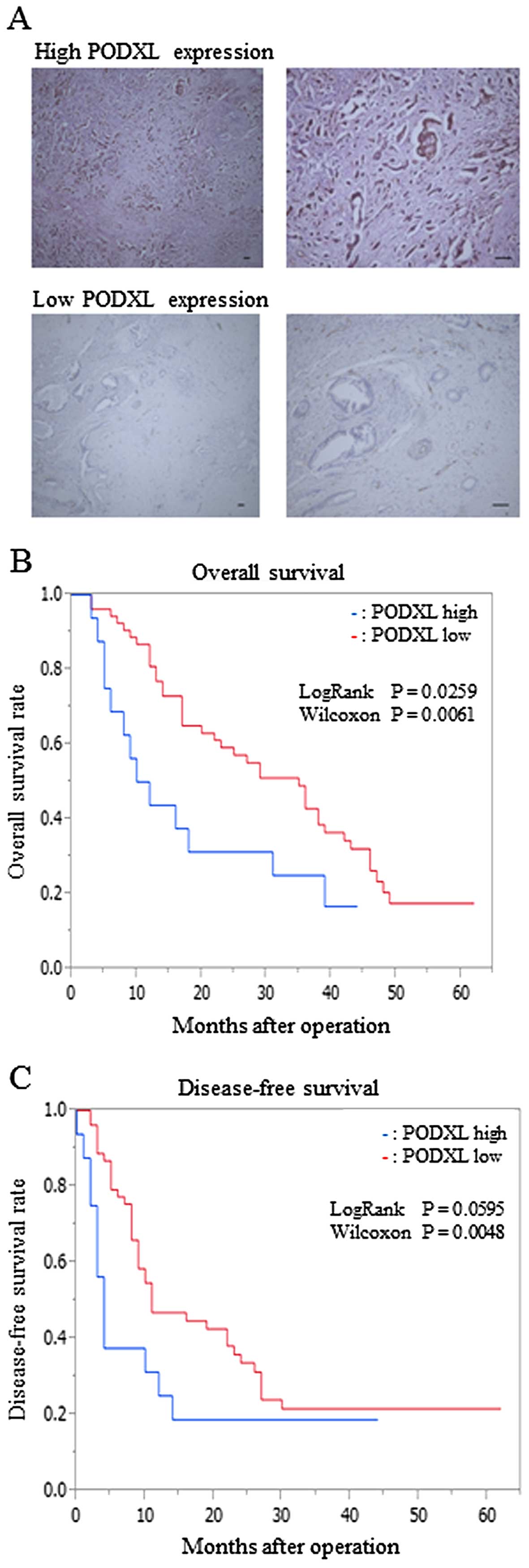
|
1
|
Singh D, Upadhyay G, Srivastava RK and
Shankar S: Recent advances in pancreatic cancer: Biology,
treatment, and prevention. Biochim Biophys Acta. 1856:13–27.
2015.PubMed/NCBI
|
|
2
|
Mirus JE, Zhang Y, Li CI, Lokshin AE,
Prentice RL, Hingorani SR and Lampe PD: Cross-species antibody
microarray interrogation identifies a 3-protein panel of plasma
biomarkers for early diagnosis of pancreas cancer. Clin Cancer Res.
21:1764–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukushige S and Horii A: Road to early
detection of pancreatic cancer: Attempts to utilize epigenetic
biomarkers. Cancer Lett. 342:231–237. 2014. View Article : Google Scholar
|
|
4
|
Güngör C, Hofmann BT, Wolters-Eisfeld G
and Bockhorn M: Pancreatic cancer. Br J Pharmacol. 171:849–858.
2014. View Article : Google Scholar :
|
|
5
|
Collins A and Bloomston M: Diagnosis and
management of pancreatic cancer. Minerva Gastroenterol Dietol.
