Introduction
Rhabdomyosarcoma (RMS) is the most common
soft-tissue sarcoma of adolescence and childhood and as reported
accounts for 5% of all malignant tumors in patients under 15 years
of age (1,2). It occurs also in older patients, and
the median age at diagnosis for all 2,600 analyzed RMS cases (birth
through 96 years) was 16 years (3). RMS belongs to the family of so-called
‘small round blue tumor cells’, which often infiltrate bone marrow
(BM) and on BM smears may sometimes be misdiagnosed as acute
leukemia cells (4). There are two
major histological subtypes of this tumor: alveolar
rhabdomyosarcoma (ARMS) and embryonal rhabdomyosarcoma (ERMS). ARMS
is associated with more aggressive behavior and a worse prognosis
than ERMS (5).
At the molecular level, ARMS is characterized by the
t(2;13)(q35;q14) translocation in 70% of cases and the variant
t(1;13)(p36;q14) in a smaller percentage of cases (6). These translocations disrupt the
PAX3 and PAX7 genes on chromosomes 2 and 1,
respectively, and the FOXO1 gene on chromosome 13,
generating PAX3-FOXO1 and PAX7-FOXO1 fusion genes.
The resulting fusion proteins, PAX3-FOXO1 and PAX7-FOXO1, have
enhanced transcriptional activity compared with wild-type PAX3 and
PAX7 and are postulated to play a role in cell survival and
dysregulation of the cell cycle in ARMS (7). Since there are also ARMS cases that
are fusion-negative and have a better outcome than ARMS cases that
are fusion-negative, it has recently been proposed that RMS be
classified into fusion-positive (PAX3-FOXO1 and
PAX7-FOXO1) and fusion-negative tumors (8).
Both pituitary peptide- and gonadal steroid-based
sex hormones play an important role in normal development and
tumorigenesis and, thus, we were interested in the role of sex
hormones in RMS pathogenesis. First, it is well known that these
hormones are involved in both developmental growth and regeneration
of skeletal muscles (9–11). For example, skeletal muscle
satellite stem cells respond to stimulation by estrogens and
androgens (9,12). Second, pediatric sarcomas,
including RMS, share several common markers with the germ
line-derived cells. For example, RMS cells express several cancer
testis antigens (CTAs) (13,14).
Moreover, changes in expression of parentally imprinted genes (e.g,
Igf2-H19, Dlk1-Meg3 and
p57KIP2) are involved in the pathogenesis
of both pediatric sarcomas and germline tumors (15–17).
Notably, 150 years ago, Virchow (18) and Conheim (19) proposed the so-called ‘embryonic
rest’ hypothesis of cancer development, in which malignancies may
develop from dormant development early embryonic cells or perhaps
cells endowed with germ line potential residing in adult tissues.
Small round blue cell tumors, including RMS, are potential
candidates for such malignancies. Interestingly, a recent report
demonstrated that the PAX7 gene, which plays an important
role in skeletal muscle development, is one of the stem cell
markers in germ cells in gonads (20).
We report here that several sex hormone receptors
are indeed expressed by RMS cells. Moreover, we demonstrate for the
first time that follicle-stimulating hormone (FSH) and luteinizing
hormone (LH) receptors are expressed in established human RMS cell
lines and, even more importantly, in primary tumor samples isolated
from patients. We also found that several human RMS cell lines
respond to pituitary and gonadal sex hormone stimulation by
enhanced proliferation, chemotaxis, cell adhesion and
phosphorylation of MAPKp42/44 and AKT. We conclude that sex
hormones are involved in the pathogenesis and progression of RMS,
and their therapeutic application should be avoided in patients
with RMS.
Materials and methods
Cell lines
We used several human RMS cell lines (provided by Dr
Peter Houghton, Nationwide Children's Cancer Center, Columbus, OH,
USA), including both fusion-positive (RH28, RH30 and RH41) and
fusion-negative (JR, RD, RH18, RH36 and SMS-CTR) cell lines. All
cell lines used in the present study were authenticated by short
tandem repeat (STR) analysis. STR profiles were compared with those
of the original cell lines, obtained in Dr Peter Houghton's
Laboratory, or with published profiles. SMS-CTR and RH36 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin
and 10 μg/ml streptomycin. All other RMS cells used for experiments
were cultured in Roswell Park Memorial Institute (RPMI)-1640
medium, supplemented with 100 IU/ml penicillin and 10 μg/ml
streptomycin in 10% heat-inactivated FBS. The cells were cultured
in a humidified atmosphere at 37°C in 5% CO2 at an
initial cell density of 2.5×104 cells/flask.
Conventional RT-PCR
Total RNA from various cells was isolated using the
RNeasy Mini kit (Qiagen Inc., Valencia CA, USA), including
treatment with DNase I (Qiagen). The mRNA was reverse-transcribed
with TaqMan Reverse Transcription reagents (Applied Biosystems,
Grand Island, NY, USA) according to the manufacturer's
instructions. The resulting cDNA fragments were amplified (1 cycle
of 8 min at 95°C, 2 cycles of 2 min at 95°C, 1 min at 60°C, 1 min
at 72°C, and subsequently 40 cycles of 30 sec at 95°C, 1 min at
60°C, 1 min at 72°C, and 1 cycle of 10 min at 72°C) using AmpliTaq
Gold polymerase with sequence-specific primers designed using the
NCBI/Primer-BLAST program. One primer in each pair was designed to
include an exon-intron boundary: β-actin: F, GGATGCAGAAGGAGATCACTG
and R, CGATCCACACGGAGTACTTG; hFSHR: F, GCTTCTGAGATCTGTGGAGGTT and
R, ACCTCAGTTCAATGGCATTCCT; hLHR: F, GGGCCGCACTCAGAGG and R,
AGGGAGGTAGGCAAGTGATAGTC; hERα: F, AGGTGCCCTACTACCTGGAG and R,
CGGTCTTTTCGTATCCCACCT; hERβ: F, TTTTTGGACACCCACTCCCC and R,
CACCTGTTGAGGAAAGCGAG; hANDR: F, CGACTTCACCGCACCTGATG and R,
CTTCTGTTTCCCTTCAGCGG; hPROGR: F, CGGACACCTTGCCTGAAGTT and R,
AGTCCGCTGTCCTTTTCTGG; hPRLR: F, GAGCTTCTTCTCACAGAGCCA and R,
AAGTTCACTTCAGGGTTCATGTGG.
