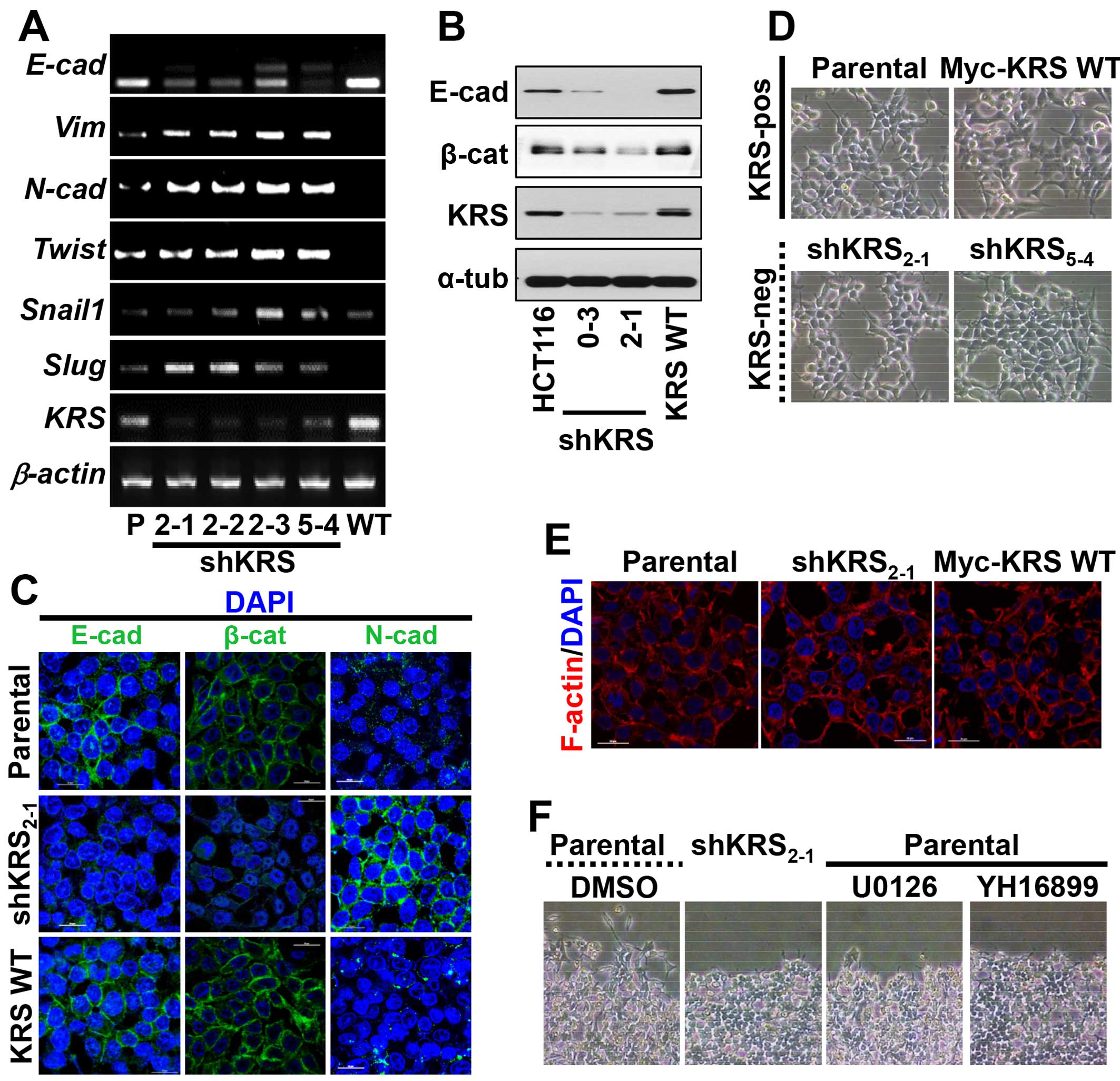
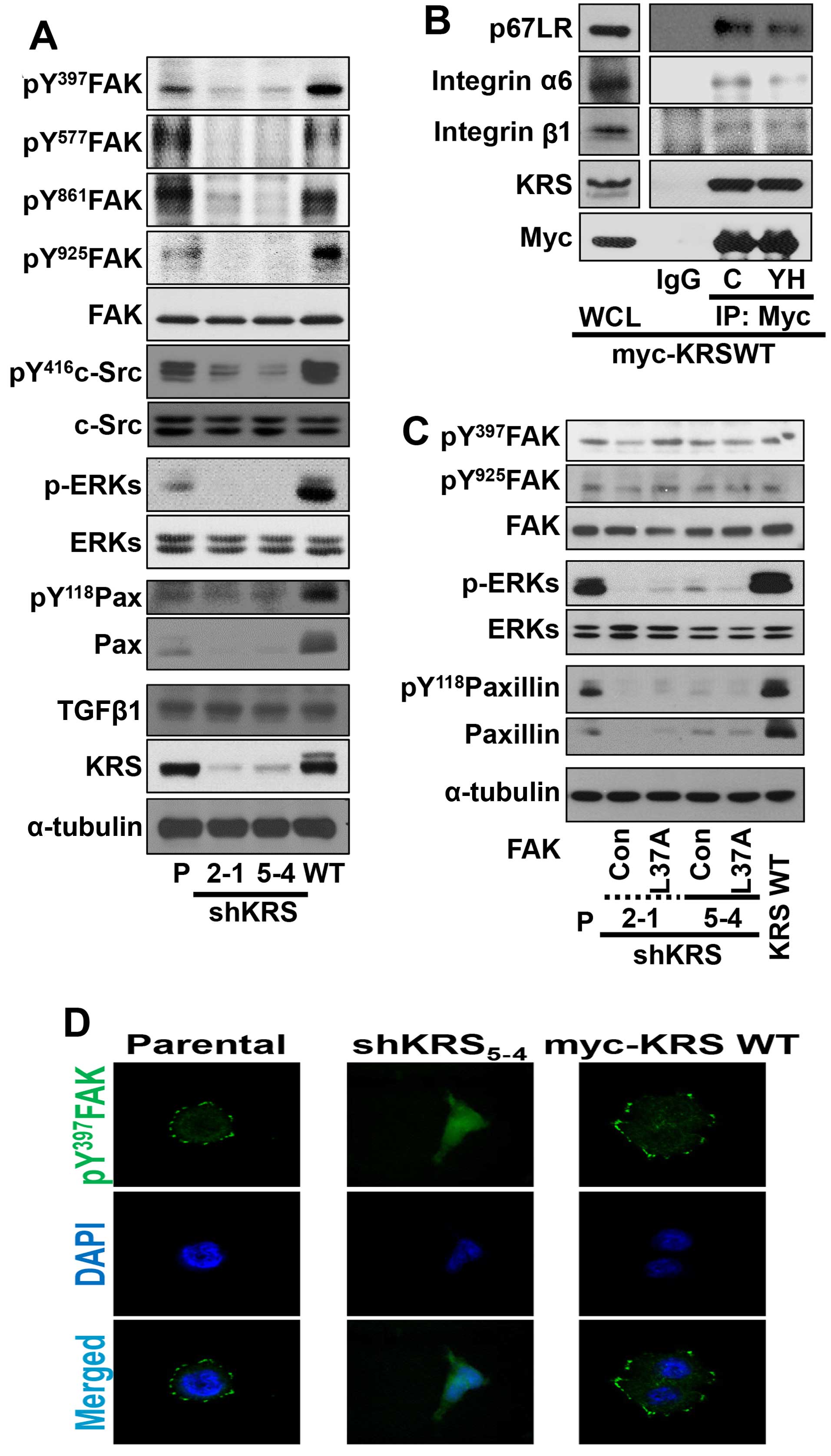
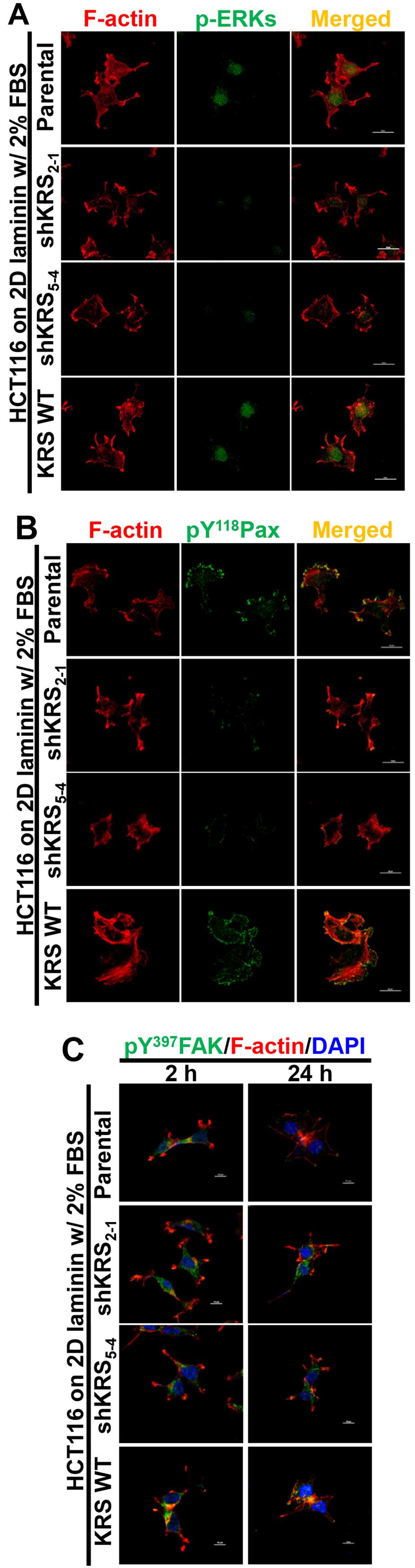
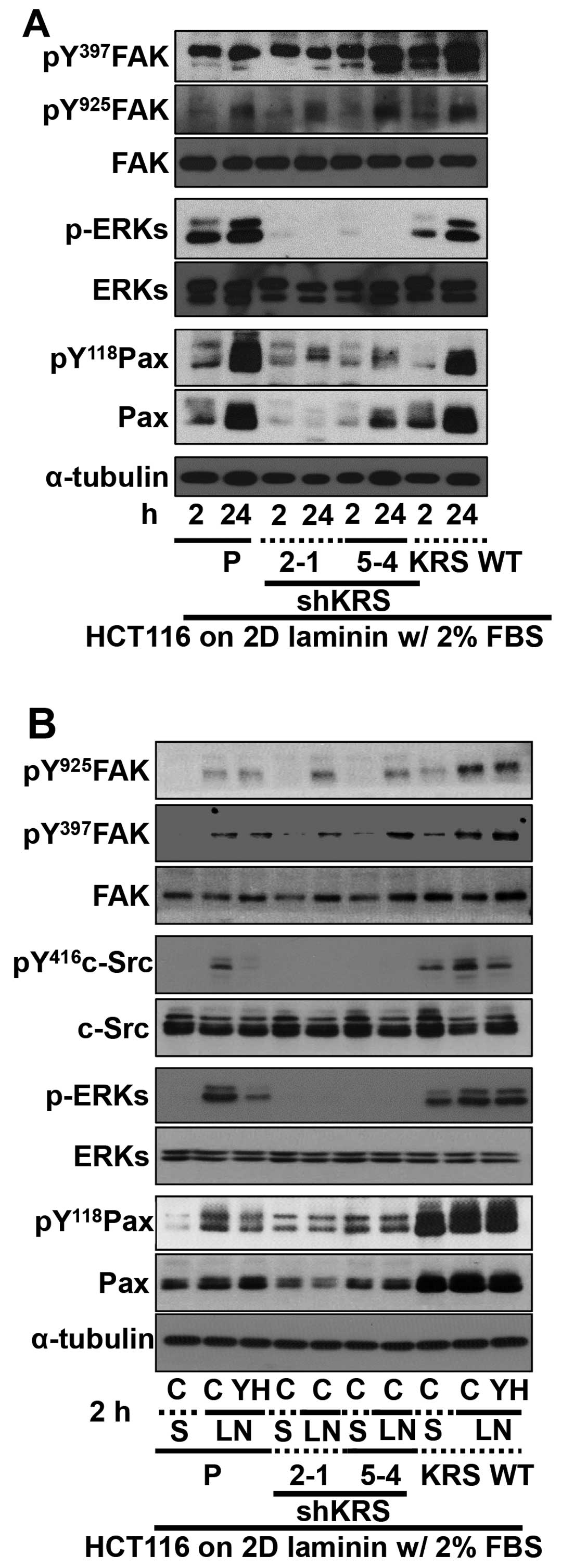
|
1
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collins C and Nelson WJ: Running with
neighbors: Coordinating cell migration and cell-cell adhesion. Curr
Opin Cell Biol. 36:62–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geiger B, Spatz JP and Bershadsky AD:
Environmental sensing through focal adhesions. Nat Rev Mol Cell
Biol. 10:21–33. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geiger T and Geiger B: Towards elucidation
of functional molecular signatures of the adhesive-migratory
phenotype of malignant cells. Semin Cancer Biol. 20:146–152. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SG, Kim HJ, Min YH, Choi EC, Shin YK,
