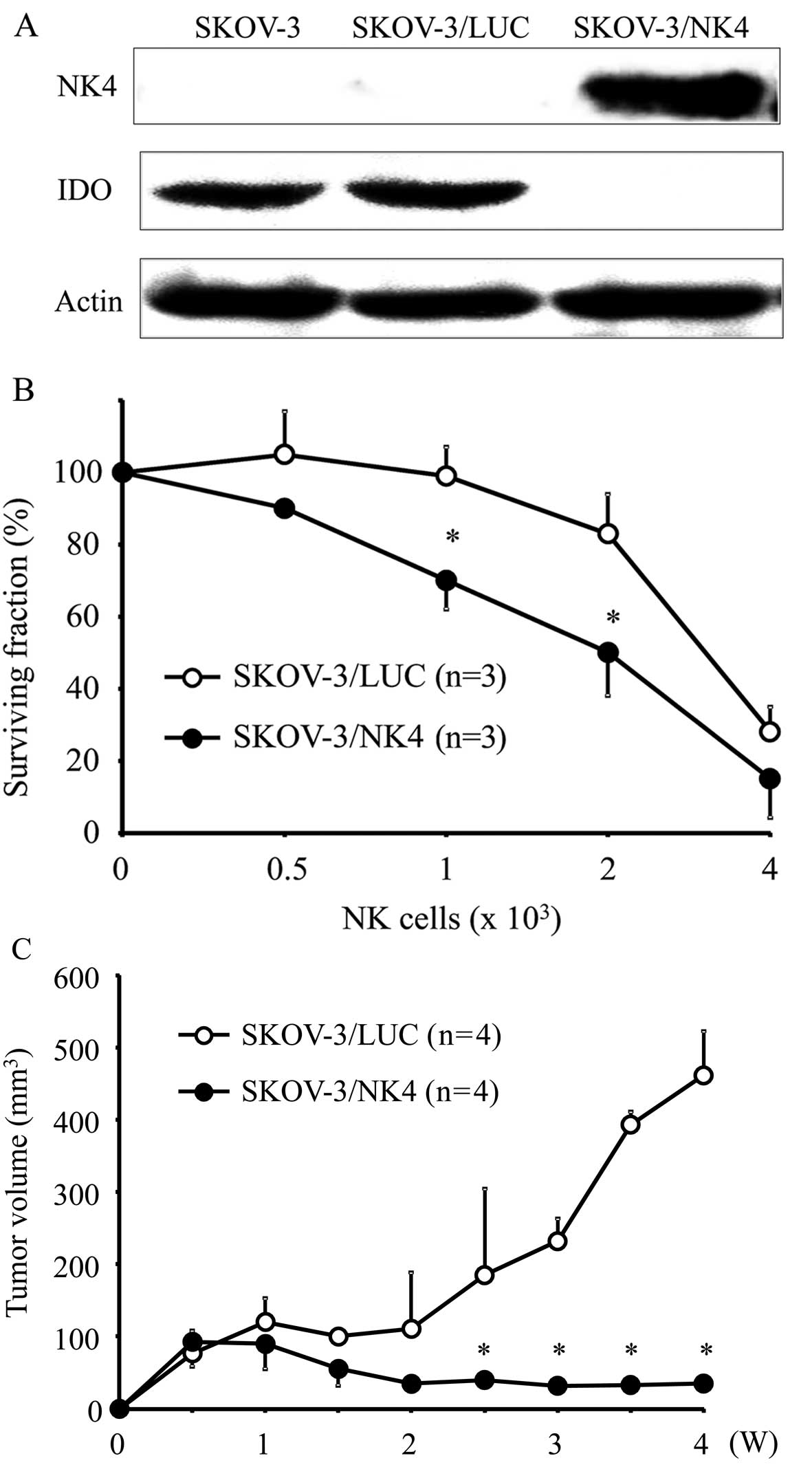
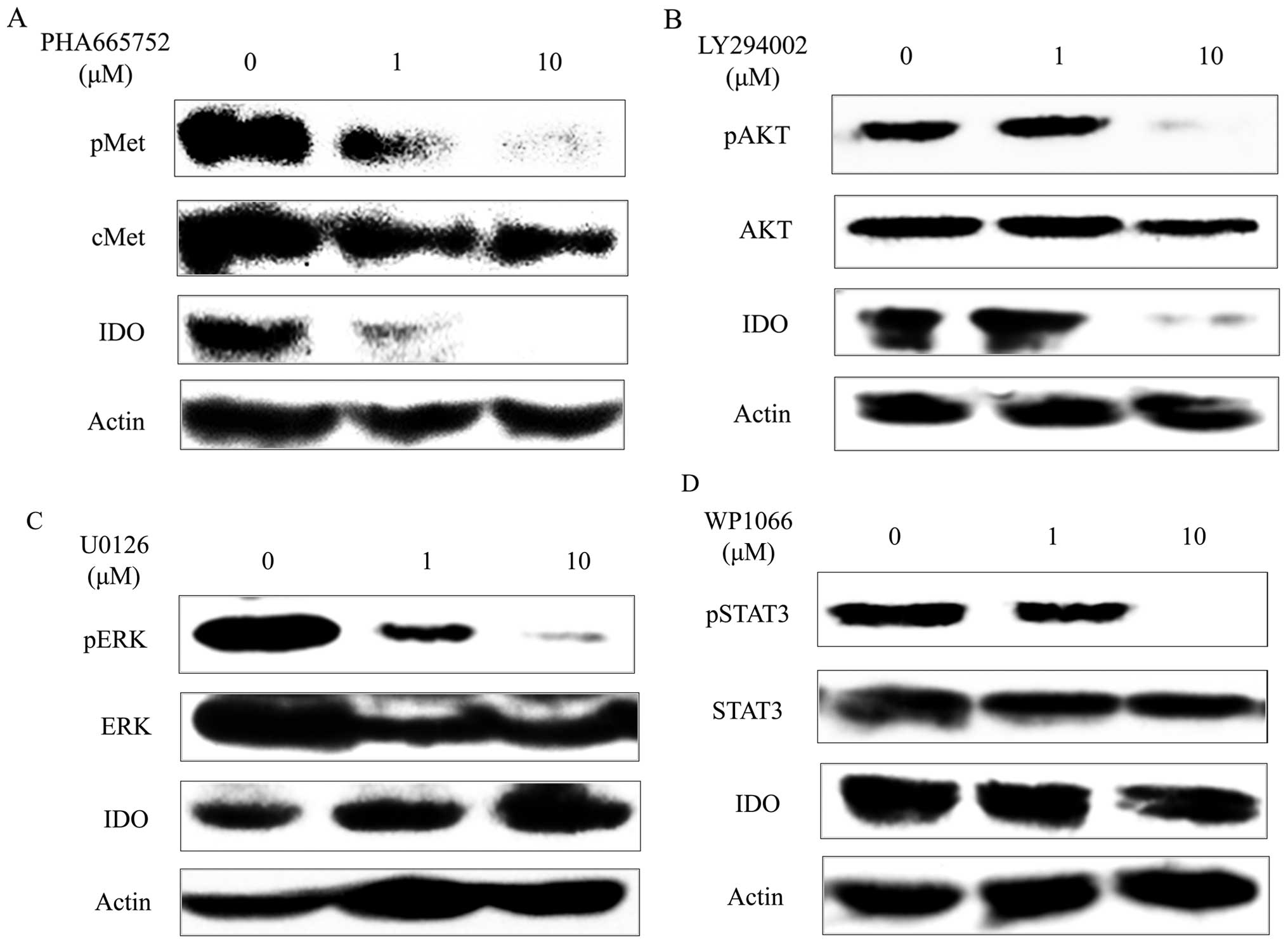
|
1
|
Higuchi K and Hayaishi O: Enzymic
formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys.
120:397–403. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto S and Hayaishi O: Tryptophan
pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme
or enzymes. J Biol Chem. 242:5260–5266. 1967.PubMed/NCBI
|
|
3
|
Shimizu T, Nomiyama S, Hirata F and
Hayaishi O: Indoleamine 2,3-dioxygenase. Purification and some
properties. J Biol Chem. 253:4700–4706. 1978.PubMed/NCBI
|
|
4
|
Uyttenhove C, Pilotte L, Théate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munn DH, Zhou M, Attwood JT, Bondarev I,
Conway SJ, Marshall B, Brown C and Mellor AL: Prevention of
allogeneic fetal rejection by tryptophan catabolism. Science.