Fluorescent staining of the
rhabdomyosarcoma cells
RH30 and RD cells were fixed in 4% paraformaldehyde
for 15 min, permeabilized by employing 0.1% Triton X-100 for 10
min, washed in PBS, preblocked with 2.5% BSA in PBS, and
subsequently stained with antibodies to follicle-stimulating
hormone receptor (FSH-R, 1:200, rabbit polyclonal antibody; Santa
Cruz Biotechnology, Santa Cruz, CA, USA), luteinizing
hormone/choriogonadotropin receptor (LH-R, 1:200, rabbit polyclonal
antibody; Santa Cruz Biotechnology), androgen receptor (Ab-2,
1:200, rabbit polyclonal antibody; Thermo Fisher Scientific,
Pittsburgh, PA, USA), and estrogen receptor (ER, Ab-11, 1:200,
mouse monoclonal IgG antibody; Thermo Fisher Scientific). Staining
was performed overnight in 4°C. Antibodies were diluted in 2.5% BSA
in PBS. Appropriate secondary antibody Texas Red were used for 2 h
at 37°C (1:400; Texas Red goat anti-rabbit IgG and Texas Red horse
anti-mouse IgG; Vector Laboratories, Inc., Burlingame, CA, USA). In
control experiments, cells were stained with secondary antibodies
only. The nuclei were labeled with DAPI. The fluorescence images
were collected with a confocal laser scanning microscope (FV1000;
Olympus).
Chemotaxis assay
Chemotaxis assays were performed in a modified
Boyden's chamber with 8-μm-pore polycarbonate membrane inserts
(Costar Transwell; Corning Incorporated-Life Sciences, Lowell, MA,
USA) as previously described. In brief, cells detached with 0.25%
trypsin were seeded into the upper chambers of inserts at a density
of 4.5×104 in 100 μl. The lower chambers were filled
with pre-warmed culture medium containing hormones: FSH (1 or 10
U/ml), LH (1 or 10 U/ml), estrogen (100 nM), progesterone (100 nM),
danazol (80 μg/ml), or prolactin (0.5 μg/ml). Medium supplemented
with 0.5% bovine serum albumin (BSA) was used as a negative
control. After 24 h, the inserts were removed from the Transwell
supports. The cells that had not migrated were scraped off with
cotton wool from the upper membrane, and the cells that had
transmigrated to the lower side of the membrane were fixed and
stained with Hema 3 fixative according to the manufacturer's
protocol (Fisher Fisher Scientific) and counted on the lower side
of the membrane using an inverted microscope. To address whether
the observed increase in motility is a result of a chemotactic or a
chemokinetic response, we performed a checkerboard assay in which
FSH (10 U/ml), LH (10 U/ml), estrogen (100 nM), progesterone (100
nM), danazol (80 μg/ml), and prolactin (0.5 μg/ml) were added at
the same time into the upper and the lower Transwell chambers.
Fibronectin adhesion assay
Ninety-six-well plates were coated with fibronectin
(10 μg/ml) overnight at 4°C and blocked with 0.5% BSA for 1 h
before the experiment. Cells were made quiescent for 3 h with 0.5%
BSA in medium before incubation with hormones: FSH (1 or 10 U/ml),
LH (1 or 10 U/ml), estrogen (100 nM), progesterone (100 nM),
danazol (80 μg/ml) or prolactin (0.5 μg/ml). Subsequently, cell
suspensions (2×103/100 μl) were added directly to
96-well plates coated with fibronectin and incubated for 5 min at
37°C. Following incubation, the plates were vigorously washed three
times to remove non-adherent cells, and the adherent cells were
counted using an inverted microscope.
Cell proliferation
Cells were grown in 24-well culture plates at an
initial density of 1.5×104 cells/well. After 24 h, the
medium was changed to new medium supplemented with hormones: FSH (1
or 10 U/ml), LH (1 or 10 U/ml), estrogen (100 nM), progesterone
(100 nM), danazol (80 μg/ml) or prolactin (0.5 μg/ml). The medium
with 0.5% BSA was used as a control. The cell number was calculated
at 24, 48 and 72 h after the change of medium.
Phosphorylation of intracellular pathway
proteins
The fusion-positive RMS cell line RH30 and the
fusion-negative cell line JR were incubated overnight in RPMI
medium containing low levels of BSA (0.5%) to render the cells
quiescent. After the cells were stimulated with hormones: FSH (1 or
10 U/ml), LH (1 or 10 U/ml), estrogen (100 nM), progesterone (100
nM), danazol (80 μg/ml), or prolactin (0.5 μg/ml) at 37°C for 5
min, the cells were lysed for 10 min on ice in RIPA lysis buffer
containing protease and phosphatase inhibitors (Santa Cruz
Biotechnology). The extracted proteins were separated on a 4–12%
SDS-PAGE gel and transferred to a PVDF membrane. Phosphorylation of
the serine/threonine kinase AKT (yielding phospho-AKT473) and
p44/42 mitogen-activated kinase (yielding phospho-p44/42 MAPK) was
detected by phospho-specific p44/42 MAPK mouse and AKT rabbit
polyclonal antibodies (Cell Signaling Technology, Danvers, MA, USA)
with HRP-conjugated goat anti-mouse and anti-rabbit secondary
antibodies (Santa Cruz Biotechnology). Equal loading in the lanes
was evaluated by stripping the blots and reprobing with anti-p42/44
MAPK monoclonal antibody (Cell Signaling Technology) and anti-AKT
polyclonal antibody (Cell Signaling Technology). The membranes were
developed with an enhanced chemiluminescence (ECL) reagent
(Amersham Life Science, Arlington Heights, IL, USA), dried, and
subsequently exposed to film (Hyperfilm; Amersham Life
Science).
In vivo experiments with transplants of
RMS cells into immunodeficient mice
This study was performed in accordance with the
guidelines of the Animal Care and Use Committee and Guide for Care
and Use of Laboratory Animals (Department of Health and Human
Services, publication no. 86–23). To evaluate the in vivo
metastatic behavior of RH30 cell lines incubated with FSH (50 U/ml)
and LH (50 U/ml), after pre-treatment and a washing step, cells
were injected intravenously (i.v.; 2.5×106 per mouse)
into SCID mice. Marrows, livers and lungs were removed 48 h after
injection of these cells, and the presence of RMS cells (i.e.,
murine-human chimerism) was evaluated by the level of human
α-satellite DNA expression. Detection of human satellite and murine
β-actin DNA levels was conducted using real-time PCR and an ABI
Prism 7500 Sequence detection system. A 25-μl reaction mixture
containing 12.5 μl SYBR-Green PCR Master Mix, 100 ng DNA template,
primers for α-satellite DNA: (forward, 5′-ACCACTCTGTGTCCTTC
GTTCG-3′ and reverse, 5′-ACTGCGCTCTCAAAAGGAG TGT-3′; and the
primers for β-actin DNA: forward, 5′-TTCAATTCCAACACTGTCCTGTCT-3′
and reverse, 5′-CTGTGGAGTGAC TAAATGGAAACC-3′) were used. The number
of human cells present in the murine organs (the degree of
chimerism) was calculated from the standard curve obtained by
mixing different numbers of human cells with a constant number of
murine cells.
Patient samples
RMS frozen primary tumor specimens (n=58) (26
PAX3-FOXO1-positive, 7 PAX7-FOXO1-positive and 25
fusion-negative) were used for microarray profiling. Total RNA was
extracted from primary tumors using RNA STAT-60 reagent (Tel-Test
Inc., Friendswood, TX, USA) in accordance with the manufacturer's
instructions. These RNA samples were analyzed on Affymetrix
GeneChip® Human Genome U133 Plus 2.0 Arrays at the
University of Pennsylvania Microarray Core Facility. The expression
array data was normalized and log2-transformed with the
RMA (robus multi-array average) algorithm in the Bioconductor
oligo-package. The expression levels of FSH-R and LH-R were
calculated using probe sets 211201_at and 207240s_at,
respectively.
