-guanine
phosphoribosyltransferase (HPRT) housekeeping gene (Table II). The amplification reactions
were performed using a DNA Engine Opticon Real-Time Thermal Cycler
(Bio-Rad Laboratories). The relative expression of Cul5 mRNA was
determined using the ΔΔCT Livak method (21) and data were reported as fold
increase calculated using the 2−ΔΔCT ± SD equation.
FCM
At 72 h after stimulation with ADMexo and
ADM22-52, the samples were collected and then analysed
by flow cytometry to measure the expression of myeloid
differentiation markers and adhesion molecules (CD45, CD34, CD66b,
CD11b, CD11c, CD14, CD31 and CD38). The samples were prepared as
described above using antibodies listed in Table I. For each marker, data were
expressed as the ratio of geometric MFI (rMFI) ± SD derived from
primed cells and relative resting samples. Statistical significance
was calculated using the Student's t-test.
Histochemistry
Air-dried slides of cells were prepared using a
Cytospin 4 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
500 rpm for 5 min. The slides were fixed in methanol
(Sigma-Aldrich) for 5 min and then stained for 5 min in 50% (v/v)
May-Grunwald stain (Merck Millipore, Billerica, MA, USA) followed
by 15 min in 10% Giemsa stain (Merck Millipore). Both stain
solutions were freshly made using Sorenson's phosphate buffer (133
mM, pH 6.6). After staining, the slides were washed and destained
for 5 min in the phosphate buffer.
Results and Discussion
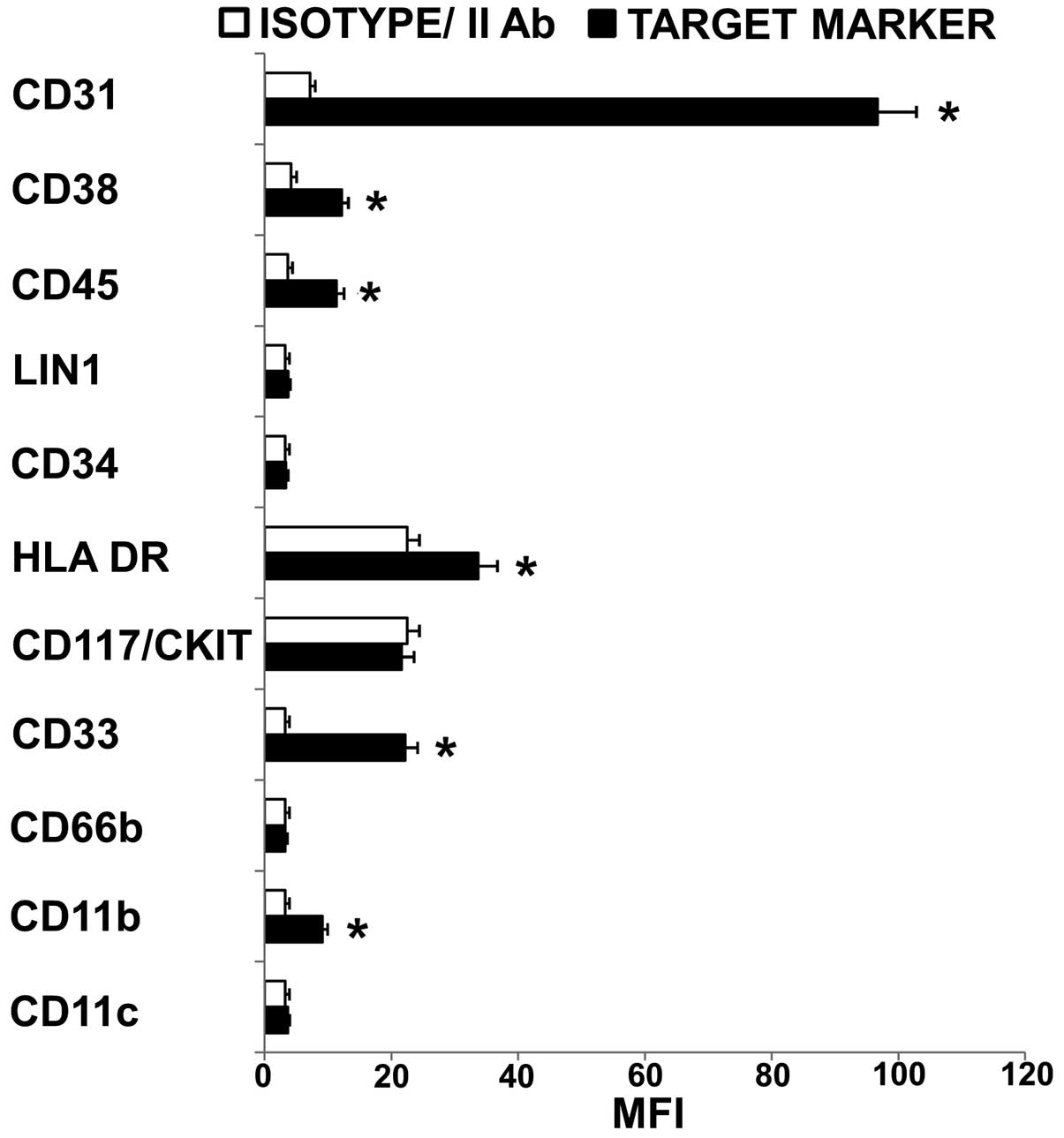
Immunophenotype of HL60 cells
Before priming with ADMexo and
ADM22-52, the immunophenotype of HL60 cells was explored
by FCM evaluating the expression level of undifferentiated and
differentiated state markers (Fig.
1). The myeloid phenotype was confirmed by the expression of
CD33 while the presence of stem cell compartment was suggested by
the absent expression of CD117/CKIT, Lin 1 and CD34 (22,23).
Moreover, the undifferentiated state of cells was demonstrated by
the negative or low fluorescence intensity of HLA-DR, CD45, CD11b,
CD11c and CD66b (24–27). Among adhesion molecules expressed
by acute myelogenous leukemia blasts, CD31 (28,29)
and CD38 (30) are reported to
play pivotal role in the interaction of tumor cells with
microenviromental elements, i.e. CD31 on the surface of marrow
endothelial cells (CD31/CD31 and CD38/CD31 interactions) and
hyaluronate (CD38/hyaluronate interactions). In the present study,
an excess of CD31 relative to CD38 was detected (CD31/CD38 MFI
ratio >1) suggesting that HL60 cells have a high potential of
transendothelial migration (31–33).
Similar expression level of CD31, CD33, CD38 in HL60 and APL cells
was considered important to define a prospective correlation
between in vitro and in vivo conditions.
