Introduction
Angiogenesis is a critical hallmark of malignancy
and is one of the features used frequently by pathologists to make
this histological diagnosis and to assess tumor grade (1). Although modulated by various
proangiogenic factors, including matrix metalloproteases (MMPS),
platelet derived growth factor-β (PDGF-β), tumor necrosis factor-α
(TNF-α), and transforming growth factor-β (TGF-β), angiogenesis is
principally driven by interactions between vascular endothelial
growth factors (VEGFs) and VEGF receptors (VEGFRs), with persistent
upregulation of this process being an important factor in the
pathology of cancer growth and metastasis (2,3). Of
seven VEGF family ligands, such as VEGF-A (VEGF), VEGF-B, VEGF-C,
VEGF-D, VEGF-E, placental growth factor (PlGF) and VEGF-F, VEGF-A
is known to be the essential regulator of tumor angiogenesis and
endothelial proliferation permeability and survival (4–6).
VEGF binds primarily to two tyrosine kinase receptors with high
affinity, VEGFR-1 and VEGFR-2 (7).
Emerging data suggest that another non-tyrosine kinase receptor
identified neuropilins are believed to function as co-receptors for
VEGFR-1 and VEGFR-2 (8,9), which trigger the full spectrum of
VEGF-induced biological modifications, including proliferation,
migration, vascular endothelial cell differentiation and
angiogenesis (10,11).
Neuropilins (NRPs) are transmembrane glycoprotein
receptors that play an important role in the development of the
neuronal and vascular systems as receptors for members of the
class-3 semaphorin family (SEMAs) of axonal guidance factors and
also for members of the vascular endothelial growth factor (VEGF)
family of angiogenesis factors (12,13).
In higher eukaryotes, two neuropilin genes, neuropilin-1 (NRP-1)
and neuropilin-2 (NRP-2), have been identified. They have
approximately 44% amino acid sequence identity and share many
structural and biological properties (13–15).
Both NRP-1 and NRP-2 contain a large extracellular region and a
short cytoplasmic tail of approximately 40 amino acids, lacking any
enzymatic activity. Their extracellular region contains three
domains: two CUB homology domains (a1a2) as SEMA 3 ligand-binding
domain, two coagulation factor V/VIII homology domains (b1b2) as
VEGF binding domain, and a MAM domain (c) involved in NRP-1
dimerization (16,17). The binding site for VEGF ligands
has been localized to the b1b2 domains of NRP-1 and NRP-2, whereas
the binding of semaphorins requires both the a1a2 and b1b2 repeats
(18). NRP-1 and NRP-2 interact
selectively with different members of the VEGF and semaphorin
families and have non-overlapping expression patterns (18). NRP-1 binds VEGF-A165, VEGF-B,
VEGF-E, PlGF, SEMA3A, SEMA3B and SEMA3C, whereas NRP-2 binds
VEGF-A165, VEGF-A145, VEGF-C, VEGF-D, SEMA3B, SEMA3C and SEMA3F
(18,19). However, VEGF-A165 binds 50-fold
more strongly to NRP-1 than NRP-2 (20). NRP-1 was found to interact with
VEGF-A165 (and other VEGFs), and to act as a VEGF co-receptor that
specifically enhances VEGFR-2 signaling to promote VEGF biological
activity, including endothelial cell migration, sprouting and
angiogenesis (21,22). Transgenic overexpression or
knockout of the NRP-1 gene results in lethal abnormalities in the
cardiovascular system, suggesting that NRP-1 plays an important
role in vasculogenesis and angiogenesis (23). Nevertheless, NRP-2 has different
(but overlapping) binding preferences for VEGF family members, and
is a co-receptor for VEGFR-3 that is involved in lymphatic
endothelial cell function (24).
NRPs are differentially expressed, with NRP-1
detected primarily in arterial endothelial cells, whereas NRP-2
expression is found in venous and lymphatic endothelium (25). Recently, both NRP-1 and NRP-2 are
reported to be upregulated in several human tumors, with NRP-1 more
preferentially upregulated than NRP-2, and therefore, each is
implicated in different aspects of tumor pathogenesis (15,26).
For example, blocking NRP-1 function with anti-NRP-1 antibodies
inhibited tumor growth (27),
whereas anti-NRP-2 antibodies did not affect primary tumor growth,
instead they reduced tumor metastasis to sentinel lymph nodes and
distant organs (28). Furthermore,
overexpression of NRP-1 is closely correlated with the infiltration
and migration of tumors, therapy resistance and poor prognosis
(29–32). These findings revealed that NRP-1
might serve as a novel target for cancer diagnosis and therapy.
NRP-1 inhibition using monoclonal antibody is considered as a
promising strategy for cancer therapy (33,34).
In vivo imaging of tumor-receptor offers a
more accurate and real-time assay of receptor expression both for
patient stratification and monitoring expression-level changes in
response to therapy, without such biopsy-associated pitfalls and
the need of repetitive invasive biopsies (35). A variety of small molecular
peptides based upon NRP-1 have been labeled with radionuclide
99mT for single photon emission computed tomography
(SPECT) molecular imaging of NRP-1 expression (36,37).
But, for the small molecules, the probes generally show rapid blood
clearance, very low tumor uptake; thus, the imaging quality is
poor. Compared with small peptides, monoclonal antibodies (mAbs)
can improve imaging of NRP-1-expression, due to their high
affinity, specificity and slow extraction. Our previous studies has
shown that a novel monoclonal antibody against NRP-1 b1b2 domain
(A6-11-26), generated by our laboratory (38), can inhibit tumor proliferation,
growth and migration, such as gliomas and breast cancer (39,40),
suggesting that A6-11-26 may be an effective agent for
NRP-1-targeted imaging and therapy. A6-11-26 specifically binds to
NRP-1 b1b2 domain, but not NRP-2 b1b2 domain (data not published),
consistent with previous reports (26,27).
Therefore, A6-11-26 might be valuable to specifically exploit NRP-1
expression, eliminating any possible undesirable effects mediated
by NRP-2. In the present study, we aimed to perform the Iodogen
strategy to label the anti-NRP-1 monoclonal antibody A6-11-26 with
iodine-131, and further determine whether the resulting SPECT
(single-photon emission computed tomography) probe
131I-A6-11-26 is a suitable agent for imaging mice
bearing NRP-1 expression glioma U87MG tumors.
Materials and methods
General
Goat anti-mouse IgG antibody was purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Iodogen-coated
tubes were purchased from Pierce Biotechnology Ltd. (Rockford, IL,
USA). Na131I was obtained from the China Institute of
Atomic Energy (Beijing, China). A PD-10 Sephadex G-25 column from
GE Healthcare Biosciences, Ltd. (Diegem, Belgium). rProtein A
Sepharose columns were purchased from GE Healthcare Bio-Sciences
Ltd. (Uppsala, Sweden). DMF-96 gamma counter from Hefei Zhongcheng
Electromechanical Technology Development, Co., Ltd. (Hefei, China).
