Introduction
Methionine adenosyltransferase (MAT) is a critical
enzyme that catalyzes the biosynthesis of S-adenosylmethionine
(SAMe), the principal biological methyl donor in all mammalian
cells (1). These reactions play a
central role in methionine cycle and are associated with several
important biological functions such as transmethylation,
trans-sulfuration and polyamine synthesis. There are three MAT
genes in mammals: two of them (MAT1A and MAT2A) encode for the
catalytic subunits (MATα1 and α2) of the different MAT isoforms,
while the third gene (MAT2B) encodes for the regulatory subunit
(MATβ) which modulate the catalytic activity of MAT. In mammals,
three distinct isoforms of MAT (MATI, MATII and MATIII) have been
identified: MAT1A, mainly expressed in normal hepatocytes, encodes
for MATα1 which constructs the tetramer (MATI) and the dimer
(MATIII); MATII, mainly expressed in extra-hepatic tissues, is a
heterotetramer composed by catalytic subunit MATα2, encoded by
MAT2A, and regulatory subunit MATβ, encoded by MAT2B (2). The MATβ subunit regulates the
catalytic subunit MATα2 by reducing the Km of MATII for methionine
and increasing the sensitivity of the enzyme to feedback inhibition
by SAMe (3). Downregulation of the
MAT β subunit expression causes a 6- to 10-fold increase in
intracellular SAMe levels (4).
Although MAT2B is known as a gene encoding
regulatory subunit for changing MATII enzymatic activity, recent
studies have reported that MAT2B also has several kinds of specific
biologic fuctions, especially in cancer. MAT2B expression is
elevated in human hepatocellular carcinoma and colon cancer which
promotes the growth advantage or cell cycle of tumor cells
(5–9). In HCC cells, MAT2B knockdown led to
growth inhibition by downregulating cyclin D1. Moreover, apoptosis
of HCC cells induced by LV-shRNA of MAT2B was involved in
downregulating BCL-XL and upregulating BCL-XS (6). In both liver and colon cancers, MAT2B
interacted with GIT1 and formed a scaffold to regulate
Ras/Raf/MEK1/2 activity and therefore promoted cell growth and
tumorigenesis (7,8). MAT2B was also identified to interact
with HuR, an mRNA-binding protein known to stabilize the mRNA of
several cyclins, to affect tumor cell proliferation and apoptosis
(9). These data suggest that MAT2B
may also have other functional roles in addition to modulating
catalytic activity of methionine adenosyltransferase. It may also
play a positive role in tumor cell proliferation. Despite several
studies supporting the biological function of MAT2B in the
tumorigenesis of human hepatocellular carcinoma and colon cancer,
its function in the proliferation of cancer cells remains unclear.
Moreover, the biofunctions of MAT2B have not been studied in human
malignant melanoma.
Malignant melanoma is the most lethal of skin
cancers and its pathogenesis is complex and heterogeneous. The
incidence of malignant melanoma continues to rise (10). The efficacy of conventional
therapeutic regimens for melanoma remains limited, so that it is
significant to explore new effective therapies such as targeted
therapy in the treatment of melanoma (11). BRAF, NRAS and KIT are three
well-known oncogenes associated with malignant melanoma. Several
targeted drugs such as vemurafenib and dabrafenib have improved
progression-free survival and overall survival in malignant
melanoma patients with BRAF V600E mutation, compared with
chemotherapy (12–14). However, the recurrences often
develop several months later and the tumor usually becomes more
resistant to the drug. The subsequent metastasis is even more
aggressive and is the critical cause of mortality. These reasons
highlight the importance of finding novel therapeutic targets to
fight against malignant melanoma. With the development of
biological research techniques and methods, further investigation
on the molecular pathogenesis of this disease is feasible.
Up to now, the expression of MAT2B in malignant
melanoma tissue and its biological functions in melanoma cells has
not been clearly demonstrated. Hence, in order to identify the role
of MAT2B in malignant melanoma tumorigenesis, we contrast the
expression profile of MAT2B in clinical malignant melanoma tissues
and benign nevus samples. Furthermore, by using lentivirus-mediated
RNAi to downregulate the expression of MAT2B in malignant melanoma
cell lines (A375 and Mel-RM), we investigated the effects of MAT2B
on cell growth, colony-formation ability and apoptosis in
vitro, as well as tumor growth of xengrafts in vivo.
Western blot analysis was further carried out to explore the
expression patterns of some apoptosis-related proteins.
Materials and methods
Cell lines, tissue samples and
animals
The human melanoma cell line A375 was kindly
provided by Shanghai Cell Bank, Chinese Academy of Science. The
human melanoma cell line Mel-RM was a gift from Professor Mian Wu
(University of Science and Technology of China, Hefei, China). Both
cell lines were cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco-BRL, Grand Island, NY, USA) plus 10% fetal bovine
serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA) in a 5%
CO2 incubator at 37°C.
Forty-nine primary melanoma tissues, 38 metastatic
melanoma tissues and 42 benign nevus samples were obtained from the
Department of Surgery, The First and Second Affiliated Hospitals of
Anhui Medical University between 2010 and 2015. The histological
diagnoses were evaluated by experienced pathologists based on
formalin-fixed paraffin-embedded (FFPE) tissue sections that were
stained with haematoxylin-eosin (H&E). Fresh tissue samples
were fixed in 4% paraformaldehyde for 12–24 h and then
paraffin-embedded to test the endogenous expression of MAT2B by
immunohistochemistry technique. This study was approved by the
Human Research Ethics Committees of the Anhui Medical University
and was performed according to the relevant regulations. Written
informed consent was obtained from each participant.
The experiments in vivo were licensed by the
Animal Research Ethics Committee of Anhui Medical University,
Hefei, China, and were conducted following the regulations of the
use and care of laboratory animals. Specific pathogen-free (SPF)
grade male BALB/c nude mice aged 4 weeks were purchased from
Shanghai SLAC laboratory Animal Co., Ltd., Shanghai, China, and
were maintained in a 12 h light/12 h dark cycle at constant
temperature.
Immunohistochemistry
Immunohistochemical staining was employed to test
the endogenous expression of MAT2B in primary, metastatic melanoma
tissues and benign nevus samples. Briefly, formalin-fixed
paraffin-embedded tissue sections were deparaffinized using xylene
at room temperature, rehydrated through graded ethanol. The
sections were then boiled using microwave for 10 min in citrate
buffer for antigen retrieval. Endogenous peroxidase activity was
blocked by 3% hydrogen peroxide for 20 min. The MAT2B antibody
(Sigma-Aldrich, St. Louis, MO, USA) was used at a dilution of
1:200, incubated for 12 h at 4°C. Finally, the slides were
incubated with secondary antibodies (ZSGB-Bio Co., Beijing, China)
for 30 min at 37°C. The tissue was visualized with
3,3′-diaminobenzidine (DAB) stain and counter-stained for
microscopic examination. Two pathologists scored the slides for the
staining intensity, independently. The scoring of the slides was
conducted blinded to the clinical information. The histological
score (H-Score) (15,16) was used to semi-quantitative analyze
the endogenous expression of MAT2B in tissue samples. The staining
intensity was rated as 0 (no staining), 1+ (weak), 2+ (moderate),
or 3+ (strong) based on the intensity of the staining pattern.
