Introduction
Agaricus blazei (A. blazei), an edible
mushroom belonging to the Agaricaceae family, has been
traditionally used as a health food supplement for the prevention
of cancer, diabetes, hyperlipidemia, arteriosclerosis, and chronic
hepatitis (1). In recent years,
the mushroom has been used as an immunity-stimulating adjuvant in
cancer chemotherapy. One of the anticancer substances was
postulated to be the β-glucan fraction (2); orally administered β-glucan extracted
from A. blazei results in tumor regression in tumor-bearing
mice (3). Some other compounds of
A. blazei, such as ergosterol (lipid fraction) and
blazeispirols (skeletal compounds), have antitumor effects due to
their anti-angiogenic activities (4,5). In
addition, recent studies have found that β-glucan from A.
blazei inhibits tumor growth not only by accelerating immune
activity but also via direct antitumor activities. An extract from
the fruiting bodies of A. blazei has been reported to induce
apoptosis in different cancer cell lines, including leukemia,
uterine cervical carcinoma, melanoma, and breast adenocarcinoma
cells (6). However, the apoptotic
mechanism in cancer cells induced by the tumoricidal components
from A. blazei is poorly understood. In this study, we show
that A. blazei extracts induced apoptosis in p53-wild-type
cell lines and p53-mutant cell lines. We further purified the
tumoricidal substance from A. blazei, an ergosterol
derivative, and named it ‘Agarol’.
For several decades, apoptosis has been considered
to be the principal mechanism of programmed cell death in mammalian
cells. Because one of the main objectives of traditional cancer
therapy is to enhance cancer cell apoptosis, there have been many
studies showing chemotherapeutic agents, including natural
compounds, inducing apoptosis by different apoptotic pathways in
cancer cells (7). Although
caspases were identified as the enzymes that orchestrate apoptotic
cell death, it soon became apparent that inhibition of caspase
activity may not necessarily preserve cell survival, even with the
processes of apoptosis effectively blocked (8). Indeed, numerous reports described
that complete caspase inhibition is not able to prevent cell death
in vitro or in vivo (9). Interestingly, a study has shown that
blazeispirol A, isolated from A. blazei, decreases the
viability of hepatoma Hep 3B cells, which is not blocked by the
pan-caspase inhibitor z-VAD-fmk. Accordingly, this form of cell
death has been called caspase-independent cell death to distinguish
it from the caspase-dependent apoptotic pathway. In general, many
cancer cells have defects in caspase signaling, which allow cancer
cells to become resistant to traditional chemotherapy drugs,
resulting in a major limitation of cancer treatment. Therefore, the
caspase-independent cell death pathway has become an attractive
alternative approach for eradicating tumor cells. Mitochondria are
central participants in apoptosis and play a direct role in cell
death signaling through the well-characterized mitochondrial
pathway, which results in the release of apoptogenic factors into
the cytosol. Apoptosis-inducing factor (AIF), a mitochondrial
protein, induces large-scale DNA fragmentation after nuclear
translocation in the caspase-independent apoptotic pathway
(10).
Numerous experimental and epidemiological studies
have shown that several plant-derived natural products may serve as
effective anticancer drugs. Betulinic acid (BetA) has comparatively
related structure to ergosterol and belongs to the group of
terpenes (11). BetA is found in
the bark of white birch trees, and inhibits numerous carcinoma cell
lines including lung, colon, liver, pancreatic, breast, ovarian,
head and neck, and renal cell lines. p53 is a well-known tumor
suppressor that plays a master role in the prevention of tumors by
regulating apoptosis. Mutations in p53 have been found in ≤50% of
all human cancers and cause an increase in oncogenic phenotypes
such as proliferation and tumorigenicity. BetA induces apoptosis
independent of wild-type p53 protein, and has a direct effect on
mitochondria, resulting in the release of soluble apoptogenic
factors (12). Although BetA was
reported to have less of a cytotoxic effect on normal cells, BetA
induced eryptosis/erythroptosis in human erythrocytes via
Ca2+ loading and membrane permeabilization.
In this study, we demonstrated that a novel
tumoricidal substance from A. blazei, Agarol, induced
apoptosis in cancer cells via differential cytotoxicity based on
the p53 status, increased reactive oxygen species (ROS) generation,
and decreased mitochondrial membrane potential (ΔΨm), which is
involved in the apoptotic pathway. In addition, treatment of cancer
cells with Agarol induced only a slight increase in caspase-3
activity, and z-VAD-fmk did not inhibit this Agarol-induced
apoptosis in A549 cells. We also observed that AIF plays an
important role in Agarol-induced apoptosis signaling in A549 cancer
cells. Further understanding of the mechanisms underlying
Agarol-induced apoptosis may reveal novel therapeutic avenues for
cancer treatment.
Materials and methods
Isolation of Agarol from A. blazei
The fruiting bodies of A. blazei were
cultivated by the Hokuto Corp. (Nagano, Japan), and 10 kg was
extracted with ethanol at room temperature for 2 days. The ethanol
extracts were fractionated by solvent partition between ethyl
acetate and water to yield an ethyl acetate soluble fraction. The
ethyl acetate extracts (20.73 g) were applied to a silica gel
column, and eluted with n-hexane-ethyl acetate-methanol. An active
fraction (250 mg), eluted with n-hexane-ethyl acetate (1:3), was
further applied to an ODS column, and eluted with
water-acetonitrile-ethyl acetate. The acetonitrile-ethyl acetate
(1:1) eluent (24.4 mg) was next applied to a silica gel column, and
eluted with chloroform-methanol, resulting in 2.6 mg of Agarol. The
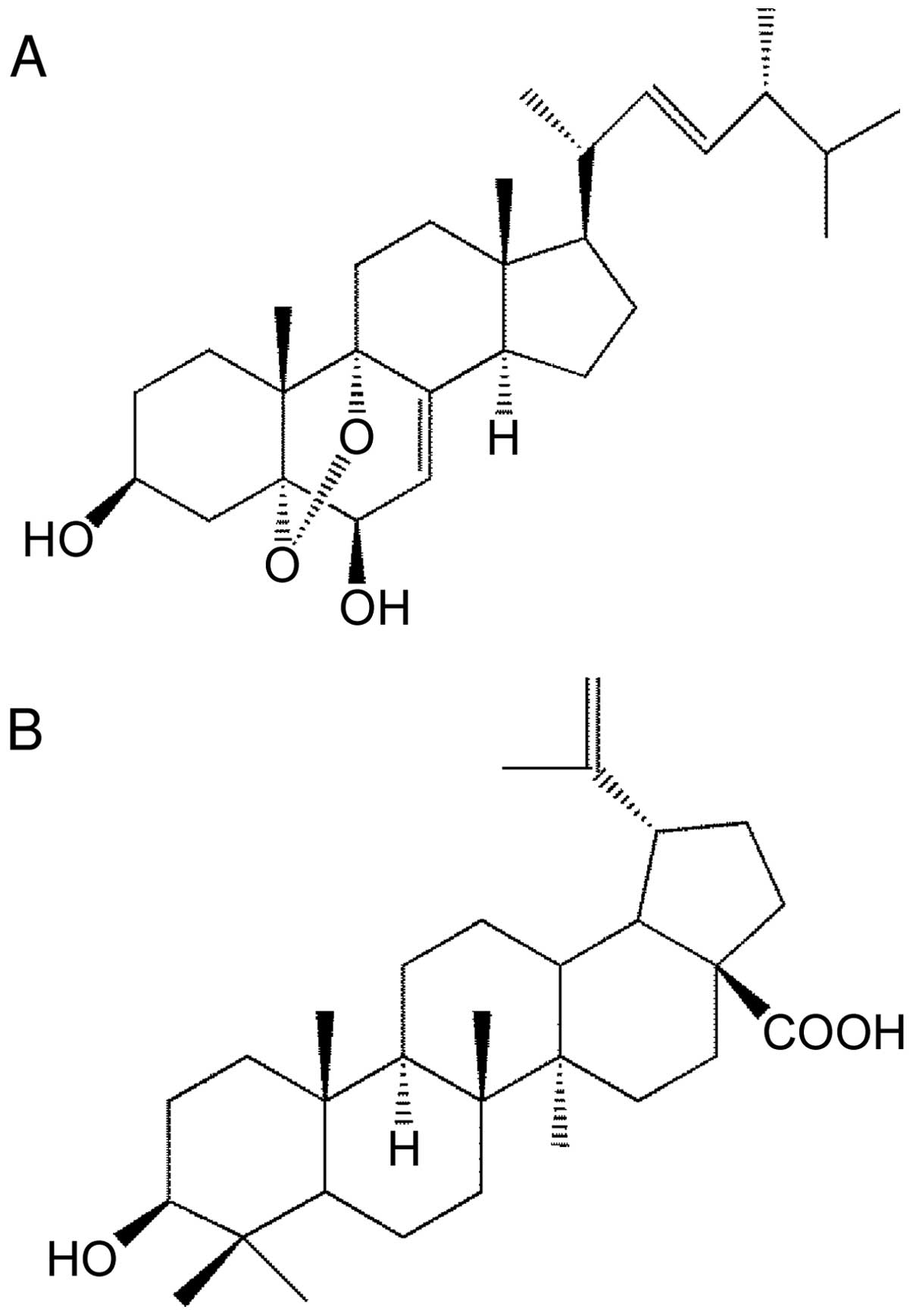
structure of the active compound was identified by its
1H and 13C NMR spectra. Its chemical
structure is 5α,9α-epidioxy-(22E)-ergosta-7,22-diene-3β,6β-diol,
which is comparatively similar to BetA (Fig. 1).