CRC-25R dose calibrator from Capintec, Inc. (Ramsey, NJ, USA).
BrightView XCT SPECT/CT from Philips Medical Systems, Inc.
(Milpitas, CA, USA). Glioma U87 MG cell line was obtained from the
Cell Culture Center of Institute of Basic Medical Sciences of
Chinese Academy of Medical Sciences (Beijing, China). Female nude
mice, 6 and 8 weeks of age, and Balb/c mice were purchased from the
Experimental Animal Center of Xiamen University (Xiamen,
China).
Production and purification of anti-NRP-1
monoclonal antibodies
An anti-NRP-1 monoclonal antibody (A6-11-26) was
produced by hybridomas derived from mice immunized with a
recombinant human NRP-1 b1b2 in our laboratory according to a
method previously described (38,40).
Briefly, 6-week-old Balb/c mice were injected with hybridoma cells
(2×105–106). Seven to 10 days later, ascites
(5–10 ml/mouse) with anti-NRP-1 b1b2 monoclonal antibodies
(A6-11-26) were centrifuged at 12,000 × g for 5 min and the
supernatant were collected. A6-11-26 were purified by rProtein A
Sepharose column chromatography as previously described (38), and diluted in PBS. The purity and
concentration of A6-11-26 were assessed by 8% SDS-PAGE gel and
Bradford assay, respectively.
Titer analysis of A6-11-26
Titer analysis of A6-11-26 was performed by indirect
ELISA according to the previously described methods (38). Briefly, 96-well plates were coated
with 10 μg/ml of NRP-1 b1b2 in carbonate buffer (pH 9.6) and
incubated overnight at 4°C. Non-specific binding was blocked with
5% non-fat dry milk in PBS (pH 7.5) for 2 h at 37°C followed by
washing three times with washing buffer (0.05% Tween-20 in PBS).
The plates were then incubated with supernatant of hybridoma cell
(ascites) or A6-11-26 or antiserum IgG of mice for 2 h at 37°C,
respectively. After washing, the plates were incubated with goat
anti-mouse IgG-HRP conjugate for 1 h at 37°C. Finally, the plates
were washed as before and o-Phenylenediamine (OPD) was added to
develop color. The optical density (OD) was determined at 450 nm by
a microplate ELISA reader (Bio-Rad Laboratories, Tokyo, Japan)
after the reaction was stopped with 2 M
H2SO4.
Cellular immunofluorescence staining
Cellular immunofluorescence staining was performed
as previously described (29).
Briefly, the cell-seeded coverslips were washed and fixed. The
diluted (1:100) A6-11-26 and the diluted (1:50) fluorescence
(TRITC)-labeled secondary antibody were added. After the U87MG
cells were fluorescence-labeled, the fluorescence-labeled secondary
antibody was discarded, eluted and stained with Hoechst 33258
staining solution, then observed under a confocal scanning
microscope, in which, the excitation wavelength for Hoechst 33258
was ~350 nm and the emission wavelength was ~460 nm; while the
maximum absorption wavelength of light for TRITC was 550 nm and the
maximum emission wavelength was 620 nm. The relevant images were
shot.
Labeling anti-NRP-1 monoclonal antibody
A6-11-26 with iodine-131
An anti-NRP-1 monoclonal antibody A6-11-26 was
labeled with Na131I by the Iodogen method according to
the previous study (41). Briefly,
100 μl 0.01 M phosphate-buffered saline (PBS, pH 7.4) and 22.8 MBq
Na131I were added into the prepared Iodogen-coated
tubes, and then 20 μg of A6-11-26 was added. Subsequently, the
mixture was incubated at room temperature for 15 min with
occasional shaking. The reaction was quenched by incubation with
150 μl 0.01 M PBS for 15 min at room temperature. Radiolabeled
antibodies were then purified by size-exclusion chromatography
using a PD-10 Sephadex G-25 column. For routine quality control of
labeling, the labeling efficiency and radiochemical purity of
radiolabeled A6-11-26 probes were calculated by paper
chromatography on Xinhua filter paper (Hangzhou Xinhua Paper
Industry, Co., Ltd., Hangzhou, China) with the mixture developed
with n-butanol:ethanol:ammonia (5:1:2) as the mobile phase.
Retention factors (Rf) were: 131I-A6-11-26=0.01, free
131I=0.9–1.0.
Immunoreactive fraction assay
Immunoreactive fraction of 131I-A6-11-26
was performed according to the previously described methods with
slight modifications (42,43). Briefly, U87MG cells were washed
three times with 0.01 M PBS (pH 7.4) and suspended in a cold PBS
with 1% bovine serum albumin (BSA) solution.
131I-A6-11-26 at a constant concentration of 50 ng/ml,
in PBS with 1% BSA solution was added to different amounts of cells
(final concentration ranging from 2.6×06 to
0.08×106 cells/ml). Cells were incubated for 2 h at 4°C
and then washed twice with 500 μl of cold PBS with 1% BSA solution,
before counting cell-associated radioactivity in a gamma counter.
The data were plotted as a double inverse plot of the applied
radiolabelled antibody over the specific binding as a function of
the inverse cell concentration. In this plot, the origin of the
abscissa represents infinite cell concentration, i.e., conditions
of infinite antigen excess.
In vitro stability analysis
In vitro stability in serum or saline was
determined by paper chromatography method using strips on Xinhua
filter paper (1 cm width and 13 cm length) as described with minor
modifications (44,45). Briefly, 131I-A6-11-26
(4.44 MBq) in 250 μl of PBS was added to 2.0 ml of mouse serum or
0.01 M PBS (pH 7.4) and was incubated at 37°C for 1, 6, 24, 48, 72
and 96 h. At each time-point, the mixture was centrifuged at 16,000
× g for 2 min. A total of 2 μl of the supernatant was placed 2 cm
above the lower edge and was allowed to evaporate spontaneously,
one strip was developed with the mixture with
n-butanol:ethanol:ammonia (5:1:2). After complete development, the
paper sheet was removed, dried, and cut into strips of 1 cm width;
and then each strip was counted in a gamma counter.
Cell assays
Cell uptake, receptor saturation and internalization
assays were performed as previously described with minor
modifications (44,46–48).
Briefly, the U87MG cell lines were cultured in DMEM supplemented
with 10% FBS and 1% penicillin-streptomycin. The cells were
maintained in a humidified atmosphere of 5% CO2 at 37°C,
with the medium changed every two days. A 70–80% confluent
monolayer was detached by 0.1% trypsin and dissociated into a
single cell suspension for further cell culture.
