Introduction
Breast cancer is one of the most common malignant
tumors in women, and its incidence rate ranks first in
gynecological tumors (1). For the
past few years, the main focus for research on the treatment of
cancer has gradually transferred from the use of cytotoxic drugs to
targeted therapies, that is, targeting specific genes or proteins
that play a key role in the growth and progression of cancer
(2). In this area, compared with a
wide range of studies on genes or proteins that are downstream of
the epidermal growth factor receptor (EGFR), corresponding work
focusing on upstream genes or proteins has rarely been carried out
(3). A disintegrin and
metalloproteinase (ADAM) family members are a series of
Zn-dependent metalloproteinases and they are, as ectodomain
sheddases, best known for their domains that function as
metalloproteases (4). ADAM exists
in a variety of organisms and is mainly distributed in the cell
membrane (5). Studies have
indicated that it is closely related to tumor invasion and
metastasis and plays a crucial role in the progression of breast
cancer (4,5). Because the 17th member of the family
is the major enzyme which takes charge of releasing soluble tumor
necrosis factor-alpha (TNF-α) from the plasmalemma, it is also
known as TNF-α converting enzyme (TACE/ADAM17) (6). Besides releasing TNF-α, ADAM17 is
also conducive to the progression of the disease by means of
processing a number of growth factors and growth factor receptors
and takes part in the activation of EGFR and related receptors,
which is causally related to the progression of different cancers
of epidermal origin (6–8). ADAM17 plays the role of signal
scissor in cancer microenvironment (9). Recently, it has been proved as the
major sheddase for a variety of EGFR pro-ligands such as
heparin-binding-EGF, transforming growth factor-alpha (TGF-α),
amphiregulin (AREG), neuregulin (NRG), epiregulin (EREG) and
betacellulin (BTC) as well as factors which are important in
inflammation, particularly TNF-α and its receptor (2,10,11).
EGFR ligand-binding leads to receptor self-dimerization,
autophosphorylation and followed activation of downstream MEK-ERK
or PI3K-AKT pathways (12,13). In the field of breast cancer, there
has been substantial research on the expression of ADAM17 based on
cells, animal models and clinical samples. ADAM17 showed a lower
expression in less aggressive rather than in highly aggressive
breast cancers and patients with low ADAM17 expression had an
obviously longer overall survival than those with high expression
(14,15). Our previous studies found that
there was a significant increase of ADAM17 expression in breast
cancers compared to normal human breast tissues (16,17),
which indicate that ADAM17 may be a potential clinical therapeutic
target for breast cancer, and that ADAM17-siRNA inhibited MCF-7
breast cancer cell proliferation and invasion in vitro
(18,19). Our present study reports that
ADAM17-siRNA inhibits MCF-7 breast cancer cell migration and
proliferation in vitro and is activated through the
EGFR-PI3K-AKT signaling pathway and that ADAM17-siRNA can inhibit
MCF-7 breast cancer in vivo.
Materials and methods
Cell line and cell culture
The MCF-7 human breast cancer cell line was obtained
from the Institute of Basic Medical Sciences, Chinese Academy of
Medical Sciences (Beijing, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS), 50 μg/ml
streptomycin and 50 units/ml penicillin, in a humidified atmosphere
incubator of 5% CO2 at 37°C. The selective EGFR blocker
AG1478, PI3K phosphorylation inhibitor LY294002, and the MEK
inhibitor PD0325901 were purchased from Sigma-Aldrich. Cells from
AG1478, LY294002 and PD0325901 group were cultured with 20 μM
AG1478, LY294002 and PD0325901 for 48 h before migration and
proliferation assay, real-time PCR and western blot analysis,
respectively.
Transfection of MCF-7 cells with
ADAM17-siRNA
ADAM17-small interference RNA (ADAM17-siRNA):
5′-TGAGGCAG TCTCTCCTATTCCTGACCAGC-3′ and nonsense siRNA:
5′-TGACCACCCTGACCTACGGCGTGCAGTGC-3′ were from Shanghai GenePharma,
Co., Ltd. (Shanghai, China). MCF-7 cells of the ADAM17-siRNA groups
and the nonsense siRNA groups were transfected with Lipofectamine
2000 (Invitrogen) following the manufacturer's instruction. The
same amount of PBS was added to the control group cells.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
According to the manufacturer's instruction, cells
were rinsed with PBS and RNA was extracted using TRIzol reagent
(Invitrogen). Subsequently, RNA was converted to cDNA with
SuperScript II reverse transcriptase (Invitrogen). Whereafter,
qRT-PCR was performed. Primers designed by Primer Premier 5
software were as follows, ADAM17: 5′-ATCAAA CCTTTCCTGCG-3′
(forward) and 5′-CAAACCCATCCTC GTCCA-3′ (reverse); β-actin:
5′-CTGGAACGGTGAAGGT GACA-3′ (forward) and 5′-AAGGGACTTCCTGTAACAATG
CA-3′ (reverse). β-actin was used as the internal control. PCR
instrument on the following cycle: 94°C for denaturing 3 min, 94°C
60 sec, 58°C 60 sec, 72°C 90 sec, a total of 35 circulations, 72°C
for 10 min, computer analysis of gene amplification, export the
corresponding threshold cycle number, and the expression of β-actin
as an internal control to calculate the relative gene expression
levels, detecting ADAM17 mRNA expression levels of MCF-7 breast
cancer cells. Each sample was detected three times with qRT-PCR,
and samples obtained from three independent experiments were used
to analyze the expression of relative genes.