Cell cultures and reagents
HSC-3 and HSC-4 cells (mutant-type p53, human oral
squamous cell carcinoma), and A549 cells (wild-type p53, human
non-small cell lung adenocarcinoma) were obtained from the RIKEN
Cell Bank (Tsukuba, Japan), and MKN45 cells (wild-type p53, human
stomach adenocarcinoma) were obtained from the Japanese Cancer
Research Resources Bank. HNG-1 (normal human fibro-blast cells),
derived from gingiva, was isolated. Cells were cultured in
RPMI-1640 (Nissui Pharmaceutical, Tokyo, Japan) medium supplemented
with 10% (v/v) heat-inactivated fetal bovine serum, 100 IU/ml
penicillin (Invitrogen, Carlsbad, CA, USA), and 100 μg/ml
streptomycin (Invitrogen). Cells were maintained in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. Pan-caspase
inhibitor z-VAD-fmk (MBL, Nagoya, Japan), p53 inhibitor pifithrin-α
(PFT-α) (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and
free radical scavenger N-acetyl-L-cysteine (NAC) (Calbiochem, La
Jolla, CA, USA) were purchased. Betulinic acid (Enzo Life Science,
NY, USA) was provided as pure substance and dissolved in dimethyl
sulfoxide. All inhibitors used in this study were incubated with
cells for 1 h, followed by each indicated treatment without
washing.
Cytotoxicity assays
The cytotoxic effects of Agarol were evaluated using
an MTT (WST-8) colorimetric assay kit (Dojindo, Kumamoto, Japan).
Apoptotic cells were assayed by the TUNEL method using the Mebstain
apoptosis kit direct (MBL) for flow cytometric analysis
(FACSCalibur; Becton-Dickinson, San Jose, CA, USA).
Caspase-3 assay
Caspase-3 activity was measured using a
CPP32/caspase-3 colorimetric protease assay kit (MBL). Absorbance
of extracts from cells treated with Agarol was measured at 405 nm
in a microplate reader.
Western blotting
SDS-PAGE and western blots for whole or nuclear
proteins were performed according to standard procedures.
Immunodetection was performed with HRP-conjugated secondary
antibodies and visualized with the chemiluminescence detection
method. Primary antibodies were anti-Bcl-2 (BD Biosciences, San
Jose, CA, USA), Bax (Cell Signaling Technology, Inc., Danvers, MA,
USA), PARP (Cell Signaling Technology), AIF (Cell Signaling),
β-actin (Funakoshi, Tokyo, Japan), and Lamin A (Biolegend, San
Diego, CA, USA). Secondary antibodies were HRP-conjugated sheep
anti-mouse IgG (GE Healthcare, Piscataway, NJ, USA) and goat
anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA,
USA).
siRNA transfection
Cells were transiently transfected with either 40 nM
negative control small interfering (si)RNA (Bioneer, Deajeon,
Korea) or 10 nM AIF siRNA (Santa Cruz Biotechnology) at 80%
confluence using Lipofectamine 2000 Transfection Reagent
(Invitrogen).
Measurement of ROS and ΔΨm
Intercellular ROS production was measured using ROS
detection reagents (Invitrogen). Cells were treated with Agarol for
4 h. After incubation, cells were exposed to
Carboxy-H2DCFDA (C400) for 30 min at 37°C, harvested,
and then analyzed using FACSCalibur. Similarly, ΔΨm was measured
using the JC-1 Mitochondrial Membrane Potential Assay kit (Cayman
Chemical Co., MI, USA).
In vivo tumor study of Agarol
Male C57BL/6 mice, 7-week-old mice, were used to
confirm the toxicity of Agarol. A series of Agarol doses in PBS
were tested. Mice were injected i.p. with 10 or 100 mg/kg/day of
Agarol every 3 days for 3 weeks. The drug-free PBS were
administered as vehicle controls. Mice were monitored and weighed
every 3 days during the course of 3 weeks. Toxicity was assessed as
a percent of weight loss. The values presented are the mean ± SE
(n=3 in each group). The animal protocol was approved by the
Institute for Animal Experimentation, Tohoku University Graduate
School of Medicine (no. 2014shidou-018).
Female severe combined immunodeficiency (SCID) mice,
5-week-old mice, were used to in vivo tumor modeling study
of Agarol. A549 cells (1×107 cells/mouse) suspended in
PBS were injected subcutaneously into the right flank of the mice.
After 2 weeks, when the size of solid tumor in tumor-bearing SCID
mice reached 150–200 mm3, the tumor-bearing SCID mice
were treated with Agarol or BetA via i.p. administration at the
dosage of 0, 10, 30 and 100 mg/kg/day with Agarol or 100 mg/kg/day
with BetA every 3 days for 3 weeks. After 3 weeks, the tumors were
resected and measured. The values presented are the mean ± SE (n=10
in each group). The animal protocol was approved by the Ethics
Committee of KAC Corporation (no. 14-0921).
In situ detection of apoptosis by the
TUNEL assay
Xenograft tumors were resected and fixed in 10%
formalin neutral buffer solution (Wako Pure Chemical Industries),
and embedded in paraffin and 5-micron sections. Apoptotic tumor
cells were determined by the TUNEL method using the Apoptotic In
Situ Detection kit Wako (Wako Pure Chemical Industries) according
to the manufacturer's instructions.
Statistical analysis
Data are given as the mean ± SE. When required,
multiple comparisons were made by Scheffe's test. P-values <0.05
were considered as statistically significant.