H-Score (range from 0 to 300) was calculated by adding the
multiplication of the different staining intensities using the
following equation. H-Score = [1 * (% cells 1+) + 2 * (% cells 2+)
+ 3 * (% cells 3+)].
Lentiviral vector construction and
transfection
The most effective MAT2B-targeted small interfering
RNA sequence (AGTTCATCACATCATTCAT) and a negative control small
interfering RNA sequence (TTCTCCGAACGTGTCACGT) were selected. Short
hairpin RNA (shRNA) was synthesized and was cloned into the
pGCSIL-GFP vector (GeneChem Co., Ltd., Shanghai, China) to
construct the recombinant plasmid. The pGCSIL-GFP vector contains
AgeI/EcoRI enzyme cleavage sites and green
fluorescent protein (GFP) reporter gene with an internal CMV
(cytomegalovirus) promoter. The MAT2B-shRNA recombinant plasmid and
assistant packaging plasmid (GeneChem) were co-transfected into
HEK293T cells. Lentivirus particles expressing MAT2B-shRNA were
collected from the supernate of the culture medium, purified by
centrifugal ultrafiltration and stored at −80°C.
Cells of human melanoma cell lines A375 and Mel-RM
were seeded in 6-well plates and allowed to grow until the density
of cells reached ~40%. Appropriate amount of lentivirus were added
into the culture medium according to the multiplicity of infection
(MOI) which was determined as 20. Culture medium was refreshed 24 h
after lentivirus infection. GFP fluorescence expression was
detected for infecting efficiency evaluation 72 h after infection
by fluorescence microscopy.
Quantitative real-time PCR (qRT-PCR)
The quantitative real-time PCR was carried out to
detect the mRNA level of MAT2B in human melanoma cell lines A375
and Mel-RM after infected by lentivirus. Cells were divided into 3
groups: mock (melanoma cells without lentivirus infection), shCtrl
(infected melanoma cells by lentivirus expressing non-silencing
shRNA) and shMAT2B (infected melanoma cells by lentivirus
expressing MAT2B-shRNA). Seventy-two hours after lentivirus
infection, the cells were collected and total RNA was extracted by
TRIzol (Invitrogen Corp., Carlsbad, CA, USA). According to the
instructions of the manufacturer, total RNA was reversely
transcripted to cDNA by M-MLV reverse transcriptase kits (Promega,
Madison, WI, USA). The SYBR-Green Master Mix kit (Takara Bio Inc.,
Shiga, Japan) was used, and quntitative real-time PCR was performed
on Agilent Mx3000P QPCR system (Agilent Technologies, Santa Clara,
CA, USA). The following primers were used: MAT2B forward primer
5′-ACAGAGAGGAAGACATACCAG-3′ and reverse primer
5′-GTTCATTGCCAGACCAGTG-3′; GAPDH forward primer
5′-TGACTTCAACAGCGACACCCA-3′ and reverse primer
5′-CACCCTGTTGCTGTAGCCAAA-3′. The cycling profile was: initial
denaturation at 95°C for 15 sec, 45 cycles consisting of 95°C for 5
sec, 60°C for 30 sec. Data were calculated using the
2−ΔΔCT method with GAPDH serving as an internal control.
All experiments were independently repeated at least 3 times.
Cell proliferation assay
Two methods were carried out to measure the cell
proliferation of human melanoma cells after lentivirus infection.
First, cell growth of human melanoma cell line A375 was measured by
the fluorescent cytometer. A375 cells were infected with shCtrl or
shMAT2B lentivirus. Seventy-two hours after infection, cells were
collected, counted and seeded into 96-well plates. All cells with
green fluorescence were counted by Nexcelom Celigo Image Cytometer
in the following 5 days. Fluorescent photomicrographs were taken
and cell growth curves were drawn. Second, in order to confirm the
inhibition effect and test whether the shCtrl lentivirus can affect
cell proliferation, we conducted the MTT assay in human melanoma
cell lines A375 and Mel-RM. Cells were divided into 3 groups as
described above: Mock, shCtrl and shMAT2B. Seventy-two hours after
lentivirus infection, cells were collected and seeded in 96-well
plates. MTT (Sigma-Aldrich) solution was added after 24, 48, 72, 96
and 120 h. The cells were then cultured for another 4 h. The
resulting formazan crystals were dissolved in DMSO and absorbance
at 490 nm was detected. The experiments were repeated at least
three times.
Colony formation assay
The colony-forming abilities of human melanoma cell
lines A375 and Mel-RM were tested. Cells were divided into 3
groups: Mock, shCtrl and shMAT2B, as described above. Seventy-two
hours after lentivirus infection, cells at the logarithmic phase
were collected and inoculated at a density of 300 cells/well in
6-well plates. Culture medium was refreshed every 3 days. After
incubating for 14 days, cells were fixed with 4% paraformaldehyde
and stained with Giemsa. Cell colonies that contained at least 50
cells were manually counted.
Apoptosis assay
The apoptosis ratio of human melanoma cell lines
A375 and Mel-RM was detected by a flow cytometer. As described
above, cells in Mock group were not infected by any lentivirus,
cells in shCtrl or shMAT2B group were infected by lentivirus
expressing non-silencing shRNA or MAT2B-shRNA. Seventy-two hours
after lentivirus infection, melanoma cells were collected, washed
and then stained by Annexin V-APC at room temperature in the dark
for 15 min. After filtered by a 50 μm mesh, apoptosis ratio of each
sample was detected by Millipore Guava easyCyte HT flow cytometer
(Millipore, Billerica, MA, USA).
Western blot analysis
Western blot analysis was conducted to measure the
variations in protein expression after MAT2B downregulation in
human melanoma cell lines A375 and Mel-RM. Cells were divided into
3 groups: Mock, shCtrl and shMAT2B. Seventy-two hours after
lentivirus infection, cells were collected. Proteins were
extracted, loaded, separated by SDS polyacrylamide gel
electrophoresis and transferred onto a PVDF (polyvinylidene
difluoride) membrane. The membrane was blocked, washed and
incubated overnight with primary antibodies against MAT2B (1:500
dilution; Sigma-Aldrich), XAF1 (1:1,000 dilution; Santa Cruz
Biotechnology, Dallas, TX, USA), BCL2 (1:1,500 dilution; Santa Cruz
Biotechnology). After that, the membrane was washed and incubated
with secondary antibodies (Santa Cruz Biotechnology) and detected
by enhanced chemiluminescence. The intensity of bands was
quantified by ImageJ software and protein expression was
standardized to GAPDH.