Statistical analysis
All results are presented as mean ± SD. Statistical
analysis of the data was done using Student's t-test for unpaired
samples, with P<0.05 considered to indicate a statistically
significant result.
Results
Human RMS cell lines express several sex
hormone receptors
First, we performed RT-PCR studies to evaluate mRNA
expression in three human fusion-positive (RH28, RH30 and RH41) as
well as five human fusion-negative (JR, RD, RH18, RH36 and SMS-CTR)
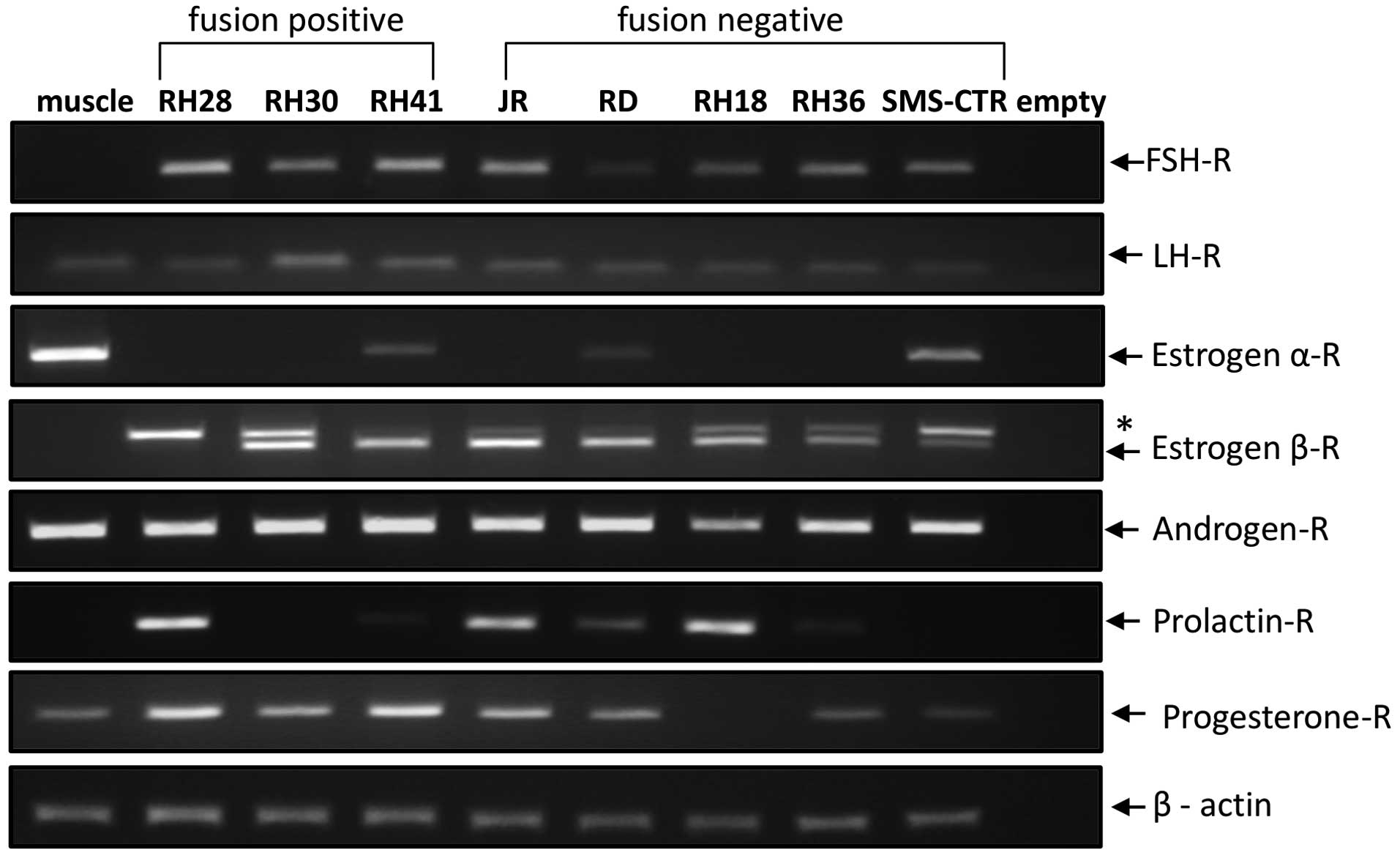
RMS cell lines. Fig. 1 shows that
we were able to detect mRNA for several sex hormone receptors by
conventional RT-PCR. All cell lines evaluated in the present study
expressed follicle-stimulating hormone receptor (FSH-R),
luteinizing hormone receptor (LH-R) and androgen receptor (AR).
While the progesterone receptor (PRG-R) was expressed in all cell
lines except the RH18 fusion-negative RMS cell line, the prolactin
receptor (PRL-R) was not expressed in RH30 and SMS-CTR cells.
Several RMS cells also expressed estrogen receptor alpha (ER-α) and
beta (ER-β). We also found that mRNA from human skeletal muscle
cells expressed all the receptors except FSH-R, PRL-R and ER-β.
To confirm expression of sex hormone receptors at
protein level we evaluated fusion positive RH30 and fusion negative
RD cells for expression of FSHR, LHR, AR and ER at protein level by
immunofluorescence staining (Fig.
2) and confirmed that these cells express sex hormone
receptors.