Park BJ, Lee SW and Kim S: Human lysyl-tRNA synthetase is secreted
to trigger proinflammatory response. Proc Natl Acad Sci USA.
102:6356–6361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yannay-Cohen N, Carmi-Levy I, Kay G, Yang
CM, Han JM, Kemeny DM, Kim S, Nechushtan H and Razin E: LysRS
serves as a key signaling molecule in the immune response by
regulating gene expression. Mol Cell. 34:603–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DG, Choi JW, Lee JY, Kim H, Oh YS, Lee
JW, Tak YK, Song JM, Razin E, Yun SH, et al: Interaction of two
translational components, lysyl-tRNA synthetase and p40/37LRP, in
plasma membrane promotes laminin-dependent cell migration. FASEB J.
26:4142–4159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DG, Lee JY, Kwon NH, Fang P, Zhang Q,
Wang J, Young NL, Guo M, Cho HY, Mushtaq AU, et al: Chemical
inhibition of prometastatic lysyl-tRNA synthetase-laminin receptor
interaction. Nat Chem Biol. 10:29–34. 2014. View Article : Google Scholar :
|
|
9
|
Canfield SM and Khakoo AY: The nonintegrin
laminin binding protein (p67 LBP) is expressed on a subset of
activated human T lymphocytes and, together with the integrin very
late activation antigen-6, mediates avid cellular adherence to
laminin. J Immunol. 163:3430–3440. 1999.PubMed/NCBI
|
|
10
|
Ardini E, Tagliabue E, Magnifico A, Butò
S, Castronovo V, Colnaghi MI and Ménard S: Co-regulation and
physical association of the 67-kDa monomeric laminin receptor and
the alpha6beta4 integrin. J Biol Chem. 272:2342–2345. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berno V, Porrini D, Castiglioni F,
Campiglio M, Casalini P, Pupa SM, Balsari A, Ménard S and Tagliabue
E: The 67 kDa laminin receptor increases tumor aggressiveness by
remodeling laminin-1. Endocr Relat Cancer. 12:393–406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carragher NO and Frame MC: Focal adhesion
and actin dynamics: A place where kinases and proteases meet to
promote invasion. Trends Cell Biol. 14:241–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scales TM and Parsons M: Spatial and
temporal regulation of integrin signalling during cell migration.
Curr Opin Cell Biol. 23:562–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albiges-Rizo C, Destaing O, Fourcade B,
Planus E and Block MR: Actin machinery and mechanosensitivity in
invado-podia, podosomes and focal adhesions. J Cell Sci.