Cell uptake assays
The U87MG cells were washed three times with 0.01 M
PBS (pH 7.4) and dissociated with 0.25% trypsin-EDTA. DMEM medium
was then added to neutralize trypsin-EDTA. Cells were spun down and
re-suspended with serum-free DMEM. Cells (0.5×106) were
incubated at 37°C for 0.25 to 2 h with 5.4×10−3 MBq,
0.02 μg 100 μl 131I-A6-11-26 in 0.5 ml serum-free DMEM
medium. The non-specific binding of the probes with U87MG cells was
determined by co-incubation with 2.0 μg unlabeled A6-11-26. The
cells were washed three times with 0.01 M PBS (pH 7.4) at room
temperature. The cells were then washed three times with chilled
PBS and spun down at a speed of 7,000–8,000 rpm. The cell pellets
at the bottom of the tube were spliced, and the radioactivity of
the pellets was measured using a gamma counter. The uptake
(counts/min) was normalized to the percentage of binding for
analysis using Excel (Microsoft Software, Inc., Redmond, WA, USA).
All experiments were performed in duplicate.
Receptor saturation assay
The U87MG cells (0.5×106) were plated on
6-well plates one day before the experiment. Cells were washed with
PBS three times. Serum-free DMEM (1 ml) was added to each well,
followed by the addition of 131I-A6-11-26
(11.1–599.4×10−3 MBq, 2–120 nM final concentration). The
non-specific binding of 131I-A6-11-26 with U87MG cells
was determined by co-incubation with 100 times excess (0.6 μM) of
A6-11-26. The plates were then put on ice for 2 h, and the cells
were washed with cold PBS three times and detached with TrypLE
Express. The radioactivity of the cells was measured using a gamma
counter. Specific binding (SB) = total binding (TB) − non-specific
binding (NSB). The data were analyzed using GraphPad Prism
(GraphPad Software, Inc., San Diego, CA, USA), and the dissociation
constant (KD value) of 131I-A6 was calculated
from a 1-site-fit binding curve. All experiments were performed in
duplicate.
Internalization assay
The U87MG cells (0.6×106) were plated on
6-well plates and incubated overnight with internalization buffer
(DMEM containing 1% fetal bovine serum) to obtain good cell
adherence. The following day, the cells were pretreated with the
internalization medium for 1 h at 37°C. 131I-A6-11-26
(5.4×10−3 MBq, 0.02 μg, 100 μl) was added to the medium,
and the cells were incubated for 1, 2, 4, 8, 16 and 24 h at 37°C
and 5% CO2. A 100-fold excess of unlabeled A6-11-26 (2.0
μg) was used to determine non-specific internalization. At each
time-point, the internalization was stopped by removal of the
medium followed by washing the cells with ice-cold 0.01 M PBS (pH
7.4). Cells were then treated for 5 min (three times) with ice-cold
glycine buffer (0.05 mol/l glycine solution, and pH was adjusted to
2.8 with 1 mol/l HCl) to distinguish between cell surface-bound
(acid-releasable) and internalized (acid-resistant) radiolabeled
antibody. Finally, cells were detached from the plates by
incubation with 1.0 M NaOH for 10 min at 37°C. The medium, the
receptor-bound and the internalized fraction of
131I-A6-11-26 were measured in a gamma counter, and the
internalized radioactivity rate was calculated and normalized to
1×106 cells/well. All experiments were performed in
duplicate.
Biodistribution study
The animal procedures were performed according to a
protocol approved by the Institutional Animal Care and Use
Committee of Zhongshan Hospital Xiamen University. Approximately
5×106 cultured U87MG cells suspended in PBS were
implanted subcutaneously in the right upper shoulders of nude mice.
Tumors were allowed to grow to ~0.8–1.0 cm in diameter (25–30 days)
and then the tumor-bearing mice were subject to in vivo
biodistribution and imaging studies.
For biodistribution studies, U87MG tumor-bearing
mice (n=5 for each group) were injected with
131I-A6-11-26 (1.2 MBq, 200 μl) through the tail vein.
At 24, 48, 72, 96 and 120 h after injection, the mice were
sacrificed, and tumors and normal tissues of interest were removed
and weighed, and their radioactivity was measured in a gamma
counter. The radioactivity uptake in the tumor and normal tissues
was expressed as a percentage of the injected radioactivity per
gram of tissue (%ID/g). In order to study the in vivo NRP-1
targeting specificity of 131I-A6-11-26, based on the
previous studies (44,49), unlabeled A6-11-26 antibody (700 μg)
was co-injected with 131I-A6-11-26 in nude mice bearing
U87MG tumors (n=5 for each group) via a tail vein, and
biodistribution studies were conducted at 120 h after
injection.
SPECT/CT imaging
SPECT/CT imaging of tumor-bearing mice was performed
on a dual-head SPECT/CT scanner. The mice bearing U87MG tumor (n=5
for each group) were injected with 131I-A6-11-26 (3.7
MBq, 200 μl) with or without co-injection of unlabeled A6-11-26
antibody (700 μg) through the tail vein. At 24, 48, 72, 96 and 120
h after injection, the mice were anesthetized with 2% isoflurane
and placed on SPECT/CT bed (ventral side down). SPECT images were
acquired in 30 projections over 15 min using a double-headed camera
with high energy, high-resolution collimators. CT images were
acquired in 30 projections with a 1000 msec exposure time using a
45 kVp X-ray source over 5 min. Whole-body radionuclide images were
reconstructed using an iterative ordered subset expectation
maximization two dimensional algorithm, and these images were fused
with CT images using Syntegra software (Philips Medical Systems).
Regions of interest (ROIs) were drawn over the tumor and
contralateral muscle, and then the ratio of tumor to contralateral
muscle (T/NT) were calculated.
Statistical methods
Statistical analysis was performed using Student’s
two-tailed t-test for unpaired data. A 95% confidence level was
chosen to determine the significance between groups, with a
P<0.05 being indicated as a significant difference.
Results
Characterization of anti-NRP-1 monoclonal
antibodies (A6-11-26)
Our previous western blot results showed that
A6-11-26 was specifically combined with both NRP-1 b1b2 recombinant
protein and whole NPR-1 (38), but
not NRP-2 b1b2 domain (data not published). To identify the purity
of the current A6-11-26 obtained from ascites, the purified
A6-11-26 was resolved by 12% SDS-PAGE (Fig. 1A). A6-11-26 purity was determined
to be >95%, as detected by Gray analysis of Quantity One
1D-analysis software (GE Healthcare), at a concentration of 4
mg/ml. Moreover, the results also showed that A6-11-26 was IgG1
isotype.
The purified A6-11-26 was then diluted to measure
the titers against NRP-1 b1b2 by indirect ELISA. As shown in
Fig. 1B, the purified A6-11-26 can
bind to synthetic immunogenic peptides with a titer of
1.28×105.
Immunofluorescence analysis was performed to
detected the U87MG cellular expression of NRP-1. As shown in
Fig. 2, A6-11-26 could bind well
with NRP-1 receptor on the surface of the U87MG cells. Thus, it
could be concluded that NRP-1 was expressed in the U87MG cells.
Radioiodination of anti-NRP-1 monoclonal
antibody A6-11-26
131I-A6-11-26 was successfully
radioiodinated. The radiolabeling efficiency, radiochemical purity
and specific activity of 131I-A6-11-26 was 95.46±3.34%,
98.23±1.41% and 180.68±21.4 MBq/μg, respectively.
