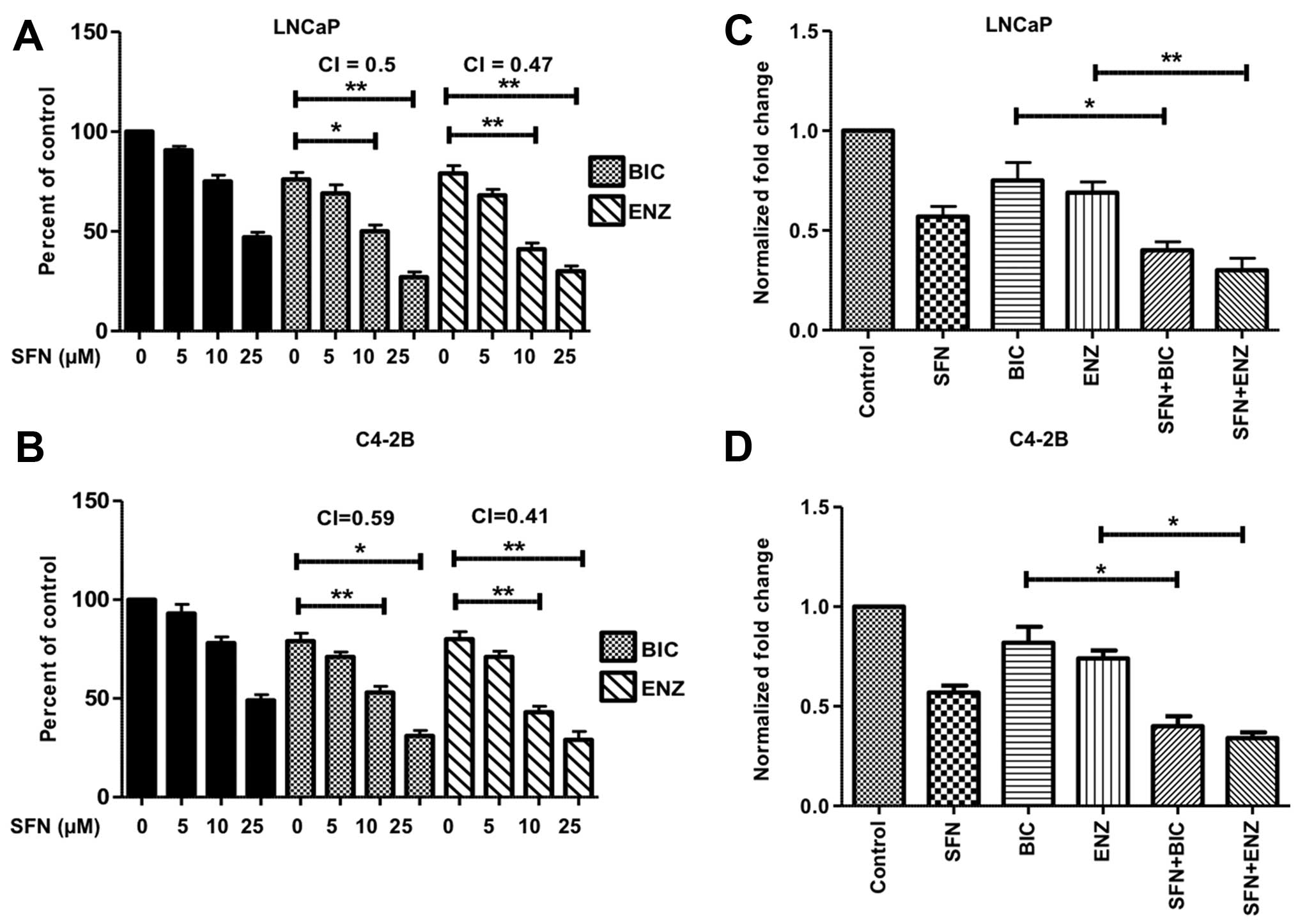
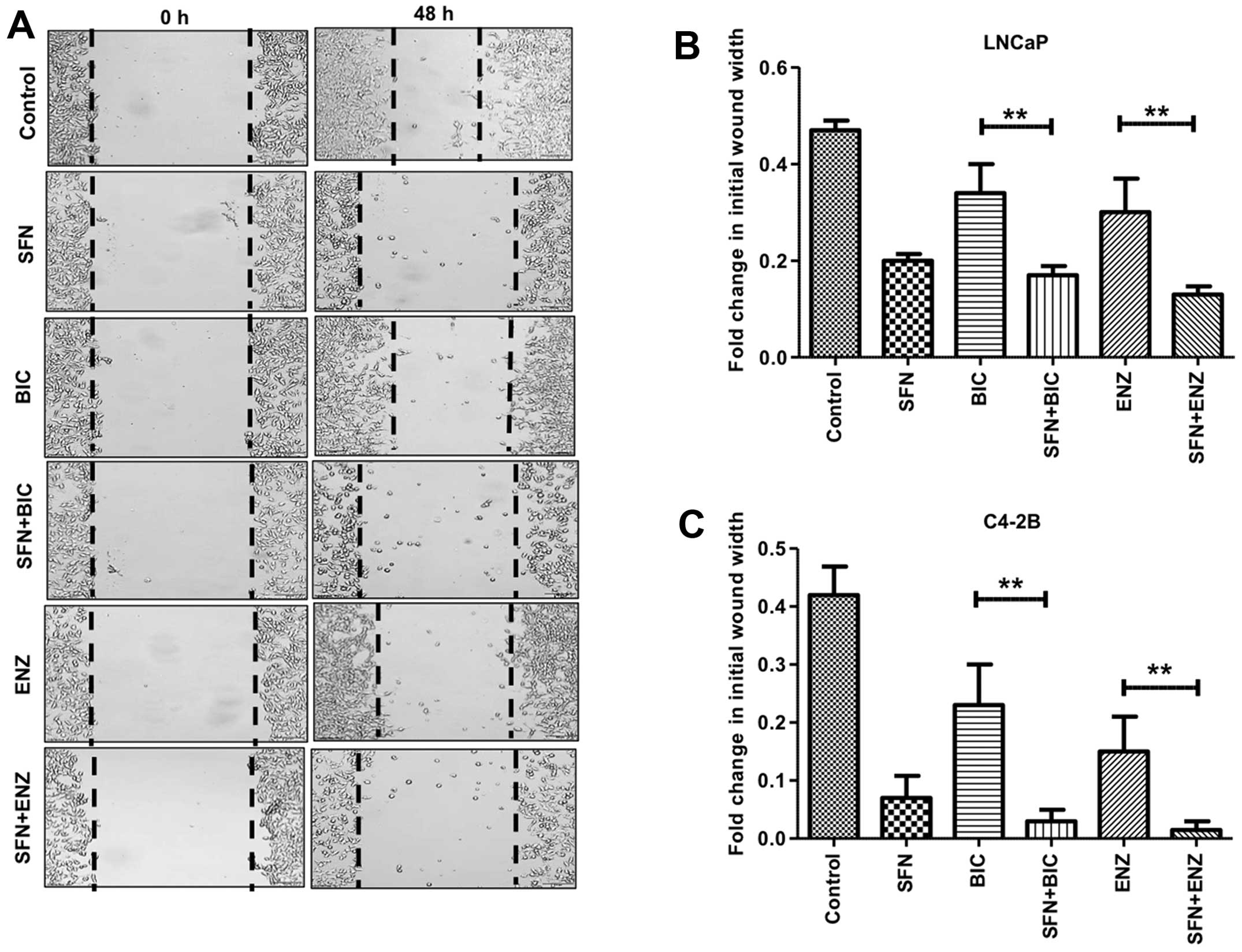
|
1
|
Yap TA, Zivi A, Omlin A and de Bono JS:
The changing therapeutic landscape of castration-resistant prostate
cancer. Nat Rev Clin Oncol. 8:597–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodgson MC, Bowden WA and Agoulnik IU:
Androgen receptor footprint on the way to prostate cancer
progression. World J Urol. 30:279–285. 2012. View Article : Google Scholar :
|
|
3
|
Chang KH, Ercole CE and Sharifi N:
Androgen metabolism in prostate cancer: From molecular mechanisms
to clinical consequences. Br J Cancer. 111:1249–1254. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan CJ, Smith A, Lal P, Satagopan J,
Reuter V, Scardino P, Gerald W and Scher HI: Persistent
prostate-specific antigen expression after neoadjuvant androgen
depletion: An early predictor of relapse or incomplete androgen
suppression. Urology. 68:834–839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harris WP, Mostaghel EA, Nelson PS and
Montgomery B: Androgen deprivation therapy: Progress in
understanding mechanisms of resistance and optimizing androgen
depletion. Nat Clin Pract Urol. 6:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Godbole AM and Njar VC: New insights into
the androgen-targeted therapies and epigenetic therapies in
prostate cancer. Prostate Cancer. 2011:9187072011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim W and Ryan CJ: Androgen receptor
directed therapies in castration-resistant metastatic prostate
cancer. Curr Treat Options Oncol. 13:189–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N,
Gaikwad NW, Evans CP and Gao AC: Intracrine androgens and AKR1C3
activation confer resistance to enzalutamide in prostate cancer.