Tumor growth experiment in vivo
For the transplanted tumor model, A375 cells were
infected by shMAT2B or shCtrl lentivirus. After lentivirus
infection, cells at the logarithmic phase were collected and
adjusted at a density of 2×107 cells/ml. The cell
suspension (250 μl) was subcutaneously injected into the right
dorsal flank of the nude mice. After implantation, tumors were
allowed to grow until their diameters reached ~5 mm. Tumor size was
measured every 4 days and tumor volumes were calculated using the
formula: V = (πab2)/6, where a was the largest
superficial diameter and b was the perpendicular diameter.
Twenty-four days later, the mice were sacrificed and the tumors
were dissected and weighted. Inhibition ratio was defined as 1 −
(tumor weight of shMAT2B group/shCtrl group). As previously
described (17), relative tumor
volume was used to create the tumor growth curve and it was
regarded as the ratio of measured tumor volume compared with the
initial tumor volume of the same mouse. Tumor growth equations were
constructed based on the Gompertz model, which was determined by
the equation: y = V0 * exp {b * [1 − exp (−a * x)]},
where y was tumor size, x was time, V0 was the initial
tumor volume, a and b were constant (18). Tumor growth time (TGT 10) was the
number of days it took the tumor to grow to 10 times the size of
its initial volume. Tumor growth delay (TGD) was regarded as the
time difference of TGT 10 between shMAT2B group and shCtrl
group.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation of three independent experiments. Statistical
analysis was performed by SPSS 13.0 software. Statistical
comparisons were performed by two-tailed t-test or one-way analysis
of variance for parameter test. The expression of MAT2B in primary
melanoma tissues, metastatic melanoma tissues and nevi tissues was
compared by Kruskal-Wallis test. Comparisons of tumor growth curves
in vivo were completed by repeated-measures variance
analysis. A P-value <0.05 was considered as statistically
significant.
Results
MAT2B expression is frequently
upregulated in malignant melanoma tissues
We examined the endogenous expression of MAT2B by
conducting immunohistochemical staining on formalin-fixed
paraffin-embedded samples from 49 patients with primary melanoma,
38 patients with metastatic melanoma and 42 patients with benign
nevus. As shown in Fig. 1A and B,
endogenous expression of MAT2B was elevated in malignant melanoma
compared to benign nevus. However, there was no significant
difference in MAT2B level between primary and metastatic melanoma.
Hierarchical analysis of MAT2B expression in melanoma tissue found
that poorly differentiated tumors frequently have higher level of
MAT2B compared with well differentiated tumors (Fig. 1C). However, there were no
significant changes in MAT2B expression within different gender,
age and primary site (data not shown). These results indicated that
the expression of MAT2B may be related to the tumorigenesis and
differentiation of melanoma.
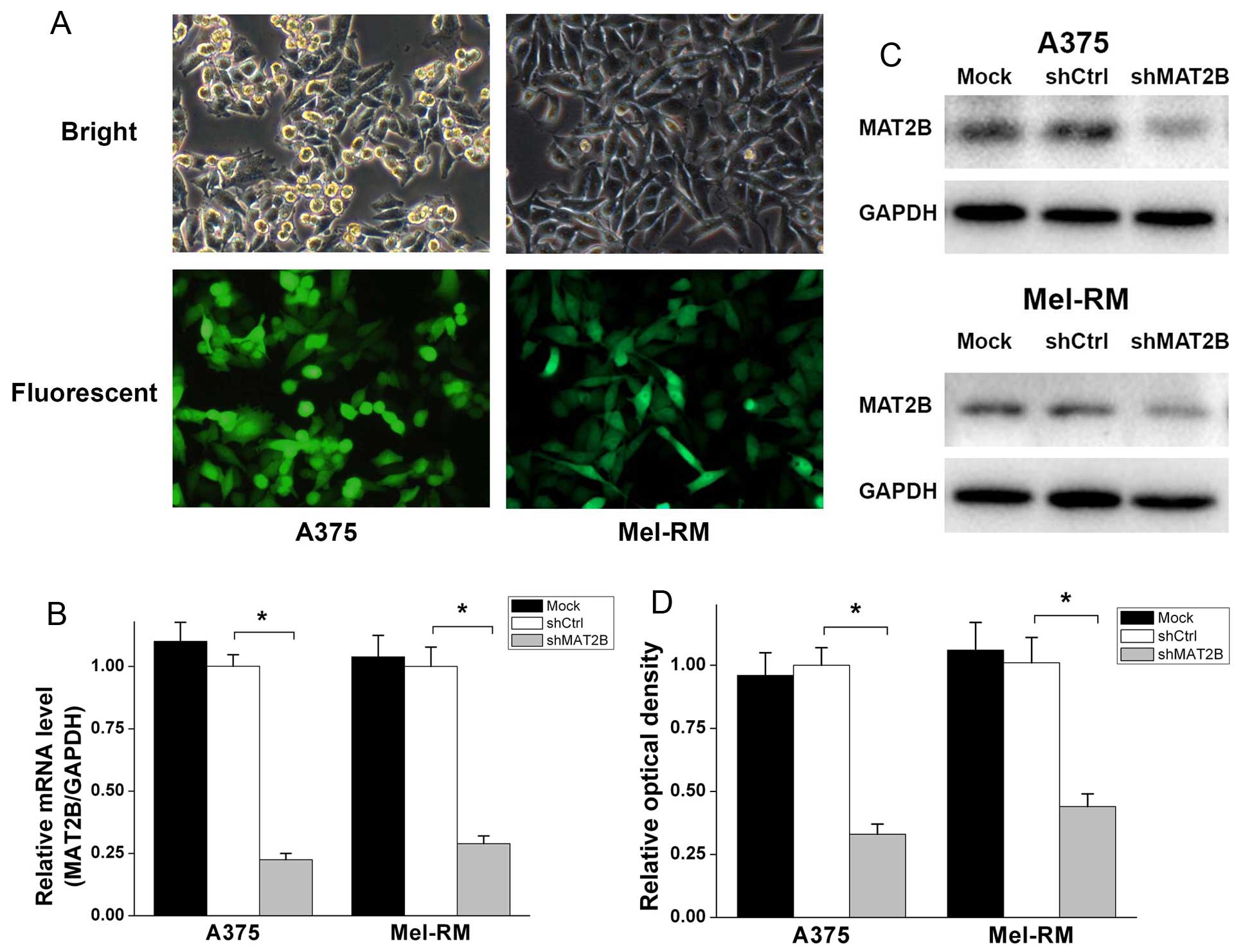
MAT2B is effectively knocked down in
human melanoma cells by MAT2B-shRNA lentivirus
MAT2B-shRNA (shMAT2B) lentivirus and non-silencing
shRNA (shCtrl) lentivirus were constructed and infected in human
melanoma cell lines A375 and Mel-RM. As shown in Fig. 2A, the infection efficiency was
>90% according to the GFP expression, 72 h after lentivirus
infection. Quantitative real-time PCR (Fig. 2B) indicated that the MAT2B mRNA
level was downregulated ~70–80% in two cell lines after shMAT2B
lentivirus infection. There was no statistical difference in the
mRNA level between Mock and shCtrl group. In terms of protein
levels, western blot analysis (Fig. 2C
and D) exhibited a similar profile. These data suggested that
MAT2B was effectively knocked down in human melanoma cell lines
A375 and Mel-RM after shMAT2B lentivirus infected, and that shCtrl
lentivirus did not change the mRNA or protein level of MAT2B in
melanoma cells.