Migration assay in vitro
Migration of cells was performed utilizing 24-well
Transwell chambers with 8.0 μm pore carrying polycarbonate membrane
(BD Biosciences, San Jose, CA, USA) following the manufacturer's
instruction. After trypsinization, cells of the ADAM17-siRNA, the
nonsense siRNA, the AG1478, the LY294002, the PD0325901 and the
control groups were suspended in DMEM, respectively and the cell
concentration of the six groups was adjusted to
5×105/ml. The concentration of AG1478, LY294002 and
PD0325901 in the relevant group was 20 μM, respectively. Suspending
cell liquid (100 μl) of each group was placed in the upper
compartment of the plates. Afterward, the lower compartment was
filled with complete medium and chemokine mixture of 500 μl. After
24 h of incubation, the chamber polycarbonate membrane was cut,
stained with eosin and mounted with neutral gum. The numbers of
adherent cells in each of five random fields for a given well were
counted under an optical microscope at 200 times magnification and
then numbers of every five fields were numerically averaged and
counted. The number of cells through the Transwell chamber was an
indicator to evaluate the tending movement ability.
Growth curve
MCF-7 cells of the ADAM17-siRNA, the nonsense siRNA,
the AG1478, the LY294002, the PD0325901 and the control groups were
seeded at a concentration of 1.5×104 cells/well in
24-well plates filled with the complete medium which refreshed
every 24 h. Thereafter, every day, three wells of total adherent
cells from each group were trypsinized and counted utilizing a
hemocytometer under the microscope, and the cell numbers of every
three wells were numerically averaged. The cell growth curve was
drawn after 7 days of a continuous count.
MTT assay for cell proliferation
The effects of different administrations on breast
cancer cell proliferation was detected by MTT assay. In brief,
MCF-7 cells during the logarithmic growth phase of the
ADAM17-siRNA, the nonsense siRNA, the AG1478, the LY294002, the
PD0325901 and the control group were trypsinized and seeded onto
96-well plates at the concentration of 1×104/well
approximately, and maintained for 24, 48 and 72 h. When the culture
finished, the medium was replaced by 200 μl fresh medium stained
with 250 μg/ml sterile MTT (Chemicon, Billerica, MA, USA), and then
the plates were incubated at 37°C for further 2 h. Afterwards, the
medium was taken out carefully and 200 μl DMSO was added, the
reaction was sustained at 37°C for 15 min. Absorbance was measured
using a microplate reader (Bio-Tek ELx800; BioTek Instruments,
Inc., Winooski, VT, USA) at a wavelength of 570 nm and subtracted
from that at 450 nm. Each experiment was conducted in triplicate
and all detections were repeated in quadruplicate.
Treatment of ADAM17-siRNA on MCF-7 breast
cancer in vivo
Female Nu/Nu athymic mice with a body weight of
15–25 g were obtained from Beijing HFK Bioscience (Beijing, China).
Estrogen 0.2 ml (0.15 mg/ml) was administered to the nude mice by
intraperitoneal injection every day until sacrifice. MCF-7 breast
cancer cells (0.2 ml) (5×107/ml) were implanted
subcutaneously in the right flank of the nude mice after 3 days of
estrogen injection. Tumor diameter was measured with calipers and
the tumor volume was calculated by the formula: (width)2
× length/2. After 2 weeks of implantation, 15 nude mice which had
developed tumors with an average diameter of 7 mm were then divided
into three groups: ADAM17-siRNA group (inject ADAM17-siRNA 10 μg
and in vivo JetPEI™ 1.5 μl), vector group (inject equal volume of
in vivo JetPEI™) and control group (injection equal volume of PBS).
Each dose was injected at different locations around the tumor
every 3 days. Mice were sacrificed on the 16th day after
treatment.
Immunohistochemistry of tumors
When mice were sacrificed, the removed breast cancer
transplanted tumors were fixed in 4% paraformaldehyde,
paraffin-embedded, sectioned and processed for hematoxylin and
eosin (H&E) staining and immunohistochemical staining to
observe under a light microscope. ADAM17 and Ki-67 were examined. A
primary rabbit monoclonal antibody anti-ADAM17 (1:100; Abcam,
Cambridge, MA, USA) and a primary rabbit polyclonal antibody
anti-Ki-67 (1:100; Cell Signaling Technology, Danvers, MA, USA)
were used, respectively, followed by HRP-conjugated secondary goat
antibodies (ZSGB-Bio Co., Ltd., Beijing, China).
Results were evaluated by three pathologists in a
blinded manner. The percentage of positive cells and intensity of
staining in each sample should be evaluated with no less than 1000
cells and 5 high power fields. The percentage of positive cells was
scored as 0 (<10%), 1 (10–30%), 2 (31–70%) and 3 (71–100%),
while the staining intensity was scored as 0 (negative), 1 (weak),
2 (moderate) and 3 (strong). The whole results of
immunohistochemical staining were calculated by the percentage
score × intensity score.
