Introduction
Angiogenesis, making new blood vessels from existing
ones, is considered as a key process in many physiological and
pathological states. Many reports have demonstrated that
angiogenesis is strongly implicated on cancer progression and
metastasis (1,2). Research on the molecular mechanisms
of angiogenesis has rapidly advanced and it has contributed to the
approval of anti-angiogenic drugs for cancer (3). Recently, a large number of candidates
composed of peptides have been developed because peptides have been
considered as secure pharmaceutical reagents against
angiogenesis-related diseases. However, there are many obstacles to
synthesize optimized peptides for cancer treatment (4,5).
Raddum et al revealed that seven different
model peptides based on Annexin A2 (AnxA2) present anti-angiogenic
effects on HUVECs. Especially, a synthetic peptide D1-P2 adopting
higher α-helical structure significantly inhibited network
formation rather than the others in the co-culture system of HUVECs
and SMCs (6). Therefore, in this
study, in order to reveal whether peptides containing α-helical
structure can affect angiogenesis, a novel cationic linear
α-helical model peptide, αAL14, was used.
Therapeutic strategies for treating diseases with
abnormal angiogenesis use control of VEGF receptor 2 because it can
modulate main angiogenic processes such as endothelial cell
proliferation, migration and tube formation by activating cellular
key kinases, ERK, FAK and AKT (7,8).
Rho GTPase family members are involved in cell
migration. Best-studied Rho GTPases, Rac1, Cdc42 and RhoA,
contribute to extend cell protrusions at the end of cell edge,
motivating cells to migrate. Formation of lamellipodia is the
primary step of cell migration. During cell migration, Rac1
activates Arp2/3 complex and WAVE2 to polymerize cytoskeletal
sheets at the front of cells (9,10).
Like cell migration, cell invasion is triggered through degradation
of ECM, secreting special proteolytic enzymes, MMPs. MMP2 and MMP9
have vital roles in the degradation of ECM during angiogenesis
(11–13).
In this study, a novel α-helical model peptide αAL14
was used to identify its anti-angiogenic properties. αAL14
inhibited angiogenesis by regulating VEGFR2-mediated signaling and
it affected expression of Rho GTPases (Rac1 and Cdc42) and MMPs.
Therefore, we can expect that this peptide can be applied for
development of therapeutic peptides to treat
angiogenesis-associated diseases such as cancer.
Materials and methods
Reagents
Matrigel was purchased from BD Bioscience (Bedford,
MA, USA). Vascular endothelial growth factor (VEGF) was from
Sigma-Aldrich (St. Louis, MO, USA). Formaldehyde (Junsei Chemical
Co., Ltd., Tokyo, Japan), Giemsa (Gurr-Giemsa, BDH Merk Ltd.,
Poole, UK) and BrdU cell proliferation assay kit (Cell Signaling
Technology, Danvers, MA, USA) were used. EGM-2 bullet kit was
obtained from Lonza (Walkersville, MD, USA). Dulbecco’s modified
Eagle’s medium (DMEM), fetal bovine serum (FBS) and antibiotics
(penicillin and streptomycin) were obtained from Corning (Manassas,
VA, USA). Hoechst 33342 and all of rabbit primary antibodies for
western blot analysis, VEGF receptor 2 (1:1,000, #9698),
phospho-VEGF receptor 2 (Tyr1175) (1:1,000, #2478), phospho-MEK1/2
(Ser217/221) (1:1,000, #9154), phospho-ERK1/2 (Thr202/Tyr204)
(1:1,000, #4370), RhoA (1:1,000, #2117), Cdc42 (1:1,000, #2466),
Rac1 (1:1,000, #2465), FAK (1:1,000, #3285), phospho-FAK (Tyr397)
(1:1,000, #8556), phospho-FAK (Tyr576/577) (1:1,000, #3281),
phospho-FAK (Tyr925) (1:1,000, #3284), phospho-Src (Tyr416)
(1:1,000, #6943), MMP2 (1:1,000, #4022), MMP9 (1:1,000, #3852),
NF-κB, phospho-NF-κB (Ser536) (1:1,000, #3033), Akt (1:1000,
#4691), phospho-Akt (Ser473) (1:1000, #4060), mTOR (1:1000, #2983),
phospho-mTOR (Ser2481) (1:1000, #2974), GAPDH (1:1,000, #2118) and
β-actin (1:1,000, #4970)) were purchased from Cell Signaling
Technology Inc. and ERK2 (1:1,000, sc-81457) for western blot
analysis was purchased from Santa Cruz Biotechnology Inc. (Dallas,
TX, USA). Secondary antibodies (horseradish peroxidase-conjugated
anti-rabbit IgG second antibodies and anti-mouse IgG second
antibodies) were purchased from Cell Signaling Technology Inc.