Cancer Res. 75:1413–1422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schrader AJ, Schrader MG and Cronauer MV:
Words of wisdom. Re: Androgen receptor splice variants mediate
enzalutamide resistance in castration-resistant prostate cancer
cell lines. Eur Urol. 64:169–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arora VK, Schenkein E, Murali R, Subudhi
SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis
C, et al: Glucocorticoid receptor confers resistance to
anti-androgens by bypassing androgen receptor blockade. Cell.
155:1309–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joseph JD, Lu N, Qian J, Sensintaffar J,
Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B, et al: A
clinically relevant androgen receptor mutation confers resistance
to second-generation anti-androgens enzalutamide and ARN-509.
Cancer Discov. 3:1020–1029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy
DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, et al: An F876L
mutation in androgen receptor confers genetic and phenotypic
resistance to MDV3100 (enzalutamide). Cancer Discov. 3:1030–1043.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawata H, Ishikura N, Watanabe M,
Nishimoto A, Tsunenari T and Aoki Y: Prolonged treatment with
bicalutamide induces androgen receptor overexpression and androgen
hypersensitivity. Prostate. 70:745–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colabufo NA, Pagliarulo V, Berardi F,
Contino M, Inglese C, Niso M, Ancona P, Albo G, Pagliarulo A and
Perrone R: Bicalutamide failure in prostate cancer treatment:
Involvement of Multi Drug Resistance proteins. Eur J Pharmacol.
601:38–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bohl CE, Gao W, Miller DD, Bell CE and
Dalton JT: Structural basis for antagonism and resistance of
bicalutamide in prostate cancer. Proc Natl Acad Sci USA.
102:6201–6206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saad F, Adachi JD, Brown JP, Canning LA,
Gelmon KA, Josse RG and Pritchard KI: Cancer treatment-induced bone
loss in breast and prostate cancer. J Clin Oncol. 26:5465–5476.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salturk Z, Çakır O, Kumral TL, Yıldırım G,
Ötünçtemur A, Aydoğdu Ï and Uyar Y: Subjective and objective
effects of androgen ablation therapy on voice. J Voice. 29:490–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McCarty MF, Hejazi J and Rastmanesh R:
Beyond androgen deprivation: Ancillary integrative strategies for
targeting the androgen receptor addiction of prostate cancer.
Integr Cancer Ther. 13:386–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamont KR and Tindall DJ: Minireview:
Alternative activation pathways for the androgen receptor in
prostate cancer. Mol Endocrinol. 25:897–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brooke GN and Bevan CL: The role of
androgen receptor mutations in prostate cancer progression. Curr
Genomics. 10:18–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armstrong CM and Gao AC: Drug resistance
in castration resistant prostate cancer: Resistance mechanisms and
emerging treatment strategies. Am J Clin Exp Urol. 3:64–76.