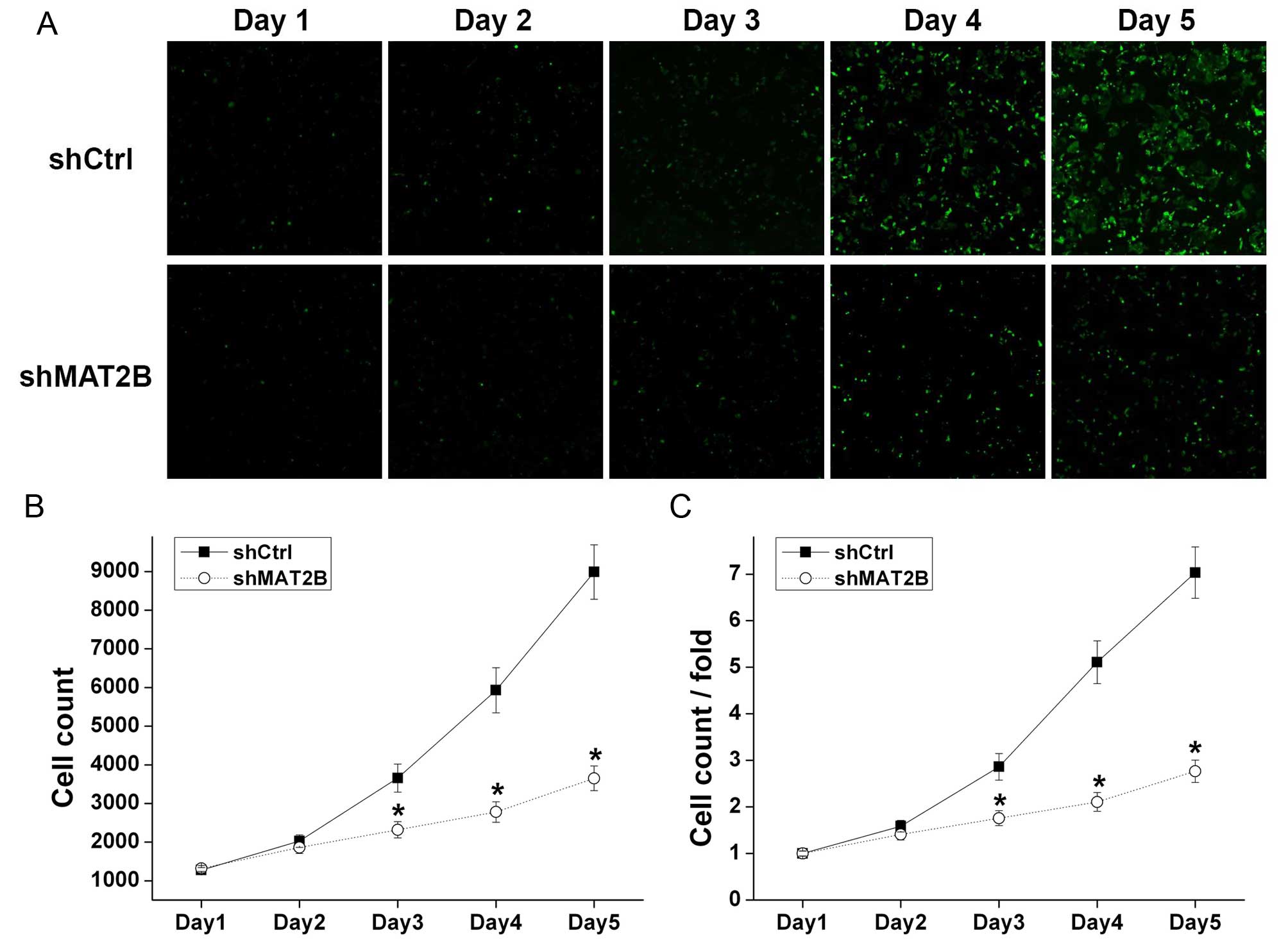
Cell proliferation is inhibited after
MAT2B downregulation in human melanoma cells
A375 cells with green fluorescence were counted by
Nexcelom Celigo Image Cytometer in continuous 5 days (Fig. 3A) and the cell growth curves were
drawn (Fig. 3B and C). After a
5-day culture, A375 cells in shCtrl and shMAT2B groups increased by
7.03 and 2.77 times, respectively, revealing that cell growth of
A375 was inhibited after shMAT2B lentivirus infection.
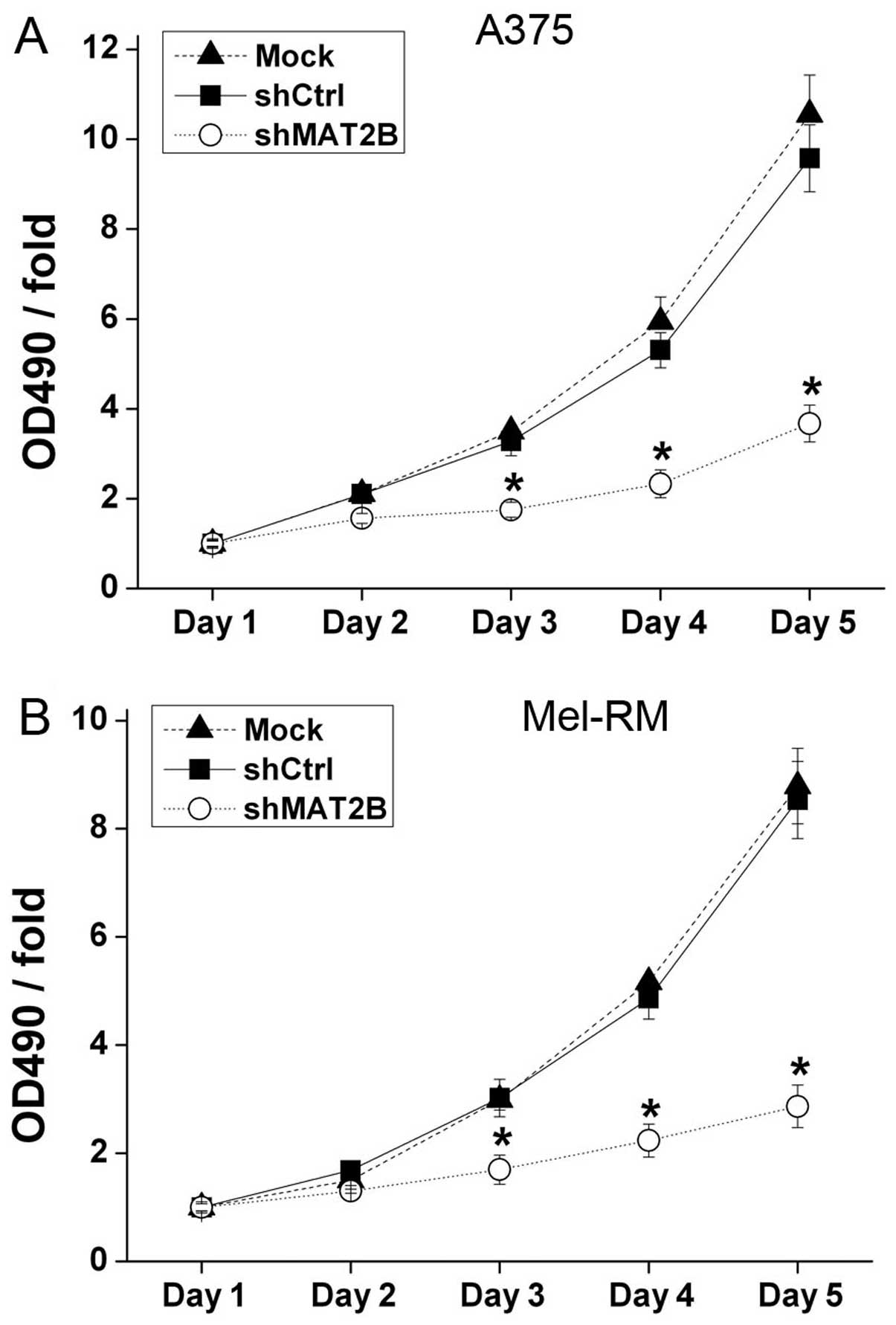
Moreover, in order to confirm the inhibition effect
and to detect whether the shCtrl lentivirus can affect cell
proliferation, MTT assays were conducted in A375 and Mel-RM, and
Mock groups were also added (Fig.