Annexin V, Alexa Fluor® 488 Conjugate, live cell imaging
solution (1X) and ProLong® Gold Antifade mounting medium
were purchased from Life Technologies (Carlsbad, CA, USA).
Peptide synthesis and structure
prediction
αAL14 was synthesized and purified as described
(14). Briefly, αAL14 was
commercially synthesized by Peptron Inc. (Daejeon, Korea) at a
purity grade of >95 % using N-(9-fluorenylmethoxycarbonyl)
(Fmoc) solid phase peptide synthesis with ASP48S. This peptide was
purified by reverse phase high performance liquid chromatography
(HPLC). Synthetic peptide was dissolved in sterilized distilled
water to obtain stock solutions of 10 mg/ml. The structure of αAL14
was predicted as described previously (14). A theoretical isoelectric point (pI)
of peptide was estimated using ExPASy’s ProtParam server
(http://www.expasy.org) and helical wheel diagram
was created by EMBOSS pepwheel sequence analysis program (European
Bioinformatics Institute, Cambridge, UK) (15).
Cell culture
Human vascular endothelial cells (HUVECs) were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA) and incubated with an endothelial basal medium-2 (EBM-2)
including EGM-2 singleQuots kit, 1% (v/v) penicillin-streptomycin
and 10% (v/v) FBS. Human keratinocytes (HaCat) and human embryonic
kidney cells (HEK-293) were obtained from Korean Cell Line Bank
(KCLB, Seoul, Korea). These two cell lines were incubated in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1%
(v/v) penicillin-streptomycin and 10% (v/v) FBS. The cells were
maintained at 37°C and 5% CO2 in a humidified
atmosphere.
BrdU cell proliferation assay
Effects of αAL14 on cell proliferation were
investigated using BrdU cell proliferation assay kit. The BrdU
assay was conducted according to the manufacturer’s instructions.
Cells (1×104 cells/well) were plated on a 96-well cell
culture plate and treated with αAL14 (0–40 μM) for 24 h. After
incubation, cells were incubated with BrdU for 4 h at 37°C. BrdU
incorporation was measured by microplate reader at 450 nm. This
assay was repeated three times.
Cell adhesion assay
Cell adhesion assay was performed as described
previously, with some modifications (16). The 96-well cell culture plates were
coated with 50 μg/ml Matrigel and left to air dry for 1 h. Wells
were blocked with 1% BSA for 1 h at room temperature and rinsed
twice with 0.1% PBS before plating cells. HUVECs were suspended
with complete EBM-2 media, and then seeded at 3.5×104
cells in each well, and then treated with various concentrations of
αAL14 for 2 h at 37°C. Cells were washed twice with 0.1% PBS. The
cells attached on a bottom were fixed with 4% formaldehyde for 15
min and stained with 5 mg/ml crystal violet for 10 min at room
temperature. Adhesive cells were observed with phase-inverted
microscope and quantitated by dye extraction with 2% SDS. The
absorbance was measured at 590 nm. Experiments were performed in
triplicates.