Results
Cytotoxic effects of Agarol in human
cancer cell lines
The structure of our novel ergosterol derivative,
Agarol, is shown in Fig. 1A, and
the chemical structure of plant-derived BetA, which has been shown
to induce cell death in various cancer cell lines, is shown in
Fig. 1B. We studied the cytotoxic
effects of Agarol in comparison with BetA for anticancer activity
of both compounds. We first examined human cancer cell lines with
either wild-type or mutant p53 for their sensitivity to Agarol
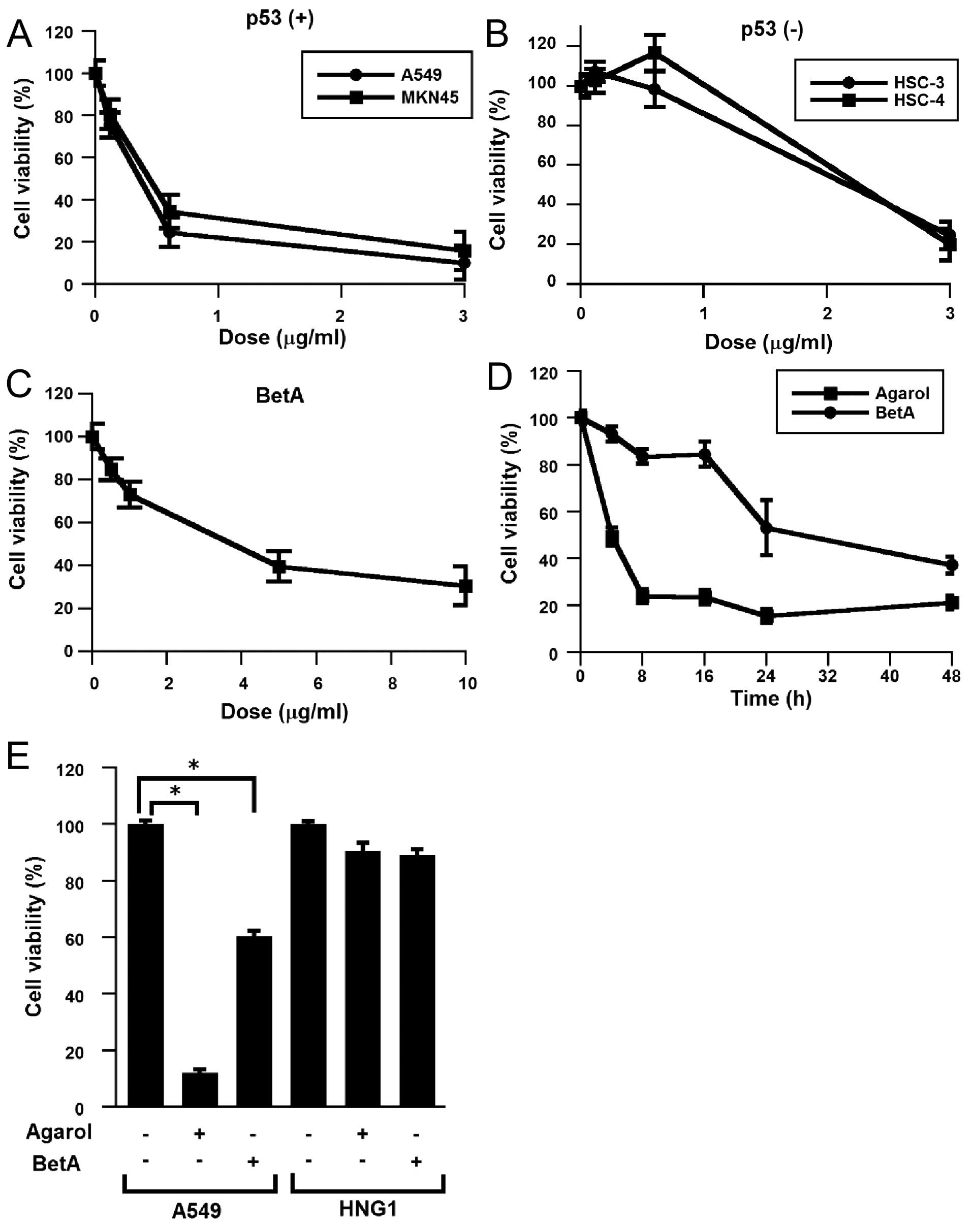
using MTT cytotoxicity assay. Each cell line was treated with
Agarol (0–3 μg/ml) for 24 h (Fig. 2A
and B). Agarol treatment decreased the viability of cells with
wild-type p53 (A549 and MKN45) in a dose-dependent analysis of cell
viability, while higher overall decrease of cell viability was
observed with high doses of Agarol in the cell lines with mutant
p53 (HSC-3 and HSC-4). IC50 values (24 h) were ~0.26
μg/ml (A549), 0.34 μg/ml (MKN45), 1.72 μg/ml (HSC-3), and 1.94
μg/ml (HSC-4). Exposure of BetA (0–10 μg/ml) to A549 cells also
triggered cell death, as indicated by a dose-dependent analysis of
cell viability (Fig. 2C). In
addition, to detect the growth inhibition of Agarol (1 μg/ml)- and
BetA (10 μg/ml)-exposed A549 cells, the cells were treated for
various times (0–48 h). Agarol showed more potent suppressive
effect than BetA (Fig. 2D). Cell
viability of normal fibroblast cells (HNG-1) was not reduced by
Agarol, similar to BetA-treated cells (Fig. 2E).
Apoptosis induced by Agarol in A549
cells
To determine whether the Agarol-induced cell death
of A549 cells showed apoptotic characteristics, we analyzed the
induction of nucleosome fragmentation by TUNEL staining. As shown
in Fig. 3A and B, Agarol (1 μg/ml)
treatment for 24 h induced apoptosis in A549 cells. In addition, we
investigated possible pathways responsible for the apoptotic
effects of Agarol, and examined the activation of caspase-3 in
Agarol- or BetA-treated A549 cells. Agarol induction was
undetectable in caspase-3 activity (Fig. 3C); and, a pan-caspase inhibitor,
z-VAD-fmk, did not inhibit the Agarol-induced apoptosis in treated
cancer cells (Fig. 3D). In
contrast, BetA induced a significant increase in caspase-3 activity
for 24 and 48 h in A549 cells, which was clearly inhibited by
z-VAD-fmk. These data indicate that Agarol induced
caspase-independent apoptosis in A549 cells.