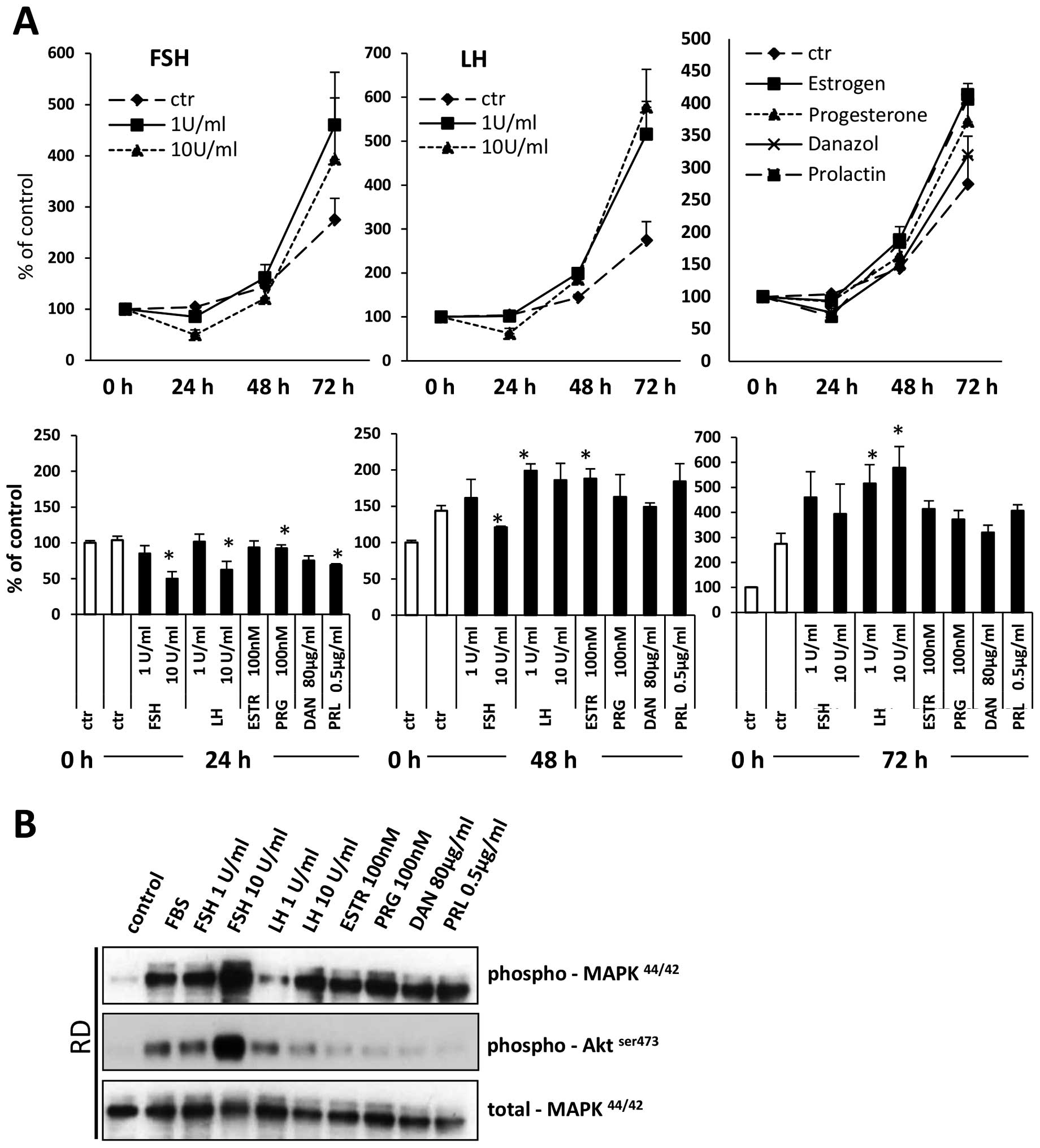
Exposure to sex hormones induces motility
and adhesion of cells in human RMS cell lines
Next, to evaluate whether sex hormones are
functional in human RMS cell lines, we performed chemotaxis and
cell adhesion assays. In Transwell chemotaxis assays, we employed
sex hormones at the indicated low and high doses to test their
chemotactic effect against fusion-positive (Fig. 3A) and fusion-negative (Fig. 3B) RMS cell lines. We found that, of
all the sex hormones tested, the strongest chemotactic activity was
observed for FSH and/or LH. Some of the cell lines, such as RH28,
RD, JR and RH36, also responded to PRL, PRG, androgen and estrogen
stimulation. To address whether the observed effect of pituitary
and gonaldal sex hormones on RMS cell motility was due to a
chemotactic or chemokinetic effect, we performed a checkerboard
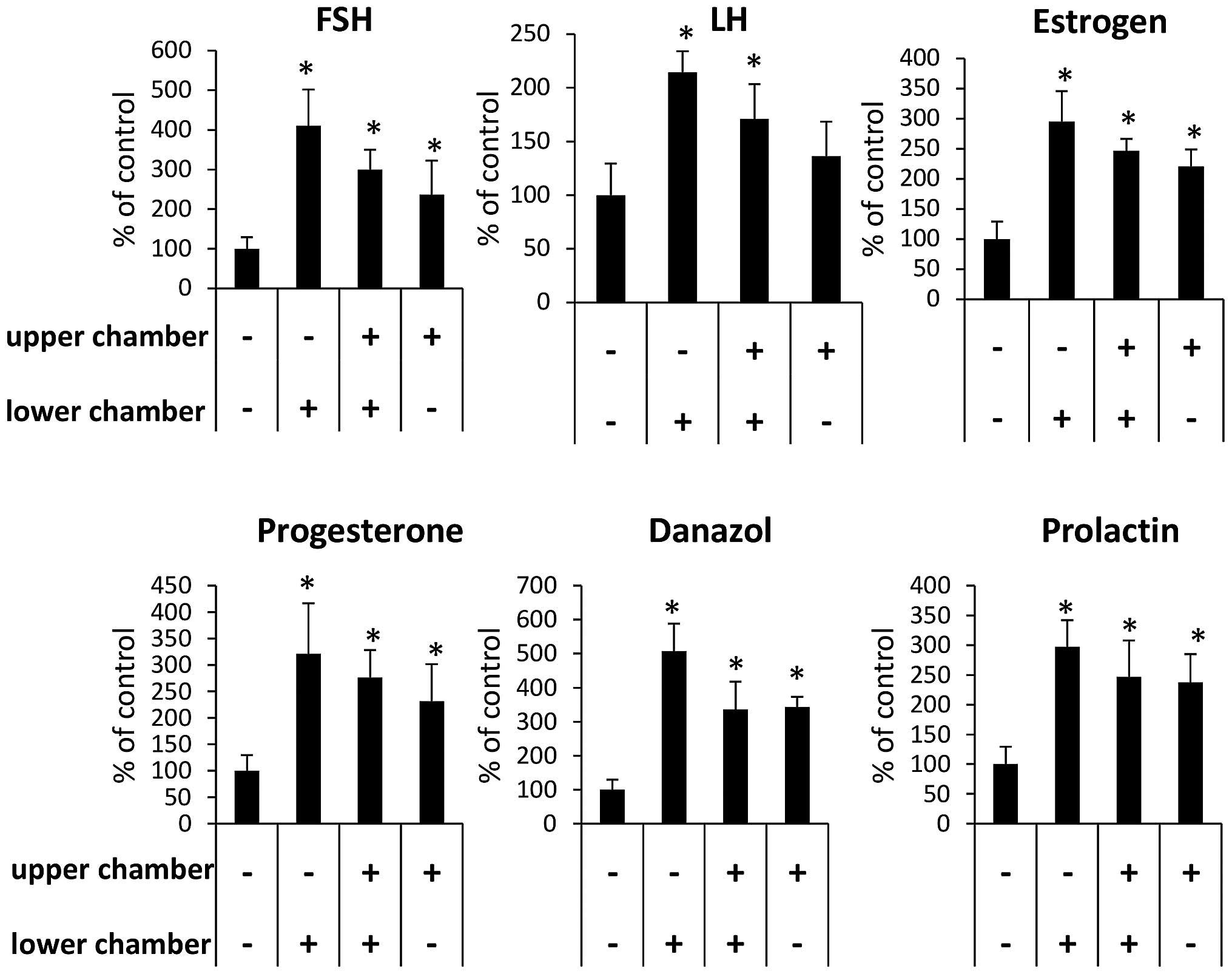
assay (Fig. 4). We found that,
while the effect of FSH and LH is both chemotactic and
chemokinetic, the effect of PRL, PRG, androgen and estrogen is
mostly chemokinetic.