Immunoreactive fraction assay
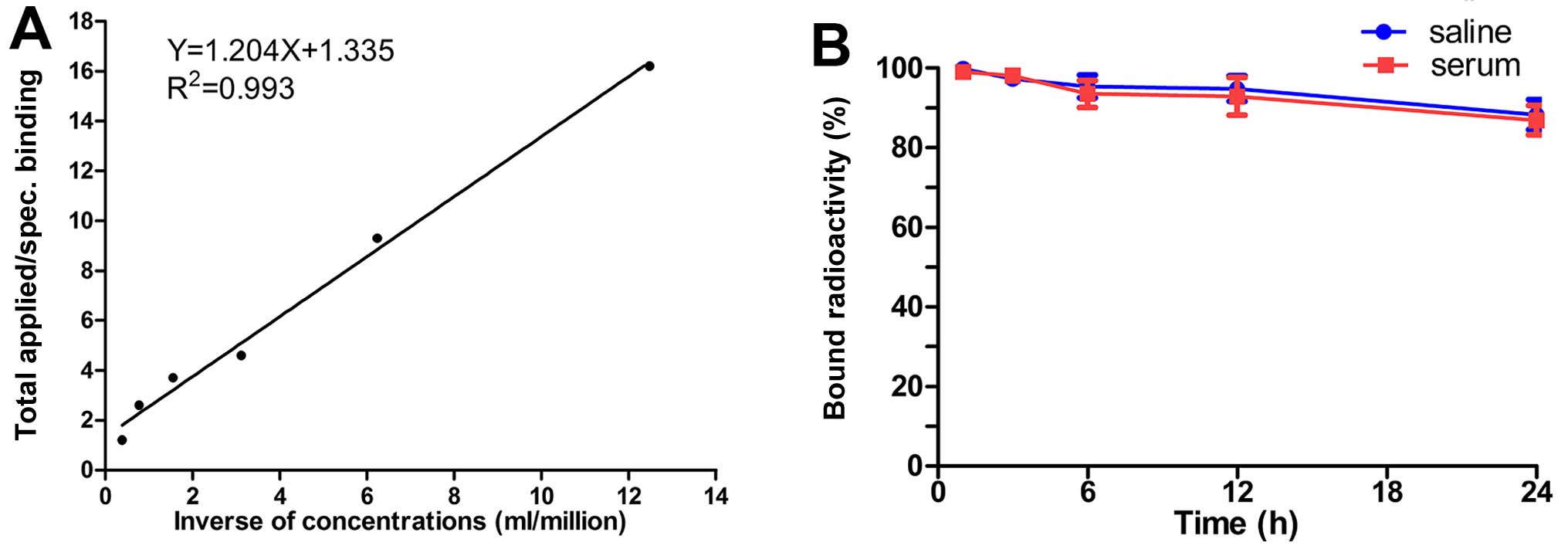
As shown in Fig.
3A, the data showed a very close linear relationship of total
applied/specific binding as a function of the inverse cell
concentration, which is based on the assumption that the total
antigen concentration (cell concentration) is a good enough
approximation for the free antigen concentration. Fitting of a
straight line to the data by means of linear regression analysis
allows an easy and precise determination of the intercept value at
the ordinate. This value equals 1/immunoreactive fraction; thus,
the immunoreactive fraction of 131I-A6-11-26 was
76.42±5.80%.
In vitro stability analysis
In vitro stability studies showed that
>85% of 131I-A6-11-26 remained intact during 1–96 h
of incubation in PBS, and declined under 80% in serum at 96 h,
indicating that 131I-A6-11-26 maintained more stable in
PBS than serum. The stability was not significantly different
between in PBS and serum (Fig.
3B).
Cell assays
Cell uptake levels for 131I-A6-11-26 are
shown in Fig. 4A.
131I-A6-11-26 quickly accumulated in U87MG cells and
reached a highest value of 15.80±1.30% of applied activity at 1 h.
When the probe was incubated with large excesses of non-radioactive
A6-11-26, its uptake levels in U87MG cells was significantly
inhibited (P<0.05) at all incubation time-points.
The binding affinity of 131I-A6-11-26 to
NRP-1 was determined through the receptor saturation assay. As
shown in Fig. 4B, the
KD value of 131I-A6-11-26 was 1.67±0.14
nM.
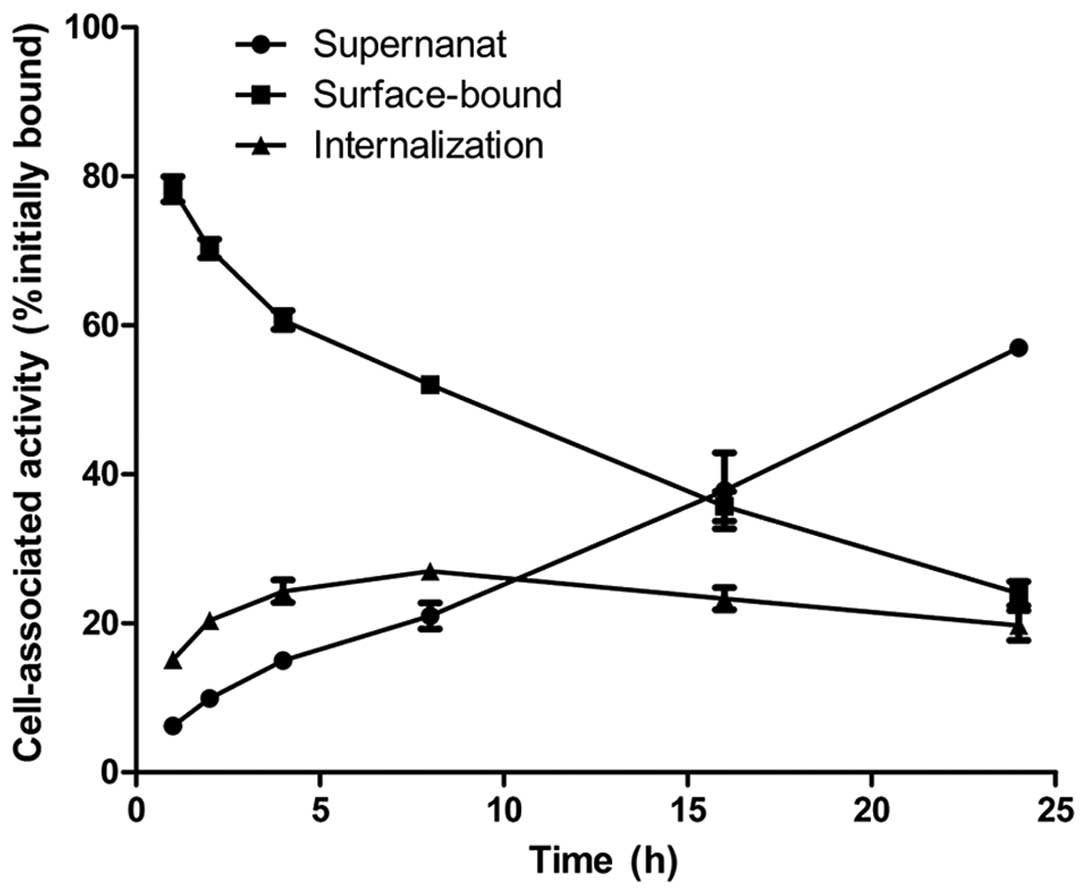
The internalization of 131I-A6-11-26 by
U87MG cells is presented in Fig.