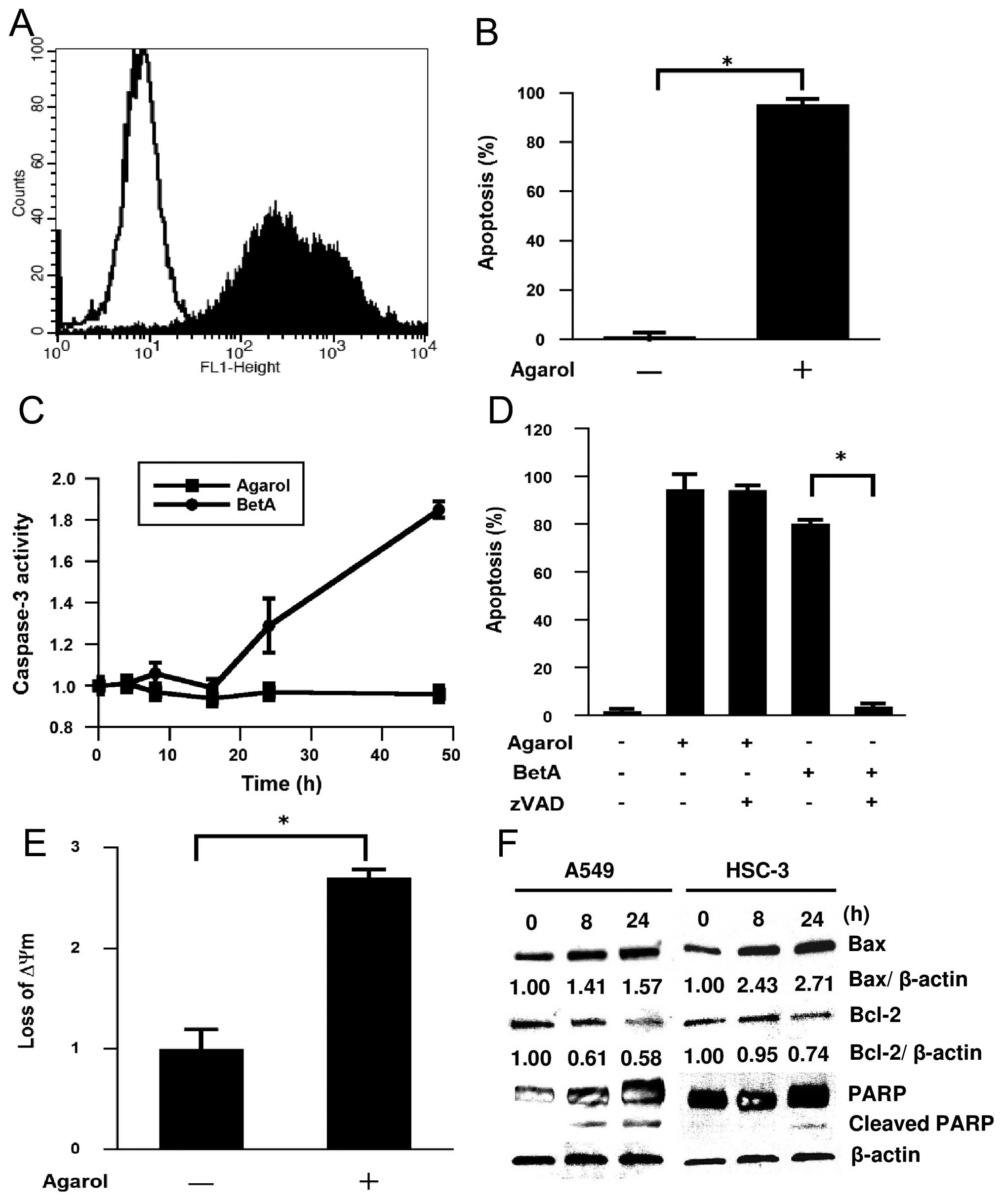 | Figure 3Induction of apoptosis by Agarol in
A549 cells. (A) Cells were treated with Agarol (1 μg/ml) for 24 h,
stained with the TUNEL method and evaluated by cytometric analysis.
Flow cytometric histograms depict apoptotic cells in untreated
control (unshaded) or Agarol-treated (shaded) cells. (B) The
percentage of apoptotic cells in triplicate was expressed (mean
value ± SE) in each group. (C) Cells were treated with Agarol (1
μg/ml) or BetA (10 μg/ml) for 48 h, and cytosolic cell lysates were
prepared at the indicated times and assayed for DEVDase activity.
Results in triplicate represent the ratio (mean value ± SE) to the
untreated control. (D) Cells were pretreated with z-VAD-fmk (20 μM)
for 1 h and then treated with Agarol or BetA for 24 h. The
percentage of apoptotic cells was determined by TUNEL staining. (E)
Cells were treated with Agarol for 4 h, incubated with JC-1 for 20
min, and then subjected to flow cytometric analysis. Results in
triplicate represent the ratio (mean value ± SE) to the untreated
control. (F) A549 cells and HSC-3 cells were treated with Agarol
(A549 cells, 1 μg/ml, HSC-3 cells, 3 μg/ml) for 0–24 h, and then
the cell lysates were harvested. Equal amounts of protein from each
sample were loaded for western blotting for determination of Bcl-2,
Bax and PARP protein expression. After immunoblotting, the film was
scanned and the magnitude of signals were quantified. β-actin was
used as a loading control. *P<0.05. |
Mitochondrial dysfunction induced by
Agarol in A549 cells
Mitochondrial dysfunction, including the loss of
ΔΨm, permeability transition, and release of apoptosis-related
factors from the mitochondria into the cytosol or nucleus, is
frequently associated with chemotherapy-induced apoptosis. Because
BetA is thought to have a direct effect on the mitochondria, we
treated A549 cells with Agarol for 4 h and observed a significant
change of ΔΨm. JC-1 staining revealed significant loss of
mitochondrial ΔΨm in the treated group (Fig. 3E). Bcl-2 and Bax have been shown to
be important regulators in the mitochondrial apoptotic pathway.
Next, the effect of Agarol on expression of Bcl-2 family proteins
was investigated in A549 and HSC-3 cells. The cytosol of
Agarol-treated cells was collected at the indicated time-points,
and expression of Bcl-2 and Bax proteins was determined by western
blot analysis. As shown in Fig.
3F, the level of Bcl-2 decreased after treatment, while Agarol
treatment increased the protein expression of Bax in a
time-dependent manner. In addition, treatment with Agarol resulted
in a time-dependent generation of cleaved PARP in A549 cells.
Agarol-induced apoptosis through
AIF-dependent pathways
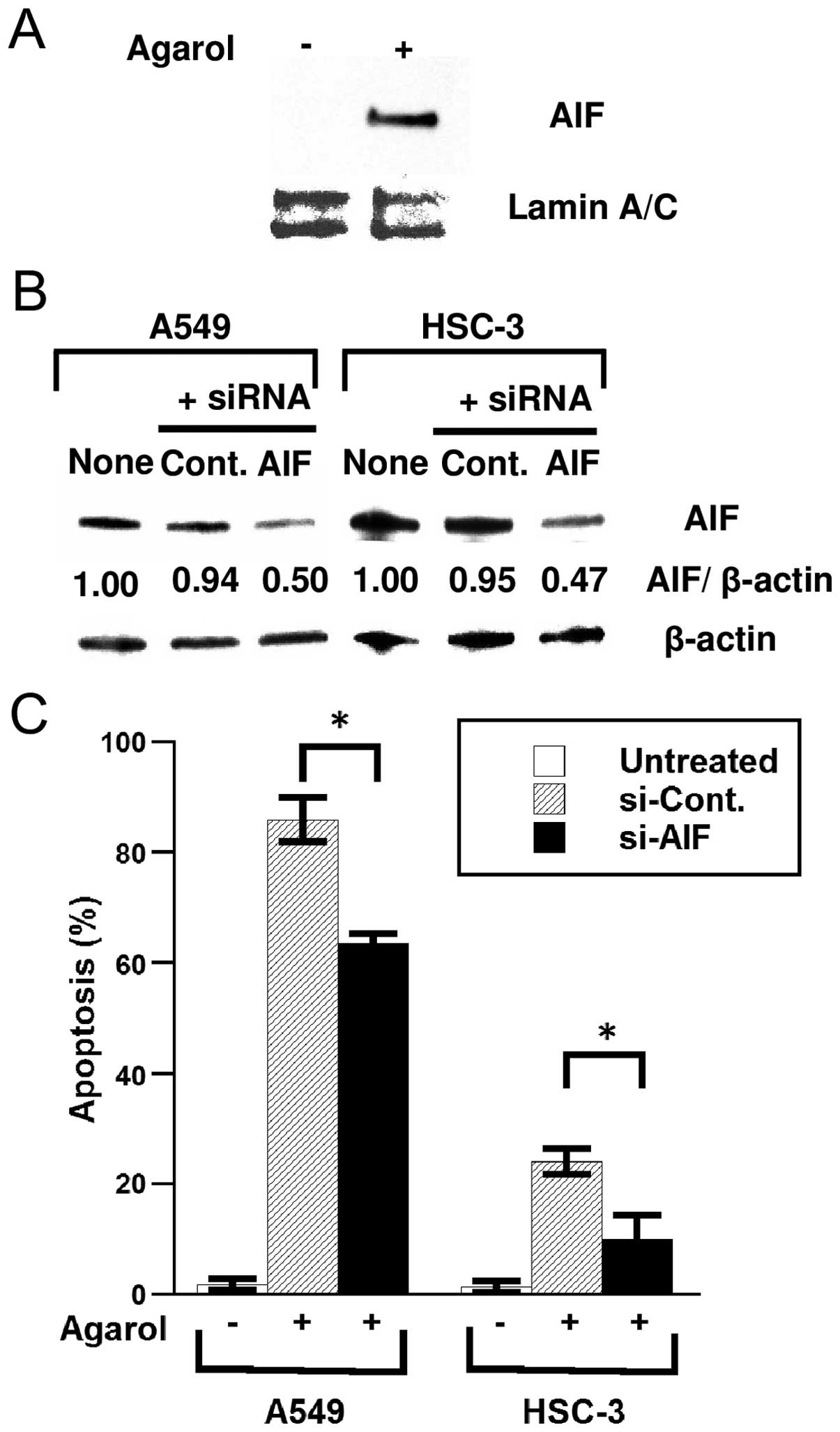
Mitochondria damage results in the release of
pro-apoptotic protein (for example, AIF), which triggers
caspase-independent cell death. To determine whether Agarol induces
the translocation of AIF from the mitochondria to the nucleus in
A549 cells, cells were treated with 1 μg/ml of Agarol for 8 h and
analyzed for AIF protein expression in nuclear fractions. Enhanced
nuclear translocation of AIF was evident after treatment (Fig. 4A). In A549 and HSC-3 cells
transfected with AIF-specific siRNA, knockdown of the AIF gene
effectively reduced the cellular level of AIF protein (Fig. 4B). Furthermore, siRNA transfection
significantly reduced Agarol-induced apoptosis in cells (Fig. 4C), suggesting that Agarol-induced
caspase-independent apoptosis is a result of the release of AIF
from the mitochondria to the nucleus in A549 and HSC-3 cells.