Western blot analysis
Cells or tissues were washed in PBS and proteins
were isolated in RIPA lysis buffer containing 2% protease inhibitor
PMSF (Sigma-Aldrich). Same concentration of proteins from each
group, as measured utilizing a BCA protein assay kit (Pierce,
Rockford, IL, USA), were loaded onto a 10% SDS-PAGE gel after being
denatured. After separation, the proteins were electro-transferred
onto PVDF membranes (Invitrogen). Subsequently, the membranes were
blocked with 5% non-fat milk powder in TBS-T (10 mM, Tris-HCl, pH
7.6 and 150 mM NaCl, 0.1% Tween-20) at room temperature for 1 h and
then incubated respectively with primary antibodies: anti-ADAM17
(1:1,000; Abcam), anti-EGFR (1:1,000; Cell Signaling Technology),
anti-phosphorylated EGFR (1:1,000; Cell Signaling Technology),
anti-AKT (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
and anti-phosphorylated AKT (1:1,000; Santa Cruz Biotechnology) at
4°C overnight. Following washing in TBS-T, the membranes were
probed with HRP-conjugated secondary antibodies (ZSGB-BIO) for 1 h
at room temperature and immunoblots were visualized using an
enhanced chemiluminescence detection kit (Amersham, Little
Chalfont, UK). The experiments were conducted in triplicate.
Statistical analysis
Results are the mean ± SD. Statistical significance
was considered at P<0.05. Comparisons among three or more groups
was made by one-way ANOVA using the SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA).
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of North China
University of Science and Technology.
Results
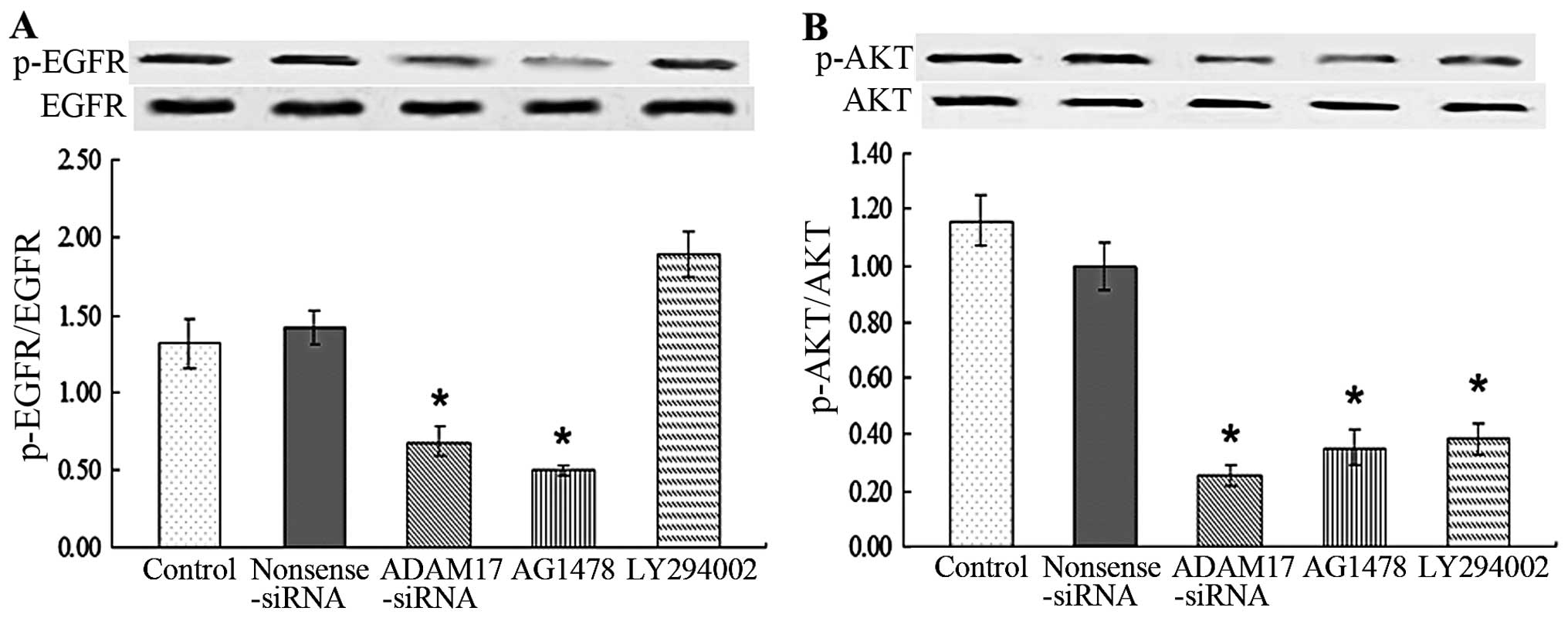
EGFR-PI3K-AKT signaling pathway is
involved in ADAM17 promoting MCF-7 cell migration and
proliferation
To detect the mechanism by which ADAM17 promotes
migration and proliferation ability of MCF-7, we evaluated the
activation of EGFR. MCF-7 had high expression of phosphorylated
EGFR (p-EGFR) while nonsense siRNA and LY294002 had no effect on
it. Administration of ADAM17-siRNA and AG1487 significantly reduced
p-EGFR compared with control (P<0.05; Fig. 1A). The data suggested that ADAM17
promotes migration and proliferation ability of MCF-7 cells by
activating EGFR.