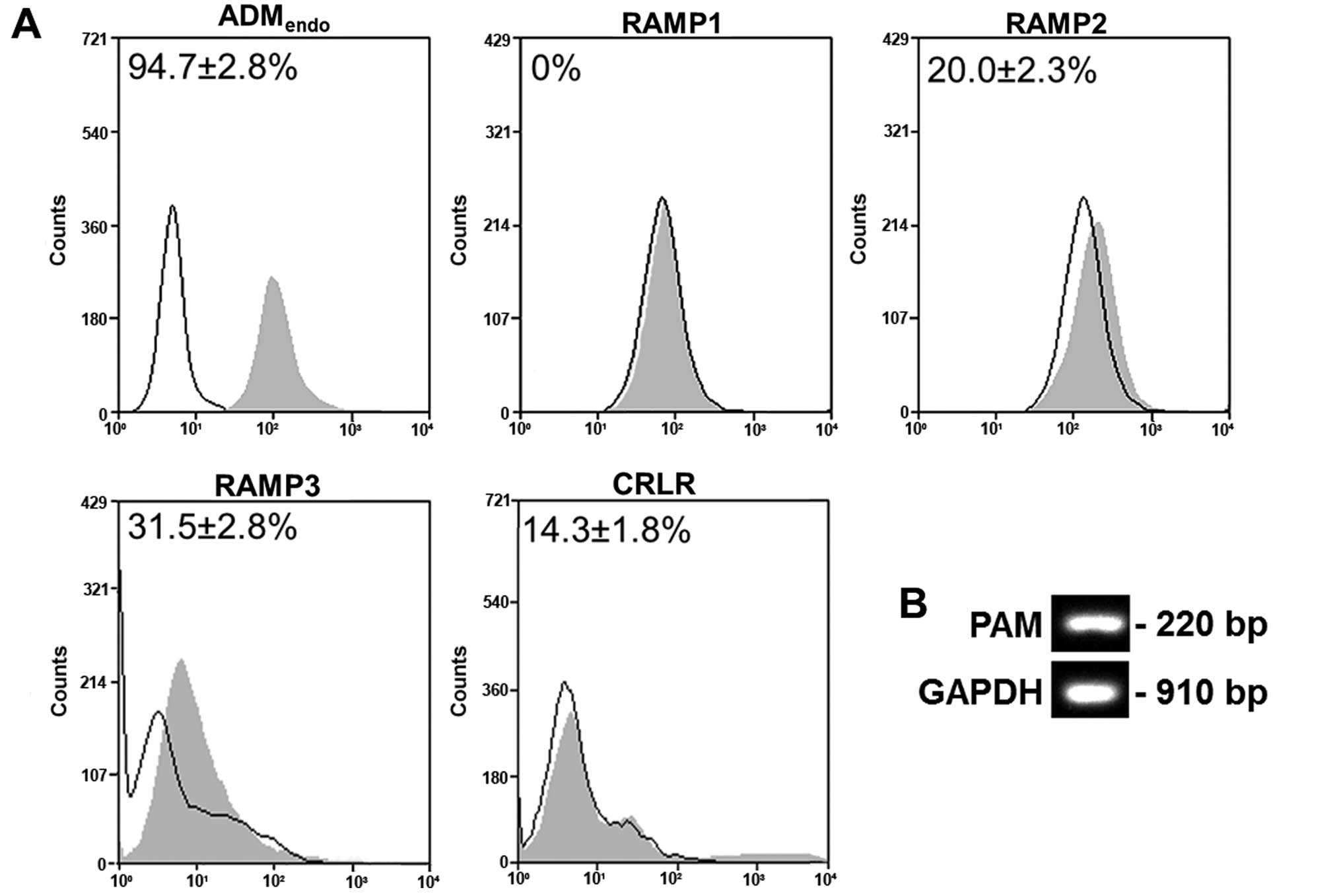
HL60 cells express ADM and its
receptors
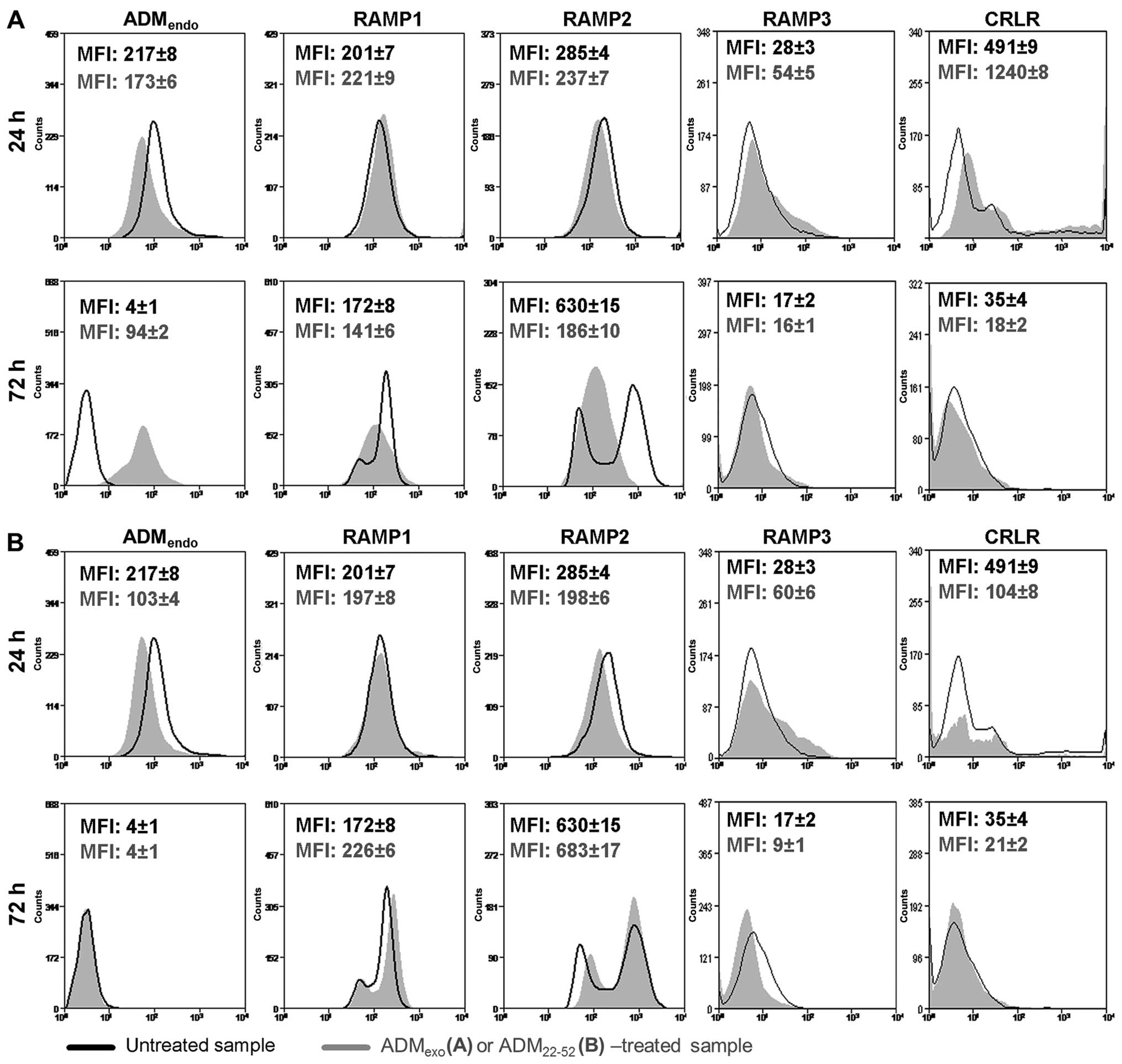
As reported in Fig.