Involvement of Agarol-induced ROS
generation in the p53 pathway
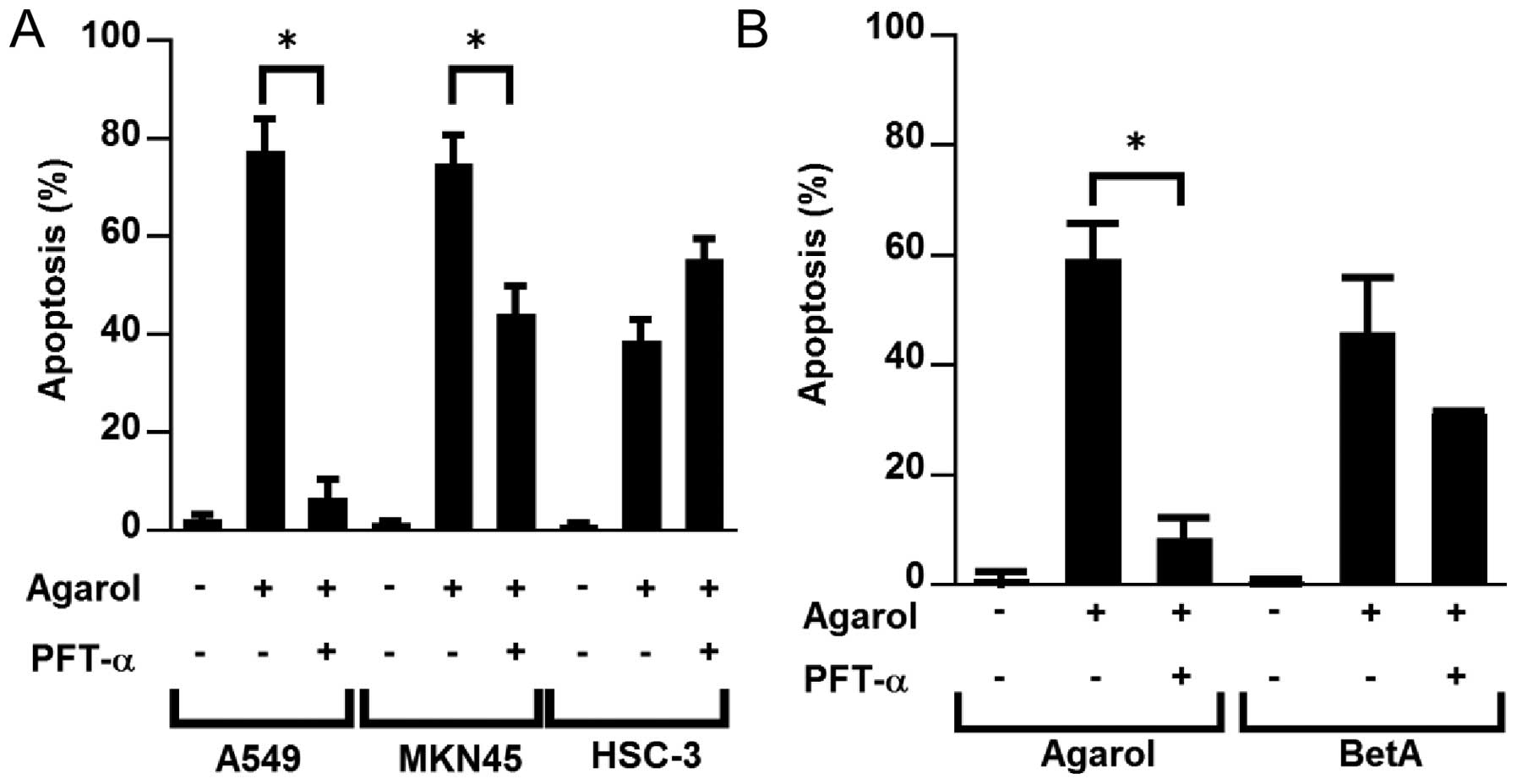
We found that wild-type p53 cells were highly
sensitive to apoptosis induced by Agarol (Fig. 2A). We examined the effect of
pifithrin-α on Agarol-treated wild-type (A549 and MKN45) and mutant
p53 cell lines (HSC-3) to confirm the possible role of p53 in
Agarol-induced apoptosis. Exposure to pifithrin-α (PFT-α, 50 μM)
for 8 h clearly reduced Agarol-induced apoptosis in wild-type p53
cells (A549 and MKN45) (Fig. 5A).
In BetA-treated A549 cells a significant effect of PFT-α was not
observed (Fig. 5B).