281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Della Chiesa M, Carlomagno S, Frumento G,
Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A and Vitale M:
The tryptophan catabolite L-kynurenine inhibits the surface
expression of NKp46- and NKG2D-activating receptors and regulates
NK-cell function. Blood. 108:4118–4125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nonaka H, Saga Y, Fujiwara H, Akimoto H,
Yamada A, Kagawa S, Takei Y, Machida S, Takikawa O and Suzuki M:
Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of
ovarian cancer through inhibition of natural killer cell function
and angiogenesis promotion. Int J Oncol. 38:113–120. 2011.
|
|
8
|
Takikawa O, Kuroiwa T, Yamazaki F and Kido
R: Mechanism of interferon-gamma action. Characterization of
indoleamine 2,3-dioxygenase in cultured human cells induced by
interferon-gamma and evaluation of the enzyme-mediated tryptophan
degradation in its anticellular activity. J Biol Chem.
263:2041–2048. 1988.PubMed/NCBI
|
|
9
|
Fujigaki S, Saito K, Sekikawa K, Tone S,
Takikawa O, Fujii H, Wada H, Noma A and Seishima M:
Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is
mediated dominantly by an IFN-gamma-independent mechanism. Eur J
Immunol. 31:2313–2318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsumoto K, Date K, Shimura H and
Nakamura T: Acquisition of invasive phenotype in gallbladder cancer
cells via mutual interaction of stromal fibroblasts and cancer
cells as mediated by hepatocyte growth factor. Jpn J Cancer Res.
87:702–710. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura T, Matsumoto K, Kiritoshi A, Tano
Y and Nakamura T: Induction of hepatocyte growth factor in
fibroblasts by tumor-derived factors affects invasive growth of
tumor cells: In vitro analysis of tumor-stromal interactions.
Cancer Res. 57:3305–3313. 1997.PubMed/NCBI
|
|
12
|
Bussolino F, Di Renzo MF, Ziche M,
Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer
A and Comoglio PM: Hepatocyte growth factor is a potent angiogenic
factor which stimulates endothelial cell motility and growth. J
Cell Biol. 119:629–641. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grant DS, Kleinman HK, Goldberg ID,
Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P and Rosen EM:
Scatter factor induces blood vessel formation in vivo. Proc Natl
Acad Sci USA. 90:1937–1941. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Nishizawa T, Hagiya M, Seki T,
Shimonishi M, Sugimura A, Tashiro K and Shimizu S: Molecular
cloning and expression of human hepatocyte growth factor. Nature.
342:440–443. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Date K, Matsumoto K, Shimura H, Tanaka M
and Nakamura T: HGF/NK4 is a specific antagonist for pleiotrophic
actions of hepatocyte growth factor. FEBS Lett. 420:1–6. 1997.
View Article : Google Scholar
|
|
16
|
Matsumoto K and Nakamura T: Mechanisms and
significance of bifunctional NK4 in cancer treatment. Biochem
Biophys Res Commun. 333:316–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuba K, Matsumoto K, Date K, Shimura H,
Tanaka M and Nakamura T: HGF/NK4, a four-kringle antagonist of
hepatocyte growth factor, is an angiogenesis inhibitor that
suppresses tumor growth and metastasis in mice. Cancer Res.
60:6737–6743. 2000.PubMed/NCBI
|
|
18
|
Saga Y, Mizukami H, Suzuki M, Urabe M,
Kume A, Nakamura T, Sato I and Ozawa K: Expression of HGF/NK4 in
ovarian cancer cells suppresses intraperitoneal dissemination and
extends host survival. Gene Ther. 8:1450–1455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomioka D, Maehara N, Kuba K, Mizumoto K,
Tanaka M, Matsumoto K and Nakamura T: Inhibition of growth,
invasion, and metastasis of human pancreatic carcinoma cells by NK4
in an orthotopic mouse model. Cancer Res. 61:7518–7524.
2001.PubMed/NCBI
|
|
20
|
Son G, Hirano T, Seki E, Iimuro Y, Nukiwa
T, Matsumoto K, Nakamura T and Fujimoto J: Blockage of HGF/c-Met
system by gene therapy (adenovirus-mediated NK4 gene) suppresses
hepatocellular carcinoma in mice. J Hepatol. 45:688–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du W, Hattori Y, Yamada T, Matsumoto K,
Nakamura T, Sagawa M, Otsuki T, Niikura T, Nukiwa T and Ikeda Y:
NK4, an antagonist of hepatocyte growth factor (HGF), inhibits
growth of multiple myeloma cells: Molecular targeting of angiogenic
growth factor. Blood. 109:3042–3049. 2007.
|
|
22
|
Kubota T, Taiyoh H, Matsumura A, Murayama
Y, Ichikawa D, Okamoto K, Fujiwara H, Ikoma H, Nakanishi M, Kikuchi
S, et al: Gene transfer of NK4, an angiogenesis inhibitor, induces
CT26 tumor regression via tumor-specific T lymphocyte activation.