4). The OD 490 nm/fold on days 3–5 showed significant
differences between shCtrl and shMAT2B groups which suggested that
downregulation of MAT2B inhibited melanoma cell proliferation.
There was no significant change in the OD 490 nm/fold between Mock
and shCtrl groups which confirmed that shCtrl lentivirus infection
could not affect cell proliferation.
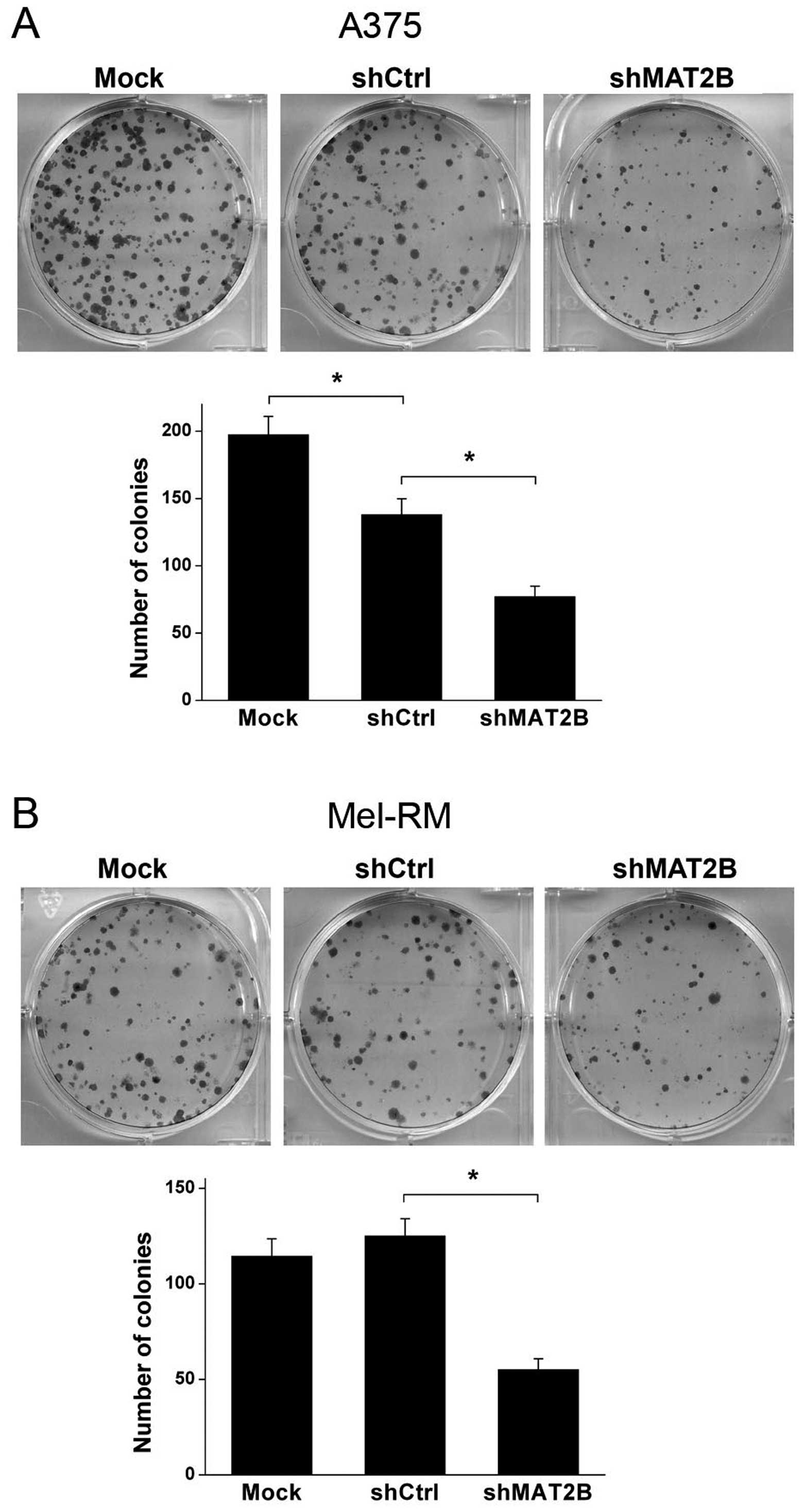
Colony formation ability is depressed
after MAT2B downregulation in human melanoma cells
Colony formation assay was conducted to test the
colony formation ability of human melanoma cell lines A375 and
Mel-RM after lentivirus infection in vitro. Data are shown
in Fig. 5. The number of colonies
was significant decreased in shMAT2B group compared with shCtrl
group in both A375 and Mel-RM cells. Another interesting result was
that shCtrl lentivirus infection inhibited the colony formation
ability in A375 cells compared with Mock group. However, the
inhibition effect in Mel-RM cells was not shown as in A375 cells.
These results suggested that despite the difference in virus
susceptibility of the 2 cell lines, downregulation of MAT2B
depressed colony formation ability of A375 and Mel-RM in
vitro.