2015.PubMed/NCBI
|
|
22
|
Elbarbry F and Elrody N: Potential health
benefits of sulforaphane: A review of the experimental, clinical
and epidemiological evidences and underlying mechanisms. J Med
Plants Res. 5:473–484. 2011.
|
|
23
|
Zhang Y and Tang L: Discovery and
development of sulforaphane as a cancer chemopreventive
phytochemical. Acta Pharmacol Sin. 28:1343–1354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clarke JD, Dashwood RH and Ho E:
Multi-targeted prevention of cancer by sulforaphane. Cancer Lett.
269:291–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheung KL and Kong AN: Molecular targets
of dietary phenethyl isothiocyanate and sulforaphane for cancer
chemoprevention. AAPS J. 12:87–97. 2010. View Article : Google Scholar :
|
|
26
|
Traka MH, Melchini A and Mithen RF:
Sulforaphane and prostate cancer interception. Drug Discov Today.
19:1488–1492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keum YS, Khor TO, Lin W, Shen G, Kwon KH,
Barve A, Li W and Kong AN: Pharmacokinetics and pharmacodynamics of
broccoli sprouts on the suppression of prostate cancer in
transgenic adenocarcinoma of mouse prostate (TRAMP) mice:
Implication of induction of Nrf2, HO-1 and apoptosis and the
suppression of Akt-dependent kinase pathway. Pharm Res.
26:2324–2331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shapiro TA, Fahey JW, Dinkova-Kostova AT,
Holtzclaw WD, Stephenson KK, Wade KL, Ye L and Talalay P: Safety,
tolerance, and metabolism of broccoli sprout glucosinolates and
isothiocyanates: a clinical phase I study. Nutr Cancer. 55:53–62.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petri N, Tannergren C, Holst B, Mellon FA,
Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, et al:
Absorption/metabolism of sulforaphane and quercetin, and regulation
of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos.
31:805–813. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh SV, Srivastava SK, Choi S, Lew KL,
Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, et
al: Sulforaphane-induced cell death in human prostate cancer cells
is initiated by reactive oxygen species. J Biol Chem.
280:19911–19924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao D, Powolny AA, Antosiewicz J, Hahm
ER, Bommareddy A, Zeng Y, Desai D, Amin S, Herman-Antosiewicz A and
Singh SV: Cellular responses to cancer chemopreventive agent
D,L-sulforaphane in human prostate cancer cells are initiated by
mitochondrial reactive oxygen species. Pharm Res. 26:1729–1738.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pei Y, Wu B, Cao Q, Wu L and Yang G:
Hydrogen sulfide mediates the anti-survival effect of sulforaphane
on human prostate cancer cells. Toxicol Appl Pharmacol.
257:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Su ZY, Khor TO, Shu L and Kong
AN: Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP
C1 cells through epigenetic regulation. Biochem Pharmacol.
85:1398–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schultz MA, Hagan SS, Datta A, Zhang Y,
Freeman ML, Sikka SC, Abdel-Mageed AB and Mondal D: Nrf1 and Nrf2
transcription factors regulate androgen receptor transactivation in
prostate cancer cells. PLoS One. 9:e872042014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kensler TW, Egner PA, Agyeman AS,
Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW and Talalay
P: Keap1-nrf2 signaling: A target for cancer prevention by
sulforaphane. Top Curr Chem. 329:163–177. 2013. View Article : Google Scholar :
|
|
36
|
Kim SH and Singh SV: D,L-Sulforaphane
causes transcriptional repression of androgen receptor in human
prostate cancer cells. Mol Cancer Ther. 8:1946–1954. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wiczk A, Hofman D, Konopa G and
Herman-Antosiewicz A: Sulforaphane, a cruciferous vegetable-derived
isothiocyanate, inhibits protein synthesis in human prostate cancer
cells. Biochim Biophys Acta. 1823:1295–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gibbs A, Schwartzman J, Deng V and Alumkal
J: Sulforaphane destabilizes the androgen receptor in prostate
cancer cells by inactivating histone deacetylase 6. Proc Natl Acad
Sci USA. 106:16663–16668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu HC, Hsieh JT, Gleave ME, Brown NM,
Pathak S and Chung LW: Derivation of androgen-independent human
LNCaP prostatic cancer cell sublines: Role of bone stromal cells.