To detect whether PI3K and AKT were downstream of
EGFR and mediated the promotive effect of ADAM17 on migration and
proliferation of MCF-7, we also measured the activation of AKT. The
data showed that MCF-7 cells had a high level of phosphorylated AKT
(p-AKT) while additional administration of ADAM17-siRNA, AG1487 and
LY294002 significantly suppressed p-AKT of MCF-7 cells compared
with control (P<0.05; Fig. 1B).
The data suggested that PI3K and AKT were activated in MCF-7 cells
following the induction of EGFR, which promoted the migration and
proliferation ability of MCF-7.
The tumor growth is inhibited by
ADAM17-siRNA
As shown in Fig. 2,
the volume of transplanted tumors in the control group (Fig. 2A) and the vector group (Fig. 2B) was significantly increased (a
and b in Fig. 2D); while in the
ADAM17-siRNA group (Fig. 2C),
tumor volume was not significantly increased (c in Fig. 2D). The tumor growth curve (Fig. 2E) was drawn according to the
average volume of transplanted tumors in nude mice of different
groups.
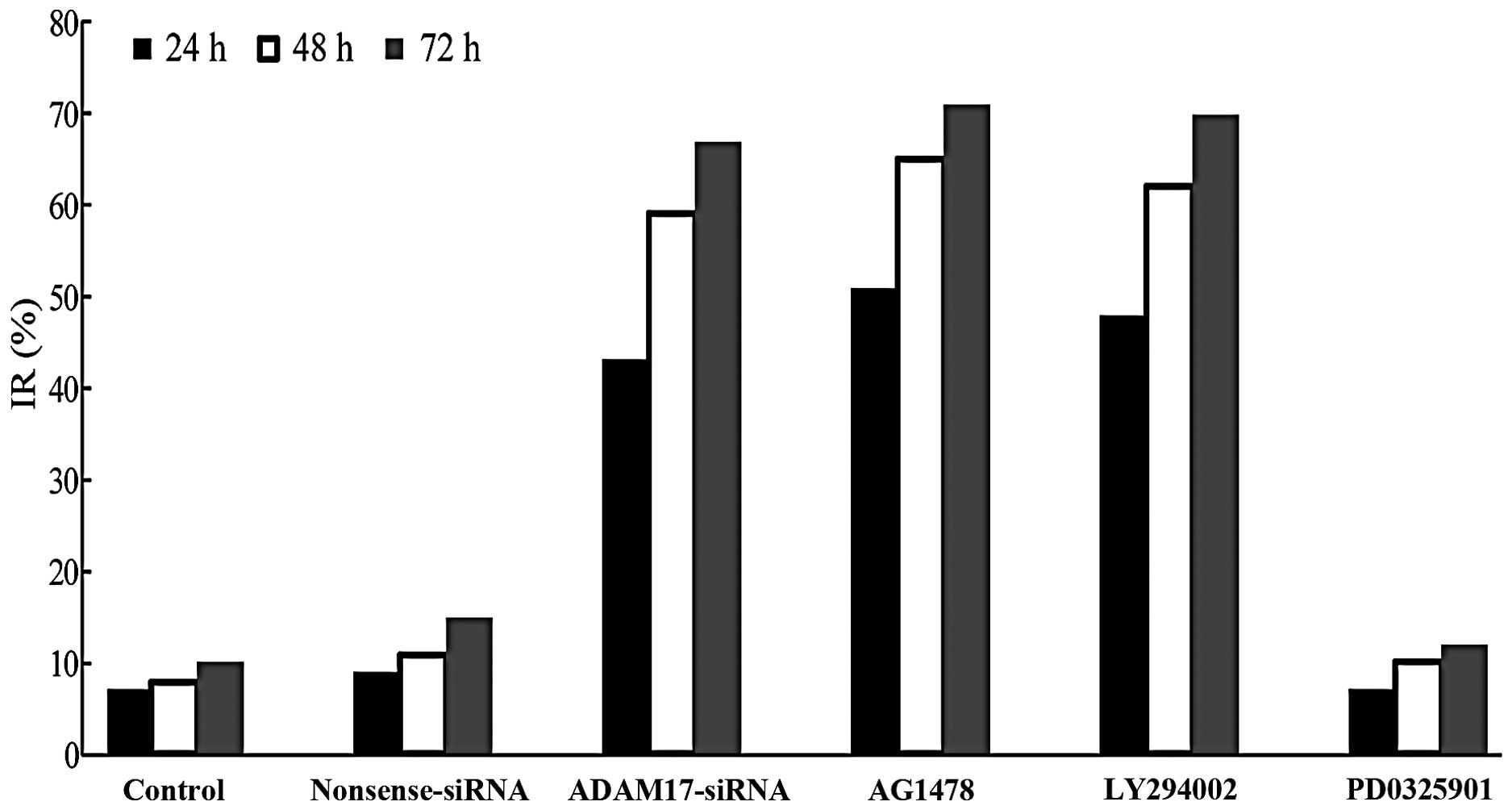
Migration of MCF-7 breast cancer
cells
Migration ability of MCF-7 cells was detected by the
Boyden chamber method. The number of migrated cells per field was
127.88±9.91, 114.96±8.08, 61.63±5.91, 54.24±6.72, 48.79±7.28 and
109.82±10.42 in the control, nonsense siRNA, ADAM17-siRNA, AG1478,
LY294002 and PD0325901 groups, respectively (Fig. 3). Compared with the control, there
was no significant difference in the nonsense and PD0325901 groups,
respectively. However, the number in the ADAM17-siRNA, AG1478 and
LY294002 groups was significantly reduced (P<0.05). The results
indicated that ADAM17 enhances MCF-7 cell migration and
EGFR-PI3K-AKT signaling pathway is also involved in the migration
process of MCF-7, but not EGFR-MEK-ERK signaling pathway.