2A, HL60 cells were shown to express ADMendo
(94.7±2.8%), RAMP2 (20.0±2.3%), RAMP3 (31.5±2.8%) and CRLR
(14.3±1.8%). In contrast, RAMP1 was not detected. The gene
expression of peptidylglycine α-amidating monooxygenase (PAM), that
is the enzyme producing α-amidated bioactive peptide from the
inactive precursor (34),
confirmed the potential of cells to synthesize the active form of
ADM (Fig. 2B). Produced by
peripheral blood mononuclear and polymorphonuclear cells, ADM is
known to regulate cell growth or differentiation through an
autocrine mode of action (15). As
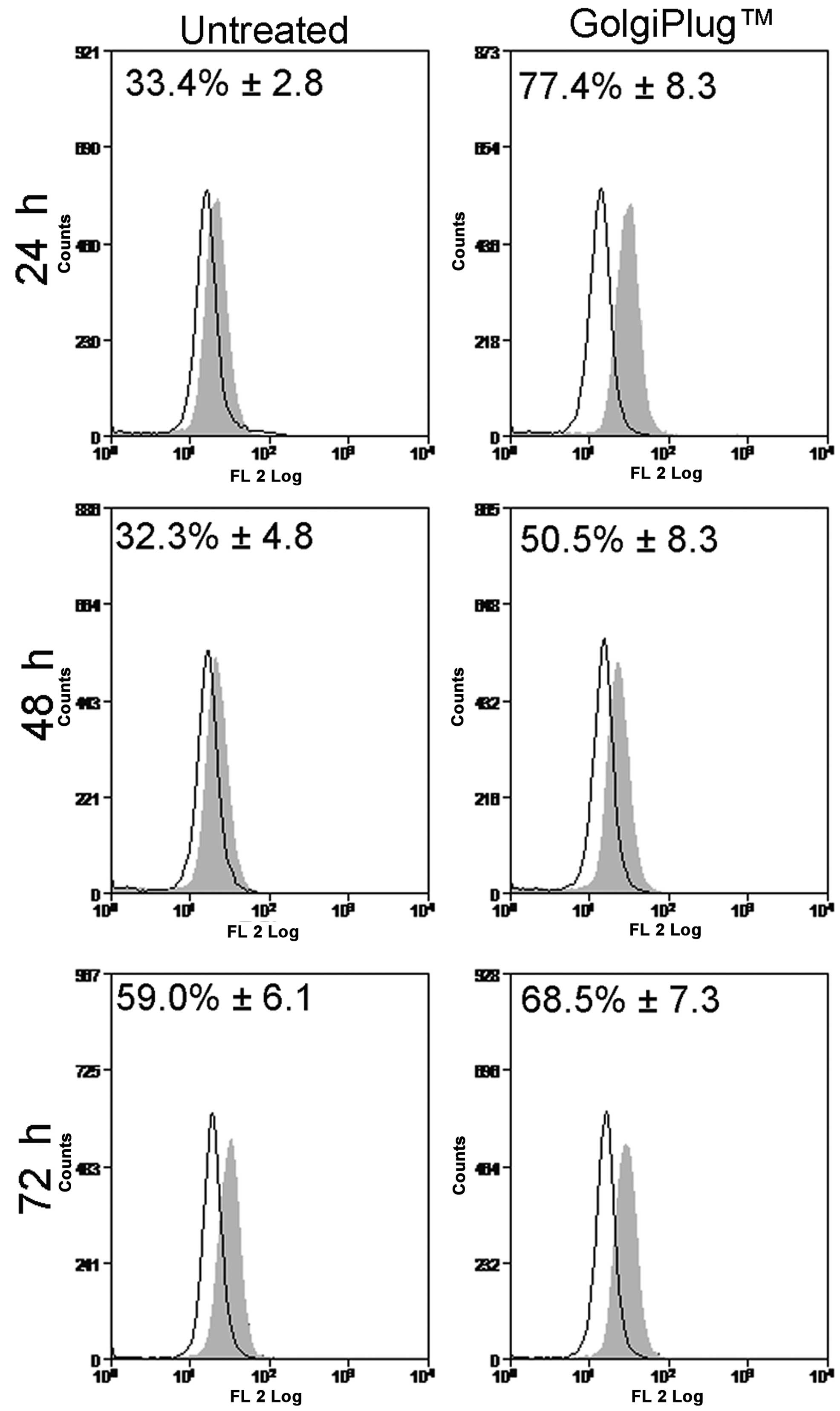
shown in Fig. 3, the release of
ADM endogenously synthetized by HL60 cells was evaluated using
Brefeldin A/GolgiPlug™ kit, a protein transport inhibitor that is
able to prevent the secretion process and to trap peptides into
intra-cellular compartments. The increased immunoreactivity for ADM
in GolgiPlug™-treated samples (24 h, 77.4±8.3%; 48 h, 50.5±8.3%;
and 72 h, 68.5±7.3%) compared to untreated cells (24 h, 33.4±2.8%;
48 h, 32.3±4.8%; and 72 h, 59.0±6.1%) was interpreted as indicative
of the cytoplasmic accumulation of ADMendo while the
decreased fluorescent signal suggested that HL60 constitutively
secrete ADM into culture medium after synthesis, as previously
reported (15).
ADMexo enhances the
proliferation of HL60 cells
It is known that ADM is produced not only by cancer
cells but also by endothelial, macrophages, and mast cells of tumor
microenvironment (35), where it
contributes to cancer pathogenesis both directly stimulating cancer
cell growth or indirectly inducing angiogenesis and reducing the
effectiveness of the immune system.
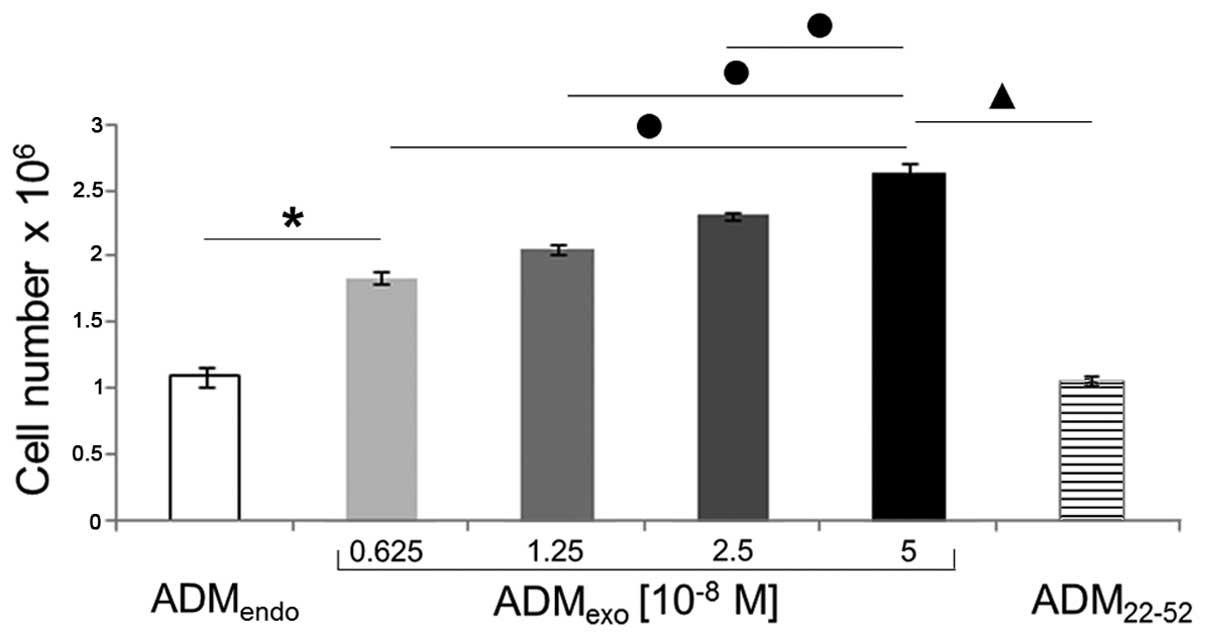
Our data (Fig. 4)
show that exogenous ADM exerted a strong proliferative effect on
HL60 cells, as already observed in several cell types (36–39).
Indeed, after a 72 h incubation period, significant increases in
cell number were detected in cultures treated with
ADMexo compared to resting cells. In particular,
5×10−8 M ADM was shown to be the most effective
concentration. In contrast, ADM22-52 demonstrated not to
affect the proliferation of HL60 cells, since total cell number in
primed cultures was comparable to that of untreated samples.
ADMexo and ADM22-52
affect the ADM system
In the present study, we explored by FCM the
modulation of adrenomedullin system (ADMendo and its
receptors) in HL60 cells treated with exogenous 5×10−8 M
ADM or 5×10−7 M ADM22-52. When HL60 cells
were in vitro cultured with ADMexo (Fig. 5A) and ADM22-52 (Fig. 5B), a different expression profile
of ADMendo was detected in comparison to resting
samples. In particular, at 24 h, a decreased immunoreactivity for
ADM was observed in primed samples suggesting that, probably due to
an increased level of intracellular Ca2+ (40), a major release of endogenous ADM
was promoted. At 72 h, the secretion rate of ADM was negatively
controlled by ADMexo (Fig.
5A) but not under treatment with ADM22-52 (Fig. 5B). It is known that the expression
of RAMP isoforms may change between physiological and pathological
conditions (41), determining the
degree of cell response to ADM. The cell sensitivity to ADM
stimulation is defined both by a balanced expression of RAMPs and
CRLR at cell membrane and their rapid rate of recycling. It is
reported that RAMPs play pivotal roles in the transport of CRLR to
plasma membrane (42). At
physiological conditions, the most abundant isoform is RAMP2. Under
pathological conditions, significant changes in RAMP expression
levels are active under a concomitant increase in plasma ADM level
(43). In the present study, the
expression of AMR1 (CRLR/RAMP2), AMR2 (CRLR/RAMP3) and CGRP
(CRLR/RAMP1) components was demonstrated to be altered following
the treatment with exogenous ADM and ADM22-52. Indeed,
at 24 h, ADMexo upregulated RAMP1, RAMP3 and CRLR
(Fig. 5A) while
ADM22-52 was effective in increasing the expression of
RAMP3, but drastically reduced CRLR (Fig. 5B). In both primed groups, probably
due to a receptor degradation after internalization (43), RAMP2 significantly decreased
(Fig. 5), suggesting that
ADMexo and ADM22-52 mediated their activity
at early phase by AMR1. These data were in accordance with the
hypothesis that an excess of ADM in bone marrow matrix could
increase the responsiveness of leukemia cells to ADM upregulating
CRLR, RAMP1 and RAMP3 (44), while
the inhibition of ADM signaling could raise the cell insensitivity
to stimulation by a significant reduction of ADM receptor system.