55:445–454. 2009.PubMed/NCBI
|
|
6
|
Michl P and Gress TM: Current concepts and
novel targets in advanced pancreatic cancer. Gut. 62:317–326. 2013.
View Article : Google Scholar
|
|
7
|
Sweeney AD, Fisher WE, Wu MF, Hilsenbeck
SG and Brunicardi FC: Value of pancreatic resection for cancer
meta-static to the pancreas. J Surg Res. 160:268–276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werner J, Combs SE, Springfeld C, Hartwig
W, Hackert T and Büchler MW: Advanced-stage pancreatic cancer:
Therapy options. Nat Rev Clin Oncol. 10:323–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khoja L, Backen A, Sloane R, Menasce L,
Ryder D, Krebs M, Board R, Clack G, Hughes A, Blackhall F, et al: A
pilot study to explore circulating tumour cells in pancreatic
cancer as a novel biomarker. Br J Cancer. 106:508–516. 2012.
View Article : Google Scholar :
|
|
10
|
Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J,
Cen P, Xu J, Liu C, Long J, et al: A preoperative serum signature
of CEA+/CA125+/CA19-9 ≥1000 U/mL indicates
poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer.
136:2216–2227. 2015. View Article : Google Scholar
|
|
11
|
Sergeant G, van Eijsden R, Roskams T, Van
Duppen V and Topal B: Pancreatic cancer circulating tumour cells
express a cell motility gene signature that predicts survival after
surgery. BMC Cancer. 12:5272012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
13
|
Chen X, Liang H, Zhang J, Zen K and Zhang
CY: Secreted microRNAs: A new form of intercellular communication.
Trends Cell Biol. 22:125–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amirkhah R, Schmitz U, Linnebacher M,
Wolkenhauer O and Farazmand A: MicroRNA-mRNA interactions in
colorectal cancer and their role in tumor progression. Genes
Chromosomes Cancer. 54:129–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reddy KB: MicroRNA (miRNA) in cancer.
Cancer Cell Int. 15:382015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen J, Stass SA and Jiang F: MicroRNAs as
potential biomarkers in human solid tumors. Cancer Lett.
329:125–136. 2013. View Article : Google Scholar :
|
|
19
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
20
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weiss FU, Marques IJ, Woltering JM,
Vlecken DH, Aghdassi A, Partecke LI, Heidecke CD, Lerch MM and
Bagowski CP: Retinoic acid receptor antagonists inhibit miR-10a
expression and block metastatic behavior of pancreatic cancer.
Gastroenterology. 137:2136–2145.e1-7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mees ST, Mardin WA, Wendel C, Baeumer N,
Willscher E, Senninger N, Schleicher C, Colombo-Benkmann M and
Haier J: EP300 - a miRNA-regulated metastasis suppressor gene in
ductal adenocarcinomas of the pancreas. Int J Cancer. 126:114–124.
2010. View Article : Google Scholar
|
|
25
|
Kerjaschki D, Sharkey DJ and Farquhar MG:
Identification and characterization of podocalyxin - the major
sialoprotein of the renal glomerular epithelial cell. J Cell Biol.
98:1591–1596. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kershaw DB, Thomas PE, Wharram BL, Goyal
M, Wiggins JE, Whiteside CI and Wiggins RC: Molecular cloning,
expression, and characterization of podocalyxin-like protein 1 from
rabbit as a transmembrane protein of glomerular podocytes and
vascular endothelium. J Biol Chem. 270:29439–29446. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan JY and Watt SM: Adhesion receptors on
haematopoietic progenitor cells. Br J Haematol. 112:541–557. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horvat R, Hovorka A, Dekan G, Poczewski H
and Kerjaschki D: Endothelial cell membranes contain podocalyxin -
the major sialoprotein of visceral glomerular epithelial cells. J
Cell Biol. 102:484–491. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doyonnas R, Nielsen JS, Chelliah S, Drew
E, Hara T, Miyajima A and McNagny KM: Podocalyxin is a CD34-related
marker of murine hematopoietic stem cells and embryonic erythroid
cells. Blood. 105:4170–4178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miettinen A, Solin ML, Reivinen J, Juvonen
E, Väisänen R and Holthöfer H: Podocalyxin in rat platelets and
megakaryocytes. Am J Pathol. 154:813–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda T, Go WY, Orlando RA and Farquhar
MG: Expression of podocalyxin inhibits cell-cell adhesion and
modifies junctional properties in Madin-Darby canine kidney cells.