Moreover, as in chemotaxis assays, our
fusion-positive (Fig. 5A) and
fusion-negative (Fig. 5B) human
RMS cell lines also responded to sex hormone stimulation in
fibronectin adhesion assays. Finally, we became interested in
whether prestimulation of RMS cells before injection into
immunodeficient mice would affect their seeding efficiency to BM,
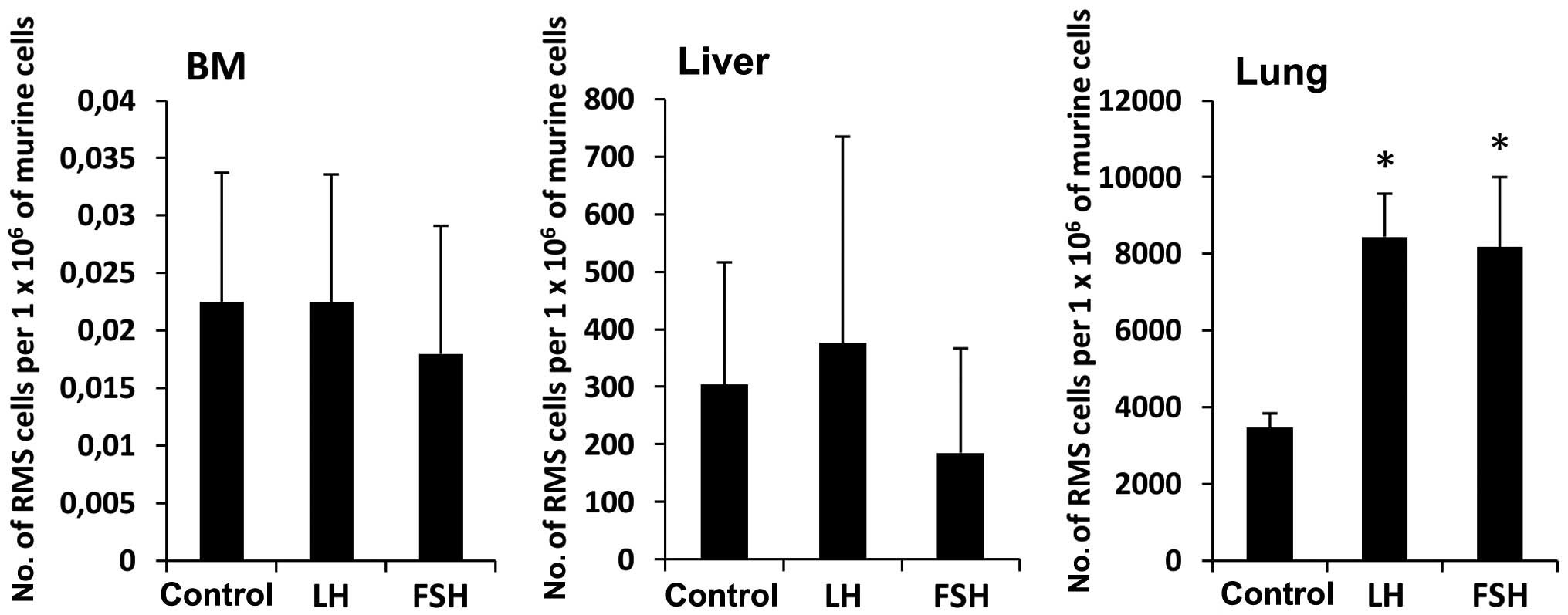
liver and lung. Fig. 6 shows that
RH30 cells stimulated by FSH or LH had higher seeding efficiency
into lung than control cells unstimulated by sex hormones.
Sex hormones induce proliferation of RMS
cells
Sex hormones have been reported to stimulate
proliferation of both normal and malignant cells. To evaluate the
effect of sex hormones on RMS cell proliferation, we exposed
fusion-positive (RH30) and fusion-negative (RD) cell lines in
response to all pituitary and gonadal hormones evaluated in the
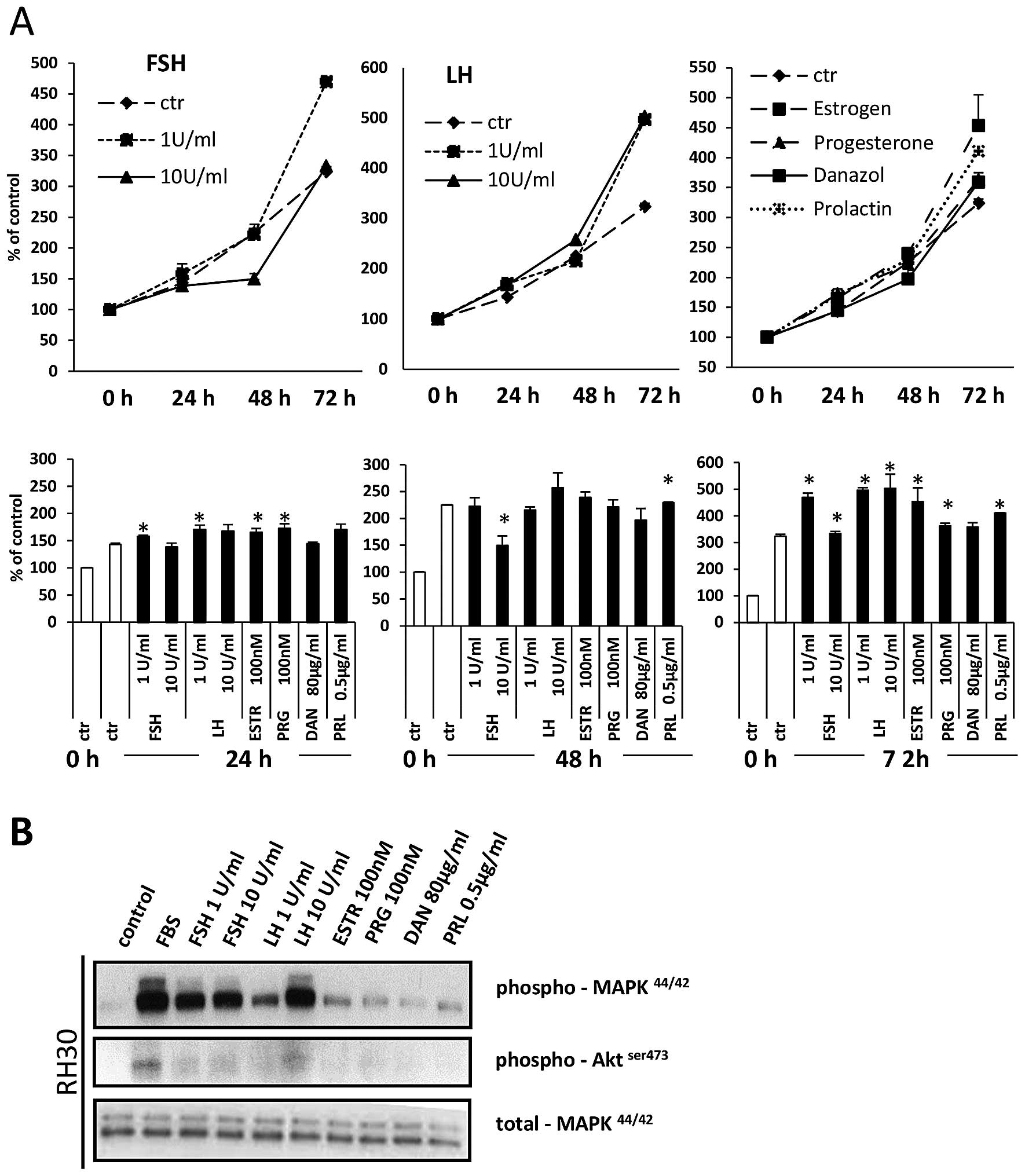
present study. Figs. 7A and
8A demonstrate that all of them
stimulate proliferation of RMS cells. This positive
pro-proliferation effect is supported by signal transduction
studies that confirm that RMS cells express functional receptors
for these hormones (Figs. 7B and
8B) and activate intracellular
pathways potentially involved in cell proliferation, chemotaxis and
adhesion.