Transwell migration/invasion assay
Cell migration and invasion assay were performed as
previously described, with some slight modifications (17). Briefly, Transwells with 8-μm pore
size membrane (Corning, Tewksbury, MA, USA) were used to examine
cell migration and invasion of HUVECs. The lower chambers were
filled with EBM-2 pure medium including 20 ng/ml VEGF. HUVECs
(3.5×104 cells/insert) suspended in EBM-2 pure medium
were added on each Transwell. The Transwells and lower chambers
contained αAL14 (0–20 μM). Migrated cells were measured after 24 h
of incubation. Cells on topside of the membrane were removed by
wiping with a cotton swab, then, the membrane was washed with 1X
PBS. Cells on bottom side of the membrane were fixed with 4%
formaldehyde for 15 min, and stained with 5 mg/ml crystal violet
for 10 min at room temperature. Images were taken using
phase-inverted microscope at a magnification of ×40. To quantify
migrated cells, 2% SDS was added to lysis of the cells for 1 h at
room temperature and an absorbance was measured at 590 nm. To
perform invasion assay, Transwells were coated with 30% Matrigel in
pure EBM-2 medium and incubated for 3 h before adding cells on the
inserts. Following procedure of this assay is the same with the
migration assay using Transwells as above. Experiments were
performed in triplicates.
Tube formation assay
The effect of αAL14 on tube formation was
investigated in accordance with the procedure described by Kim
et al (16). The 96-well
cell culture plates were coated with 60 μl of Matrigel per well,
which was allowed to solidify at 37°C for 30 min. HUVECs were
seeded at a density of 2×104 cells per well on the
Matrigel and incubated with complete EBM-2 medium containing
different concentrations of αAL14 (0–20 μM). After 18 h, tubule
structures were observed using a phase-inverted microscope.
Quantification of total tube length was analyzed using Wimasis
image analysis service, WimTube (Wimasis GmbH, Munich, Germany).
Experiments were performed in triplicates.
Immunofluorescence staining and
localization of peptide
Immunofluorescence staining was performed as
described previously with some modifications (18). Briefly, HUVECs seeded on glass
coverslips were treated with 15 μM αAL14 for 30 min, fixed with 4%
formaldehyde for 15 min at room temperature, and blocked with 2%
BSA in PBS with 0.3% Triton X-100. These cells were then incubated
with specific rabbit primary antibodies at 4°C. Phospho-VEGF
receptor 2 (Tyr1175) (1:100, #2478), phospho-ERK1/2 (Thr202/Tyr204)
(1:200, #4370), phospho-Akt (Ser473) (1:200, #4060) and Rac1 (1:25,
#2467) were purchased from Cell Signaling Technology, Inc. and
phospho-FAK (Tyr397) (1:100, sc-11765) was purchased from Santa
Cruz Biotechnology, Inc. The cells were incubated with 0.1 μg/ml of
anti-rabbit IgG (H+L), F(ab′)2 fragment (Alexa Fluor 488 conjugate)
for 1 h at room temperature. Cells were stained with DAPI for 20
min at 37°C. The cells were mounted using ProLong Gold Antifade
mounting medium. Immunofluorescence was observed and imaged using a
laser scanning confocal microscope (Carl Zeiss LSM 700; Carl Zeiss,
Jena, Germany). To detect localization of αAL14, HUVECs were seeded
on glass coverslips for 24 h. After incubation, cells were treated
with FITC-tagged αAL14 for the indicated time period. Cells were
washed with live cell imaging buffer and stained with Hoechst 33342
for 10 min at 37°C. After mounting, the localization of peptide in
HUVECs was observed using a laser scanning confocal microscope.
Annexin V-FITC detection
To detect early apoptosis in αAL14-treated HUVECs,
Annexin V-FITC detection was performed according to the
manufacturer’s instructions. Briefly, HUVECs were seeded on glass
coverslips and treated with αAL14 for 24 h. After incubation, cells
were washed with cold 1X PBS and incubated with Hoechst 33342 and
Annexin V conjugate for 15 min at room temperature. After mounting,
Annexin-V-positive cells were observed using a laser scanning
confocal microscope.