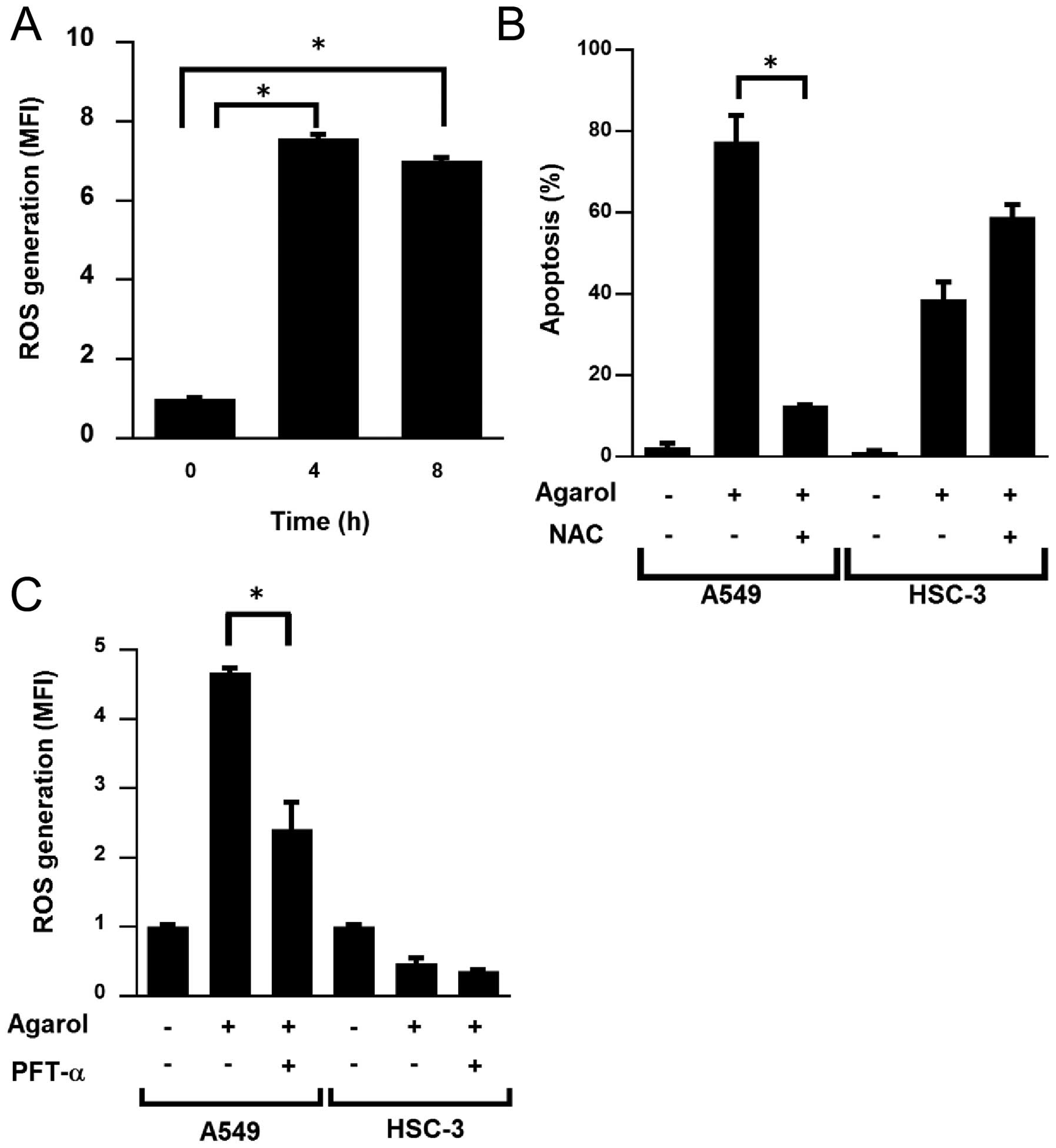
We investigated whether Agarol-induced apoptosis is
involved in ROS generation in A549 cells. Cells were examined for
evidence of oxidative stress using a peroxide-DCF fluorescence
assay. ROS accumulation was observed at 4 and 8 h of treatment with
Agarol in A549 cells (Fig. 6A). In
A549 and HSC-3 cells pretreated for 1 h with 5 mM NAC, a ROS
scavenger, followed by Agarol treatment, NAC reduced Agarol-induced
apoptosis in A549 cells, but not in HSC-3 cells (Fig. 6B). Because p53 influences
mitochondrial ROS generation, we investigated whether the
Agarol-induced ROS generation was associated with the p53 pathway
in A549 cells by pretreating cells for 1 h with PFT-α, and observed
that the Agarol-induced ROS generation was significantly decreased
in A549 cells, but not in HSC-3 cells (Fig. 6C). These results indicated that ROS
generation is involved in Agarol-induced apoptosis, and the p53
pathway is involved upstream of this activity in A549 cells.
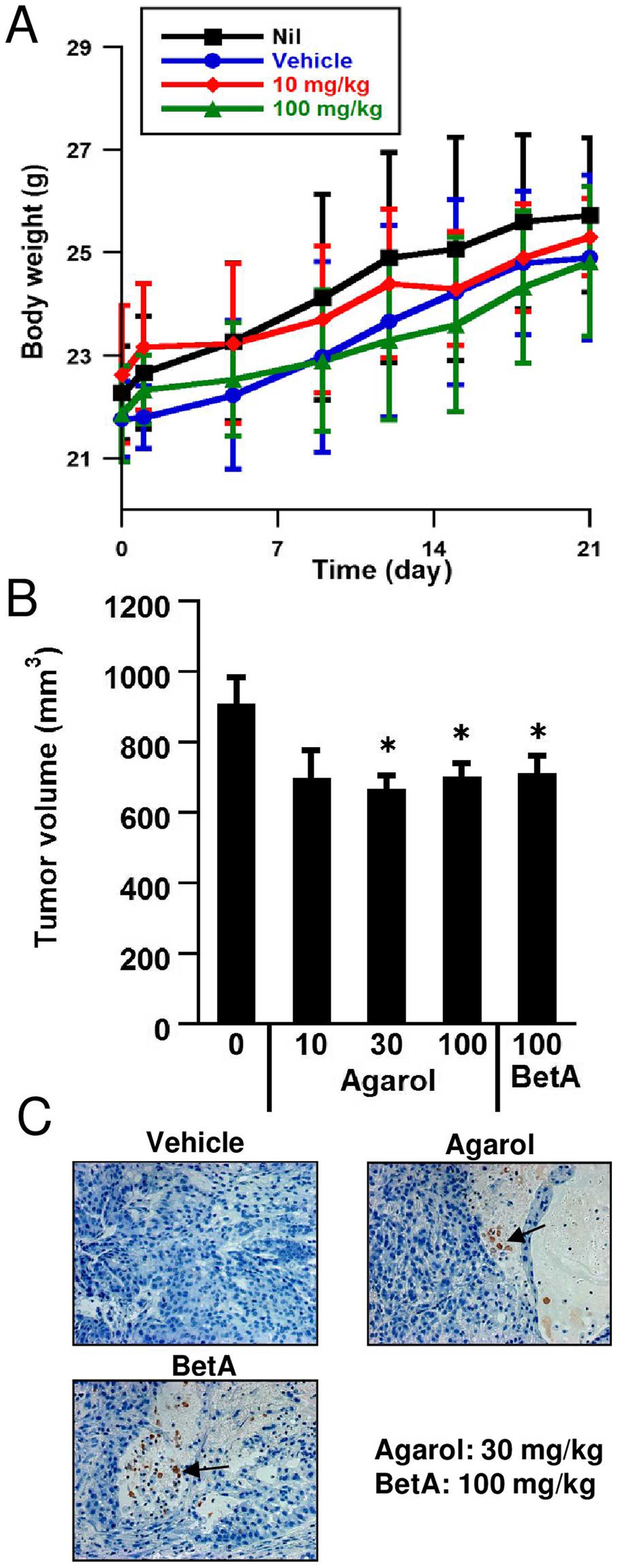
Anticancer effects of Agarol in a mouse
xenograft model with A549
When Agarol was tested for in vivo activity
of C57BL/6 mice, the top dosage employed of 100 mg/kg/day every 3
days i.p. administration for 3 weeks exhibited only weak toxicity
at reducing body weight (Fig. 7A).
To evaluate the mono-therapeutic efficacy of Agarol against human
cancer in vivo, a SCID model was adopted. Fig. 7B demonstrates that single-agent
Agarol substantially inhibited A549 human lung adenocarcinoma
growth as compared with vehicle control mice (P<0.05). The
treatment with Agarol delayed the growth of the cancer in xenograft
mice, but did not cause complete remissions. As seen in Fig. 7C, treatment with Agarol (30
mg/kg/day) or BetA (100 mg/kg/day) every 3 days for 3 weeks, showed
apoptotic features (arrow) in tumor-bearing SCID mice.