Detection of mRNA for pituitary sex
hormone receptors FSH-R and LH-R in primary patient RMS
samples
Finally, we assessed FSH-R and LH-R expression by
microarray analyses of 26 PAX3-FOXO1-positive, 7
PAX7-FOXO1-positive, and 25 fusion-negative patient samples.
We chose these receptors, because we found that they exhibited the
strongest responsiveness of our cell lines to FSH and LH
stimulation. In all these primary patient tumor samples, we were
able to detect FSH-R and LH-R expression (Fig. 9A). However, we did not observe
significant differences between fusion-positive RMS subtypes,
PAX3-FOXO1 (FP-P3) and PAX7-FOXO1 (FP-P7) and
fusion-negative subtypes.
Discussion
The salient observation of the present study is that
human RMS cells express several pituitary and gonadal sex hormone
receptors. Importantly, in assays employed in our studies these
receptors were functional and stimulated migration, proliferation
and adhesion of RMS cells.
Evidence has accumulated that sex hormones could be
involved in pathogenesis of female and male gonadal tumors as well
as breast and prostate cancer (21–23).
In addition, non-gonadal tumors, including stomach, lung and liver
cancer and melanomas, were also found to express sex hormone
receptors (24–27). In addition, they express cancer
testis antigens (CTA), which could indicate a potential
developmental link between these tumors and the germ line-derived
cells (28–31). Similarly, RMS cells were also
reported to express CTA (13,14).
Rare cases of coexistence of RMS with adenocarcionoma have also
been described in endometriotic cysts of the ovary (32) and with Mullerian adenosarcomas in
the uterus (33). Interestingly,
as we reported recently human and murine germline tumors and RMS
cells share functional erythropoietin receptors (34).
RMS is a tumor in which cells share several
characteristics with skeletal muscle cells, while, it is also well
known that sex hormones regulate the development of skeletal
muscles and play a role in their regeneration (9–11).
Moreover, decrease in the level of sex hormones with age is
responsible for the development of sarcopenia in 30% of individuals
aged over 60 years (35,36). Furthermore, results obtained from
rodent models demonstrated that androgens and estrogens augment
skeletal muscle satellite stem cell activation and modulate
inflammatory processes during skeletal muscle regeneration
(9,12). Despite the fact that gonadal sex
hormones affect expansion of skeletal muscle cells, very little is
known about the role of gonadal and pituitary sex hormones in RMS
pathogenesis. To shed more light on this process, we tested several
established human RMS cell lines as well as primary patient samples
for expression of functional sex hormone receptors.
RMS is a tumor that mostly develops in children and
young adults (3). Thus, an open
question remains whether sex hormones are involved in RMS
development at the levels observed in these patients. Our results
clearly show that sex hormones stimulate proliferation and regulate
motility of RMS cells. Expression of their receptors was detectable
in established human cell lines as well as in primary patient
samples. The presence of these receptors on RMS cells suggests that
in particular pituitary sex hormone therapy in RMS survivors may
lead to reactivation of ‘dormant’ malignant cells. In fact,
recurrence of RMS in young women 25 years after estrogen and
progesterone therapy has been reported (37). The tumor appeared 3 months after
commencing hormonal therapy, and primary and recurrent tumors were
immunohistochemically identical (37). Notably, our observation here that
both androgen and estrogens simulate proliferation of RMS cells may
explain why there is no significant sex-dependent predisposition
for RMS development (38).
The recurrence of tumor growth after successful
initial treatment and the fatal tendency of cancerous cells to
spread and metastasize to different vital organs are major problems
affecting the survival of cancer patients, including those
afflicted with RMS (4). The
tropism of cancer cells to metastasize to selected organs requires
the involvement of organ-specific factors that direct metastasis.
These factors promote the formation of a pre-metastatic niche that
provides metastasizing tumor cells with a favorable growth and
survival environment (39,40). An important step in the metastatic
process is egress of cancer cells from the primary tumor (41). This egress could be the result of
random motility of cancer cells, a process known as chemokinesis,
or the result of directed gradient-mediated migration, known as
chemotaxis (42). Our results
indicate that both processes, chemokinesis and chemo-taxis, occur
in RMS cells upon stimulation by sex hormones, and, for the first
time, we identified them as a potential prometastatic factors for
RMS cells. The metastasis of RMS cells, however, is directed by
several other factors, including growth factors (e.g., hepatocyte
growth factor and insulin-like growth factors 1 and 2) (43–45)
chemokines (stromal-derived factor 1 alpha, and macrophage
inhibitory factor) (46,47), cytokines (leukemia inhibitory
factor) (48), as well as several
bioactive lipids (sphingosine-1-phosphate, ceramide-1-phosphate-1
(49), lysophosphatidylocholine
and lysophosphatidic acid) (50).
Since sex hormones increase the motility of RMS cells, they may
promote their egress from the primary tumor and direct their spread
to distant locations. Further work is also needed to address their
cooperative, pro-metastatic effect in combination with other
pro-metastatic factors.
It is known that pituitary and gonadal sex hormone
receptors are expressed by cells from the germ lineage beginning at
the very early stages of embryogenesis (51,52).
As demonstrated in this study, RMS cells also express receptors
both for pituitary peptide and gonadal steroid sex hormones.
Interestingly, as mentioned above, Virchow (18) and Connheim (19) proposed that some malignancies
develop from dormant embryonic or germ cells residing in adult
tissues. We have recently reported the existence of very small
embryonic-like stem cells (VSELs), which express several
embryonic/germline markers residing in adult tissues, including
skeletal muscle (53). One can
speculate that these or closely related cells could theoretically
be the source of some tumors, including pediatric sarcomas. In
support of this possibility, VSELs express several sex hormone
receptors (54) as well as genes
involved in primordial germ cells and skeletal muscle development
(53,55). This interesting hypothesis,
however, requires more experimental evidence.