At 72 h, a correlation between the increased expression level of
RAMP1/2 and the release of ADM was observed in resting samples
suggesting that HL60 cells could combine the release of ADM with
the exposure of ADM receptors at cell membrane to control their
growth. Notably, the treatment of HL60 cells with exogenous ADM
reduced the release of ADMendo and the expression of
RAMP1 and RAMP2 while no change was induced by ADM22-52
compared to resting cells. The expression of RAMP3 and CRLR was
drastically reduced at 72 h in all experimental groups. Unlike
previously reported by Poyner et al (42), ADM22-52 exerted
inhibitory activity of ADM signaling in leukemia cells through a
negative regulation of CRLR expression, either by an increased
receptor internalization followed by degradation or a block of
receptor transport to plasma membrane.
AKT and MAPK signaling pathways were
differently modulated by ADMexo and
ADM22-52
Pleiotropic effects of ADM are reported to be
mediated through several intracellular signal transduction pathways
(43). Using HL60 leukemia cells,
we explored by FCM the modulation exerted by 5×10−8 M
ADM and 5×10−7 M ADM22-52 on PI3K/Akt and
ERK/MAPK signaling pathways, that are involved in several
haematopoiesis malignancies, including AML (45–49).
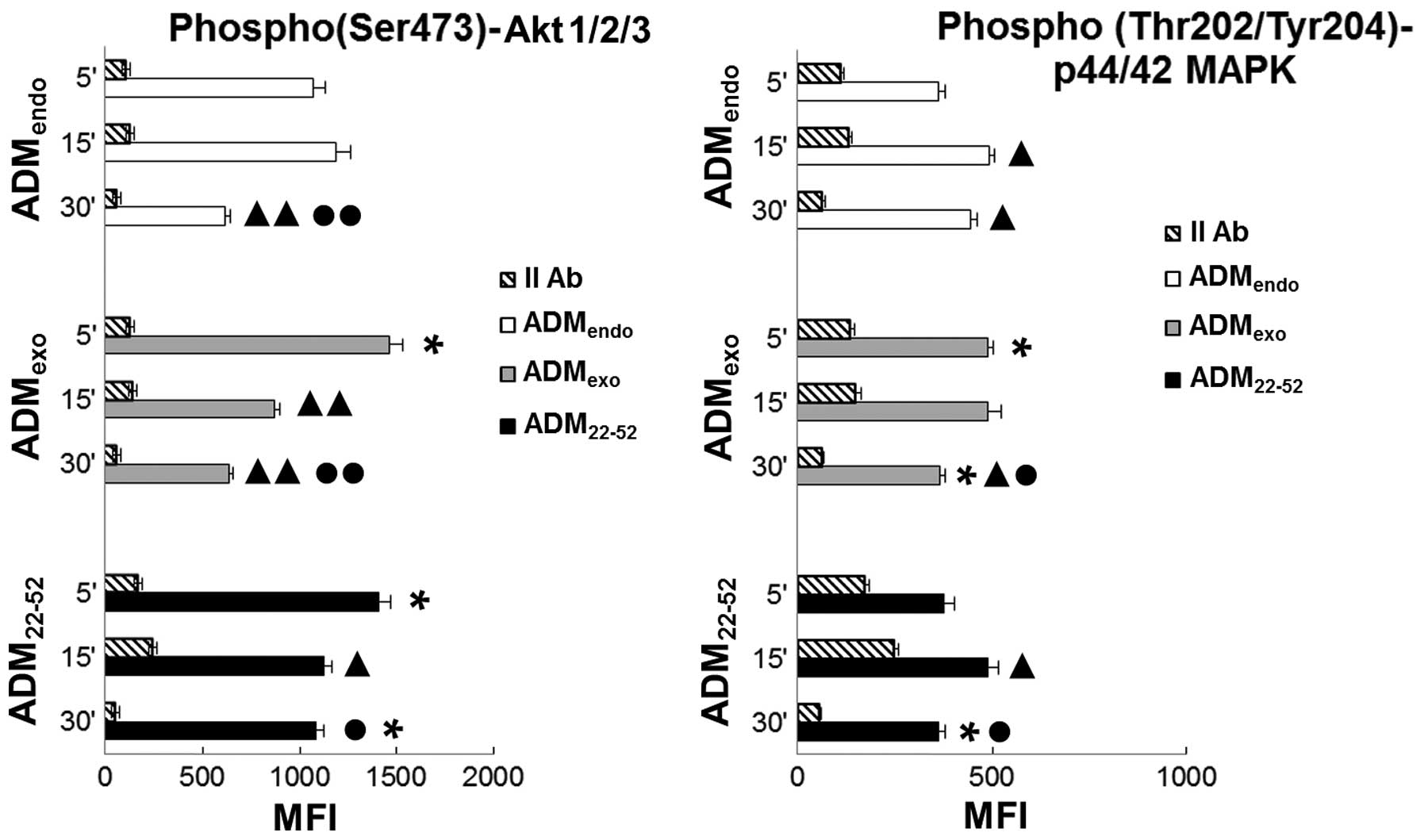
As shown in Fig. 6, the
simultaneous detection of p(Ser473)-Akt1/2/3 and
phospho(Thr202/Tyr204)-p44/42 MAPK in resting cells
(ADMendo) suggested a constitutive activity of both
pathways in HL60 cells. Hayashi and colleagues (50) reported that PI3K/Akt positively
regulates the MAPK cascade, facilitating maximal ERK responses to
physiological stimuli, whereas activated ERK, in turn, negatively
controls the PI3K/Akt pathway. In the present study (Fig. 6), the phosphorylation level of Akt
in ADMendo reached the maximum value at 5 to 15 min and
decreased at 30 min. In parallel, the upregulation in MAPK activity
from 15 to 30 min could be indicative of a reciprocal regulation
loop between Akt and MAPK. Notably, the stimulation of HL60 with
ADMexo and ADM22-52 differently affected the
activity of both pathways. Under stimulation with
ADMexo, an increase in the expression level of
p(Ser473)-Akt and phospho(Thr202/Tyr204)-p44/42 MAPK was observed
at 5 min indicating that HL60 cells were responsive to exogenous
ADM via the PI3K/Akt and ERK/MAPK cascade. In samples treated with
ADMexo compared to ADMendo group, the activation level
of MAPK unchanged from 5 to 15 min while a significant decrease in
phosphorylated Akt expression was observed. In contrast, the
stimulation with ADM22-52 promoted the maximum
activation of Akt at 5 to 30 min and a downregulation of MAPK
activity at 30 min. Based on this evidence, we hypothesized that
the activity of ADMexo was mainly mediated by the
ERK/MAPK pathway while that of ADM22-52 was especially
involved in PI3K/Akt signaling.