Growth curve
Compared with the control, there was no significant
difference in the nonsense and PD0325901 groups, respectively.
However, the number of the ADAM17-siRNA, AG1478 and LY294002 groups
was significantly reduced (P<0.05; Fig. 4).
MTT assay for cell proliferation
With the extension of culture time, the
proliferation ability of cells in the control group gradually
enhanced. Compared with the control, there was no significant
difference in the nonsense and PD0325901 groups, respectively.
However, the numbers in the ADAM17-siRNA, AG1478 and LY294002
groups were significantly reduced (P<0.05; Fig. 5). The results indicated that ADAM17
promotes the proliferation of MCF-7 breast cancer cells and
EGFR-PI3K-AKT signaling pathway is also involved in the
proliferation of MCF-7, but not EGFR-MEK-ERK signaling pathway.
ADAM17 expression in MCF-7 cells and
changes induced by ADAM17-siRNA, AG1478 and LY294002
adminstration
Since ADAM17 was involved in the migration and
proliferation process of MCF-7, we detected the levels of ADAM17
mRNA and protein and observed whether ADAM17 expression was
affected by ADAM17-siRNA, AG1478 and LY294002 administration. As
MEK had no effect on migration and proliferation of MCF-7, it was
omitted in the following study. qRT-PCR analysis was used to
measure mRNA levels. The Ct values of ADAM17 mRNA expression were
corrected by β-actin and the ratio of the control group was
considered as 100%. Our results showed that MCF-7 cells (control)
expressed ADAM17 mRNA at a high level. Compared with the control,
nonsense sequence siRNA, AG1478 and LY294002 did not change ADAM17
mRNA expression, but ADAM17-siRNA significantly inhibited it
(P<0.05; Fig. 6A). The changes
of ADAM17 protein expression were detected by western blot
analysis. As shown in Fig. 6B,
ADAM17 protein was highly expressed in MCF-7 cells. Similar to the
changes of mRNA expression, ADAM17-siRNA significantly decreased
the level of ADAM17 protein compared with control group (P<0.05)
although there was no difference in the nonsense sequence siRNA,
AG1478 and LY294002 groups. The data suggest that ADAM17-siRNA
successfully inhibits ADAM17 expression in both mRNA and protein
levels, while inhibition of EGFR and PI3K has no such effect.
Pathological observation of breast cancer
tissues after H&E staining
H&E staining showed that the transplanted tumor
was characterized by typical human breast invasive ductal
carcinoma. Compared with the control group and the vector group,
the tumor tissues in the ADAM17-siRNA group developed large areas
of necrosis, where the cells were destroyed and the cell structures
disappeared; there was no significant difference between the vector
group and the control group (Fig.
7).
Expression of ADAM17 and Ki-67 in
immunohistochemistry of tumors
ADAM17 was mainly expressed in cytoplasm, as
positive brown staining. The transplanted tumor staining index
score in the control group was 5.57±1.8, and in vector group was
5.29±1.89, thus, there was no significant difference between the
two groups; while the staining index of the transplanted tumor in
the ADAM17-siRNA group was 1.71±1.80, compared with the control
group and the vector group, the difference had statistical
significance (P=0.001; Fig. 8A).
The data showed that ADAM17-siRNA inhibits the expression of ADAM17
in the transplanted tumor.
Ki-67 was mainly expressed in the nucleus, as
positive brown staining. The transplanted tumor staining index
score in the control group was 7.00±1.64, and in vector group was
6.57±1.60, thus, there was no significant difference between the
two groups; while the staining index of the transplanted tumor in
the ADAM17-siRNA group was 2.29±1.03, compared with the control
group and the vector group, the difference had statistical
significance (P<0.001; Fig.
8B). The data showed that ADAM17-siRNA inhibits the expression
of Ki-67 in the transplanted tumor.
ADAM17 protein expression in tumor
tissues is reduced by ADAM17-siRNA
The value of ADAM17/β-actin in the control group was
0.74±0.10, and in the vector group was 0.77±0.05, thus the
difference between the two was not statistically significant; while
the value of ADAM17/β-actin in ADAM17-siRNA group was 0.46±0.07,
compared with the control and the vector groups, the difference had
statistical significance (P<0.001; Fig. 9). This data proved that
ADAM17-siRNA successfully inhibits the expression of ADAM17 protein
in the transplanted tumor.
Discussion
ADAM17 is one of the most important members of the
ADAM family. Besides releasing TNF-α, ADAM17 is also conducive to
the progression of disease by means of processing a number of
growth factors and growth factor receptors (6). ADAM17, also known as tumor necrosis
factor-α-converting enzyme, participates in the activation of EGFR
and related receptors, which is causally related to the progression
of different cancers of epidermal origin (6–8).