In conclusion, the presence of functional sex
hormone receptors in RMS cells indicates that sex hormone
supplementation, e.g., in RMS survivors, may result in the unwanted
side-effect of tumor recurrence. Finally, like the expression of
CTA antigens (13,14), the presence of functional sex
hormone receptors in RMS cells is consistent with the hypothesis
that some pediatric sarcomas could develop in adult tissues from
embryonic remnants that are endowed with germline potential.
Acknowledgements
We would like to thank Dr Sylwia Rzeszotek from
Pomeranian Medical University for her help with immunofluorescence
staining. The present study was supported by the NIH grants 2R01
DK074720 and R01HL112788, the Stella and Henry Endowment, the
Maestro grant 2011/02/A/NZ4/00035 and the NCN Harmonia Grant
UMO-2014/14/M/NZ3/00475 to M.Z.R. The work by F.G.B. and W.S. was
supported by the Intramural Research Program of the National Cancer
Institute.
References
|
1
|
Gurney JG, Severson RK, Davis S and
Robison LL: Incidence of cancer in children in the United States.
Sex-, race-, and 1-year age-specific rates by histologic type.
Cancer. 75:2186–2195. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huh WW and Skapek SX: Childhood
rhabdomyosarcoma: New insight on biology and treatment. Curr Oncol
Rep. 12:402–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sultan I, Qaddoumi I, Yaser S,
Rodriguez-Galindo C and Ferrari A: Comparing adult and pediatric
rhabdomyosarcoma in the surveillance, epidemiology and end results
program, 1973 to 2005: An analysis of 2,600 patients. J Clin Oncol.
27:3391–3397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandberg AA, Stone JF, Czarnecki L and
Cohen JD: Hematologic masquerade of rhabdomyosarcoma. Am J Hematol.
68:51–57. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newton WA Jr, Gehan EA, Webber BL, Marsden
HB, van Unnik AJ, Hamoudi AB, Tsokos MG, Shimada H, Harms D,
Schmidt D, et al: Classification of rhabdomyosarcomas and related
sarcomas. Pathologic aspects and proposal for a new classification
- an Intergroup Rhabdomyosarcoma Study. Cancer. 76:1073–1085. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis RJ, D'Cruz CM, Lovell MA, Biegel JA
and Barr FG: Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14)
translocation in alveolar rhabdomyosarcoma. Cancer Res.
54:2869–2872. 1994.PubMed/NCBI
|
|
7
|
Collins MH, Zhao H, Womer RB and Barr FG:
Proliferative and apoptotic differences between alveolar
rhabdomyosarcoma subtypes: A comparative study of tumors containing
PAX3-FKHR or PAX7-FKHR gene fusions. Med Pediatr Oncol. 37:83–89.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kashi VP, Hatley ME and Galindo RL:
Probing for a deeper understanding of rhabdomyosarcoma: Insights
from complementary model systems. Nat Rev Cancer. 15:426–439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
MacKrell JG, Yaden BC, Bullock H, Chen K,
Shetler P, Bryant HU and Krishnan V: Molecular targets of androgen
signaling that characterize skeletal muscle recovery and
regeneration. Nucl Recept Signal. 13:e0052015.PubMed/NCBI
|
|
10
|
Carson JA and Manolagas SC: Effects of sex
steroids on bones and muscles: Similarities, parallels, and
putative interactions in health and disease. Bone. 80:67–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Velders M and Diel P: How sex hormones
promote skeletal muscle regeneration. Sports Med. 43:1089–1100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reiter BC, Kamanga-Sollo E, Pampusch MS,
White ME and Dayton WR: Epidermal growth factor receptor is
required for estradiol-stimulated bovine satellite cell
proliferation. Domest Anim Endocrinol. 48:48–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dalerba P, Frascella E, Macino B,
Mandruzzato S, Zambon A, Rosolen A, Carli M, Ninfo V and Zanovello
P: MAGE, BAGE and GAGE gene expression in human rhabdomyosarcomas.
Int J Cancer. 93:85–90. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lifantseva N, Koltsova A, Krylova T,
Yakovleva T, Poljanskaya G and Gordeeva O: Expression patterns of
cancer-testis antigens in human embryonic stem cells and their cell
derivatives indicate lineage tracks. Stem Cells Int.
2011:7952392011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderson J, Gordon A, McManus A, Shipley J
and Pritchard-Jones K: Disruption of imprinted genes at chromosome
region 11p15.5 in paediatric rhabdomyosarcoma. Neoplasia.
1:340–348. 1999. View Article : Google Scholar
|
|
16
|
Roeb W, Boyer A, Cavenee WK and Arden KC:
PAX3-FOXO1 controls expression of the p57Kip2 cell-cycle regulator
through degradation of EGR1. Proc Natl Acad Sci USA.
104:18085–18090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider G, Bowser MJ, Shin DM, Barr FG
and Ratajczak MZ: The paternally imprinted DLK1-GTL2 locus is
differentially methylated in embryonal and alveolar
rhabdomyosarcomas. Int J Oncol. 44:295–300. 2014.
|
|
18
|
Virchow R: Editorial Archive fuer
pathologische. Anatomie und Physiologie fuer klinische Medizin.
8:23–54. 1855.(In German).
|
|
19
|
Conheim J: Congenitales, quergestreiftes
muskelsarkon der nireren. Virchows Arch. 65:64–69. 1875.(In
German). View Article : Google Scholar
|
|
20
|
Aloisio GM, Nakada Y, Saatcioglu HD, Peña
CG, Baker MD, Tarnawa ED, Mukherjee J, Manjunath H, Bugde A,
Sengupta AL, et al: PAX7 expression defines germline stem cells in
the adult testis. J Clin Invest. 124:3929–3944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Kruchten M, van der Marel P, de Munck
L, Hollema H, Arts H, Timmer-Bosscha H, de Vries E, Hospers G and
Reyners A: Hormone receptors as a marker of poor survival in
epithelial ovarian cancer. Gynecol Oncol. 138:634–639. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lønning PE: Poor-prognosis estrogen
receptor- positive disease: Present and future clinical solutions.
Ther Adv Med Oncol. 4:127–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García-Cruz E, Piqueras M, Huguet J, Peri
L, Izquierdo L, Musquera M, Franco A, Alvarez-Vijande R, Ribal MJ
and Alcaraz A: Low testosterone levels are related to poor
prognosis factors in men with prostate cancer prior to treatment.
BJU Int. 110:E541–E546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW,
Park SH, Kim SJ, Mok YJ and Kim CS: Expression of estrogen
receptors in gastric cancer and their clinical significance. J Surg
Oncol. 106:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang AG, Lee KY, Kim SY, Choi JY, Lee KH,
Kim WH, Wang HJ, Kim JM, Park MG, Yeom YI, et al: The expression of
estrogen receptors in hepatocellular carcinoma in Korean patients.
Yonsei Med J. 47:811–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siegfried JM, Hershberger PA and Stabile
LP: Estrogen receptor signaling in lung cancer. Semin Oncol.
36:524–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou JH, Kim KB, Myers JN, Fox PS, Ning J,
Bassett RL, Hasanein H and Prieto VG: Immunohistochemical
expression of hormone receptors in melanoma of pregnant women,
nonpregnant women, and men. Am J Dermatopathol. 36:74–79. 2014.