Discussion
The well-known component of A. blazei,
β-glucan (β1–3, β1–6 linked), inhibits the growth of sarcoma in
mice and improves the quality of life via elevating immune activity
in cancer patients (13). In
addition, the polysaccharide-protein complex also shows antitumor
activities due to immunological host-mediated mechanisms (14). There is some evidence that the
β-glucan from A. blazei inhibits tumor growth through its
direct antitumor activity against aberrantly activated signaling
pathways in cancer cells (15).
Most studies concerning the mechanisms of β-glucans have
demonstrated that the major antitumor activity of A. blazei
extracts may be the induction of apoptosis and cell cycle arrest.
Treatment with β-D-glucan extracted from A. blazei directly
stimulates apoptotic signaling in HRA ovarian cancer cells
(16). Proteoglycans from A.
blazei induce cell cycle arrest and apoptosis in gastric cancer
cells and leukemia U937 cells (17). Among other compounds found in A.
blazei, it was demonstrated that blazeispirol A, which is the
active anti-hepatoma compound in an ethanolic extract of an A.
blazei fermentation product, decreases the viability of
hepatoma Hep 3B cells by inducing both caspase-dependent and
caspase-independent cell death (5). The pro-vitamin D2, ergosterol, is
abundant in mushrooms and has been shown to have chemoprevention
activity. Ergosterol peroxide attenuates the growth of prostate
cells triggering an apoptotic process. However, detailed research
of the antitumor components isolated using different extraction and
purification methods is lacking. In this study, we used a novel
naturally-occurring compound, Agarol, isolated from an extract of
A. blazei.
In this study, Agarol at concentrations of 3 μg/ml
and above significantly inhibited the growth and viability of human
tumor cells with different genetic backgrounds and status of p53.
The sensitivity of these cells to Agarol is variable; A549 and
MKN45 cells are sensitive to Agarol, whereas HSC-3 and HSC-4 cells
are moderately resistant (as shown by MTT cell viability assays in
Fig. 2A and B). The presence of
wild-type p53 in cancer cells facilitated apoptotic cell death in
response to Agarol treatment as determined by TUNEL staining,
whereas cancer cells with a mutated form of p53 appeared to some
degree resistant to Agarol-induced apoptosis. In contrast to
Agarol, it has been shown that BetA mediated tumor cell death in 3
different adherent human melanomas, irrespective of p53 status
(18). p53 regulates the
transcription of genes that play an important role in apoptosis.
Apoptosis can be induced by p53 through several pathways, one of
which involves the Bcl-2 family (19). Bax protein is a p53 target and
promotes the release of cytochrome c from the mitochondria.
On the other hand, anti-apoptotic proteins, such as Bcl-2, are
transcriptionally suppressed by p53, and suppress the release of
cytochrome c that activates the effectors of apoptosis. The
ethanolic extracts of A. blazei, which are enriched in
blazeispirols (A and C), upregulated expression of pro-apoptotic
Bax protein and downregulated expression of anti-apoptotic Bcl-2
protein in human hepatocellular carcinoma cell lines (20). It was shown that blazeispirol A (4
μg/ml) decreased Bcl-2 and Bcl-xL expression and increased Bax
expression in a time-dependent manner (5). Agarol-induced apoptosis may be, at
least in part, regulated by p53 function, via apoptotic promotion
by Bax and Bcl-2.
Some reports suggest a role for ROS as a potential
mediator of p53-dependent apoptosis. For example, excessive calcium
has been reported to induce permeabilization of the mitochondrial
outer membrane and, in turn, increase the release of mitochondrial
ROS, further contributing to apoptotic death (21). Agarol seems to increase the
activation of the apoptotic pathway in cancer cells by reducing the
mitochondrial transmembrane potential with the concomitant release
of ROS. Intracellular production of ROS may activate and modulate
apoptosis by regulation of p53 activity and accelerate
mitochondrial depolarization during the effector phase of apoptosis
(22). In turn, activation of p53
induces the production of ROS by activation or repression of genes
that regulate production of ROS during apoptosis (23). Apoptosis induced by water-soluble
proteoglycan is associated with the mitochondrial pathway, and is
mediated by ROS generation and prolonged JNK activation (24). BetA is known to be a
mitochondriotoxic drug and also induces ROS in RKO colon cancer
cells (25). MKN45 cells seem to
be able to activate distinct apoptotic pathways without dependence
on ROS release as an apoptotic signaling amplification mechanism
(data not shown).
Although caspase-dependent apoptosis is the main
pathway of cell death, there is considerable evidence suggesting
that caspase-independent pathways are also important. It has
previously been shown that A. blazei polysaccharides
effectively induce apoptosis in HL-60 cells via a
mitochondria-caspase-3-dependent signaling cascade (26). An A. blazei extract by Jin
et al (17), comprised of
proteoglycans with a ratio (74:26) of polysaccharides to peptides,
induced apoptosis in U937 cells via a caspase-3-dependent pathway.
Mitochondria contain several potentially apoptogenic factors,
including cytochrome c, procaspases-2, −3 and −9, AIF and
Endo G, all of which have crucial roles in caspase-dependent or
caspase-independent apoptosis (8,9). In
particular, translocation of AIF, a mitochondrial flavoprotein
which normally resides in the inner mitochondrial membrane, to the
cytosol and nucleus results in caspase-independent apoptosis in a
number of model systems (10).
Importantly, various pro-apoptotic effects of AIF are not inhibited
by pharmacological caspase inhibitors such as z-VAD-fmk, indicating
that AIF is able to induce apoptosis in a caspase-independent
manner (10). We believe that
Agarol is similarly capable of inducing the translocation of AIF
into the nucleus during progression of apoptosis in a
caspase-independent manner, due to the failures of the pan-caspase
inhibitor to attenuate Agarol-induced AIF translocation and to
rescue the cells from Agarol-mediated cell death. Several reports
indicate that a generation of ROS is necessary for AIF release from
the mitochondria (27,28). Blazeispirol A has been shown to
induce caspase-independent cell death, because it is not blocked by
z-VAD-fmk, and AIF is translocated from the mitochondria to the
cytosol after treatment (5).
The oral administration of A. blazei Murrill
does not prevent tumor growth in SCID mice inoculated with HT-29
human colon cancer cells; however, compared with the control group,
these mice showed a dose-dependent reduction in tumor growth
(29). Wu et al also
evaluated the A. blazei Murrill extract-dependent reduction
of hepatoma formation by Smmu 7721 cells in SCID mice and
metastasis formation by B16F10 melanoma cells in C57BL/6 mice
(29). In vivo assays
suggest a role for A. blazei-derived β-glucan in the initial
steps of the metastatic process or intravasation in lung cancer
(16). In addition, β-glucan
reduced tumor burden associated with peritoneally disseminated
metastasis from ovarian cancer (16). Several studies have demonstrated
that various mushroom species can enhance immunity (so-called
immunomodulators) through activation of natural killer cells and
modulation of lymphocyte number and activity (30,31).
In contrast, it has been demonstrated that the purified components
of A. blazei Murill primarily consists of β-glucan, and
sensitizes doxorubicin-mediated apoptotic signaling by enhancing
the accumulation of intracellular doxorubicin via the inhibition of
NF-κB activity (32). Therefore,
β-glucan, when combined with low doses of doxorubicin, has the
potential to provide more efficient therapeutic effects against
drug-resistant human hepatocellular carcinoma (32). In xenograft mono-therapeutic
experiments, there was still not a clear trend and it is probable
that the animal protocol used was not sufficient to detect
significant effects of Agarol. Careful animal studies are still
necessary to determine whether Agarol can provide similar antitumor
activities.
In conclusion, while the underlying mechanisms and
actions of the newly identified Agarol remain to be elucidated,
mitochondria-mediated downstream molecular events, including AIF
release without activated caspase-3 expression during induction of
apoptosis, as well as other related mechanisms, should be further
investigated. With additional insight into the mechanisms of these
compounds derived from A. blazei, current findings suggest
that Agarol could be a promising modality for solid cancers.