Western blot analysis
Western blot analysis was performed as previously
described with some modifications (18). Briefly, after treatments, HUVECs
were washed once with cold 1X PBS and then lysed with lysis buffer
containing 50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM DTT, 0.5%
NP-40, 1% Triton X-100, 1% deoxycholate, 0.1% SDS and proteinase
inhibitors (PMSF, EDTA, Aprotinin, Leupeptin, Prostatin A) (Intron
Biotechnology, Gyeonggi, Korea). After 30 min on ice, lysates were
centrifuged at 14,000 rpm for 20 min at 4°C to remove cell debris
and collect proteins. Proteins were separated by 12%
SDS-polyacrylamide gel electrophoresis. The proteins were
transferred to nitrocellulose membrane. The membranes were blocked
with 5% skim milk in 1X PBST. After blocking, the proteins were
treated with primary antibodies (1:1,000) overnight at 4°C. The
proteins were incubated with horseradish peroxidase-conjugated
anti-rabbit IgG second antibodies (1:2,000) or anti-mouse IgG
second antibodies (1:2,000) for 1 h at room temperature. The
membranes were then washed in 1X PBST and then developed by
enhanced chemiluminescence ECL® (AbFontier, Gyeonggi,
Korea). Band intensities were quantified using ImageJ.
Reverse transcriptase PCR
Total RNA from HUVECs (1.0×107 cells) was
extracted using RNeasy Mini kit (Qiagen, Venlo, KJ, The
Netherlands) according to the manufacturer’s protocol and
quantified by Qubit (Life Technologies). RNA (1 μg) template in 20
μl reaction volume was converted into a cDNA using
AccuPower® RT PreMix from Bioneer (Daejeon, Korea). cDNA
(2 μl) was amplified in 20 μl reaction volume for 25 cycles.
Primers for human MMP2 (forward, 5′-TTGAC GGTAAGGACGGACTC-3′ and
reverse, 5′-ACTTGCAGTA CT CCCCATCG-3′), human MMP9 (forward,
5′-TTGACAGC GACAAGAAGTGG-3′ and reverse, 5′-CCCTCAGTGAAGC
GGTACAT-3′) and human GAPDH (forward, 5′-CGGGAAA CTGTGGCGTGAT-3′
and reverse, 5′-AGTGGGTGT CGCTG TTGAAGT-3′), were used. PCR product
(5 μl) was run in 2% agarose gel and stained with ethidium bromide
solution and band intensities were quantified using ImageJ.
Statistical analysis
The prism 6.0 software (Graphpad, CA, USA) for
window was used to confirm the statistical significance of
differences between values for various experimental and control
groups. Determinations were performed in triplicate and results are
expressed as mean ± standard deviation (SD) and analyzed using
one-way ANOVA test. Subsequently, Dunnett’s multiple comparison
test were performed for statistical analysis. p<0.001 was
considered as statistically significant.
Results
Amphiphilic α-helical structure of α
AL14
The sequence of the novel model peptide we
synthesized is AAWKLLKALAKAAL. It is composed of abundant
hydrophobic and basic amino acids without disulfide bonds.
According to some research (19,20),
peptides including these features tend to be cationic linear
amphiphilic peptides. Basic linear peptides take an amphiphilic
α-helical structure and their amphiphilicity presents distinct
biological activities (21–23).
In light of these facts, it is predicted that the synthesized
peptide adopts an amphiphilic α-helical conformation because of its
amino acid arrangement. According to helical wheel representation,
all hydrophobic amino acid residues, A1, A2, L5, L6, A8, L9, A10,
A12, A13, and L14 were located on one side, whereas hydrophilic
amino acid residues, K4, K7, and K11, were located on the other
side of the helix (Fig. 1).
Therefore, we suggest that this peptide can be classified into the
basic linear α-helical peptides and we named it as αAL14 based on
the number of amino acids in this peptide and its α-helical
property.
