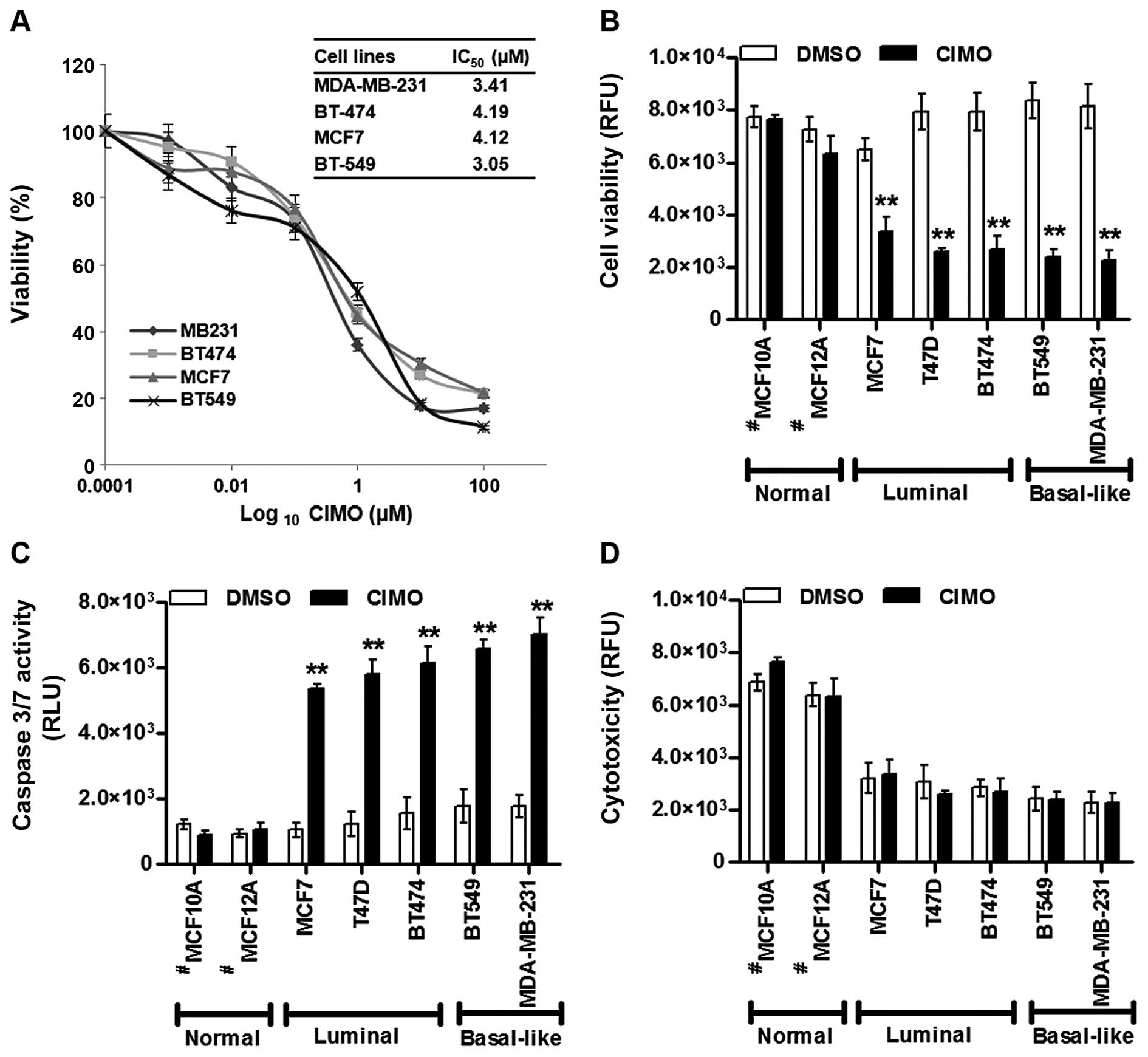
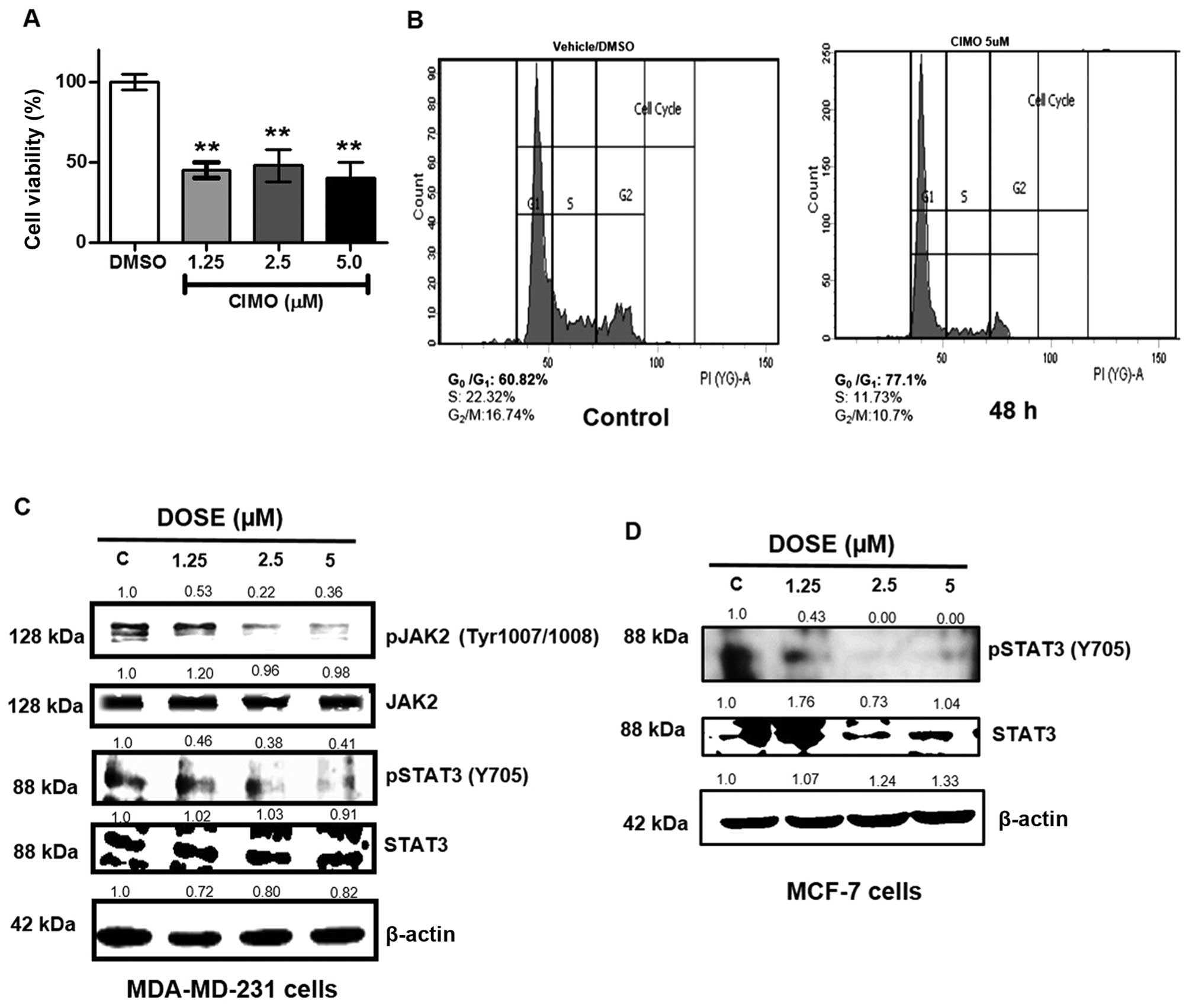
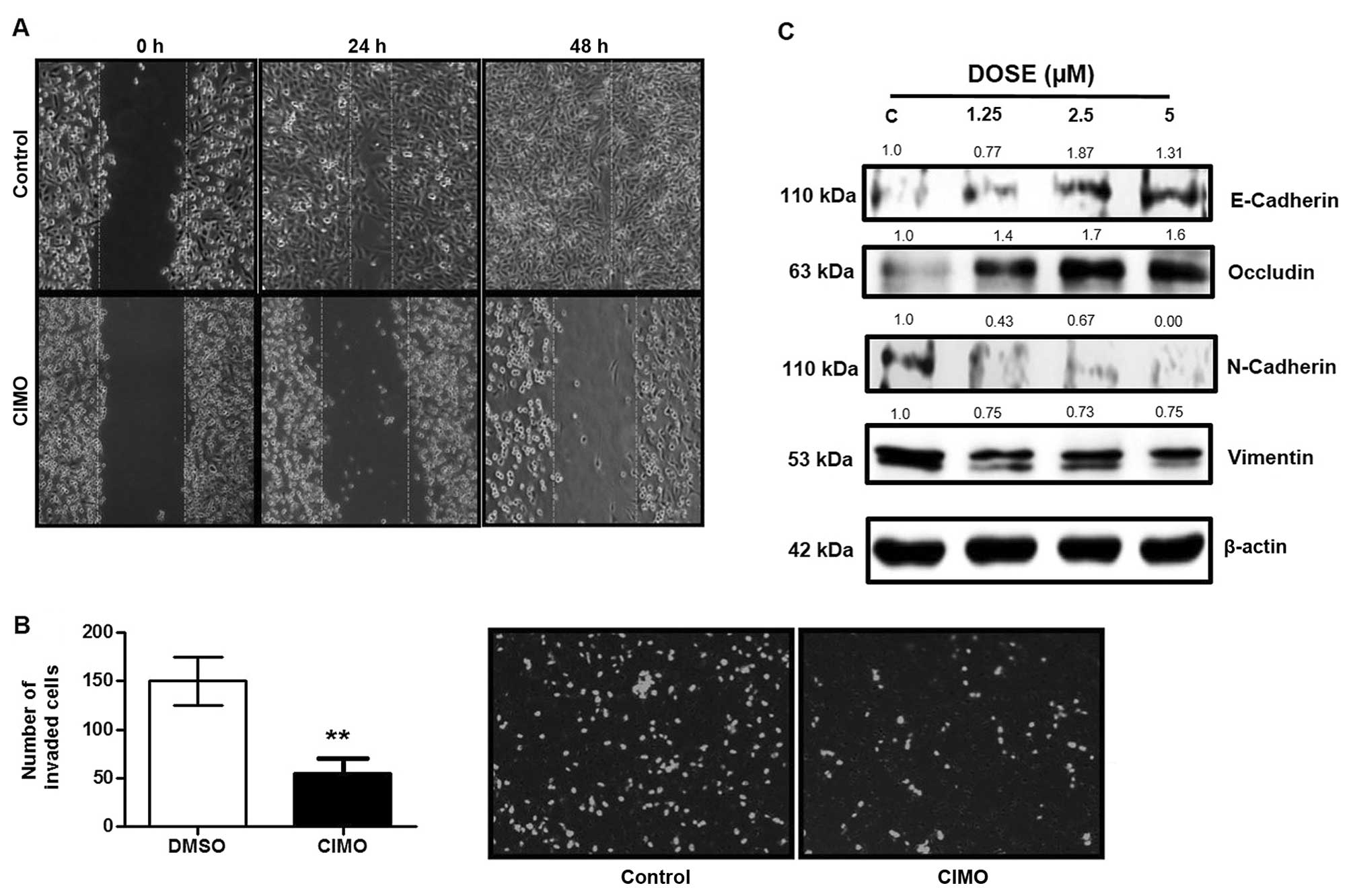
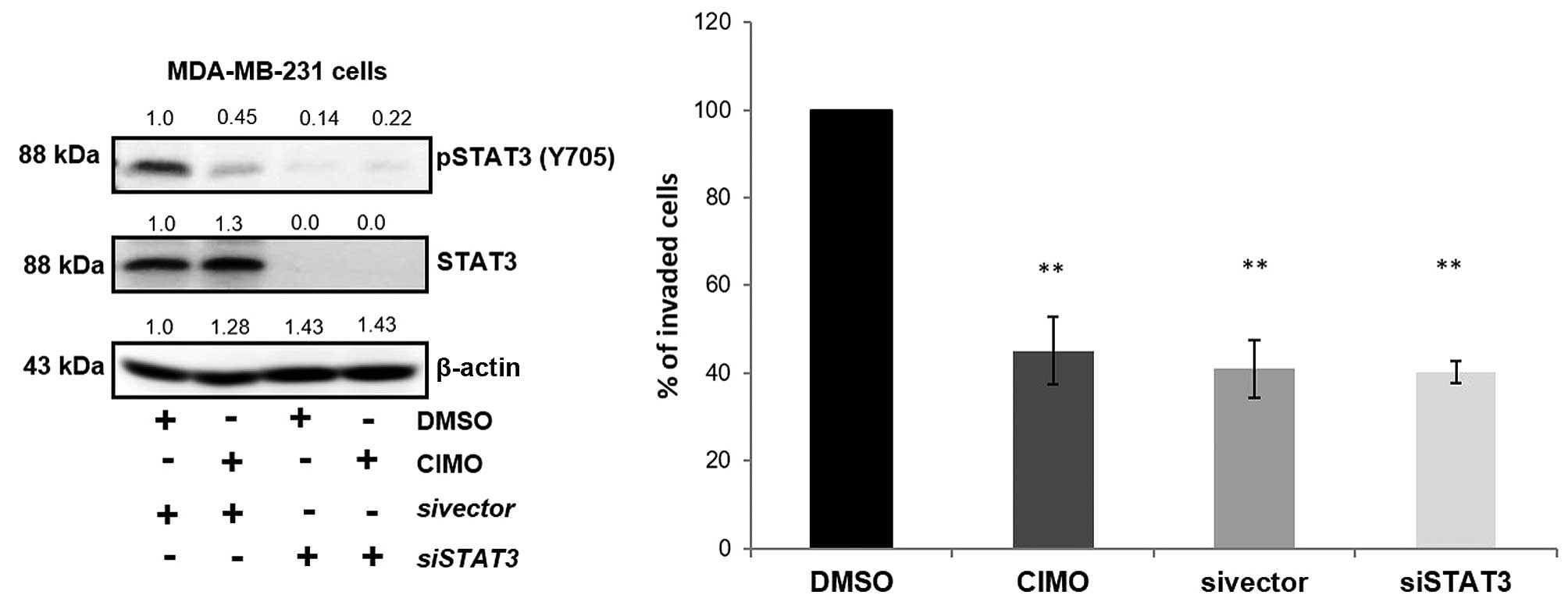
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narod SA, Dubé M-P, Klijn J, Lubinski J,
Lynch HT, Ghadirian P, Provencher D, Heimdal K, Moller P, Robson M,
et al: Oral contraceptives and the risk of breast cancer in BRCA1
and BRCA2 mutation carriers. J Natl Cancer Inst. 94:1773–1779.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donovan M, Tiwary CM, Axelrod D, Sasco AJ,
Jones L, Hajek R, Sauber E, Kuo J and Davis DL: Personal care
products that contain estrogens or xenoestrogens may increase
breast cancer risk. Med Hypotheses. 68:756–766. 2007. View Article : Google Scholar
|
|
4
|
Ronckers CM, Erdmann CA and Land CE:
Radiation and breast cancer: A review of current evidence. Breast
Cancer Res. 7:21–32. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srinivasa V, Li F, Siveen KS, et al:
Synthesis and biological evaluation of tetrahydropyridinepyrazoles
(‘PFPs’) as inhibitors of STAT3 phosphorylation. MedChemComm.