Mol Biol Cell. 11:3219–3232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dallas MR, Chen SH, Streppel MM, Sharma S,
Maitra A and Konstantopoulos K: Sialofucosylated podocalyxin is a
functional E- and L-selectin ligand expressed by metastatic
pancreatic cancer cells. Am J Physiol Cell Physiol. 303:C616–C624.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thomas SN, Schnaar RL and Konstantopoulos
K: Podocalyxin-like protein is an E-/L-selectin ligand on colon
carcinoma cells: Comparative biochemical properties of selectin
ligands in host and tumor cells. Am J Physiol Cell Physiol.
296:C505–C513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konstantopoulos K and Thomas SN: Cancer
cells in transit: The vascular interactions of tumor cells. Annu
Rev Biomed Eng. 11:177–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cipollone JA, Graves ML, Köbel M, Kalloger
SE, Poon T, Gilks CB, McNagny KM and Roskelley CD: The
anti-adhesive mucin podocalyxin may help initiate the
transperitoneal metastasis of high grade serous ovarian carcinoma.
Clin Exp Metastasis. 29:239–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Somasiri A, Nielsen JS, Makretsov N, McCoy
ML, Prentice L, Gilks CB, Chia SK, Gelmon KA, Kershaw DB, Huntsman
DG, et al: Overexpression of the anti-adhesin podocalyxin is an
independent predictor of breast cancer progression. Cancer Res.
64:5068–5073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forse CL, Yilmaz YE, Pinnaduwage D,
O'Malley FP, Mulligan AM, Bull SB and Andrulis IL: Elevated
expression of podocalyxin is associated with lymphatic invasion,
basal-like phenotype, and clinical outcome in axillary lymph
node-negative breast cancer. Breast Cancer Res Treat. 137:709–719.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaprio T, Fermér C, Hagström J, Mustonen
H, Böckelman C, Nilsson O and Haglund C: Podocalyxin is a marker of
poor prognosis in colorectal cancer. BMC Cancer. 14:4932014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Larsson A, Johansson ME, Wangefjord S,
Gaber A, Nodin B, Kucharzewska P, Welinder C, Belting M, Eberhard
J, Johnsson A, et al: Overexpression of podocalyxin-like protein is
an independent factor of poor prognosis in colorectal cancer. Br J
Cancer. 105:666–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saukkonen K, Hagström J, Mustonen H, Juuti
A, Nordling S, Fermér C, Nilsson O, Seppänen H and Haglund C:
Podocalyxin is a marker of poor prognosis in pancreatic ductal
adenocarcinoma. PLoS One. 10:e01290122015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Du P, Kibbe WA and Lin SM: lumi: A
pipeline for processing Illumina microarray. Bioinformatics.