EGFR generally has a wide range of expression in
most cell types but in hematopoietic cells, moreover, its
activation plays an important role in normal cell physiology
processes (20,21). Nonetheless, unduly increased EGFR
signaling is always bound up with the growth of various malignant
tumors including breast cancer (20,22–24).
EGFR ligand-binding leads to receptor self-dimerization,
autophosphorylation and followed activation of downstream MEK-ERK
or PI3K-AKT signal pathways, which could contribute to the
progression of a tumor (12,13).
There is plenty of research on targeting EGFR in the treatment of
breast cancer (25–27). AG1478, as a specific inhibitor of
EGFR tyrosine kinase, has been widely used in laboratory studies to
demonstrate its antitumor function (28). Both in vitro cell models and
in vivo mouse models have AG1478 prominent
anti-proliferative efficacities and in some tumor cells, moreover,
can heighten antitumor effects of the monoclonal antibody 806 (an
anti-EGFR antibody) and cytotoxic drugs (29,30).
Our western blot results showed that ADAM17-siRNA and AG1478 could
significantly decrease expression of p-EGFR.
PI3K, a heterodimer made up of a p110 catalytic
subunit and a p85 regulatory subunit, has been confirmed to
phosphorylate the serine/threonine kinase (AKT) (31). As a downstream effector of PI3K,
AKT is closely related to cell survival and anti-apoptotic
signaling (32,33). A recent study proved that cell
signaling is mediated by PI3K-AKT through induction of ADAM17,
which is involved in proliferation and migration of cancer cells
(14). LY294002, which
specifically inhibits the PI3K-AKT signal pathway, may compete with
ATP for binding sites on PI3K, thereby suppressing AKT activation
and blocking signal transduction (34). Our western blot results showed that
ADAM17-siRNA, AG1478 and LY294002 significantly decreased
expression of p-AKT.
Tumor cell proliferation and migration are crucial
factors for the growth of malignant tumors, which are complex
processes being regulated and controlled by many genes (35). Our previous results showed
ADAM17-siRNA inhibited MCF-7 breast cancer cell proliferation and
invasion in vitro (18,19).
In this project, we continued to employ MCF-7 human breast cancer
cell line in investigating the effect of ADAM17 on proliferation
and migration of breast cancer in vitro. Our data
demonstrated that reduction of ADAM17 by siRNA significantly
decreased cancer cell proliferation and migration. EGF has been
demonstrated to excite the migration and proliferation of both
normal and cancer cells, including normal breast epithelial cells
and breast cancer cells (36,37).
EGFR is activated by binding ligands, such as EGF, TNF-α, to
stimulate proliferation, migration and metastasis and ADAM17 has
been shown to hydrolyze these ligands (6–8).
Thus, we further explored the contribution of EGFR-PI3K-AKT signal
pathway or EGFR-Ras-Raf-MEK-ERK signal pathway to the ADAM17
induced migration and proliferation of breast cancer cells. Boyden
chamber assay showed that AG1478, and LY294002 effectively blocked
migration of MCF-7 cells, but PD0325901 could not affect migration
of MCF-7 cells. Similarly, MTT assay showed that AG1478, and
LY294002 also effectively reduced proliferation of MCF-7 cells, but
PD0325901 could not affect proliferation of MCF-7 cells. These
results indicated that ADAM17-siRNA inhibiting MCF-7 breast cancer
cell migration and proliferation may be through EGFR-PI3K-AKT
signal pathway, but not EGFR-Ras-Raf-MEK-ERK signal pathway.
We further confirmed the effect of EGFR-PI3K-AKT
signal pathway on the ADAM17 induced migration and proliferation of
breast cancer cells. As a competitive inhibitor of ATP binding site
in the kinase domain, the quinazoline derivative AG1478 is a highly
effective and specific reversible tyrosine kinase inhibitor of the
EGFR (29,30). We found that the activation of EGFR
and its downstream AKT signal pathway could be efficiently
inhibited by AG1478. Specific suppression of EGFR-PI3K-AKT signal
pathway by inhibitor LY294002 also weakened the migration and
proliferation ability of MCF-7 cells. The results suggested that
ADAM17 contributed to cancer development via activation of
EGFR-PI3K-AKT signal pathway. Kenny and Bissel (38) also reported similar findings.
ADAM17 has been shown to play a critical role in the progression of
EGFR dependent malignant tumors. Besides, our previous study proved
ADAM17-siRNA inhibited proliferation and invasion of MCF-7 breast
cancer cells in vitro (18,19).
These data imply that ADAM17-siRNA inhibits metastasis of breast
cancer cells towards other organs.
Subsequently, we tested the antitumor effect of
ADAM17-siRNA in vivo. We established transplanted tumor
models of MCF-7 breast cancer cells in nude mice and used a
multi-point injection of JetPEI™-ADAM17-siRNA in the tumor for
treatment. The results showed that ADAM17-siRNA can inhibit tumor
growth and cause tissue necrosis of a tumor. Immunohistochemistry
showed that the expression of ADAM17 and Ki-67 in tumor tissues was
decreased. Ki-67, a kind of alkaline protein with protease
properties, as a common malignant tumor biological indicator, can
be used to determine the cell proliferation activity of tumor
cells, to judge the malignant degree of the tumor and to evaluate
the prognosis of patients (39).