View Article : Google Scholar
|
|
28
|
Nakamura Y, Tanaka F, Nagahara H, Ieta K,
Haraguchi N, Mimori K, Sasaki A, Inoue H, Yanaga K and Mori M: Opa
interacting protein 5 (OIP5) is a novel cancer-testis specific gene
in gastric cancer. Ann Surg Oncol. 14:885–892. 2007. View Article : Google Scholar
|
|
29
|
Song MH, Choi KU, Shin DH, Lee CH and Lee
SY: Identification of the cancer/testis antigens AKAP3 and CTp11 by
SEREX in hepatocellular carcinoma. Oncol Rep. 28:1792–1798.
2012.PubMed/NCBI
|
|
30
|
Su C, Xu Y, Li X, Ren S, Zhao C, Hou L, Ye
Z and Zhou C: Predictive and prognostic effect of CD133 and
cancer-testis antigens in stage Ib-IIIA non-small cell lung cancer.
Int J Clin Exp Pathol. 8:5509–5518. 2015.PubMed/NCBI
|
|
31
|
Sigalotti L, Fratta E, Coral S, Tanzarella
S, Danielli R, Colizzi F, Fonsatti E, Traversari C, Altomonte M and
Maio M: Intratumor heterogeneity of cancer/testis antigens
expression in human cutaneous melanoma is methylation-regulated and
functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res.
64:9167–9171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuoka H, Tsujimoto M, Fujita S, Yamasaki
T, Kashihara H, Nishio Y, Kodama R, Inagaki M, Oishi H, Sanke T, et
al: Coexistence of a clear cell adenocarcinoma and an adenosarcoma
with a heterologous rhabdomyosarcoma in an endometriotic cyst of
the ovary: A case study. Int J Gynecol Pathol. 28:362–366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim DW, Shin JH, Lee HJ, Hong YO, Joo JE
and Kim EK: Spindle cell rhabdomyosacoma of uterus: A case study.
Korean J Pathol. 47:388–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poniewierska-Baran A, Suszynska M, Sun W,
Abdelbaset-Ismail A, Schneider G, Barr FG and Ratajczak MZ: Human
rhabdomyosarcoma cells express functional erythropoietin receptor:
Potential therapeutic implications. Int J Oncol. 47:1989–1997.
2015.PubMed/NCBI
|
|
35
|
Kamel HK, Maas D and Duthie EH Jr: Role of
hormones in the pathogenesis and management of sarcopenia. Drugs
Aging. 19:865–877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumgartner RN, Koehler KM, Gallagher D,
Romero L, Heymsfield SB, Ross RR, Garry PJ and Lindeman RD:
Epidemiology of sarcopenia among the elderly in New Mexico. Am J
Epidemiol. 147:755–763. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zacharin M, Waters K, Chow CW, Crock P and
McKelvie P: Recurrent rhabdomyosarcoma after 25 years: A possible
association with estrogen and progestogen therapy. J Pediatr
Hematol Oncol. 19:477–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dagher R and Helman L: Rhabdomyosarcoma:
An overview. Oncologist. 4:34–44. 1999.PubMed/NCBI
|
|
39
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coghlin C and Murray GI: Current and
emerging concepts in tumour metastasis. J Pathol. 222:1–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petrie RJ, Doyle AD and Yamada KM: Random
versus directionally persistent cell migration. Nat Rev Mol Cell
Biol. 10:538–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jankowski K, Kucia M, Wysoczynski M, Reca
R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, et
al: Both hepatocyte growth factor (HGF) and stromal-derived
factor-1 regulate the metastatic behavior of human rhabdomyosarcoma
cells, but only HGF enhances their resistance to radiochemotherapy.
Cancer Res. 63:7926–7935. 2003.PubMed/NCBI
|
|
44
|
Kalebic T, Tsokos M and Helman LJ: In vivo
treatment with antibody against IGF-1 receptor suppresses growth of
human rhabdomyosarcoma and down-regulates p34cdc2. Cancer Res.
54:5531–5534. 1994.PubMed/NCBI
|
|
45
|
Rezvani G, Lui JC, Barnes KM and Baron J:
A set of imprinted genes required for normal body growth also
promotes growth of rhabdomyosarcoma cells. Pediatr Res. 71:32–38.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Libura J, Drukala J, Majka M, Tomescu O,
Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG,
Janowska-Wieczorek A, et al: CXCR4-SDF-1 signaling is active in
rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and
adhesion. Blood. 100:2597–2606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tarnowski M, Grymula K, Liu R, Tarnowska
J, Drukala J, Ratajczak J, Mitchell RA, Ratajczak MZ and Kucia M:
Macrophage migration inhibitory factor is secreted by
rhabdomyosarcoma cells, modulates tumor metastasis by binding to
CXCR4 and CXCR7 receptors and inhibits recruitment of
cancer-associated fibroblasts. Mol Cancer Res. 8:1328–1343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wysoczynski M, Miekus K, Jankowski K,
Wanzeck J, Bertolone S, Janowska-Wieczorek A, Ratajczak J and
Ratajczak MZ: Leukemia inhibitory factor: A newly identified
metastatic factor in rhabdomyosarcomas. Cancer Res. 67:2131–2140.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schneider G, Bryndza E, Abdel-Latif A,
Ratajczak J, Maj M, Tarnowski M, Klyachkin YM, Houghton P, Morris
AJ, Vater A, et al: Bioactive lipids S1P and C1P are prometastatic
factors in human rhabdomyosarcoma, and their tissue levels increase
in response to radio/chemotherapy. Mol Cancer Res. 11:793–807.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schneider G, Sellers ZP, Abdel-Latif A,
Morris AJ and Ratajczak MZ: Bioactive lipids, LPC and LPA, are
novel prometastatic factors and their tissue levels increase in
response to radio/chemotherapy. Mol Cancer Res. 12:1560–1573. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hou Q and Gorski J: Estrogen receptor and
progesterone receptor genes are expressed differentially in mouse
embryos during preimplantation development. Proc Natl Acad Sci USA.
90:9460–9464. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Crocoll A, Zhu CC, Cato AC and Blum M:
Expression of androgen receptor mRNA during mouse embryogenesis.
Mech Dev. 72:175–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ratajczak MZ, Zuba-Surma EK, Wysoczynski
M, Ratajczak J and Kucia M: Very small embryonic-like stem cells:
Characterization, developmental origin, and biological
significance. Exp Hematol. 36:742–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mierzejewska K, Borkowska S, Suszynska E,
Suszynska M, Poniewierska-Baran A, Maj M, Pedziwiatr D, Adamiak M,
Abdel-Latif A, Kakar SS, et al: Hematopoietic stem/progenitor cells
express several functional sex hormone receptors-novel evidence for
a potential developmental link between hematopoiesis and primordial
germ cells. Stem Cells Dev. 24:927–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zuba-Surma EK, Kucia M, Wu W, Klich I,
Lillard JW Jr, Ratajczak J and Ratajczak MZ: Very small
embryonic-like stem cells are present in adult murine organs:
ImageStream-based morphological analysis and distribution studies.
Cytometry A. 73A:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|