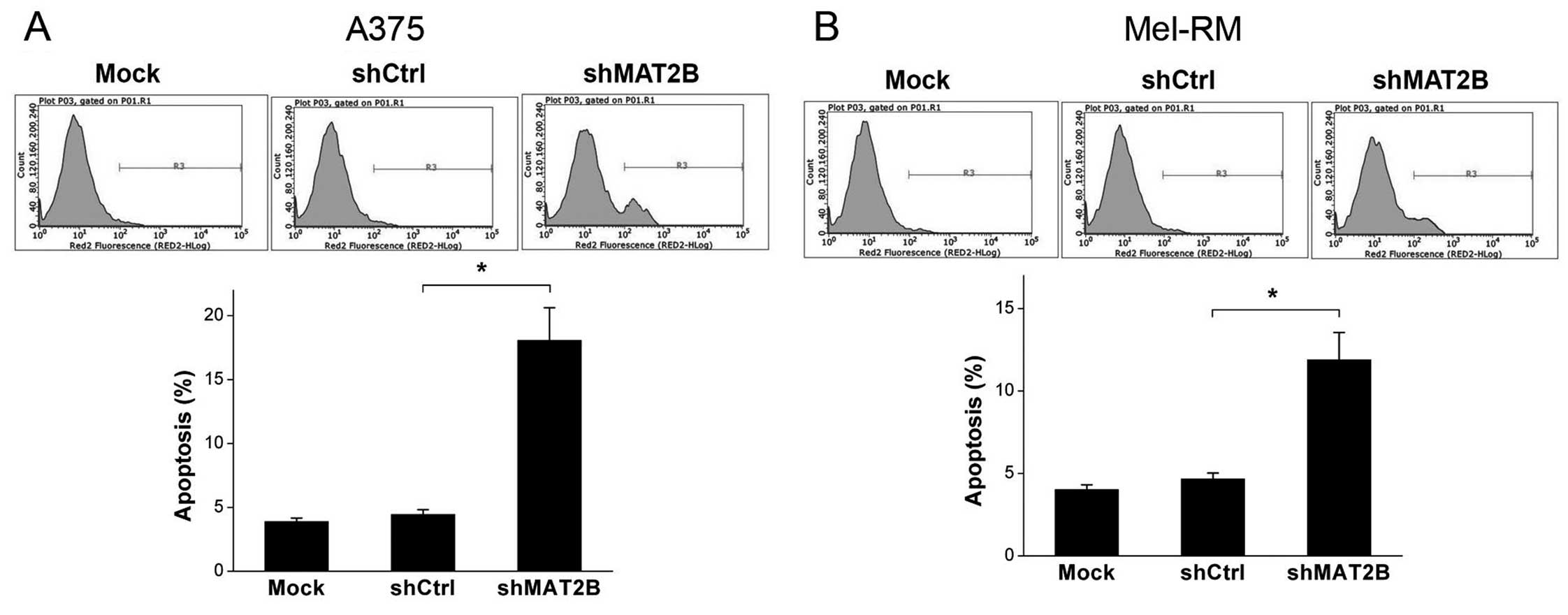
Apoptosis ratio is increased after MAT2B
downregulation in human melanoma cells
The apoptosis ratio of each sample was detected with
Annexin V-APC staining by a flow cytometer. The results were shown
in Fig. 6. The apoptosis ratios of
A375 and Mel-RM in shMAT2B group were increased compared with those
in shCtrl group, from 4.45 to 18.07 and 4.66 to 11.89%,
respectively. Furthermore, there were no significant differences in
the apoptosis ratios of these two cell lines between Mock and
shCtrl groups. This suggested that MAT2B knockdown promoted
apoptosis of human melanoma cells in vitro.
Protein expression levels of BCL2 and
XAF1 are significantly changed after MAT2B downregulation in human
melanoma cells
Protein expression levels of BCL2 and XAF1, which
were closely related to apoptosis of tumor cells, were analyzed by
western blot analysis. As shown in Fig. 7, XAF1 was significantly upregulated
while BCL2 protein expression was significantly downregulated after
MAT2B was knocked down in human melanoma cell lines A375 and
Mel-RM.
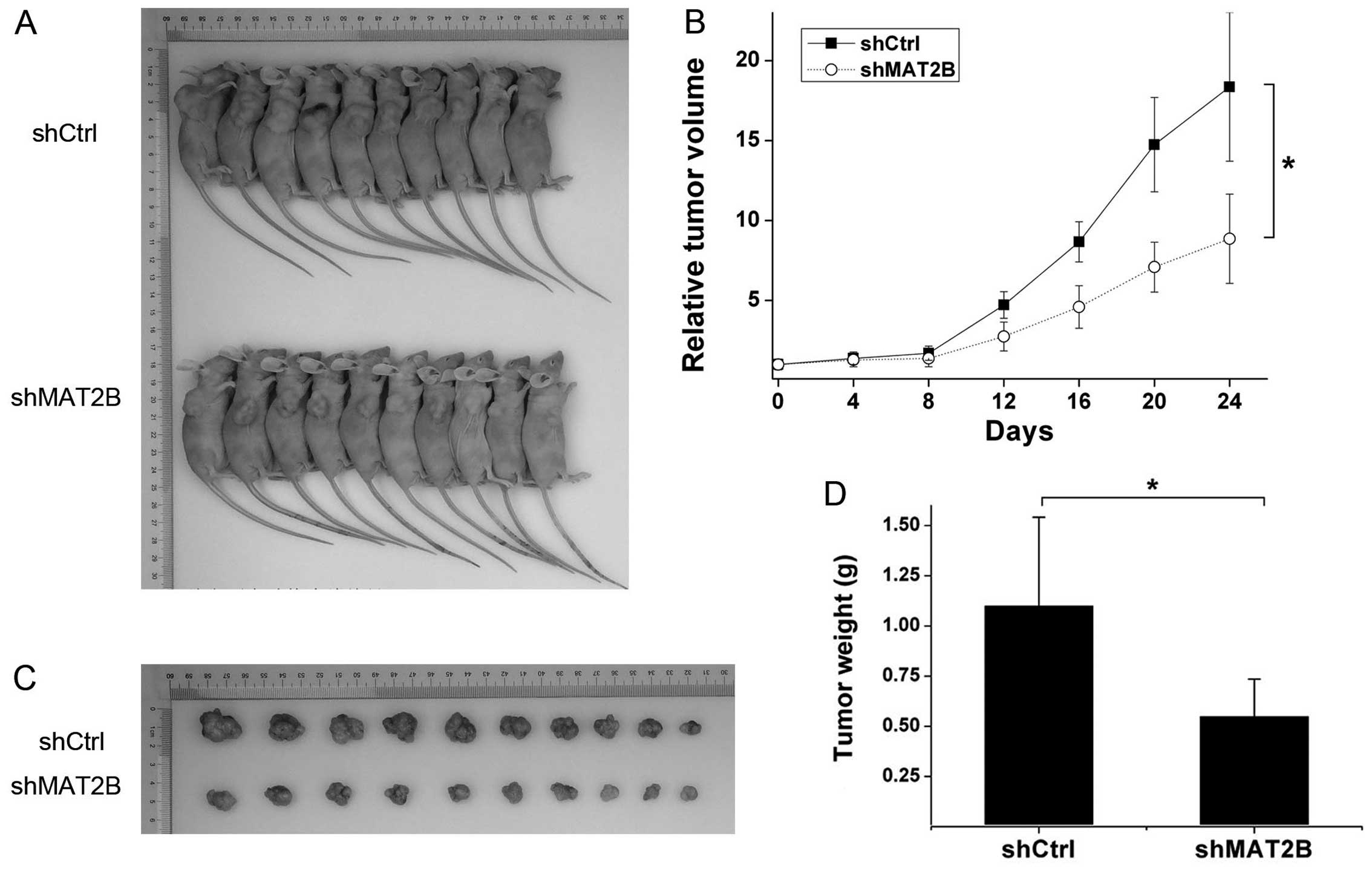
MAT2B downregulation delays tumor growth
in vivo
To investigate the effect of MAT2B on tumor growth
in vivo, a transplanted tumor model was established by
subcutaneously injecting A375 (infected by shCtrl or shMAT2B
lentivirus) into the right dorsal flank of the BALB/c nude mice.