Int J Cancer. 57:406–412. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uygur B and Wu WS: SLUG promotes prostate
cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol
Cancer. 10:1392011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Horoszewicz JS, Leong SS, Chu TM, Wajsman
ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, et
al: The LNCaP cell line - a new model for studies on human
prostatic carcinoma. Prog Clin Biol Res. 37:115–132. 1980.
|
|
43
|
Cunningham D and You Z: In vitro and in
vivo model systems used in prostate cancer research. J Biol
Methods. 2:pii. e172015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Agoulnik IU, Vaid A, Bingman WE, Erdeme H,
Frolov A, Smith CL, Ayala G, Ittmann MM and Weigel NL: Role of
SRC-1 in the promotion of prostate cancer cell growth and tumor
progression. Cancer Res. 65:7959–7967. 2005.PubMed/NCBI
|
|
45
|
He S, Zhang C, Shafi AA, Sequeira M,
Acquaviva J, Friedland JC, Sang J, Smith DL, Weigel NL, Wada Y, et
al: Potent activity of the Hsp90 inhibitor ganetespib in prostate
cancer cells irrespective of androgen receptor status or variant
receptor expression. Int J Oncol. 42:35–43. 2013.
|
|
46
|
Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson
JB and Wang Z: HDAC6 regulates androgen receptor hypersensitivity
and nuclear localization via modulating Hsp90 acetylation in
castration-resistant prostate cancer. Mol Endocrinol. 23:1963–1972.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Myzak MC, Tong P, Dashwood WM, Dashwood RH
and Ho E: Sulforaphane retards the growth of human PC-3 xenografts
and inhibits HDAC activity in human subjects. Exp Biol Med
(Maywood). 232:227–234. 2007.
|
|
48
|
Shiota M, Yokomizo A and Naito S:
Oxidative stress and androgen receptor signaling in the development
and progression of castration-resistant prostate cancer. Free Radic
Biol Med. 51:1320–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Demir A, Cecen K, Karadag MA, Kocaaslan R
and Turkeri L: The course of metastatic prostate cancer under
treatment. Springerplus. 3:7252014. View Article : Google Scholar
|
|
50
|
Lee JG, Zheng R, McCafferty-Cepero JM,
Burnstein KL, Nanus DM and Shen R: Endothelin-1 enhances the
expression of the androgen receptor via activation of the c-myc
pathway in prostate cancer cells. Mol Carcinog. 48:141–149. 2009.
View Article : Google Scholar
|
|
51
|
Mostaghel EA and Nelson PS: Intracrine
androgen metabolism in prostate cancer progression: Mechanisms of
castration resistance and therapeutic implications. Best Pract Res
Clin Endocrinol Metab. 22:243–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mostaghel EA, Page ST, Lin DW, Fazli L,
Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM,
et al: Intraprostatic androgens and androgen-regulated gene
expression persist after testosterone suppression: Therapeutic
implications for castration-resistant prostate cancer. Cancer Res.
67:5033–5041. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huo C, Kao YH and Chuu CP: Androgen
receptor inhibits epithelial-mesenchymal transition, migration, and
invasion of PC-3 prostate cancer cells. Cancer Lett. 369:103–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Singh SV, Warin R, Xiao D, Powolny AA,
Stan SD, Arlotti JA, Zeng Y, Hahm ER, Marynowski SW, Bommareddy A,
et al: Sulforaphane inhibits prostate carcinogenesis and pulmonary
metastasis in TRAMP mice in association with increased cytotoxicity
of natural killer cells. Cancer Res. 69:2117–2125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aapro MS, Eliason JF, Krauer F and Alberto
P: Colony formation in vitro as a prognostic indicator for primary
breast cancer. J Clin Oncol. 5:890–896. 1987.PubMed/NCBI
|
|
56
|
Kallifatidis G1, Rausch V, Baumann B, Apel
A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G,
et al: Sulforaphane targets pancreatic tumour-initiating cells by
NF-κB-induced antiapoptotic signaling. Gut. 58:949–963. 2009.
View Article : Google Scholar
|