5. The cell-surface-bound counts gradually decreased,
accompanied by a slow increase in counts in the cell culture
supernatant. The intracellular trapped radioactivity of
131I-A6-11-26 in U87MG cells gradually increased to a
maximum of 27.00±1.00% at 8 h.
Overall, these results strongly suggested that SPECT
probe 131I-A6-11-26 had high NRP-1 binding specificity,
affinity and low internalization by U87MG cells, which warranted
their further evaluation in vivo.
Biodistribution study
As shown in Fig. 6
and Table I
131I-A6-11-26 exhibited high accumulation at the tumor
bearing U87 MG cells. At 24 h after administration, the tumor
uptake was 6.00±1.24%ID/g, significantly higher than that in the
other organs except for liver (7.68±1.56%ID/g) and blood
(8.00±1.42%ID/g). Moreover, at 48, 72, 96 and 120 h,
131I-A6-11-26 in the tumor still remained at high level,
significantly higher than that in the other organs including the
liver and blood except thyroid, and in lung and bone had moderate
levels. 131I-A6-11-26 provided significantly higher
tumor-to-muscle ratios and lower tumor-to-liver and tumor to kidney
ratios (Fig. 7A). At 24 h, the
ratio of tumor to muscle (T/M=3.20±0.30) was the highest among the
tumors to liver (T/L=0.78±0.20), tumor to blood (T/B=0.78±0.10),
tumor to kidney (T/K=1.45±0.41), and tumor to lung (T/L=1.79±0.42).
Moreover, during 48 to 120 h, the T/M ratio increased gradually
over time.
 | Table IComparison of biodistribution for
131I-A6-11-26 in U87MG xenogratfs between 0 μg (unblock)
and 700 μg (block). |
Table I
Comparison of biodistribution for
131I-A6-11-26 in U87MG xenogratfs between 0 μg (unblock)
and 700 μg (block).
| Organ (%ID/g)
(spiked dose) |
131I-A6-11-26 (120 h) |
|---|
|
|---|
| 0 μg (unblock) | 700 μg (block) |
|---|
| Tumor | 1.60±0.24a | 0.52±0.11a |
| Heart | 0.87±0.23 | 0.6±0.15 |
| Liver | 0.98±0.16 | 0.87±0.25 |
| Lung | 1.70±0.14a | 0.64±0.07a |
| Spleen | 0.75±0.17 | 0.56±0.14 |
| Kidney | 0.74±0.18 | 0.69±0.21 |
| Brain | 0.35±0.05 | 0.30±0.07 |
| Stomach | 0.57±0.13 | 0.40±0.11 |
| Intestine | 0.46±0.10 | 0.34±0.12 |
| Blood | 0.80±0.15 | 0.61±0.09 |
| Bone | 1.24±0.27 | 0.58±0.10a |
| Muscle | 0.27±0.10 | 0.19±0.08 |
| Pancreas | 0.60±0.11 | 0.42±0.13 |
| Thyroid | 2.89±0.58 | 2.04±0.46 |
| Uptake ratio |
| Tumor to
blood | 1.87±0.50 | 0.85±0.22 |
| Tumor to
muscle | 6.13±0.24a | 2.53±0.86a |
For in vivo blocking study (Table I), 131I-A6-11-26 was
coinjected with a large excess (700 μg) of the unlabeled A6-11-26
to saturate endogenous and overexpressed NRP-1. The coinjection of
A6-11-26 significantly reduced the tumor, lung and bone uptake of
131I-A6-11-26 at 120 h after injection (P<0.05),
whereas the liver, kidney, blood, spleen and muscle uptake are not
significantly changed in the blocking group (P>0.05).
SPECT/CT imaging
SPECT/CT images acquired at 24, 48, 72, 96 and 120 h
after injection of 131I-A6-11-26 are shown in Fig. 8A. 131I-A6-11-26
accumulated in the U87MG tumor at 24 h and then showed a gradual
increase of uptake. During 72–120 h after injection, U87MG tumors
were clearly visible, with good tumor to background contrast. Also
observed were high levels of radioactivity accumulation in the
kidneys, liver and lungs. However, when coinjected with unlabeled
A6-11-26 antibody (700 μg), the tumor was barely visible on SPECT
images at 24–120 h after injection (Fig. 8B). Regions of interest (ROIs)
analysis of SPECT showed significantly lower ratio of tumor to
contralateral muscle (T/NT) for mice injected with 700 μg blocking
dose compared to unblocking dose (P<0.05) at 72–120 h
post-injection (Fig. 7B).
Discussion
As a co-receptor for vascular endothelial growth
factor (VEGF), neuropilin-1 (NRP-1) plays an essential role in the
development, progression, invasion of various types of cancers.
Inhibition of NRP-1 expression thus appears to be a promising
approach for cancer therapy. Several NRP-1 targeting strategies,
such as monoclonal antibodies and small-molecule peptides, are
being investigated in phases I and Ib clinical trials. Patients
with cancer lesions that express NRP-1 may benefit from NRP-1
targeted therapy (33,34). Clinical trials have shown that
there is an urgent unmet clinical need for the development of
predictive biomarkers permitting patient selection for such
therapy. Non-invasive molecular imaging, including SPECT imaging,
is an ideal method, since it can offer a more accurate and
real-time assay of NRP-1 expression, without such biopsy-associated
pitfalls and the need of repetitive invasive biopsies. It has been
reported that anti-NRP-1 peptides, such as ATWLPPR and CK3, labeled
with radionuclide 99mTc generally showed rapid blood
clearance, but very low tumor uptake and poor tumor imaging quality
(36,37). To develop an imaging agent for
NRP-1 expression is very important since our goal is to ultimately
apply antibody-based SPECT probes for imaging patients.
Due to their highly specific targeting ability,
monoclonal antibodies (mAbs) have been considered attractive
candidates for targeted therapy and diagnostics in a broad range of
medical indications, but especially in oncology. Until now, five
technetium-99m (99mTc) or indium-111
(111In)-labeled mAbs have been approved by the U.S. Food
and Drug Administration (FDA) for SPECT diagnostic imaging, among
which four are for the imaging of cancer (50). The global sales of mAbs have
reached 48 billion dollars in 2011, then in 2015, it is estimated
that the sales of antibody drugs only in China are expected to rise
spectacularly to 4.6–9.3 billion dollars (51). Hundreds of new mAbs are under
development worldwide.