Anti-proliferative effects of αAL14 on
HUVECs
As angiogenesis is intimately associated with
endothelial cell proliferation, and most angiogenesis inhibitors
affect endothelial cell proliferation, we investigated whether
αAL14 can inhibit growth of endothelial cells. HUVECs, HEK-293
cells and HaCat cells were treated with αAL14 for 24 h. In HUVECs,
αAL14 did not exert an inhibitory effect on cell proliferation at ≤
25 μM but cell proliferation was decreased with a statistical
significance when HUVECs was incubated with >30 μM of αAL14
(Fig. 2A). In contrast, HEK-293
cells and HaCat cells were not affected by αAL14 treatment
(Fig. 2A). Moreover, in order to
identify whether αAL14 can trigger apoptosis in HUVECs, a marker of
early apoptosis, phosphatidylserine presentation, was detected
staining with Annexin V. As shown in Fig. 2B, Annexin V-positive cells were
observed when HUVECs were treated with >25 μM of αAL14 for 24 h
(Fig. 2B). Therefore, our data
suggest that although HUVECs are more sensitive to αAL14 than the
other two cell lines, αAL14 tends to show anti-proliferative effect
at high concentrations. Therefore, based on this result, we used
concentrations ≤20 μM of αAL14 for further studies to identify
biological activities of αAL14 excluding any inhibitory effects on
cell proliferation or cell survival in HUVECs.
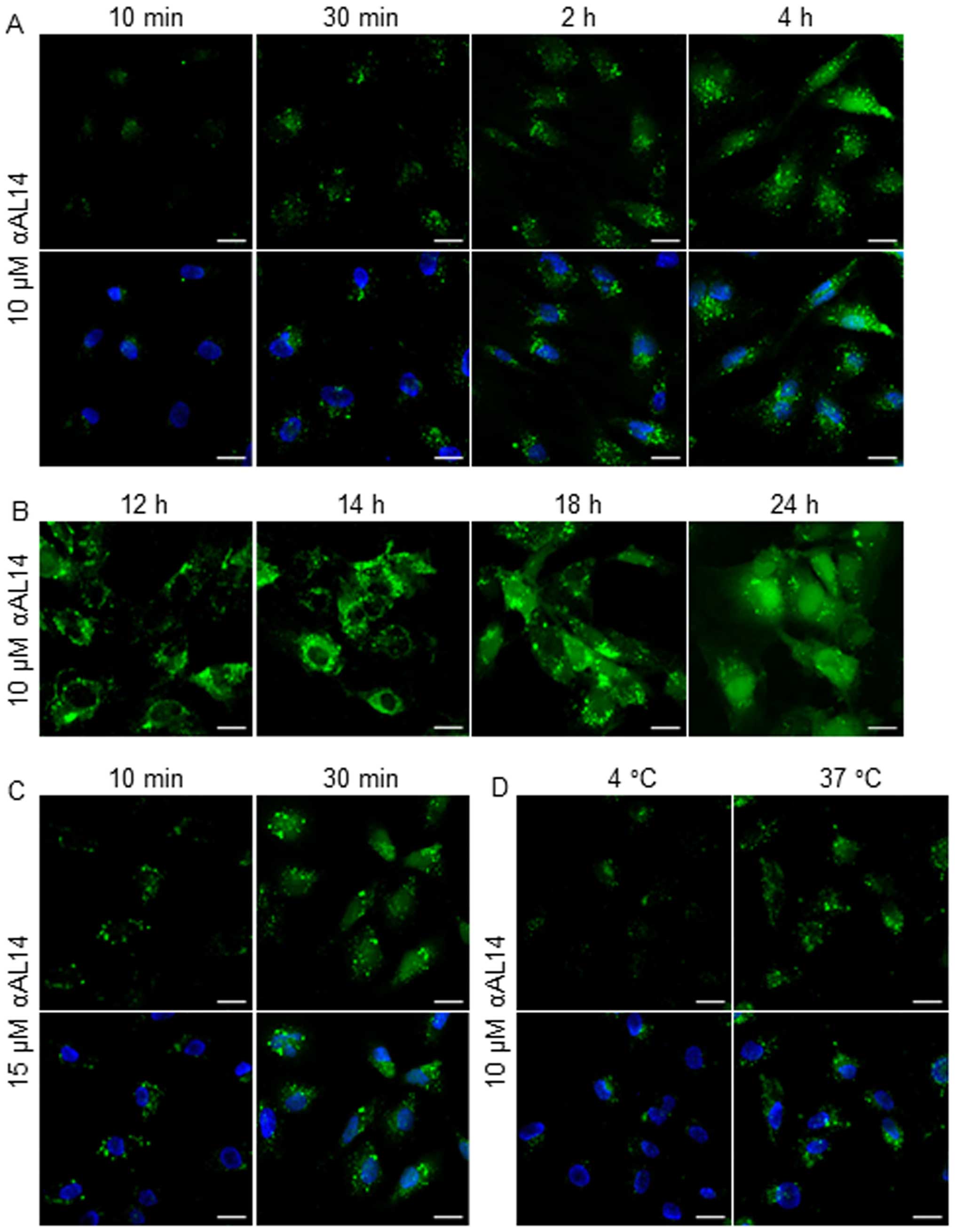
A localization of αAL14 in HUVECs
As αAL14 presented hydrophobic property, we
expected that this peptide penetrates preferentially into the
cytoplasm. To prove our hypothesis, we treated HUVECs with
FITC-tagged αAL14 for the indicated time period. Even though HUVECs
were treated with αAL14 for a relatively short time, αAL14 rapidly
internalized cytoplasm within 10 min and its FITC signal gradually
increased (Fig. 3A). αAL14 was
distributed throughout the cytoplasm and translocated into the
nucleus when cells were treated for comparative long time (Fig. 3B). Accumulation of internalized
αAL14 was increased in a dose- and temperature-dependent manner
(Fig. 3C and D). However, no
interactions between αAL14 and cell membrane were detected in
HUVECs.