Acknowledgements
We thank Mr. D. Mrozek for editing the
manuscript.
Abbreviations:
|
A. blazei
|
Agaricus blazei
|
|
AIF
|
apoptosis-inducing factor
|
|
BetA
|
betulinic acid
|
|
ΔΨm
|
mitochondria membrane potential
|
|
PFT-α
|
pifithrin-α
|
|
ROS
|
reactive oxygen species
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Xu T, Beelman RB and Lambert JD: The
cancer preventive effects of edible mushrooms. Anticancer Agents
Med Chem. 12:1255–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kidd PM: The use of mushroom glucans and
proteoglycans in cancer treatment. Altern Med Rev. 5:4–27.
2000.PubMed/NCBI
|
|
3
|
Ohno N, Furukawa M, Miura NN, Adachi Y,
Motoi M and Yadomae T: Antitumor beta glucan from the cultured
fruit body of Agaricus blazei. Biol Pharm Bull. 24:820–828. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takaku T, Kimura Y and Okuda H: Isolation
of an antitumor compound from Agaricus blazei Murill and its
mechanism of action. J Nutr. 131:1409–1413. 2001.PubMed/NCBI
|
|
5
|
Su Z-Y, Tung Y-C, Hwang LS and Sheen L-Y:
Blazeispirol A from Agaricus blazei fermentation product induces
cell death in human hepatoma Hep 3B cells through caspase-dependent
and caspase-independent pathways. J Agric Food Chem. 59:5109–5116.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ziliotto L, Pinheiro F, Barbisan LF and
Rodrigues MAM: Screening for in vitro and in vivo antitumor
activities of the mushroom Agaricus blazei. Nutr Cancer.
61:245–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki M, Endo M, Shinohara F, Echigo S
and Rikiishi H: Differential apoptotic response of human cancer
cells to organoselenium compounds. Cancer Chemother Pharmacol.
66:475–484. 2010. View Article : Google Scholar
|
|
8
|
Kroemer G and Martin SJ:
Caspase-independent cell death. Nat Med. 11:725–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirose T and Horvitz HR: An Sp1
transcription factor coordinates caspase-dependent and -independent
apoptotic pathways. Nature. 500:354–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim E-A, Jang J-H, Lee Y-H, Sung EG, Song
IH, Kim JY, Kim S, Sohn HY and Lee TJ: Dioscin induces
caspase-independent apoptosis through activation of
apoptosis-inducing factor in breast cancer cells. Apoptosis.
19:1165–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yogeeswari P and Sriram D: Betulinic acid
and its derivatives: A review on their biological properties. Curr
Med Chem. 12:657–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y and Luo W: Betulinic acid induces
Bax/Bak-independent cytochrome c release in human nasopharyngeal
carcinoma cells. Mol Cells. 33:517–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hetland G, Johnson E, Lyberg T and
Kvalheim G: The mushroom Agaricus blazei murill elicits medicinal
effects on tumor, infection, allergy, and inflammation through its
modulation of innate immunity and amelioration of Th1/Th2 imbalance
and inflammation. Adv Pharmacol Sci. 2011:1570152011.PubMed/NCBI
|
|
14
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu C-H, Kan S-F, Shu C-H, Lu T-J,
Sun-Hwang L and Wang PS: Inhibitory mechanisms of Agaricus blazei
Murill on the growth of prostate cancer in vitro and in vivo. J
Nutr Biochem. 20:753–764. 2009. View Article : Google Scholar
|
|
16
|
Kobayashi H, Yoshida R, Kanada Y, Fukuda
Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, et
al: Suppressing effects of daily oral supplementation of
beta-glucan extracted from Agaricus blazei Murill on spontaneous
and peritoneal disseminated metastasis in mouse model. J Cancer Res
Clin Oncol. 131:527–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin C-Y, Choi YH, Moon D-O, Park C, Park
YM, Jeong SC, Heo MS, Lee TH, Lee JD and Kim GY: Induction of G2/M
arrest and apoptosis in human gastric epithelial AGS cells by
aqueous extract of Agaricus blazei. Oncol Rep. 16:1349–1355.
2006.PubMed/NCBI
|
|
18
|
Rieber M and Rieber MS: Signalling
responses linked to betulinic acid-induced apoptosis are
antagonized by MEK inhibitor U0126 in adherent or 3D spheroid
melanoma irrespective of p53 status. Int J Cancer. 118:1135–1143.
2006. View Article : Google Scholar
|
|
19
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tung Y-C, Su Z-Y, Kuo M-L and Sheen L-Y:
Ethanolic extract of Agaricus blazei fermentation product inhibits
the growth and invasion of human hepatoma HA22T/VGH and SK-Hep-1
cells. J Tradit Complement Med. 2:145–153. 2012.PubMed/NCBI
|
|
21
|
Hajnóczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holley AK, Dhar SK and St Clair DK:
Manganese superoxide dismutase versus p53: The mitochondrial
center. Ann NY Acad Sci. 1201:72–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maillet A and Pervaiz S: Redox regulation
of p53, redox effectors regulated by p53: A subtle balance.
Antioxid Redox Signal. 16:1285–1294. 2012. View Article : Google Scholar
|
|
24
|
Kim M-O, Moon D-O, Jung JM, Lee WS, Choi
YH and Kim G-Y: Agaricus blazei extract induces apoptosis through
ROS-dependent JNK activation involving the mitochondrial pathway
and suppression of constitutive NF-κB in THP-1 cells. Evid Based
Complement Alternat Med. 2011:8381722011. View Article : Google Scholar
|
|
25
|
Chintharlapalli S, Papineni S, Lei P,
Pathi S and Safe S: Betulinic acid inhibits colon cancer cell and
tumor growth and induces proteasome-dependent and -independent
downregulation of specificity proteins (Sp) transcription factors.
BMC Cancer. 11:3712011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Zhao X, Wang H, Han J and Liu L: A
polysaccharide from the fruiting bodies of Agaricus blazei Murill
induces caspase-dependent apoptosis in human leukemia HL-60 cells.
Tumour Biol. 35:8963–8968. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie J, Xu Y, Huang X, Chen Y, Fu J, Xi M
and Wang L: Berberine-induced apoptosis in human breast cancer
cells is mediated by reactive oxygen species generation and
mitochondrial-related apoptotic pathway. Tumour Biol. 36:1279–1288.
2015. View Article : Google Scholar
|
|
28
|
Su J, Cheng H, Zhang D, Wang M, Xie C, Hu
Y, Chang HC and Li Q: Synergistic effects of 5-fluorouracil and
gambogenic acid on A549 cells: Activation of cell death caused by
apoptotic and necroptotic mechanisms via the ROS-mitochondria
pathway. Biol Pharm Bull. 37:1259–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu M-F, Chen Y-L, Lee M-H, Shih YL, Hsu
YM, Tang MC, Lu HF, Tang NY, Yang ST, Chueh FS, et al: Effect of
Agaricus blazei Murrill extract on HT-29 human colon cancer cells
in SCID mice in vivo. In Vivo. 25:673–677. 2011.PubMed/NCBI
|
|
30
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nozaki H, Itonori S, Sugita M, Nakamura K,
Ohba K, Suzuki A and Kushi Y: Mushroom acidic glycosphingolipid
induction of cytokine secretion from murine T cells and
proliferation of NK1.1 α/β TCR-double positive cells in vitro.
Biochem Biophys Res Commun. 373:435–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JS and Hong EK: Agaricus blazei Murill
enhances doxorubicin-induced apoptosis in human hepatocellular
carcinoma cells by NFκB-mediated increase of intracellular
doxorubicin accumulation. Int J Oncol. 38:401–408. 2011.
|