Our previous studies have shown that a novel
anti-NRP-1 b1b2 monoclonal antibody A6-11-26, developed by our
laboratory, can inhibit tumor proliferation, growth, and migration
(39,40), indicating that A6-11-26 may be an
effective agent for NRP-1-targeted imaging and therapy. To further
study A6-11-26 imaging performance for targeting NRP-1 herein, we
first re-generated monoclonal antibodies A6-11-26 by hybridoma.
SDS-PAGE indicated the successful production and purification
(>95%) of A6-11-26 sufficient for in vitro and in
vivo cancer research, Furthermore immunofluorescence analysis
showed that U87MG cells highly expressed NRP-1, consistent with
previous reports (39). Next,
A6-11-26 was labeled with 131I using an Iodogen method,
and then measured the binding specificity and affinity to NRP-1.
The probe 131I-A6-11-26 showed good binding affinity to
the U87MG cell NRP-1 with a KD of 1.67±0.14 nM (Fig. 4B). In vitro cell uptake
experiments showed that 131I-A6-11-26 had rapid
accumulation in the U87MG cells. The uptake reached a plateau in 1
h. This accumulation is NRP-1 specific receptor binding since the
rapid cellular uptake of the tracer could be effectively blocked by
cold A6-11-26 (Fig. 4A),
suggesting that labeling has not influenced the ability of A6-11-26
to bind specifically to NRP-1. These results warranted further
evaluation of the probe for in vivo NRP-1-targeted tumor
imaging.
Our previous study showed that the
fluorescence-labeled A6-11-26 could gather at the sites with the
transplantation of U87MG tumor cells (39). In the present study, the
immunoreactive fraction assay demonstrated that 76.42±5.80% of the
antibodies remain immunoreactive even after the radiolabelling
procedure (Fig. 3A).
131I-A6-11-26 mainly localized in U87MG tumors and
showed good tumor uptake, retention, and tumor-to-muscle ratios
(Figs. 6 and 8). U87MG tumors could be clearly
visualized with good contrast by SPECT at 24–120 h after injection,
especially at 72–120 h. It is also interesting to find out that the
tumor uptake of the 131I-A6-11-26, and tumor to muscle
ratio are higher than those of the [99mTc]Tc-MA-ATWLPPR
(36) and 99mTc-CK3
(37). Evaluation of the probe in
mice demonstrated that 131I-A6-11-26 is a promising
agent for NRP-1 imaging.
In the present study, the liver, blood and kidney
showed high uptake at 24 h after administration.
131I-A6-11-26 was enriched more in the lung, liver and
kidney, because of the high natural expression of NRP-1 in the
liver (52) and mAbs metabolism
through the liver and kidney. The high level of
131I-A6-11-26 in the blood is also possibly due to long
circulating mAbs (33,34). Whereas, at 48, 72, 96 and 120 h
after injection of 131I-A6-11-26, with
131I-A6-11-26 clearance from blood, the level of the
tumor uptake still remained higher than that in the other organs
including the liver and blood except thyroid. Moreover, in
agreement with previous studies (36,37),
radioactivity was found in the lung and bone, since the two normal
organs have moderate NRP-1 expression. A high expression of the
target in normal organ might appreciable influence the imaging
results, especially when the target level in the tumor is low.
After optimization of spiking doses, administration to saturate the
target expression in normal organ, an increase tumor-normal ratio
could be achieved (44,46). Bumbaca et al (49) reported that the radiolabelled
anti-NRP-1 antibody (MNRP1685A) was co-dosed at 0, 0.1, 0.3, 0.5,
1, 2.5, 5, 7.5, 10, 15 and 25 mg·kg−1 unlabelled
antibody, at 24 h post dose, a dose-dependent increase in
radioactivity was observed in the tumors up to ~2.5–5
mg·kg−1, after which the radioactivity appeared to reach
a plateau. The tumor-plasma ratios also increased with dose before
reaching a plateau starting with the 2.5 mg·kg−1 dose.
Thus, saturation of non-tumor tissue uptake is required in order to
achieve tumor uptake (49). In the
study, the in vivo NRP-1 binding specificity of
131I-A6-11-26 was also verified. When 700 μg of
unlabeled A6-11-26 was coinjected, uptakes in high NRP-1 expression
organs/tissues, such as the tumor, lung and bone, were both
significantly reduced (P<0.05) (Figs. 7B and 8).
However, low tumor accumulation, slow clearance
from the circulation, and high energy iodine-131 may hamper its
clinical applications. We have currently undertaken studies to
improve these parameters. For example, the antibody fragments or
anti-NRP-1 affibody molecules and the way of labeling with low
energy 99mTc or 111In could increase rapidly
NRP-1 positive tumor targeting ability and gain high imaging
contrast within a short period after injection.
Imaging of NRP-1 expression in vivo is not
only value for treatment optimization of cancer patients, but also
may be useful for identifying the sensitivity to chemotherapy in
the patients with pancreatic, breast cancer, osteosarcoma, gliomas
that are resistant to gemcitabine, 5-fluorouracil (5-FU) or
doxorubicin through this mechanism, because NRP-1 overexpression
increases constitutive mitogen activated protein kinase (MAPK)
signalling through both the ERK and JNK pathways. These pathways
appear to promote survival of the pancreatic cancer cell,
specifically against anoikis and chemotherapy induced apoptosis
(53–55). Furthermore, NRP-1 signaling has
been suggested to be involved in development of sorafenib
resistance in squamous cell carcinoma of the head and neck (SCCHN)
patients (56). Thus, further
research for imaging of NRP-1 expression has high clinical
translational ability and will likely find broad applications in
patient therapy and management for targeting the expression of
NRP-1 and cross-talk between MAPK, HER2 and NRP-1 signaling.
In conclusion, an anti-NRP-1 monoclonal antibody
A6-11-26 has been easily and successfully radiolabeled with
iodine-131. The in vitro and in vivo study showed the
potential of 131I-A6-11-26 as a promising SPECT probe
for imaging NRP-1-positive tumor and encouraged further
investigation. Nevertheless, since A6-11-26 mAb has a large
molecular weight and an immunogenicity that may hinder its
application in the clinic, it remains a great challenge to explore
a novel small fragment of mAbs or affibody molecules with improved
imaging of NRP-1-expression.
Acknowlegements
The present study was supported by grants from the
National Natural Science Foundation of China (NSFC) (81571707,
81071182 and 81172970), the Program for Training Young Talents of
Fujian Health (2014-ZQN-ZD-35), the Natural Science Foundation of
Fujian (2015J01519, 2013J01384), the Technology Foundation for
Selected Overseas Chinese Scholar, Ministry of Human Resources and
Social Security of China (2014-240) and the Scientific Research
Foundation for the Returned Overseas Chinese Scholars, Ministry of
Education of China (2014-1685).