Twenty-four days later, tumors were dissected and weighed, the
shMAT2B group formed smaller and lighter tumors than the shCtrl
group (Fig. 8A, C and D). Tumor
inhibition ratio was 50.09%. Tumor growth curves are shown in
Fig. 8B. Tumor growth equations
were constructed according to the Gompertz model and used to
calculate parameters such as tumor growth time (TGT 10) and tumor
growth delay (TGD). The TGT 10 was 17.05 and 27.76 days in shCtrl
and shMAT2B groups, respectively. TGD was 10.71 days between the
two groups. Through comparing tumor growth between shCtrl and
shMAT2B groups by repeated-measures variance analysis, we observed
that downregulation of MAT2B extended the transplanted tumor growth
in vivo.
Discussion
Recent studies have suggested that MAT2B may also
have other functional roles in addition to modulating catalytic
activity of methionine adenosyltransferase. In the present study,
in order to explore the expression profiles and biological
functions of MAT2B in human malignant melanoma, we investigated the
expression profiles of MAT2B in primary and metastatic malignant
melanoma compared with benign nevus by immunohistochemistry
technique, and selected lentivirus-mediated RNAi for MAT2B
downregulation. We found that MAT2B was expressed at higher levels
in primary and metastatic melanoma tissues compared with nevus.
Lentivirus-mediated downregulation of MAT2B suppressed cell growth
and colony formation abilities, enhanced apoptosis in human
malignant melanoma cell lines A375 and Mel-RM in vitro. In
addition, we provided evidence that downregulation of MAT2B
inhibited tumor growth of xenografts in vivo. Then protein
expression of BCL2 and XAF1, which were closely related to
apoptosis of tumor cells, were analyzed by western blot analysis.
The results implied that some of the pro-apoptotic genes were
upgraded and anti-apoptotic genes downgraded after MAT2B knocked
down in human melanoma cell lines A375 and Mel-RM.
Apoptosis plays a crucial role in maintaining proper
tissue development and homeostasis. Apoptosis escaping by cancer
cells is a strategy of their adaption to microenvironment and
treatment (19). Anti-apoptotic
molecules can protect tumor cells from apoptosis and mediate some
other processes, combination with lacking of pro-apoptotic
molecules, result in an enhanced aggressive phenotype (20,21).
The BCL2 family is considered as the most important
and potent mediators of apoptosis and survival in human cancers
including melanoma (22). Since it
was discovered in the late 1980s, their mechanisms of action and
interactions in apoptosis have been elucidated in exquisite
molecular detail (23). BCL2 is
the core member of the family with the functions of promoting cell
survival and inhibiting apoptosis. The BCL2 anti-apoptotic gene is
overexpressed in many human cancers, and leads to aggressive
disease course and poor survival in patients with different
cancers. This overexpression can result from chromosomal
translocations, gene amplification, increased gene transcription,
altered post-translational processing and promoter hypomethylation
(24). In the present study, the
expression level of BCL2 anti-apoptotic gene was attenuated after
MAT2B downregulation which induced apoptosis and inhibited cell
proliferation both in vitro and in vivo.
X-linked inhibitor of apoptosis protein (XIAP) is
the most potent member in the inhibitors of apoptosis (IAP) family.
It is well known as an inhibitor of apoptosis by binding to
caspase-3, -7 and -9 to suppress their activities (25). XIAP-associated factor 1 (XAF1) was
identified as a XIAP-binding protein and could directly bind
preferentially to XIAP and antagonize the anti-caspase activity of
XIAP to induce apoptosis (26).
Pro-apoptotic gene XAF1 was constantly expressed in normal and
fetal tissues but weakly expressed in most human cancer cell lines
and human cancer tissues such as ovarian, hepatocellular, colon,
pancreatic cancer and melanoma (27–31).
Recent studies had shown that XAF1 expression was relatively low in
hepatocellular carcinoma cell lines and hepatoma tissues.
Adenovirus-mediated XAF1 overexpression inhibited cell
proliferation and induced apoptosis, and prolonged the survival of
tumor-bearing mice, suggested that XAF1 could be associated with
tumor growth by inducing apoptosis and inhibiting cell
proliferation (28). In the
present study, the pro-apoptotic gene XAF1 expression was
significantly augmented after MAT2B downregulation, then lead to
similar tumor-depressing functions in melanoma cell lines in
vitro and A375 transplanted tumor model in vivo.
Another interesting result was observed in colony
formation assay. The number of colonies was significant decreased
in shCtrl group compared with Mock group in A375 cells. Suggesting
that shCtrl lentivirus infection inhibited the colony formation
ability in A375, but it did not show the same inhibition effect in
Mel-RM cells in colony formation assay. In MTT assay, however, the
OD 490 nm/fold showed no significant difference between Mock and
shCtrl groups in both A375 and Mel-RM cells, since the cell density
was different between colony-forming assay and MTT assay. These
results implied that, different cell lines have various
susceptibilities to virus infection, and possibly A375 in a low
density would become more sensitive to virus infection.
In the present study, we only used single shRNA
sequence to detect the bio-functions of MAT2B. It may bring out the
off-target effects of RNA interference. In order to eliminate the
off-target effects, we selected two malignant melanoma cell lines
to confirm the functional results because these effects were
probably different between A375 and Mel-RM cell lines. Employing
multiple shRNA sequences in RNA interference may present stricter
results.
In conclusion, our data showed that MAT2B expression
was elevated in malignant melanoma compared with benign nevus and
was associated with tumor differentiation status. Knockdown of
MAT2B suppressed cell growth, colony formation ability and induced
apoptosis in vitro, as well as extended the transplanted
tumor growth in vivo. The possible mechanisms could be
associated with the upregulation of some pro-apoptotic genes
expression such as XAF1 and downregulation of some anti-apoptotic
genes expression such as BCL2. However, the particular mechanisms
still need to be confirmed. These results indicated that MAT2B was
critical for melanoma cell proliferation and tumorigenicity. It is
a probable target of anti-melanoma therapy.