5:32–40. 2014. View Article : Google Scholar
|
|
6
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: Clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohan CD, Bharathkumar H, Bulusu KC,
Pandey V, Rangappa S, Fuchs JE, Shanmugam MK, Dai X, Li F,
Deivasigamani A, et al: Development of a novel azaspirane that
targets the Janus kinase-signal transducer and activator of
transcription (STAT) pathway in hepatocellular carcinoma in vitro
and in vivo. J Biol Chem. 289:34296–34307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
9
|
Lee JH, Kim C, Sethi G and Ahn KS:
Brassinin inhibits STAT3 signaling pathway through modulation of
PIAS-3 and SOCS-3 expression and sensitizes human lung cancer
xenograft in nude mice to paclitaxel. Oncotarget. 6:6386–6405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strati P, Kantarjian H, Ravandi F, Nazha
A, Borthakur G, Daver N, Kadia T, Estrov Z, Garcia-Manero G,
Konopleva M, et al: Phase I/II trial of the combination of
midostaurin (PKC412) and 5-azacytidine for patients with acute
myeloid leukemia and myelodysplastic syndrome. Am J Hematol.
90:276–281. 2015. View Article : Google Scholar :
|
|
12
|
M.D. Anderson Cancer Center. Study of
atiprimod treatment for patients with advanced cancer. https://clinicaltrials.gov/ct2/show/NCT00430014.
Verified February 2012.
|
|
13
|
Aubert L, Guilbert M, Corbet C, Génot E,
Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magné N, Le
Bourhis X, et al: NGF-induced TrkA/CD44 association is involved in
tumor aggressiveness and resistance to lestaurtinib. Oncotarget.
6:9807–9819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Millward MJ, House C, Bowtell D, Webster
L, Olver IN, Gore M, Copeman M, Lynch K, Yap A, Wang Y, et al: The
multikinase inhibitor midostaurin (PKC412A) lacks activity in
metastatic melanoma: A phase IIA clinical and biologic study. Br J
Cancer. 95:829–834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stone RM, DeAngelo DJ, Klimek V, Galinsky
I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, et al:
Patients with acute myeloid leukemia and an activating mutation in
FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor,
PKC412. Blood. 105:54–60. 2005. View Article : Google Scholar
|
|
16
|
Quintás-Cardama A, Manshouri T, Estrov Z,
Harris D, Zhang Y, Gaikwad A, Kantarjian HM and Verstovsek S:
Preclinical characterization of atiprimod, a novel JAK2 AND JAK3
inhibitor. Invest New Drugs. 29:818–826. 2011. View Article : Google Scholar
|
|
17
|
Knapper S, Burnett AK, Littlewood T, Kell
WJ, Agrawal S, Chopra R, Clark R, Levis MJ and Small D: A phase 2
trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line
treatment for older patients with acute myeloid leukemia not
considered fit for intensive chemotherapy. Blood. 108:3262–3270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hexner EO, Serdikoff C, Jan M, Swider CR,
Robinson C, Yang S, Angeles T, Emerson SG, Carroll M, Ruggeri B, et
al: Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses
JAK2/STAT5 signaling and the proliferation of primary erythroid
cells from patients with myeloproliferative disorders. Blood.
111:5663–5671. 2008. View Article : Google Scholar
|
|
19
|
Children’s Oncology Group. Combination
chemotherapy with or without lestaurtinib in treating younger
patients with newly diagnosed acute lymphoblastic leukemia.
https://clinicaltrials.gov/ct2/show/NCT00557193.
Verified September 2015.
|
|
20
|
Tapley P, Lamballe F and Barbacid M: K252a
is a selective inhibitor of the tyrosine protein kinase activity of
the trk family of oncogenes and neurotrophin receptors. Oncogene.
7:371–381. 1992.PubMed/NCBI
|
|
21
|
Moon C, Yoo J-Y, Matarazzo V, Sung YK, Kim
EJ and Ronnett GV: Leukemia inhibitory factor inhibits neuronal
terminal differentiation through STAT3 activation. Proc Natl Acad
Sci USA. 99:9015–9020. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bharathkumar H, Mohan CD, Rangappa S, Kang
T, Keerthy HK, Fuchs JE, Kwon NH, Bender A, Kim S, Basappa, et al:
Screening of quinoline, 1,3-benzoxazine, and 1,3-oxazine-based
small molecules against isolated methionyl-tRNA synthetase and A549
and HCT116 cancer cells including an in silico binding mode
analysis. Org Biomol Chem. 13:9381–9387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basappa, Sugahara K, Thimmaiah KN, Bid HK,
Houghton PJ and Rangappa KS: Anti-tumor activity of a novel
HS-mimetic-vascular endothelial growth factor binding small
molecule. PLoS One. 7:e394442012. View Article : Google Scholar :
|
|
24
|
Rakesh KS, Jagadish S, Vinayaka AC,
Hemshekhar M, Paul M, Thushara RM, Sundaram MS, Swaroop TR, Mohan
CD, Basappa, et al: A new ibuprofen derivative inhibits platelet
aggregation and ROS mediated platelet apoptosis. PLoS One.