122:3037–3049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JW and Juliano R: Mitogenic signal
transduction by integrin-and growth factor receptor-mediated
pathways. Mol Cells. 17:188–202. 2004.PubMed/NCBI
|
|
17
|
Valdembri D and Serini G: Regulation of
adhesion site dynamics by integrin traffic. Curr Opin Cell Biol.
24:582–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam SH, Kim D, Lee MS, Lee D, Kwak TK,
Kang M, Ryu J, Kim HJ, Song HE, Choi J, et al: Noncanonical roles
of membranous lysyl-tRNA synthetase in transducing cell-substrate
signaling for invasive dissemination of colon cancer spheroids in
3D collagen I gels. Oncotarget. 6:21655–21674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SA, Kim YM, Kwak TK, Kim HJ, Kim S, Ko
W, Kim SH, Park KH, Kim HJ, Cho M, et al: The extracellular loop 2
of TM4SF5 inhibits integrin alpha2 on hepatocytes under collagen
type I environment. Carcinogenesis. 30:1872–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung O, Choi S, Jang SB, Lee SA, Lim ST,
Choi YJ, Kim HJ, Kim DH, Kwak TK, Kim H, et al: Tetraspan
TM4SF5-dependent direct activation of FAK and metastatic potential
of hepatocarcinoma cells. J Cell Sci. 125:5960–5973. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dumbauld DW, Lee TT, Singh A, Scrimgeour
J, Gersbach CA, Zamir EA, Fu J, Chen CS, Curtis JE, Craig SW, et
al: How vinculin regulates force transmission. Proc Natl Acad Sci
USA. 110:9788–9793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clark K, Howe JD, Pullar CE, Green JA,
Artym VV, Yamada KM and Critchley DR: Tensin 2 modulates cell
contractility in 3D collagen gels through the RhoGAP DLC1. J Cell
Biochem. 109:808–817. 2010.PubMed/NCBI
|
|
23
|
Chua KN, Poon KL, Lim J, Sim WJ, Huang RY
and Thiery JP: Target cell movement in tumor and cardiovascular
diseases based on the epithelial-mesenchymal transition concept.
Adv Drug Deliv Rev. 63:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang RY, Wong MK, Tan TZ, Kuay KT, Ng AH,
Chung VY, Chu YS, Matsumura N, Lai HC, Lee YF, et al: An EMT
spectrum defines an anoikis-resistant and spheroidogenic
intermediate mesenchymal state that is sensitive to e-cadherin
restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell
Death Dis. 4:e9152013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP and Lim CT: Tumor dissemination:
An EMT affair. Cancer Cell. 23:272–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Panetti TS: Tyrosine phosphorylation of
paxillin, FAK, and p130CAS: Effects on cell spreading and
migration. Front Biosci. 7:d143–d150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Webb DJ, Donais K, Whitmore LA, Thomas SM,
Turner CE, Parsons JT and Horwitz AF: FAK-Src signalling through
paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell
Biol. 6:154–161. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schlaepfer DD, Hanks SK, Hunter T and van
der Geer P: Integrin-mediated signal transduction linked to Ras
pathway by GRB2 binding to focal adhesion kinase. Nature.
372:786–791. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin TH, Aplin AE, Shen Y, Chen Q, Schaller
M, Romer L, Aukhil I and Juliano RL: Integrin-mediated activation
of MAP kinase is independent of FAK: Evidence for dual integrin
signaling pathways in fibroblasts. J Cell Biol. 136:1385–1395.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wary KK, Mainiero F, Isakoff SJ,
Marcantonio EE and Giancotti FG: The adaptor protein Shc couples a
class of integrins to the control of cell cycle progression. Cell.
87:733–743. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wary KK, Mariotti A, Zurzolo C and
Giancotti FG: A requirement for caveolin-1 and associated kinase
Fyn in integrin signaling and anchorage-dependent cell growth.
Cell. 94:625–634. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu KK, Armstrong SE, Ginnan R and Singer
HA: Adhesion-dependent activation of CaMKII and regulation of ERK
activation in vascular smooth muscle. Am J Physiol Cell Physiol.
289:C1343–C1350. 2005. View Article : Google Scholar : PubMed/NCBI
|