References
|
1
|
Gilbert MR: Renewing interest in targeting
angiogenesis in glioblastoma. Lancet Oncol. 15:907–908. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spratlin JL, Mulder KE and Mackey JR:
Ramucirumab (IMC-1121B): A novel attack on angiogenesis. Future
Oncol. 6:1085–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue M, Hager JH, Ferrara N, Gerber HP
and Hanahan D: VEGF-A has a critical, nonredundant role in
angiogenic switching and pancreatic beta cell carcinogenesis.
Cancer Cell. 1:193–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otrock ZK, Makarem JA and Shamseddine AI:
Vascular endothelial growth factor family of ligands and receptors:
Review. Blood Cells Mol Dis. 38:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cudmore MJ, Hewett PW, Ahmad S, Wang KQ,
Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, et al: The
role of heterodimerization between VEGFR-1 and VEGFR-2 in the
regulation of endothelial cell homeostasis. Nat Commun. 3:9722012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mac Gabhann F and Popel AS: Interactions
of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: A
computational model of human skeletal muscle. Am J Physiol Heart
Circ Physiol. 292:H459–H474. 2007. View Article : Google Scholar
|
|
9
|
Gluzman-Poltorak Z, Cohen T, Herzog Y and
Neufeld G: Neuropilin-2 is a receptor for the vascular endothelial
growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected]. J
Biol Chem. 275:18040–18045. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhakal HP, Naume B, Synnestvedt M, Borgen
E, Kaaresen R, Schlichting E, Wiedswang G, Bassarova A, Holm R,
Giercksky KE, et al: Expression of vascular endothelial growth
factor and vascular endothelial growth factor receptors 1 and 2 in
invasive breast carcinoma: Prognostic significance and relationship
with markers for aggressiveness. Histopathology. 61:350–364. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakayama T, Cho YC, Mine Y, Yoshizaki A,
Naito S, Wen CY and Sekine I: Expression of vascular endothelial
growth factor and its receptors VEGFR-1 and 2 in gastrointestinal
stromal tumors, leiomyomas and schwannomas. World J Gastroenterol.
12:6182–6187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Staton CA, Kumar I, Reed MW and Brown NJ:
Neuropilins in physiological and pathological angiogenesis. J
Pathol. 212:237–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagri A, Tessier-Lavigne M and Watts RJ:
Neuropilins in tumor biology. Clin Cancer Res. 15:1860–1864. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pellet-Many C, Frankel P, Jia H and
Zachary I: Neuropilins: Structure, function and role in disease.
Biochem J. 411:211–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prud’homme GJ and Glinka Y: Neuropilins
are multifunctional coreceptors involved in tumor initiation,
growth, metastasis and immunity. Oncotarget. 3:921–939. 2012.
View Article : Google Scholar
|
|
16
|
Chaudhary B, Khaled YS, Ammori BJ and
Elkord E: Neuropilin 1: Function and therapeutic potential in
cancer. Cancer Immunol Immunother. 63:81–99. 2014. View Article : Google Scholar
|
|
17
|
Plein A, Fantin A and Ruhrberg C:
Neuropilin regulation of angiogenesis, arteriogenesis, and vascular
permeability. Microcirculation. 21:315–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sulpice E, Plouët J, Bergé M, Allanic D,
Tobelem G and Merkulova-Rainon T: Neuropilin-1 and neuropilin-2 act
as coreceptors, potentiating proangiogenic activity. Blood.
111:2036–2045. 2008. View Article : Google Scholar
|
|
19
|
Geretti E, Shimizu A and Klagsbrun M:
Neuropilin structure governs VEGF and semaphorin binding and
regulates angiogenesis. Angiogenesis. 11:31–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parker MW, Xu P, Li X and Vander Kooi CW:
Structural basis for selective vascular endothelial growth factor-A
(VEGF-A) binding to neuropilin-1. J Biol Chem. 287:11082–11089.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuh G, Garcia KC and de Vos AM: The
interaction of neuropilin-1 with vascular endothelial growth factor
and its receptor flt-1. J Biol Chem. 275:26690–26695.
2000.PubMed/NCBI
|
|
23
|
Kawasaki T, Kitsukawa T, Bekku Y, Matsuda
Y, Sanbo M, Yagi T and Fujisawa H: A requirement for neuropilin-1
in embryonic vessel formation. Development. 126:4895–4902.
1999.PubMed/NCBI
|
|
24
|
Kärpänen T, Heckman CA, Keskitalo S,
Jeltsch M, Ollila H, Neufeld G, Tamagnone L and Alitalo K:
Functional interaction of VEGF-C and VEGF-D with neuropilin
receptors. FASEB J. 20:1462–1472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herzog Y, Kalcheim C, Kahane N, Reshef R
and Neufeld G: Differential expression of neuropilin-1 and
neuropilin-2 in arteries and veins. Mech Dev. 109:115–119. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim YJ, Bae J, Shin TH, Kang SH, Jeong M,
Han Y, Park JH, Kim SK and Kim YS: Immunoglobulin Fc-fused,
neuropilin-1-specific peptide shows efficient tumor tissue
penetration and inhibits tumor growth via anti-angiogenesis. J
Control Release. 216:56–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan Q, Chanthery Y, Liang WC, Stawicki S,
Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, et al:
Blocking neuropilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caunt M1, Mak J, Liang WC, Stawicki S, Pan
Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, et al: Blocking
neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell.
13:331–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Liu P, Jiang Y, Dou X, Yan J, Ma
C, Fan Q, Wang W, Su F, Tang H, et al: High expression of
neuropilin-1 associates with unfavorable clinicopathological
features in hepatocellular carcinoma. Pathol Oncol Res. 22:367–375.
2016. View Article : Google Scholar
|
|
30
|
Xu Y, Li P, Zhang X, Wang J, Gu D and Wang
Y: Prognostic implication of neuropilllin-1 up-regulation in human
nasopharyngealc arcinoma. Diagn Pathol. 8:1552013. View Article : Google Scholar
|
|
31
|
Cheng W, Fu D, Wei ZF, Xu F, Xu XF, Liu
YH, Ge JP, Tian F, Han CH, Zhang ZY, et al: NRP-1 expression in
bladder cancer and its implications for tumor progression. Tumour
Biol. 35:6089–6094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu H, Cai H, Tang M and Tang J:
Neuropilin-1 is overexpressed in osteosarcoma and contributes to
tumor progression and poor prognosis. Clin Transl Oncol.
16:732–738. 2014. View Article : Google Scholar
|
|
33
|
Weekes CD, Beeram M, Tolcher AW,
Papadopoulos KP, Gore L, Hegde P, Xin Y, Yu R, Shih LM, Xiang H, et
al: A phase I study of the human monoclonal anti-NRP1 antibody
MNRP1685A in patients with advanced solid tumors. Invest New Drugs.
32:653–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patnaik A, LoRusso PM, Messersmith WA,
Papadopoulos KP, Gore L, Beeram M, Ramakrishnan V, Kim AH, Beyer
JC, Mason Shih L, et al: A Phase Ib study evaluating MNRP1685A, a
fully human anti-NRP1 monoclonal antibody, in combination with
bevacizumab and paclitaxel in patients with advanced solid tumors.