Int J Cancer. 125:2879–2886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki Y, Sakai K, Ueki J, Xu Q, Nakamura
T, Shimada H, Nakamura T and Matsumoto K: Inhibition of Met/HGF
receptor and angiogenesis by NK4 leads to suppression of tumor
growth and migration in malignant pleural mesothelioma. Int J
Cancer. 127:1948–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto G, Omi Y, Lee U, Kubota E and
Tabata Y: NK4 gene therapy combined with cisplatin inhibits tumour
growth and metastasis of squamous cell carcinoma. Anticancer Res.
31:105–111. 2011.PubMed/NCBI
|
|
25
|
Koblish HK, Hansbury MJ, Bowman KJ, Yang
G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, et
al: Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase
potently suppress systemic tryptophan catabolism and the growth of
IDO-expressing tumors. Mol Cancer Ther. 9:489–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
27
|
Yagita M, Huang CL, Umehara H, Matsuo Y,
Tabata R, Miyake M, Konaka Y and Takatsuki K: A novel natural
killer cell line (KHYG-1) from a patient with aggressive natural
killer cell leukemia carrying a p53 point mutation. Leukemia.
14:922–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christensen JG, Schreck R, Burrows J,
Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, et
al: A selective small molecule inhibitor of c-Met kinase inhibits
c-Met-dependent phenotypes in vitro and exhibits cytoreductive
antitumor activity in vivo. Cancer Res. 63:7345–7355.
2003.PubMed/NCBI
|
|
29
|
Guo M, Joiakim A and Reiners JJ Jr:
Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated
aryl hydrocarbon receptor transformation and CYP1A1 induction by
the phosphatidylinositol 3-kinase inhibitor
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002).
Biochem Pharmacol. 60:635–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Favata MF, Horiuchi KY, Manos EJ, Daulerio
AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F,
et al: Identification of a novel inhibitor of mitogen-activated
protein kinase kinase. J Biol Chem. 273:18623–18632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar
|
|
32
|
Saga Y, Mizukami H, Suzuki M, Kohno T,
Urabe M, Ozawa K and Sato I: Overexpression of PTEN increases
sensitivity to SN-38, an active metabolite of the topoisomerase I
inhibitor irinotecan, in ovarian cancer cells. Clin Cancer Res.
8:1248–1252. 2002.PubMed/NCBI
|
|
33
|
Saga Y, Mizukami H, Takei Y, Ozawa K and
Suzuki M: Suppression of cell migration in ovarian cancer cells
mediated by PTEN overexpression. Int J Oncol. 23:1109–1113.
2003.PubMed/NCBI
|
|
34
|
Takei Y, Saga Y, Mizukami H, Takayama T,
Ohwada M, Ozawa K and Suzuki M: Overexpression of PTEN in ovarian
cancer cells suppresses i.p. dissemination and extends survival in
mice. Mol Cancer Ther. 7:704–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Urabe M, Hasumi Y, Ogasawara Y, Matsushita
T, Kamoshita N, Nomoto A, Colosi P, Kurtzman GJ, Tobita K and Ozawa
K: A novel dicistronic AAV vector using a short IRES segment
derived from hepatitis C virus genome. Gene. 200:157–162. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Saga Y, Mizukami H, Sato N, Nonaka
H, Fujiwara H, Takei Y, Machida S, Takikawa O, Ozawa K, et al:
Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that
inhibits natural killer cell function, as a useful target for
ovarian cancer therapy. Int J Oncol. 40:929–934. 2012.
|
|
37
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Balachandran VP, Cavnar MJ, Zeng S,
Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C,
Rossi F, et al: Imatinib potentiates antitumor T cell responses in
gastrointestinal stromal tu mor through the inhibition of Ido. Nat
Med. 17:1094–1100. 2011. View
Article : Google Scholar : PubMed/NCBI
|