Ki-67 is expressed in many kinds of tumors, especially in breast
cancer tissues, and its expression level in breast cancer tissues
was much higher than that in normal breast tissues (40). Our western blot results showed that
ADAM17 protein expression in tumor tissues was reduced by
ADAM17-siRNA. These results indicated that ADAM17-siRNA can
significantly inhibit breast cancer in vivo. The way of
multi-point injection in tumor is feasible in the experimental
animals, but not suitable for clinical application, therefore, in a
future study, we will employ bone marrow mesenchymal stem cells
(BMSCs) as the carrier, to observe whether it can transport the
ADAM17-siRNA to the tumor and the effect of this method on the
biological behavior of breast cancer and provide a new treatment
method for targeted therapy. The treatment of breast cancer
targeting ADAM17 is worthy of further study.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Hebei Province, P.R. China (no.
C2010001767) and the Tangshan Science & Technology Bureau of
Hebei Province, P.R. China (no. 14130256B).
References
|
1
|
Krieger N, Bassett MT and Gomez SL: Breast
and cervical cancer in 187 countries between 1980 and 2010. Lancet.
379:1391–1392. 2012. View Article : Google Scholar
|
|
2
|
Caiazza F, McGowan PM, Mullooly M, Murray
A, Synnott N, O'Donovan N, Flanagan L, Tape CJ, Murphy G, Crown J,
et al: Targeting ADAM-17 with an inhibitory monoclonal antibody has
antitumour effects in triple-negative breast cancer cells. Br J
Cancer. 112:1895–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ocaña A, Amir E, Seruga B, Martin M and
Pandiella A: The evolving landscape of protein kinases in breast
cancer: Clinical implications. Cancer Treat Rev. 39:68–76. 2013.
View Article : Google Scholar
|
|
4
|
Zhang P, Shen M, Fernandez-Patron C and
Kassiri Z: ADAMs family and relatives in cardiovascular physiology
and pathology. J Mol Cell Cardiol. 93:186–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bolger JC and Young LS: ADAM22 as a
prognostic and therapeutic drug target in the treatment of
endocrine-resistant breast cancer. Vitam Horm. 93:307–321. 2013.
View Article : Google Scholar
|
|
6
|
Rose-John S: ADAM17, shedding, TACE as
therapeutic targets. Pharmacol Res. 71:19–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Y, Jiang F, Zheng X, Katakowski M,
Buller B, To SS and Chopp M: TGF-β1 promotes motility and
invasiveness of glioma cells through activation of ADAM17. Oncol
Rep. 25:1329–1335. 2011.PubMed/NCBI
|
|
8
|
Santiago-Josefat B, Esselens C, Bech-Serra
JJ and Arribas J: Post-transcriptional up-regulation of ADAM17 upon
epidermal growth factor receptor activation and in breast tumors. J
Biol Chem. 282:8325–8331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy G: The ADAMs: Signalling scissors
in the tumour microenvironment. Nat Rev Cancer. 8:929–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rego SL, Helms RS and Dréau D: Tumor
necrosis factor-alpha-converting enzyme activities and
tumor-associated macrophages in breast cancer. Immunol Res.
58:87–100. 2014. View Article : Google Scholar
|
|
11
|
Maretzky T, Zhou W, Huang XY and Blobel
CP: A transforming Src mutant increases the bioavailability of EGFR
ligands via stimulation of the cell-surface metalloproteinase
ADAM17. Oncogene. 30:611–618. 2011. View Article : Google Scholar
|
|
12
|
Zheng X, Jiang F, Katakowski M, Lu Y and
Chopp M: ADAM17 promotes glioma cell malignant phenotype. Mol
Carcinog. 51:150–164. 2012. View
Article : Google Scholar
|
|
13
|
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou
HF, Guo L, Liu W, Wang SJ and Yu XG: ADAM17 targets MMP-2 and MMP-9
via EGFR-MEK-ERK pathway activation to promote prostate cancer cell
invasion. Int J Oncol. 40:1714–1724. 2012.
|
|
14
|
Zheng X, Jiang F, Katakowski M, Zhang ZG,
Lu QE and Chopp M: ADAM17 promotes breast cancer cell malignant
phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther.
8:1045–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGowan PM, McKiernan E, Bolster F, Ryan
BM, Hill AD, McDermott EW, Evoy D, O'Higgins N, Crown J and Duffy
MJ: ADAM-17 predicts adverse outcome in patients with breast
cancer. Ann Oncol. 19:1075–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han X, Yang XF, Zhang XP, Sun Y, Zhao JH
and Zhao GM: Expression of ADAM17 in tissue of breast cancer and
its clinical significance. Modern Journal of Integrated Traditional
Chinese and Western Medicine. 19:2486–2488. 2010.
|
|
17
|
Yang XF, Zhang XP, Zhao GM, Sun Y and Han
X: Clinical significance of ADAM-17 protein expression in invasive
breast cancer. Shandong Med J. 51:84–85. 2011.
|
|
18
|
Yang WJ, Zhang XP, Jiao JM, Zhao GM, Lu YQ
and Hu BS: Inhibitory effects of ADAM17-siRNA on the invasion of
human MCF-7 breast cancer. Tianjin Med J. 39:1045–1407. 2011.
|
|
19
|
Lu YQ, Zhao GM and Zhang XP: Effects of
ADAM17-siRNA on the proliferation of human MCF-7 breast cancer.