Cancer Chemother Pharmacol. 73:951–960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tolmachev V, Stone-Elander S and Orlova A:
Current approaches to the use of radiolabeled tyrosine
kinase-targeting drugs for patient stratification and treatment
response monitoring: Prospects and pitfalls. Lancet Oncol.
11:992–1000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perret GY, Starzec A, Hauet N, Vergote J,
Le Pecheur M, Vassy R, Léger G, Verbeke KA, Bormans G, Nicolas P,
et al: In vitro evaluation and biodistribution of a
99mTc-labeled anti-VEGF peptide targeting neuropilin-1.
Nucl Med Biol. 31:575–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng GK, Liu RB, Zhang MQ, Ye XX, Zhong Q,
Xia YF, Li MZ, Wang J, Song EW, Zhang X, et al: SPECT and
near-infrared fluorescence imaging of breast cancer with a
neuropilin-1-targeting peptide. J Control Release. 192:236–242.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Luo F, Wang S, Ni E, Tang X, Lv H,
Chen X, Chen L and Yan J: Monoclonal antibody against NRP-1 b1b2.
Hybridoma (Larchmt). 30:369–373. 2011. View Article : Google Scholar
|
|
39
|
Chen L, Miao W, Tang X, Zhang H, Wang S,
Luo F and Yan J: Inhibitory effect of neuropilin-1 monoclonal
antibody (NRP-1 MAb) on glioma tumor in mice. J Biomedical
Nanotechnol. 9:551–558. 2013. View Article : Google Scholar
|
|
40
|
Zeng F, Luo F, Lv S, Zhang H, Cao C, Chen
X, Wang S, Li Z, Wang X, Dou X, et al: A monoclonal antibody
targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer
cells to fibronectin by suppressing the FAK/p130cas signaling
pathway. Anticancer Drugs. 25:663–672. 2014.PubMed/NCBI
|
|
41
|
Yang C, Yun Q, Sun H, Yang G, Liang T,
Zhang C, Song J, Han J and Hou G: Non-invasive imaging of Toll-like
receptor 5 expression using 131I-labeled mAb in the mice
bearing H22 tumors. Oncol Lett. 7:1919–1924. 2014.PubMed/NCBI
|
|
42
|
Lindmo T, Boven E, Cuttitta F, Fedorko J
and Bunn PA Jr: Determination of the immunoreactive fraction of
radiolabeled monoclonal antibodies by linear extrapolation to
binding at infinite antigen excess. J Immunol Methods. 72:77–89.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Malviya G1, Anzola KL, Podestà E, Laganà
B, Del Mastro C, Dierckx RA, Scopinaro F and Signore A:
99mTc-labeled rituximab for imaging B lymphocyte
infiltration in inflammatory autoimmune disease patients. Mol
Imaging Biol. 14:637–646. 2012. View Article : Google Scholar
|
|
44
|
Su X, Cheng K, Jeon J, Shen B, Venturin
GT, Hu X, Rao J, Chin FT, Wu H and Cheng Z: Comparison of two
site-specifically 18F-labeled affibodies for PET imaging
of EGFR positive tumors. Mol Pharm. 11:3947–3956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Q, Yan P, Wang RF, Zhang CL, Li L and
Yin L: A novel 99mTc-labeled molecular probe for tumor
angiogenesis imaging in hepatoma xenografts model: A pilot study.
PLoS One. 8:e610432013. View Article : Google Scholar
|
|
46
|
Su X, Cheng K, Liu Y, Hu X, Meng S and
Cheng Z: PET imaging of insulin-like growth factor type 1 receptor
expression with a 64Cu-labeled Affibody molecule. Amino
Acids. 47:1409–1419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kato Y, Vaidyanathan G, Kaneko MK, Mishima
K, Srivastava N, Chandramohan V, Pegram C, Keir ST, Kuan CT, Bigner
DD, et al: Evaluation of anti-podoplanin rat monoclonal antibody
NZ-1 for targeting malignant gliomas. Nucl Med Biol. 37:785–794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Aldrich MB, Marshall MV and
Sevick-Muraca EM: Preclinical characterization and validation of a
dual-labeled trastuzumab-based imaging agent for diagnosing breast
cancer. Chin J Cancer Res. 27:74–82. 2015.PubMed/NCBI
|
|
49
|
Bumbaca D, Xiang H, Boswell CA, Port RE,
Stainton SL, Mundo EE, Ulufatu S, Bagri A, Theil FP, Fielder PJ, et
al: Maximizing tumour exposure to anti-neuropilin-1 antibody
requires saturation of non-tumour tissue antigenic sinks in mice.
Br J Pharmacol. 166:368–377. 2012. View Article : Google Scholar :
|
|
50
|
van Dongen GA, Visser GW, Lub-de Hooge MN,
de Vries EG and Perk LR: Immuno-PET: A navigator in monoclonal
antibody development and applications. Oncologist. 12:1379–1389.
2007. View Article : Google Scholar
|
|
51
|
Li X, Gong M, Xu W and Tang L: Market
dynamics of antibody drugs in both domestic and abroad. Drugs Clin.
27:185–191. 2012.
|
|
52
|
Bergé M, Allanic D, Bonnin P, de Montrion
C, Richard J, Suc M, Boivin JF, Contrerès JO, Lockhart BP, Pocard
M, et al: Neuropilin-1 is upregulated in hepatocellular carcinoma
and contributes to tumour growth and vascular remodelling. J
Hepatol. 55:866–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han Z, Jiang G, Zhang Y, Xu J, Chen C,
Zhang L, Xu Z and Du X: Effects of RNA interference-mediated NRP-1
silencing on the proliferation and apoptosis of breast cancer
cells. Mol Med Rep. 12:513–519. 2015.PubMed/NCBI
|
|
54
|
Wey JS, Gray MJ, Fan F, Belcheva A,
McCarty MF, Stoeltzing O, Somcio R, Liu W, Evans DB, Klagsbrun M,
et al: Overexpression of neuropilin-1 promotes constitutive MAPK
signalling and chemoresistance in pancreatic cancer cells. Br J
Cancer. 93:233–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yue B, Ma JF, Yao G, Yang MD, Cheng H and
Liu GY: Knockdown of neuropilin-1 suppresses invasion,
angiogenesis, and increases the chemosensitivity to doxorubicin in
osteosarcoma cells: an in vitro study. Eur Rev Med Pharmacol Sci.
18:1735–1741. 2014.
|
|
56
|
Mehta S, Moon J, Hashmi M, Leblanc M,
Huang CH, Rinehart E, Wolf GT, Urba SG, Banerjee SK and Williamson
S: Predictive factors in patients with advanced and metastatic
squamous cell carcinoma of the head and neck: A study based on SWOG
protocol S0420. Oncol Rep. 29:2095–2100. 2013.PubMed/NCBI
|