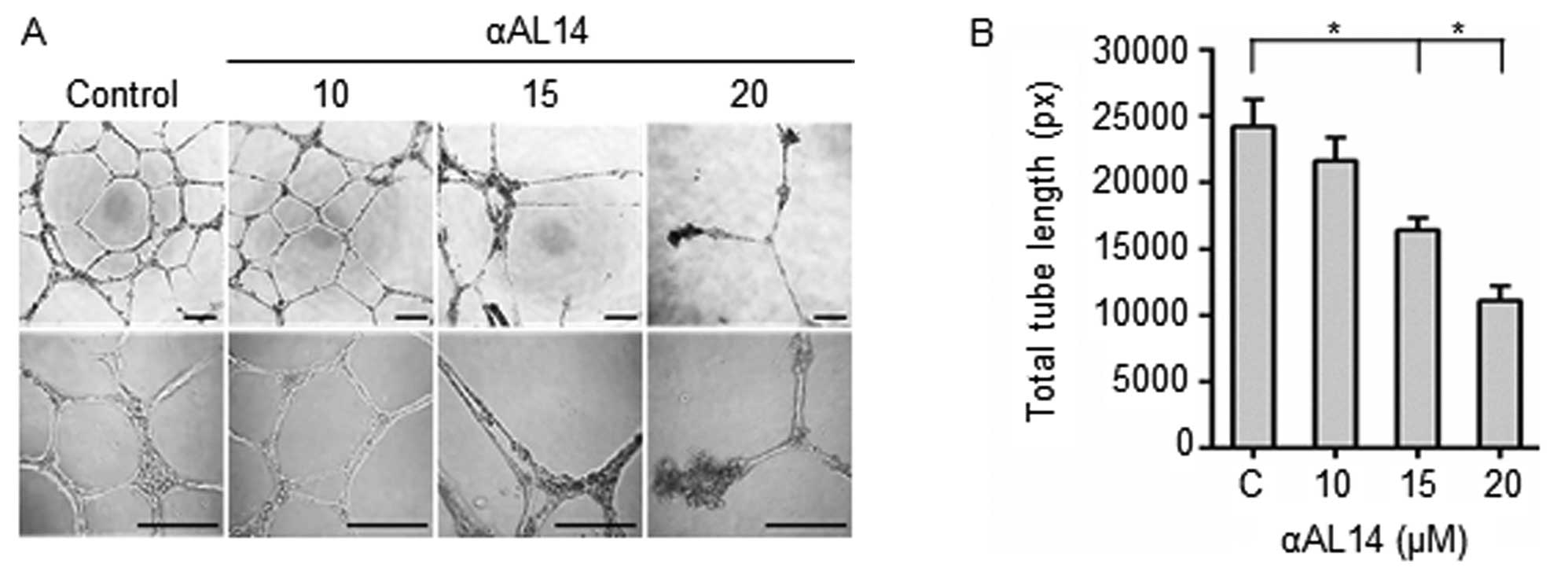
Inhibitory effects of αAL14 in tube
formation of HUVECs
Effects of αAL14 on tubular morphogenesis of HUVECs
were examined because several essential multiple steps including
endothelial cell adhesion, invasion and migration are involved in
tube formation (24). Incomplete
discontinuous tubule structures were present in αAL14-treated
HUVECs. It indicates that the formation of tubule structures was
disturbed by αAL14 treatment (Fig.