Acknowledgements
The present study is supported by the Fund for
Program of Collaborative Innovation Center for Complex and Severe
Skin Diseases, Anhui Medical University (4601001116).
References
|
1
|
Lu SC and Mato JM: S-adenosylmethionine in
liver health, injury, and cancer. Physiol Rev. 92:1515–1542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halim AB, LeGros L, Geller A and Kotb M:
Expression and functional interaction of the catalytic and
regulatory subunits of human methionine adenosyltransferase in
mammalian cells. J Biol Chem. 274:29720–29725. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeGros HL Jr, Halim AB, Geller AM and Kotb
M: Cloning, expression, and functional characterization of the β
regulatory subunit of human methionine adenosyltransferase (MAT
II). J Biol Chem. 275:2359–2366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LeGros L, Halim AB, Chamberlin ME, Geller
A and Kotb M: Regulation of the human MAT2B gene encoding the
regulatory beta subunit of methionine adenosyltransferase, MAT II.
J Biol Chem. 276:24918–24924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Ara AI, Magilnick N, Xia M, Ramani
K, Chen H, Lee TD, Mato JM and Lu SC: Expression pattern,
regulation, and functions of methionine adenosyltransferase 2beta
splicing variants in hepatoma cells. Gastroenterology. 134:281–291.
2008. View Article : Google Scholar
|
|
6
|
Wang Q, Liu QY, Liu ZS, Qian Q, Sun Q and
Pan DY: Lentivirus mediated shRNA interference targeting MAT2B
induces growth-inhibition and apoptosis in hepatocelluar carcinoma.
World J Gastroenterol. 14:4633–4642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng H, Dara L, Li TW, Zheng Y, Yang H,
Tomasi ML, Tomasi I, Giordano P, Mato JM and Lu SC: MAT2B-GIT1
interplay activates MEK1/ERK 1 and 2 to induce growth in human
liver and colon cancer. Hepatology. 57:2299–2313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng H, Li TW, Yang H, Moyer MP, Mato JM
and Lu SC: Methionine adenosyltransferase 2B-GIT1 complex serves as
a scaffold to regulate Ras/Raf/MEK1/2 activity in human liver and
colon cancer cells. Am J Pathol. 185:1135–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia M, Chen Y, Wang LC, Zandi E, Yang H,
Bemanian S, Martínez-Chantar ML, Mato JM and Lu SC: Novel function
and intracellular localization of methionine adenosyltransferase
2beta splicing variants. J Biol Chem. 285:20015–20021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Russo A, Ficili B, Candido S, Pezzino FM,
Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA and
Libra M: Emerging targeted therapies for melanoma treatment
(Review). Int J Oncol. 45:516–524. 2014.PubMed/NCBI
|
|
12
|
Wilmott JS, Menzies AM, Haydu LE, Capper
D, Preusser M, Zhang YE, Thompson JF, Kefford RF, von Deimling A,
Scolyer RA, et al: BRAF(V600E) protein expression and outcome from
BRAF inhibitor treatment in BRAF(V600E) metastatic melanoma. Br J
Cancer. 108:924–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sosman JA, Kim KB, Schuchter L, Gonzalez
R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ,
Flaherty KT, et al: Survival in BRAF V600-mutant advanced melanoma
treated with vemurafenib. N Engl J Med. 366:707–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ascierto PA, Minor D, Ribas A, Lebbe C,
O’Hagan A, Arya N, Guckert M, Schadendorf D, Kefford RF, Grob JJ,
et al: Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib
(GSK2118436) in patients with metastatic melanoma. J Clin Oncol.
31:3205–3211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Budwit-Novotny DA, McCarty KS, Cox EB,
Soper JT, Mutch DG, Creasman WT, Flowers JL and McCarty KS Jr:
Immunohistochemical analyses of estrogen receptor in endometrial
adenocarcinoma using a monoclonal antibody. Cancer Res.
46:5419–5425. 1986.PubMed/NCBI
|
|
16
|
Specht E, Kaemmerer D, Sänger J, Wirtz RM,
Schulz S and Lupp A: Comparison of immunoreactive score, HER2/neu
score and H score for the immunohistochemical evaluation of
somatostatin receptors in bronchopulmonary neuroendocrine
neoplasms. Histopathology. 67:368–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei Y, Li HX, Jin WS, Peng WR, Zhang CJ,
Bu LJ, Du YY, Ma T and Sun GP: The radiosensitizing effect of
Paeonol on lung adenocarcinoma by augmentation of radiation-induced
apoptosis and inhibition of the PI3K/Akt pathway. Int J Radiat
Biol. 89:1079–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rygaard K and Spang-Thomsen M:
Quantitation and gompertzian analysis of tumor growth. Breast
Cancer Res Treat. 46:303–312. 1997. View Article : Google Scholar
|
|
19
|
Kocab AJ and Duckett CS: Inhibitor of
apoptosis proteins as intracellular signaling intermediates. FEBS
J. 283:221–231. 2016. View Article : Google Scholar
|
|
20
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35(Suppl): S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hartman ML and Czyz M: Anti-apoptotic
proteins on guard of melanoma cell survival. Cancer Lett.
331:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moldoveanu T, Follis AV, Kriwacki RW and
Green DR: Many players in BCL-2 family affairs. Trends Biochem Sci.
39:101–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silke J and Vucic D: IAP family of cell
death and signaling regulators. Methods Enzymol. 545:35–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liston P, Fong WG, Kelly NL, Toji S,
Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW and Korneluk
RG: Identification of XAF1 as an antagonist of XIAP anti-Caspase
activity. Nat Cell Biol. 3:128–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao WJ, Deng BY, Wang XM, Miao Y and Wang
JN: XIAP associated factor 1 (XAF1) represses expression of
X-linked inhibitor of apoptosis protein (XIAP) and regulates
invasion, cell cycle, apoptosis, and cisplatin sensitivity of
ovarian carcinoma cells. Asian Pac J Cancer Prev. 16:2453–2458.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY,
Sun PH, Y, Qiao MM, Wu YL, Jiang SH, et al: Tumor suppressor XAF1
induces apoptosis, inhibits angiogenesis and inhibits tumor growth
in hepatocellular carcinoma. Oncotarget. 5:5403–5415. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ju WC, Huang GB, Luo XY, Ren WH, Zheng DQ,
Chen PJ, Lou YF and Li B: X-linked inhibitor of
apoptosis-associated factor l (XAFl) enhances the sensitivity of
colorectal cancer cells to cisplatin. Med Oncol. 31:2732014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang J, Yao WY, Zhu Q, Tu SP, Yuan F,
Wang HF, Zhang YP and Yuan YZ: XAF1 as a prognostic biomarker and
therapeutic target in pancreatic cancer. Cancer Sci. 101:559–567.
2010. View Article : Google Scholar
|
|
31
|
Ng KC, Campos EI, Martinka M and Li G:
XAF1 expression is significantly reduced in human melanoma. J
Invest Dermatol. 123:1127–1134. 2004. View Article : Google Scholar : PubMed/NCBI
|