Modern J Integrated Traditional Chinese and Western Med.
20:3003–3005. 2011.
|
|
20
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar
|
|
21
|
Dienstmann R, Braña I, Rodon J and
Tabernero J: Toxicity as a biomarker of efficacy of molecular
targeted therapies: Focus on EGFR and VEGF inhibiting anticancer
drugs. Oncologist. 16:1729–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azuaje F, Tiemann K and Niclou SP:
Therapeutic control and resistance of the EGFR-driven signaling
network in glioblastoma. Cell Commun Signal. 13:232015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CC, Shiao HY, Wang WC and Hsieh HP:
Small-molecule EGFR tyrosine kinase inhibitors for the treatment of
cancer. Expert Opin Investig Drugs. 23:1333–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lorusso V, Forcignano R, Cinieri S,
Tinelli A, Porcelli L, Quatrale AE and Chiuri VE: Which role for
EGFR therapy in breast cancer? Front Biosci (Schol Ed). 4:31–42.
2012. View Article : Google Scholar
|
|
25
|
Lluch A, Eroles P and Perez-Fidalgo JA:
Emerging EGFR antagonists for breast cancer. Expert Opin Emerg
Drugs. 19:165–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Howe LR and Brown PH: Targeting the
HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res
(Phila). 4:1149–1157. 2011. View Article : Google Scholar
|
|
27
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P, Torossian A, Zhang Q, Xu WC and Fu
S: Inhibition of phosphoinositide 3-kinase enhances the
cytotoxicity of AG1478, an epidermal growth factor receptor
inhibitor, in breast cancer cells. Med Oncol. 29:3258–3264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caja L, Sancho P, Bertran E, Ortiz C,
Campbell JS, Fausto N and Fabregat I: The tyrphostin AG1478
inhibits proliferation and induces death of liver tumor cells
through EGF receptor-dependent and independent mechanisms. Biochem
Pharmacol. 82:1583–1592. 2011. View Article : Google Scholar
|
|
30
|
Zhang YG, Du Q, Fang WG, Jin ML and Tian
XX: Tyrphostin AG1478 suppresses proliferation and invasion of
human breast cancer cells. Int J Oncol. 33:595–602. 2008.PubMed/NCBI
|
|
31
|
Ke XY, Wang Y, Xie ZQ, Liu ZQ, Zhang CF,
Zhao Q and Yang DL: LY294002 enhances inhibitory effect of
gemcitabine on proliferation of human pancreatic carcinoma PANC-1
cells. J Huazhong Univ Sci Technolog Med Sci. 33:57–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian S, Chang W, Du H, Bai J, Sun Z, Zhang
Q, Wang H, Zhu G, Tao K and Long Y: The interplay between GRP78
expression and Akt activation in human colon cancer cells under
celecoxib treatment. Anticancer Drugs. 26:964–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W, et al: MiR-20a induces cell
radioresistance by activating the PTEN/PI3K/Akt signaling pathway
in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen P, Wu J, Yuan Q, Jiang X and Huang H:
The synergistic killing of AML cells co-cultured with HS-5 bone
marrow stromal cells by As2O3 and the PI3K/Akt signaling pathway
inhibitor LY294002. Pharmazie. 70:322–327. 2015.PubMed/NCBI
|
|
35
|
Wang Y and Lazo JS: Metastasis-associated
phosphatase PRL-2 regulates tumor cell migration and invasion.
Oncogene. 31:818–827. 2012. View Article : Google Scholar
|
|
36
|
Whitsett TG, Cheng E, Inge L, Asrani K,
Jameson NM, Hostetter G, Weiss GJ, Kingsley CB, Loftus JC, Bremner
R, et al: Elevated expression of Fn14 in non-small cell lung cancer
correlates with activated EGFR and promotes tumor cell migration
and invasion. Am J Pathol. 181:111–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han J, Xie Y, Lan F, Yu Y, Liu W, Chen J,
Zheng F, Ouyang X, Lin X, Lin Y, et al: Additive effects of EGF and
IL-1β regulate tumor cell migration and invasion in gastric
adenocarcinoma via activation of ERK1/2. Int J Oncol. 45:291–301.
2014.PubMed/NCBI
|
|
38
|
Kenny PA and Bissell MJ: Targeting
TACE-dependent EGFR ligand shedding in breast cancer. J Clin
Invest. 117:337–345. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanei T, Shimomura A, Shimazu K, Nakayama
T, Kim SJ, Iwamoto T, Tamaki Y and Noguchi S: Prognostic
significance of Ki67 index after neoadjuvant chemotherapy in breast
cancer. Eur J Surg Oncol. 37:155–161. 2011. View Article : Google Scholar
|
|
40
|
Yoshioka T, Hosoda M, Yamamoto M, Taguchi
K, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y and Yamashita H:
Prognostic significance of pathologic complete response and Ki67
expression after neoadjuvant chemotherapy in breast cancer. Breast
Cancer. 22:185–191. 2015. View Article : Google Scholar
|