ADM22-52 induces a strong
differentiative induction on HL60 cells
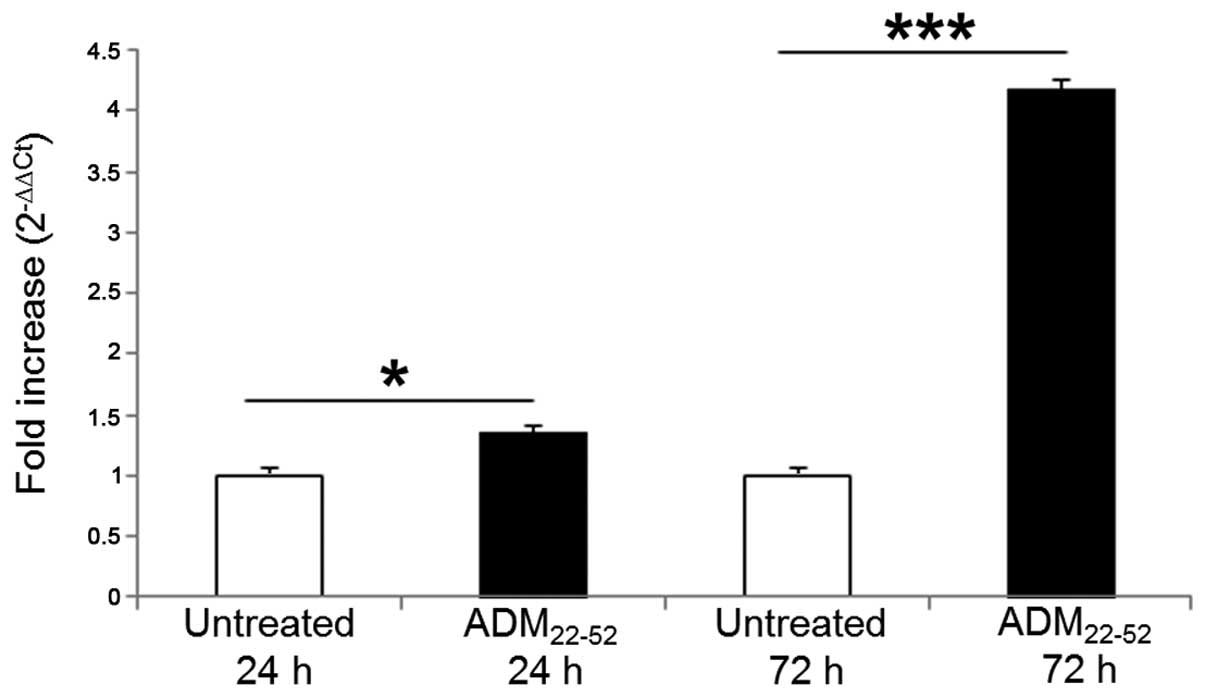
qPCR
To verify the differentiative induction after the
inhibition of ADM system, the expression of Cul5 was assessed by
quantitative RT-PCR (Fig. 7). Cul5
belongs to the Cullins gene family, including 7 isoforms
identified in mammalians. Cul5, Asb-2 (ankyrin repeat SOCS box 2),
a RING finger protein (ROC/Rbx), and an adapter protein complex
(elongin B/C) form an E3 ubiquitin ligase complex that, due to an
E2 ubiquitin-conjugating enzyme, leading to the degradation of
target proteins by 26S proteasome (51). As component of this complex, Cul5
brings the enzyme the substrate recognition components (52). Furthermore, it seems to act as
tumor suppressor in breast cancer (53) and its expression decreases in
B-cell chronic lymphocytic leukemia (54). At 24 and 72 h after stimulation
with ADM22-52, Cul5 mRNA increased 1.35- and 4.18-fold,
respectively, in primed cells vs. resting cultures. Our data were
in agreement with the increase of Cul5 mRNA and protein detected by
Baxter et al (19) during
ATRA-mediated differentiation of HL60 cells.
FCM
Several reports highlighted that PI3K/Akt and
ERK/MAPK signaling pathways control the self-renewal and
differentiation of leukemia cells. Akt was demonstrated to exert
its activity (55) activating
nuclear factor-kappa B (NF-κB) (56), phosphorylating GSK-3 (57) and inhibiting transcription factors
of FOXO family (58). Marcinkowska
et al (59) demonstrated
that ERK/MAPK pathway stimulates the monocytic differentiation of
HL60 cells through the phosphorylation of transcription factor
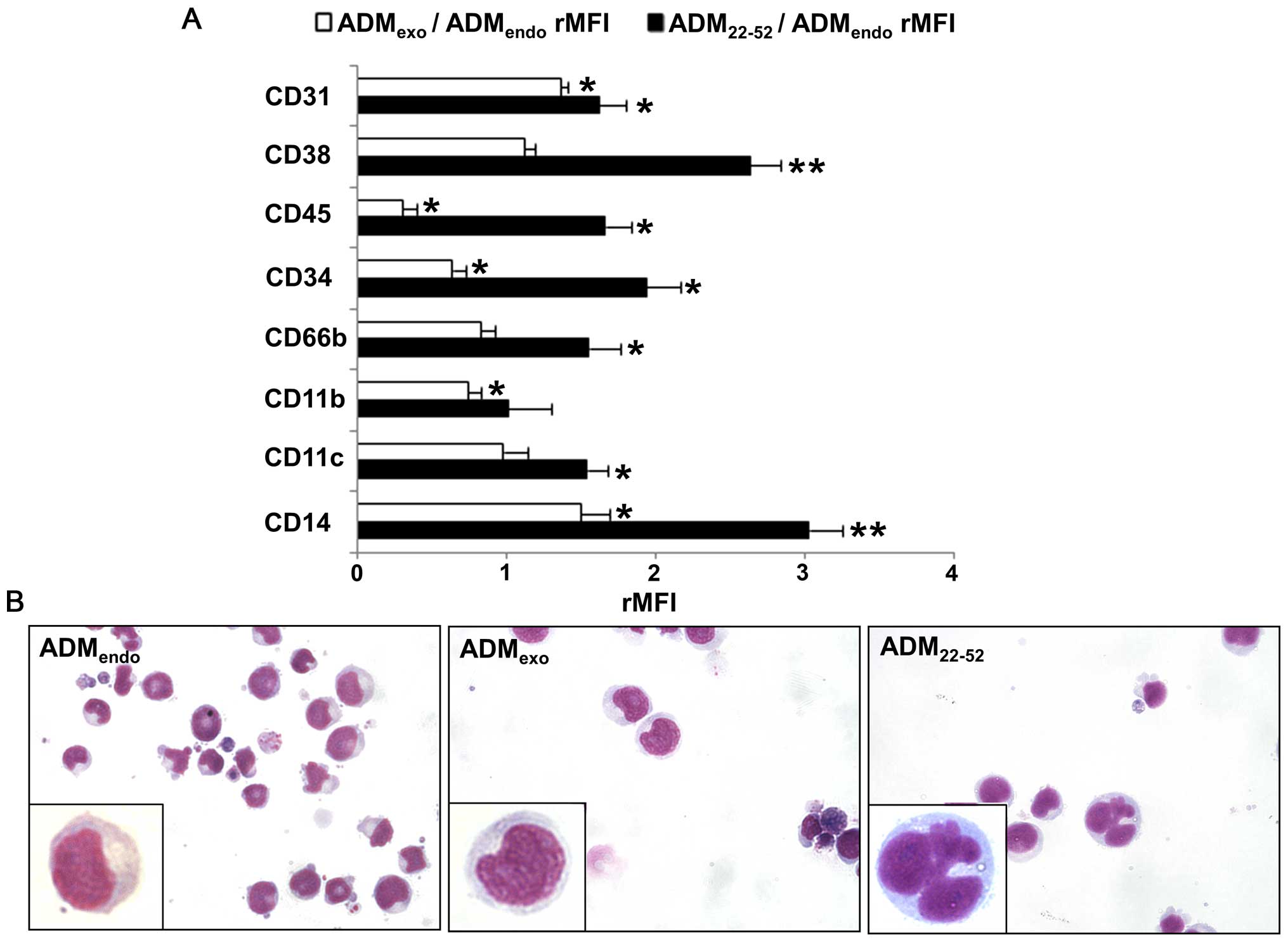
C/EBPβ. As shown in Fig. 8A, the
treatment with 5×10−8 M ADMexo was
accompanied by an upregulation in the expression level of CD14 and
CD31, which are associated with monocytic differentiation of HL60
cells (59,60). Interestingly, a decreased
(rMFI<1) or unchanged (rMFI=1) expression level of other markers
was observed in comparison to resting samples (ADMendo),
suggesting that ADMexo could exert a negative control on
the granulocytic differentiation process. In samples treated for 72
h with ADM22-52, FCM analysis evidenced a significant
increase in the expression level of CD11b, a subunit of
αMβ2 integrin, CD11c, an integrin αX, and
CD66b, a granulocyte-specific activation antigen expressed on
secondary granule membranes, that are all associated with
granulocytic differentiation of HL60 cells (21,61,62).