9:e1071822014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anusha S, Anandakumar BS, Mohan CD, et al:
Preparation and use of combustion-derived Bi2O3 for the synthesis
of heterocycles with anti-cancer properties by Suzuki-coupling
reactions. RSC Advances. 4:52181–52188. 2014.
|
|
26
|
Bharathkumar H, Mohan CD, Ananda H, Fuchs
JE, Li F, Rangappa S, Surender M, Bulusu KC, Girish KS, Sethi G, et
al: Microwave-assisted synthesis, characterization and cytotoxic
studies of novel estrogen receptor α ligands towards human breast
cancer cells. Bioorg Med Chem Lett. 25:1804–1807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anilkumar NC, Sundaram MS, Mohan CD,
Rangappa S, Bulusu KC, Fuchs JE, Girish KS, Bender A, Basappa and
Rangappa KS: A one pot synthesis of novel bioactive
tri-substitute-condensed-imidazopyridines that targets snake venom
phospholipase A2. PLoS One. 10:e01318962015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keerthy HK, Mohan CD, Sivaraman Siveen K,
Fuchs JE, Rangappa S, Sundaram MS, Li F, Girish KS, Sethi G,
Basappa, et al: Novel synthetic biscoumarins target tumor necrosis
factor-α in hepatocellular carcinoma in vitro and in vivo. J Biol
Chem. 289:31879–31890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anusha S, Mohan CD, Ananda H, Baburajeev
CP, Rangappa S, Mathai J, Fuchs JE, Li F, Shanmugam MK, Bender A,
et al: Adamantyl-tethered-biphenylic compounds induce apoptosis in
cancer cells by targeting Bcl homologs. Bioorg Med Chem Lett.
26:1056–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anusha S, Cp B, Mohan CD, Mathai J,
Rangappa S, Mohan S, Chandra, Paricharak S, Mervin L, Fuchs JE, et
al: A Nano-MgO and ionic liquid-catalyzed ‘green’ synthesis
protocol for the development of adamantyl-imidazolo-thiadiazoles as
anti-tuberculosis agents targeting sterol 14α-demethylase (CYP51).
PLoS One. 10:e01397982015. View Article : Google Scholar
|
|
31
|
Baburajeev CP, Dhananjaya Mohan C, Ananda
H, Rangappa S, Fuchs JE, Jagadish S, Sivaraman Siveen K,
Chinnathambi A, Ali Alharbi S, Zayed ME, et al: Development of
novel triazolo-thiadiazoles from heterogeneous ‘Green’ catalysis as
protein tyrosine phosphatase 1B inhibitors. Sci Rep. 5:141952015.
View Article : Google Scholar
|
|
32
|
Ashwini N, Garg M, Mohan CD, Fuchs JE,
Rangappa S, Anusha S, Swaroop TR, Rakesh KS, Kanojia D, Madan V, et
al: Synthesis of 1,2-benzisoxazole tethered 1,2,3-triazoles that
exhibit anticancer activity in acute myeloid leukemia cell lines by
inhibiting histone deacetylases, and inducing p21 and tubulin
acetylation. Bioorg Med Chem. 23:6157–6165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pandey V, Perry JK, Mohankumar KM, Kong
XJ, Liu SM, Wu ZS, Mitchell MD, Zhu T and Lobie PE: Autocrine human
growth hormone stimulates oncogenicity of endometrial carcinoma
cells. Endocrinology. 149:3909–3919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neelgundmath M, Dinesh KR, Mohan CD, Li F,
Dai X, Siveen KS, Paricharak S, Mason DJ, Fuchs JE, Sethi G, et al:
Novel synthetic coumarins that targets NF-κB in hepatocellular
carcinoma. Bioorg Med Chem Lett. 25:893–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bharathkumar H, Paricharak S, Dinesh KR,
et al: Synthesis, biological evaluation and in silico and in vitro
mode-of-action analysis of novel dihydropyrimidones targeting
PPAR-γ. RSC Advances. 4:45143–45146. 2014.