24:1547–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligo-nucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quackenbush J: Microarray data
normalization and transformation. Nat Genet. 32(Suppl): 496–501.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khan S, Ansarullah, Kumar D, Jaggi M and
Chauhan SC: Targeting microRNAs in pancreatic cancer: Microplayers
in the big game. Cancer Res. 73:6541–6547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh S, Chitkara D, Kumar V, Behrman SW
and Mahato RI: miRNA profiling in pancreatic cancer and restoration
of chemo-sensitivity. Cancer Lett. 334:211–220. 2013. View Article : Google Scholar
|
|
48
|
Shi S, Lu Y, Qin Y, Li W, Cheng H, Xu Y,
Xu J, Long J, Liu L, Liu C, et al: miR-1247 is correlated with
prognosis of pancreatic cancer and inhibits cell proliferation by
targeting neuropilins. Curr Mol Med. 14:316–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ben Q, Zheng J, Fei J, An W, Li P, Li Z
and Yuan Y: High neuropilin 1 expression was associated with
angiogenesis and poor overall survival in resected pancreatic
ductal adenocarcinoma. Pancreas. 43:744–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao Y, Hoeppner LH, Bach S, EG, Guo Y,
Wang E, Wu J, Cowley MJ, Chang DK, Waddell N, et al: Neuropilin-2
promotes extravasation and metastasis by interacting with
endothelial α5 integrin. Cancer Res. 73:4579–4590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shen J, Wan R, Hu G, Yang L, Xiong J, Wang
F, Shen J, He S, Guo X, Ni J, et al: miR-15b and miR-16 induce the
apoptosis of rat activated pancreatic stellate cells by targeting
Bcl-2 in vitro. Pancreatology. 12:91–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Batchu RB, Gruzdyn OV, Qazi AM, Kaur J,
Mahmud EM, Weaver DW and Gruber SA: Enhanced phosphorylation of p53
by microRNA-26a leading to growth inhibition of pancreatic cancer.
Surgery. 158:981–986; discussion 986–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ali S, Ahmad A, Aboukameel A, Bao B,
Padhye S, Philip PA and Sarkar FH: Increased Ras GTPase activity is
regulated by miRNAs that can be attenuated by CDF treatment in
pancreatic cancer cells. Cancer Lett. 319:173–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bera A, Venkata Subba Rao K, Manoharan MS,
Hill P and Freeman JW: A miRNA signature of chemoresistant
mesenchymal phenotype identifies novel molecular targets associated
with advanced pancreatic cancer. PLoS One. 9:e1063432014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang H, Jiang Y, Wang Y, Chen T, Yang L,
He H, Lin Z, Liu T, Yang T, Kamp DW, et al: miR-5100 promotes tumor
growth in lung cancer by targeting Rab6. Cancer Lett. 362:15–24.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Snyder KA, Hughes MR, Hedberg B, Brandon
J, Hernaez DC, Bergqvist P, Cruz F, Po K, Graves ML, Turvey ME, et
al: Podocalyxin enhances breast tumor growth and metastasis and is
a target for monoclonal antibody therapy. Breast Cancer Res.
17:462015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sizemore S, Cicek M, Sizemore N, Ng KP and
Casey G: Podocalyxin increases the aggressive phenotype of breast
and prostate cancer cells in vitro through its interaction with
ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin CW, Sun MS and Wu HC: Podocalyxin-like
1 is associated with tumor aggressiveness and metastatic gene
expression in human oral squamous cell carcinoma. Int J Oncol.
45:710–718. 2014.PubMed/NCBI
|
|
59
|
Wu H, Yang L, Liao D, Chen Y, Wang W and
Fang J: Podocalyxin regulates astrocytoma cell invasion and
survival against temozolomide. Exp Ther Med. 5:1025–1029.
2013.PubMed/NCBI
|
|
60
|
Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT,
Chen CL, Wu YY, Chen JY, Chen TH and Jou TS: Podocalyxin EBP50
ezrin molecular complex enhances the metastatic potential of renal
cell carcinoma through recruiting Rac1 guanine nucleotide exchange
factor ARHGEF7. Am J Pathol. 176:3050–3061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin CW, Sun MS, Liao MY, Chung CH, Chi YH,
Chiou LT, Yu J, Lou KL and Wu HC: Podocalyxin-like 1 promotes
invadopodia formation and metastasis through activation of
Rac1/Cdc42/cortactin signaling in breast cancer cells.
Carcinogenesis. 35:2425–2435. 2014. View Article : Google Scholar : PubMed/NCBI
|