Moreover, we observed the upregulation of CD45 and CD38, that are
reported to increase during myeloid maturation (63,64)
and ATRA-induced granulocytic/monocytic differentiation (24,64).
The increased MFI value of CD14 in ADM22-52 compared to
that of ADMendo-samples further confirmed the
granulocytic/monocytic maturation of HL60 cells (65). It is known that the expression of
CD31 and CD38 on leukemic cells could exert a balanced control
between extramedullary dissemination and marrow retention of
leukemic cells. As previously demonstrated in ATRA-induced HL60
differentiation (66), an excess
of CD38 relative to CD31 (CD31/CD38 MFI ratio<1) was detected in
ADM22-52-treated cells, suggesting a higher retention of
cells in bone marrow through the interaction with hyaluronate. In
contrast, the excess of CD31 relative to CD38 (CD31/CD38 MFI ratio
>1) in samples treated with ADMexo evidenced an
increased ability of transendothelial migration.
Histochemistry
To investigate HL60 cell differentiation, nuclear
morphology of cells stained with May-Grunwald-Giemsa was performed
(Fig. 8B). In
ADMexo-treated cells, only ovoid and intended nuclei
were detected, whereas ADM22-52 determined the
appearance of differentiated cells characterized by several
multilobulated segmented nuclei.
Conclusions
Understanding the aberrant expression of
self-renewal pathways in myeloid malignancies is an emerging area
of investigation for the development of novel treatment strategies.
Extrinsic factors driven by bone marrow microenvironment are
reported to regulate the growth of hematopoietic stem cells
(67) within supportive
osteoblastic and vascular niches (68). Among the soluble factors secreted
in BM niches by stem cells (69)
and stromal compartment (10), ADM
synergizes with stem cell factor and Flt-3 (FMS-like tyrosine
kinase-3) ligand to induce the proliferation of primitive human
CD34+CD38−lin−cells and to promote
the expansion of CD34+ progenitors in culture (70). The development of hematopoietic
malignancies causes a remodeling of BM microenvironment followed by
the alteration of HSC function, leukemia survival, protection of
cancer cells from apoptotic stimuli and development of
drug-resistant phenotype (67,71).
Hypoxic conditions in leukemia niche are demonstrated to support
the progression of cancer and to upregulate ADM (72), thus, suggesting a correlation
between the survival of leukemia cells and the increased release of
ADM in tumor microenviroment (10). Consistent with the above evidence,
and studies in several human tumor cell lines, including HL60 and
chronic monocytic leukemia (U937) (73), our results suggest for ADM a
regulatory role in the proliferation of APL by paracrine effects,
as already demonstrated for ADM in modulating the activity of the
hypothalamo-pituitary-adrenal axis (74), the growth of cardiac and vascular
smooth muscle cells (75), the
hemodynamics of brain, lungs, and kidneys (76), physiological and pathological
angiogenesis (12). Using the
in vitro model of HL60 cells, we demonstrated that
exogenously administered ADM preserves promyelocytic leukemia cells
from differentiation as shown under ADM signaling inhibition with
ADM22-52 by the detection of typical differentiation
features such as multilobulated segmented nuclei, Cul5 expression
and increased expression level of granulocytic and monocytic
antigens. As the treatment with exogenous ADM significantly
stimulated the growth of HL60 cells, we speculated that paracrine
ADM acts in APL niche supporting the survival of leukemia cells and
promoting their transendothelial migration upregulating the
cellular expression of CD31 (64).
Based on the consideration that ADM22-52 stimulates both
the maturation and the retention in the bone marrow of leukemia
cells through an increased expression of CD38, as previously
demonstrated in HL60 and human primary APL cells treated with ATRA
(64), our data provide strong
evidence for the therapeutical potential that the inhibition of ADM
signaling could have in the treatment of APL. A potentially
important therapeutic effect of the blockade of
ADM22-52-sensitive receptors has been already
demonstrated for endotoxic shock (77) and expansion of keratinocytes,
fibro-blasts (78) and adrenal
cortical cells (79).
Further studies will be necessary to verify ADM
expression in bone marrow microenvironment and its involvement in
the induction and progression of hematopoietic malignancy.
Acknowledgements
The authors are grateful to Teresa Mariotto Pelos
and Italian Leukemia-Lymphoma-Myeloma ONLUS Association, Section of
Treviso (Italy) for the financial support.
References
|
1
|
Korf K, Wodrich H, Haschke A, Ocampo C,
Harder L, Gieseke F, Pollmann A, Dierck K, Prall S, Staege H, et
al: The PML domain of PML-RARα blocks senescence to promote
leukemia. Proc Natl Acad Sci USA. 111:12133–12138. 2014. View Article : Google Scholar
|
|
2
|
Nitto T and Sawaki K: Molecular mechanisms
of the anti-leukemia activities of retinoid and arsenic. J
Pharmacol Sci. 126:179–185. 2014. View Article : Google Scholar
|
|
3
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: A novel
hypotensive peptide isolated from human pheochromocytoma. 1993.
Biochem Biophys Res Commun. 425:548–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitamura K, Kangawa K and Eto T:
Adrenomedullin and PAMP: Discovery, structures, and cardiovascular
functions. Microsc Res Tech. 57:3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andreis PG, Mazzocchi G, Rebuffat P and
Nussdorfer GG: Effects of adrenomedullin and proadrenomedullin
N-terminal 20 peptide on rat zona glomerulosa cells. Life Sci.
60:1693–1697. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickerson IM: Role of CGRP-receptor
component protein (RCP) in CLR/RAMP function. Curr Protein Pept
Sci. 14:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shindo T, Sakurai T, Kamiyoshi A,
Ichikawa-Shindo Y, Shimoyama N, Iinuma N, Arai T and Miyagawa S:
Regulation of adrenomedullin and its family peptide by RAMP system:
lessons from genetically engineered mice. Curr Protein Pept Sci.
14:347–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tixier E, Leconte C, Touzani O, Roussel S,
Petit E and Bernaudin M: Adrenomedullin protects neurons against
oxygen glucose deprivation stress in an autocrine and paracrine
manner. J Neurochem. 106:1388–1403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shichiri M and Hirata Y: Regulation of
cell growth and apoptosis by adrenomedullin. Hypertens Res.
26(Suppl): S9–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larráyoz IM, Martínez-Herrero S,
García-Sanmartín J, Ochoa-Callejero L and Martínez A:
Adrenomedullin and tumour microenvironment. J Transl Med.
12:3392014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berenguer-Daizé C, Boudouresque F, Bastide
C, Tounsi A, Benyahia Z, Acunzo J, Dussault N, Delfino C, Baeza N,
Daniel L, et al: Adrenomedullin blockade suppresses growth of human
hormone-independent prostate tumor xenograft in mice. Clin Cancer
Res. 19:6138–6150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikitenko LL, Fox SB, Kehoe S, Rees MC and
Bicknell R: Adrenomedullin and tumour angiogenesis. Br J Cancer.
94:1–7. 2006. View Article : Google Scholar
|
|
13
|
Rullé S, Ah Kioon MD, Asensio C, Mussard
J, Ea HK, Boissier MC, Lioté F and Falgarone G: Adrenomedullin, a
neuropeptide with immunoregulatory properties induces semi-mature
tolerogenic dendritic cells. Immunology. 136:252–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hojo Y, Ikeda U, Ohya K, Ichida M, Kario
K, Takahashi M, Ikeda M, Minota S, Isumi Y, Minamino N, et al:
Interaction between monocytes and vascular endothelial cells
induces adrenomedullin production. Atherosclerosis. 155:381–387.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubo A, Minamino N, Isumi Y, Kangawa K,
Dohi K and Matsuo H: Adrenomedullin production is correlated with
differentiation in human leukemia cell lines and peripheral blood
monocytes. FEBS Lett. 426:233–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakayama M, Takahashi K, Murakami O,
Murakami H, Sasano H, Shirato K and Shibahara S: Adrenomedullin in
monocytes and macrophages: Possible involvement of
macrophage-derived adrenomedullin in atherogenesis. Clin Sci
(Lond). 97:247–251. 1999. View Article : Google Scholar
|
|
17
|
Del Pup L, Belloni AS, Carraro G, De
Angeli S, Parnigotto PP and Nussdorfer GG: Adrenomedullin is
expressed in cord blood hematopoietic cells and stimulates their
clonal growth. Int J Mol Med. 11:157–160. 2003.PubMed/NCBI
|
|
18
|
De Angeli S, Del Pup L, Febas E, Conconi
MT, Tommasini M, Di Liddo R, Albertin G, Parnigotto PP and
Nussdorfer GG: Adrenomedullin and endothelin-1 stimulate in vitro
expansion of cord blood hematopoietic stem cells. Int J Mol Med.