4A). Consistent with this result, in quantitative analysis,
total tube length decreased more than half degree in 20 μM
αAL14-treated cells compared to control (Fig. 4B). Taken together, the results
indicate that αAL14 is able to inhibit tubule formation on HUVECs
at concentrations >15 μM.
Effects of αAL14 on endothelial cell
adhesion, migration and invasion in HUVECs
In order to identify which steps of angiogenesis can
be regulated by αAL14 treatment, effects of αAL14 on endothelial
cell adhesion, migration and cell invasion were evaluated. First,
inhibitory effects of αAL14 on endothelial cell adhesive ability
were investigated. Adherent cells were decreased as much as
negative control in 20 μM of αAL14-treated HUVECs (Fig. 5A). In Transwell migration assay,
αAL14 significantly decreased VEGF-stimulated HUVEC migration
(Fig. 5B). αAL14 (15 μM) reduced
VEGF-stimulated cell migration about half degree compared to
positive control in HUVECs. Following this, in Transwell invasion
assay, αAL14 remarkably inhibited VEGF-stimulated HUVEC invasion
compared to positive control (Fig.
5C). Therefore, the results suggest that αAL14 can inhibit
multiple biological processes including cell adhesion, migration
and invasion in endothelial cells during angiogenesis.
Effects of αAL14 on VEGFR2-mediated
signaling in HUVECs
VEGF receptor 2 is a major receptor for regulating
angiogenesis, which activates various downstream signaling pathways
(7). In order to identify
αAL14-induced anti-angiogenic effects on VEGFR2-mediated signaling
pathways, protein expression level of VEGFR2 was investigated. As a
result, αAL14 significantly reduced phosphorylated VEGFR2 at Tyr
1175, but did not affect total VEGFR2 protein expression (Fig. 6A). In addition, fluorescent signal
of phosphorylated VEGFR2 was diminished in response to αAL14
treatment (Fig. 7A). Upon these
results, we hypothesized that αAL14 is able to downregulate VEGFR2
downstream factors and we investigated expression level of VEGFR2
downstream factors including MEK1/2, ERK1/2, Src and Akt.
Consistent with our hypothesis, active form of phosphorylated
MEK1/2 and ERK1/2 was significantly decreased while total ERK 1/2
was not altered by αAL14 treatment (Fig. 6B). In addition, αAL14 inhibited
activation of Src and FAK (Fig.
6C), Akt and mTOR (Fig. 6D) in
a dose-dependent manner, and most of the factors we examined were
affected with statistical significance at concentration of >15
μM. Altered localization of phosphorylated ERK1/2, FAK and Akt
verified regulatory effects of αAL14 on these three factors
(Fig. 7B–D). Taken together, our
results indicate that αAL14 provokes its anti-angiogenic activity
through downregulating the activation of VEGFR2-mediated downstream
signaling pathway in HUVECs.