|
|
36
|
Morin PJ: Drug resistance and the
microenvironment: Nature and nurture. Drug Resist Updat. 6:169–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rajendran P, Li F, Manu KA, Shanmugam MK,
Loo SY, Kumar AP and Sethi G: γ-Tocotrienol is a novel inhibitor of
constitutive and inducible STAT3 signalling pathway in human
hepatocellular carcinoma: Potential role as an antiproliferative,
pro-apoptotic and chemosensitizing agent. Br J Pharmacol.
163:283–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Firestone GL and Sundar SN: Anticancer
activities of artemisinin and its bioactive derivatives. Expert Rev
Mol Med. 11:e322009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Castellano M, Pollock PM, Walters MK,
Sparrow LE, Down LM, Gabrielli BG, Parsons PG and Hayward NK:
CDKN2A/p16 is inactivated in most melanoma cell lines. Cancer Res.
57:4868–4875. 1997.PubMed/NCBI
|
|
41
|
Keerthy HK, Garg M, Mohan CD, Madan V,
Kanojia D, Shobith R, Nanjundaswamy S, Mason DJ, Bender A, Basappa,
et al: Synthesis and characterization of novel
2-amino-chromene-nitriles that target Bcl-2 in acute myeloid
leukemia cell lines. PLoS One. 9:e1071182014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singhai R, Patil VW, Jaiswal SR, Patil SD,
Tayade MB and Patil AV: E-Cadherin as a diagnostic biomarker in
breast cancer. N Am J Med Sci. 3:227–233. 2011. View Article : Google Scholar
|
|
44
|
Martin TA, Mansel RE and Jiang WG: Loss of
occludin leads to the progression of human breast cancer. Int J Mol
Med. 26:723–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhuo H, Jiang K, Dong L, Zhu Y, Lü L, Lü
Y, Zhang YB, Zhang H, Ye YJ and Wang S: Overexpression of
N-cadherin is correlated with metastasis and worse survival in
colorectal cancer patients. Chin Sci Bull. 58:3529–3534. 2013.
View Article : Google Scholar
|
|
46
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lieblein JC, Ball S, Hutzen B, Sasser AK,
Lin HJ, Huang TH, Hall BM and Lin J: STAT3 can be activated through
paracrine signaling in breast epithelial cells. BMC Cancer.
8:3022008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB
and Chin YE: Central role of the threonine residue within the p+1
loop of receptor tyrosine kinase in STAT3 constitutive
phosphorylation in metastatic cancer cells. Mol Cell Biol.
24:9390–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang W, Qian P, Zhang X, Zhang M, Wang H,
Wu M, Kong X, Tan S, Ding K, Perry JK, et al: Autocrine/paracrine
human growth hormone-stimulated microRNA 96-182-183 cluster
promotes epithelial-mesenchymal transition and invasion in breast
cancer. J Biol Chem. 290:13812–13829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Y, Diab I, Zhang X, Izmailova ES and
Zehner ZE: Stat3 enhances vimentin gene expression by binding to
the antisilencer element and interacting with the repressor
protein, ZBP-89. Oncogene. 23:168–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elkholi R, Floros KV and Chipuk JE: The
role of BH3-only proteins in tumor cell development, signaling, and
treatment. Genes Cancer. 2:523–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Crown J, O’Shaughnessy J and Gullo G:
Emerging targeted therapies in triple-negative breast cancer. Ann
Oncol. (Suppl 6): vi56–65. 2012.PubMed/NCBI
|