14:1083–1086. 2004.PubMed/NCBI
|
|
19
|
Baxter SS, Carlson LA, Mayer AM, Hall ML
and Fay MJ: Granulocytic differentiation of HL-60 promyelocytic
leukemia cells is associated with increased expression of Cul5. In
Vitro Cell Dev Biol Anim. 45:264–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ziolkowska A, Budzynska K, Trejter M,
Tortorella C, Belloni AS and Malendowicz LK: Effects of
adrenomedullin and its fragment 22–52 on basal and ACTH-stimulated
secretion of cultured rat adrenocortical cells. Int J Mol Med.
11:613–615. 2003.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Paietta E, Goloubeva O, Neuberg D, Bennett
JM, Gallagher R, Racevskis J, Dewald G, Wiernik PH and Tallman MS;
Eastern Cooperative Oncology Group. A surrogate marker profile for
PML/RAR alpha expressing acute promyelocytic leukemia and the
association of immunophenotypic markers with morphologic and
molecular subtypes. Cytometry B Clin Cytom. 59:1–9. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guglielmi C, Martelli MP, Diverio D, Fenu
S, Vegna ML, Cantù-Rajnoldi A, Biondi A, Cocito MG, Del Vecchio L,
Tabilio A, et al: Immunophenotype of adult and childhood acute
promyelocytic leukaemia: Correlation with morphology, type of PML
gene breakpoint and clinical outcome. A cooperative Italian study
on 196 cases. Br J Haematol. 102:1035–1041. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taetle R, Ostergaard H, Smedsrud M and
Trowbridge I: Regulation of CD45 expression in human leukemia
cells. Leukemia. 5:309–314. 1991.PubMed/NCBI
|
|
25
|
Carrigan SO, Weppler AL, Issekutz AC and
Stadnyk AW: Neutrophil differentiated HL-60 cells model Mac-1
(CD11b/CD18)-independent neutrophil transepithelial migration.
Immunology. 115:108–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KH, Seoh JY and Cho SJ: Phenotypic and
functional analysis of HL-60 cells used in opsonophagocytic-killing
assay for Streptococcus pneumoniae. J Korean Med Sci. 30:145–150.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Veselská R, Zitterbart K, Auer J and
Neradil J: Differentiation of HL-60 myeloid leukemia cells induced
by all-trans retinoic acid is enhanced in combination with caffeic
acid. Int J Mol Med. 14:305–310. 2004.PubMed/NCBI
|
|
28
|
Newman PJ, Berndt MC, Gorski J, White GC
II, Lyman S, Paddock C and Muller WA: PECAM-1 (CD31) cloning and
relation to adhesion molecules of the immunoglobulin gene
superfamily. Science. 247:1219–1222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brouwer RE, Hoefnagel J, Borger van Der
Burg B, Jedema I, Zwinderman KH, Starrenburg IC, Kluin-Nelemans HC,
Barge RM, Willemze R and Falkenburg JH: Expression of
co-stimulatory and adhesion molecules and chemokine or apoptosis
receptors on acute myeloid leukaemia: High CD40 and CD11a
expression correlates with poor prognosis. Br J Haematol.
115:298–308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Howard M, Grimaldi JC, Bazan JF, Lund FE,
Santos-Argumedo L, Parkhouse RM, Walseth TF and Lee HC: Formation
and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen
CD38. Science. 262:1056–1059. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muller WA, Weigl SA, Deng X and Phillips
DM: PECAM-1 is required for transendothelial migration of
leukocytes. J Exp Med. 178:449–460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deaglio S, Morra M, Mallone R, Ausiello
CM, Prager E, Garbarino G, Dianzani U, Stockinger H and Malavasi F:
Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an
Ig superfamily member. J Immunol. 160:395–402. 1998.PubMed/NCBI
|
|
33
|
Dianzani U, Funaro A, DiFranco D,
Garbarino G, Bragardo M, Redoglia V, Buonfiglio D, De Monte LB,
Pileri A and Malavasi F: Interaction between endothelium and
CD4+CD45RA+ lymphocytes. Role of the human
CD38 molecule. J Immunol. 153:952–959. 1994.PubMed/NCBI
|
|
34
|
Rocchi P, Boudouresque F, Zamora AJ,
Muracciole X, Lechevallier E, Martin PM and Ouafik L: Expression of
adrenomedullin and peptide amidation activity in human prostate
cancer and in human prostate cancer cell lines. Cancer Res.
61:1196–1206. 2001.PubMed/NCBI
|
|
35
|
Zudaire E, Martínez A, Garayoa M, Pío R,
Kaur G, Woolhiser MR, Metcalfe DD, Hook WA, Siraganian RP, Guise
TA, et al: Adrenomedullin is a cross-talk molecule that regulates
tumor and mast cell function during human carcinogenesis. Am J
Pathol. 168:280–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyashita K, Itoh H, Sawada N, Fukunaga Y,
Sone M, Yamahara K, Yurugi T and Nakao K: Adrenomedullin promotes
proliferation and migration of cultured endothelial cells.
Hypertens Res. 26(Suppl): S93–S98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Belloni AS, Trejter M, Malendowicz LK and
Nussdorfer GG: Adrenomedullin stimulates proliferation and inhibits
apoptosis of immature rat thymocytes cultured in vitro. Peptides.
24:295–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iwasaki H, Eguchi S, Shichiri M, Marumo F
and Hirata Y: Adrenomedullin as a novel growth-promoting factor for
cultured vascular smooth muscle cells: Role of tyrosine
kinase-mediated mitogen-activated protein kinase activation.
Endocrinology. 139:3432–3441. 1998.PubMed/NCBI
|
|
39
|
Andreis PG, Markowska A, Champion HC,
Mazzocchi G, Malendowicz LK and Nussdorfer GG: Adrenomedullin
enhances cell proliferation and deoxyribonucleic acid synthesis in
rat adrenal zona glomerulosa: Receptor subtype involved and
signaling mechanism. Endocrinology. 141:2098–2104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikeda U, Kanbe T, Kawahara Y, Yokoyama M
and Shimada K: Adrenomedullin augments inducible nitric oxide
synthase expression in cytokine-stimulated cardiac myocytes.
Circulation. 94:2560–2565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jacob A, Wu R and Wang P: Regulation of
RAMP expression in diseases. Adv Exp Med Biol. 744:87–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Poyner DR, Sexton PM, Marshall I, Smith
DM, Quirion R, Born W, Muff R, Fischer JA and Foord SM:
International Union of Pharmacology. XXXII. The mammalian
calcitonin gene-related peptides, adrenomedullin, amylin, and
calcitonin receptors. Pharmacol Rev. 54:233–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gibbons C, Dackor R, Dunworth W, Fritz-Six
K and Caron KM: Receptor activity-modifying proteins: RAMPing up
adrenomedullin signaling. Mol Endocrinol. 21:783–796. 2007.
View Article : Google Scholar
|
|
44
|
McLatchie LM, Fraser NJ, Main MJ, Wise A,
Brown J, Thompson N, Solari R, Lee MG and Foord SM: RAMPs regulate
the transport and ligand specificity of the
calcitonin-receptor-like receptor. Nature. 393:333–339. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
47
|
Hsu J, Shi Y, Krajewski S, Renner S,
Fisher M, Reed JC, Franke TF and Lichtenstein A: The AKT kinase is
activated in multiple myeloma tumor cells. Blood. 98:2853–2855.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakatani K, Thompson DA, Barthel A, Sakaue
H, Liu W, Weigel RJ and Roth RA: Up-regulation of Akt3 in estrogen
receptor-deficient breast cancers and androgen-independent prostate
cancer lines. J Biol Chem. 274:21528–21532. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hayashi H, Tsuchiya Y, Nakayama K, Satoh T
and Nishida E: Down-regulation of the PI3-kinase/Akt pathway by ERK
MAP kinase in growth factor signaling. Genes Cells. 13:941–947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kohroki J, Nishiyama T, Nakamura T and
Masuho Y: ASB proteins interact with Cullin5 and Rbx2 to form E3
ubiquitin ligase complexes. FEBS Lett. 579:6796–6802. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kile BT, Schulman BA, Alexander WS, Nicola
NA, Martin HM and Hilton DJ: The SOCS box: A tale of destruction
and degradation. Trends Biochem Sci. 27:235–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fay MJ, Longo KA, Ka rathanasis GA, Shope
DM, Mandernach CJ, Leong JR, Hicks A, Pherson K and Husain A:
Analysis of CUL-5 expression in breast epithelial cells, breast
cancer cell lines, normal tissues and tumor tissues. Mol Cancer.
2:402003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kalla C, Scheuermann MO, Kube I, Schlotter
M, Mertens D, Döhner H, Stilgenbauer S and Lichter P: Analysis of
11q22-q23 deletion target genes in B-cell chronic lymphocytic
leukaemia: Evidence for a pathogenic role of NPAT, CUL5, and
PPP2R1B. Eur J Cancer. 43:1328–1335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abdullah M and Seman Z: Role of signaling
pathways in acute myeloid leukemia. Myeloid Leukemia-Basic
Mechanisms of Leukemogenesis. Koschmieder S and Krug U: InTech;
Rijeka: pp. 429–448. 2011
|
|
56
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fukumoto S, Hsieh CM, Maemura K, Layne MD,
Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, et al:
Akt participation in the Wnt signaling pathway through Dishevelled.
J Biol Chem. 276:17479–17483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sykes SM, Lane SW, Bullinger L,
Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H,
Brumme KM, et al: AKT/FOXO signaling enforces reversible
differentiation blockade in myeloid leukemias. Cell. 146:697–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marcinkowska E, Garay E, Gocek E, Chrobak
A, Wang X and Studzinski GP: Regulation of C/EBPbeta isoforms by
MAPK pathways in HL60 cells induced to differentiate by
1,25-dihy-droxyvitamin D3. Exp Cell Res. 312:2054–2065. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trayner ID, Bustorff T, Etches AE, Mufti
GJ, Foss Y and Farzaneh F: Changes in antigen expression on
differentiating HL60 cells treated with dimethylsulphoxide,
all-trans retinoic acid, alpha1,25-dihydroxyvitamin D3
or 12-O-tetradecanoyl phorbol-13-acetate. Leuk Res. 22:537–547.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bellón T, López-Rodríguez C, Rubio MA,
Jochems G, Bernabeu C and Corbi AL: Regulated expression of p150,95
(CD11c/CD18; αX/β2) and VLA-4 (CD49d/CD29; α4/β1) integrins during
myeloid cell differentiation. Eur J Immunol. 24:41–47. 1994.
View Article : Google Scholar
|
|
62
|
Park DJ, Chumakov AM, Vuong PT, Chih DY,
Gombart AF, Miller WH Jr and Koeffler HP: CCAAT/enhancer binding
protein epsilon is a potential retinoid target gene in acute
promyelocytic leukemia treatment. J Clin Invest. 103:1399–1408.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van Lochem EG, van der Velden VH, Wind HK,
te Marvelde JG, Westerdaal NA and van Dongen JJ: Immunophenotypic
differentiation patterns of normal hematopoiesis in human bone
marrow: Reference patterns for age-related changes and
disease-induced shifts. Cytometry B Clin Cytom. 60:1–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lewandowski D, Linassier C, Iochmann S,
Degenne M, Domenech J, Colombat P, Binet C and Hérault O:
Phosphatidylinositol 3-kinases are involved in the all-trans
retinoic acid-induced upregulation of CD38 antigen on human
haematopoietic cells. Br J Haematol. 118:535–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hansen PB, Kjaersgaard E, Johnsen HE, Gram
J, Pedersen M, Nikolajsen K and Hansen NE: Different membrane
expression of CD11b and CD14 on blood neutrophils following in vivo
administration of myeloid growth factors. Br J Haematol. 85:50–56.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gallay N, Anani L, Lopez A, Colombat P,
Binet C, Domenech J, Weksler BB, Malavasi F and Herault O: The role
of platelet/endothelial cell adhesion molecule 1 (CD31) and CD38
antigens in marrow microenvironmental retention of acute
myelogenous leukemia cells. Cancer Res. 67:8624–8632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lemischka IR: Microenvironmental
regulation of hematopoietic stem cells. Stem Cells. 15(Suppl 1):
63–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chiarini F, Lonetti A, Evangelisti C,
Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM,
McCubrey JA and Martelli AM: Advances in understanding the acute
lymphoblastic leukemia bone marrow microenvironment: From biology
to therapeutic targeting. Biochim Biophys Acta. ppi:
S0167-4889(15)00293-1. 2015 View Article : Google Scholar
|
|
69
|
Bakondi B, Shimada IS, Perry A, Munoz JR,
Ylostalo J, Howard AB, Gregory CA and Spees JL: CD133 identifies a
human bone marrow stem/progenitor cell sub-population with a
repertoire of secreted factors that protect against stroke. Mol
Ther. 17:1938–1947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chute JP, Muramoto GG, Dressman HK, Wolfe
G, Chao NJ and Lin S: Molecular profile and partial functional
analysis of novel endothelial cell-derived growth factors that
regulate hematopoiesis. Stem Cells. 24:1315–1327. 2006. View Article : Google Scholar
|
|
71
|
Williams CA and Lavik EB: Engineering the
CNS stem cell microenvironment. Regen Med. 4:865–877. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kocemba KA, van Andel H, de Haan-Kramer A,
Mahtouk K, Versteeg R, Kersten MJ, Spaargaren M and Pals ST: The
hypoxia target adrenomedullin is aberrantly expressed in multiple
myeloma and promotes angiogenesis. Leukemia. 27:1729–1737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Takahashi K, Morimoto R, Hirose T, Satoh F
and Totsune K: Adrenomedullin 2/intermedin in the
hypothalamopituitary-adrenal axis. J Mol Neurosci. 43:182–192.
2011. View Article : Google Scholar
|
|
74
|
Tsuruda T, Kato J, Kitamura K, Kuwasako K,
Imamura T, Koiwaya Y, Tsuji T, Kangawa K and Eto T: Adrenomedullin:
A possible autocrine or paracrine inhibitor of hypertrophy of
cardiomyocytes. Hypertension. 31:505–510. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shichiri M, Fukai N, Ozawa N, Iwasaki H
and Hirata Y: Adrenomedullin is an autocrine/paracrine growth
factor for rat vascular smooth muscle cells. Regul Pept.
112:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lah JJ and Frishman WH: Adrenomedullin: A
vasoactive and natriuretic peptide with therapeutic potential.
Heart Dis. 2:259–265. 2000.
|
|
77
|
Mazzocchi G, Albertin G and Nussdorfer GG:
Adrenomedullin (ADM), acting through ADM(22-52)-sensitive
receptors, is involved in the endotoxin-induced hypotension in
rats. Life Sci. 66:1445–1450. 2000. View Article : Google Scholar
|
|
78
|
Albertin G, Carraro G, Parnigotto PP,
Conconi MT, Ziolkowska A, Malendowicz LK and Nussdorfer GG: Human
skin keratinocytes and fibroblasts express adrenomedullin and its
receptors, and adrenomedullin enhances their growth in vitro by
stimulating proliferation and inhibiting apoptosis. Int J Mol Med.
11:635–639. 2003.PubMed/NCBI
|
|
79
|
Rebuffat P, Macchi C, Malendowicz LK and
Nussdorfer GG: Up-regulation of adrenomedullin gene expression in
the regenerating rat adrenal cortex. Int J Mol Med. 20:551–555.
2007.PubMed/NCBI
|