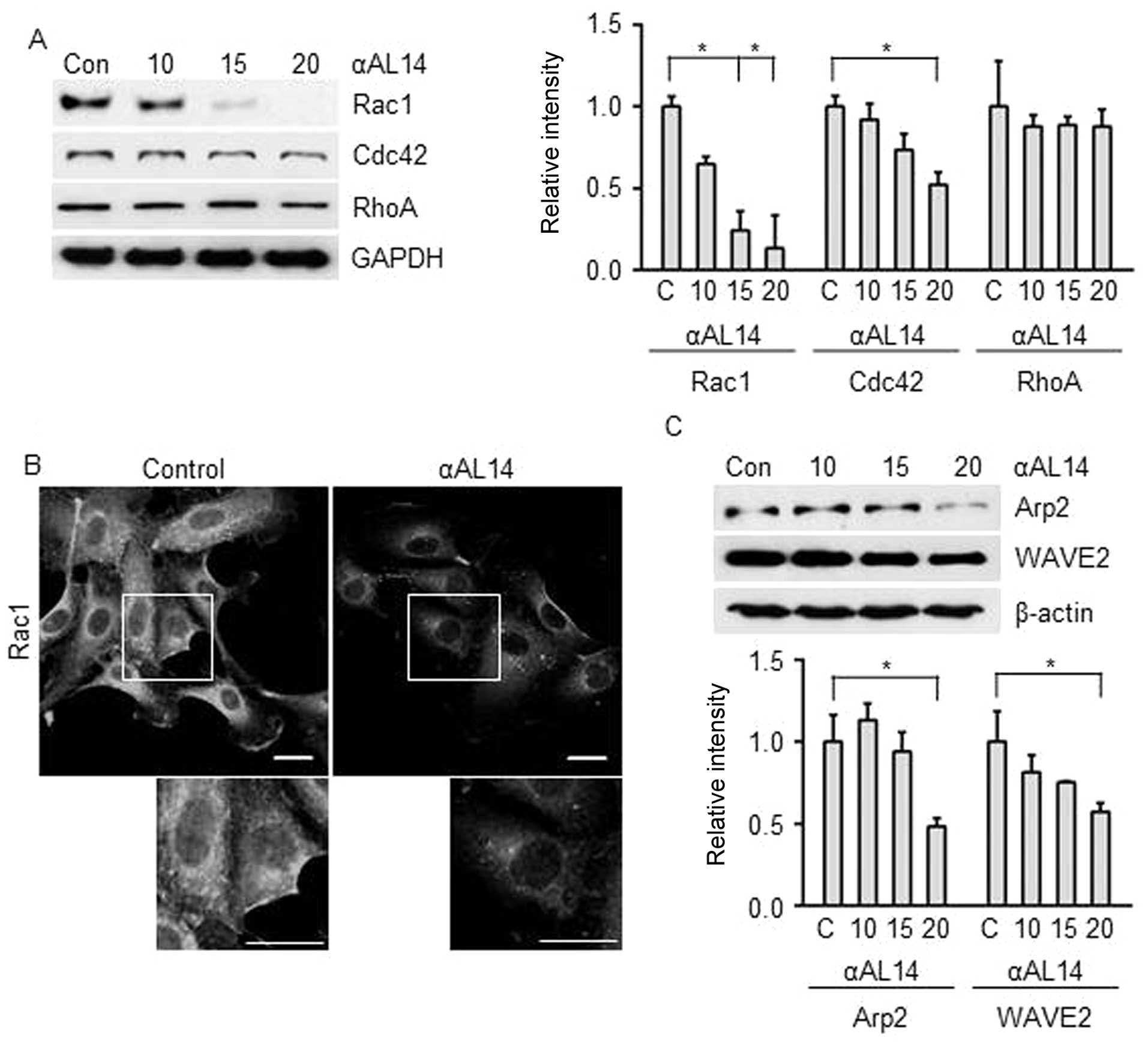
Effects of αAL14 on factors for actin
polymerization in HUVECs
As actin polymerization is an essential step in
endothelial cell migration, we investigated whether αAL14 has
regulatory effect on actin polymerization-related factors Rho
GTPase. Although αAL14 inhibited Rac1 and Cdc42, Rac1 was much
effectively downregulated than Cdc42. In contrast, RhoA was not
affected in αAL14-treated condition (Fig. 8) Thus, we hypothesized that αAL14
is capable of regulating lamellipodia because Rac1 is involved in
the formation of lamellipodia at the edge of cells (9). αAL14 relocated Rac1 away from cell
membrane where lamellipodia formed (Fig. 8B), and suppressed expression level
of Arp2/3 and WAVE2 (Fig. 8C)
which are activated by Rac1 to extend lamellipodium at cell
membrane (25). Taken together,
our results demonstrate that αAL14 may induce the disorganization
of lamillipodium extension via impairing Rac1 signaling in
HUVECs.
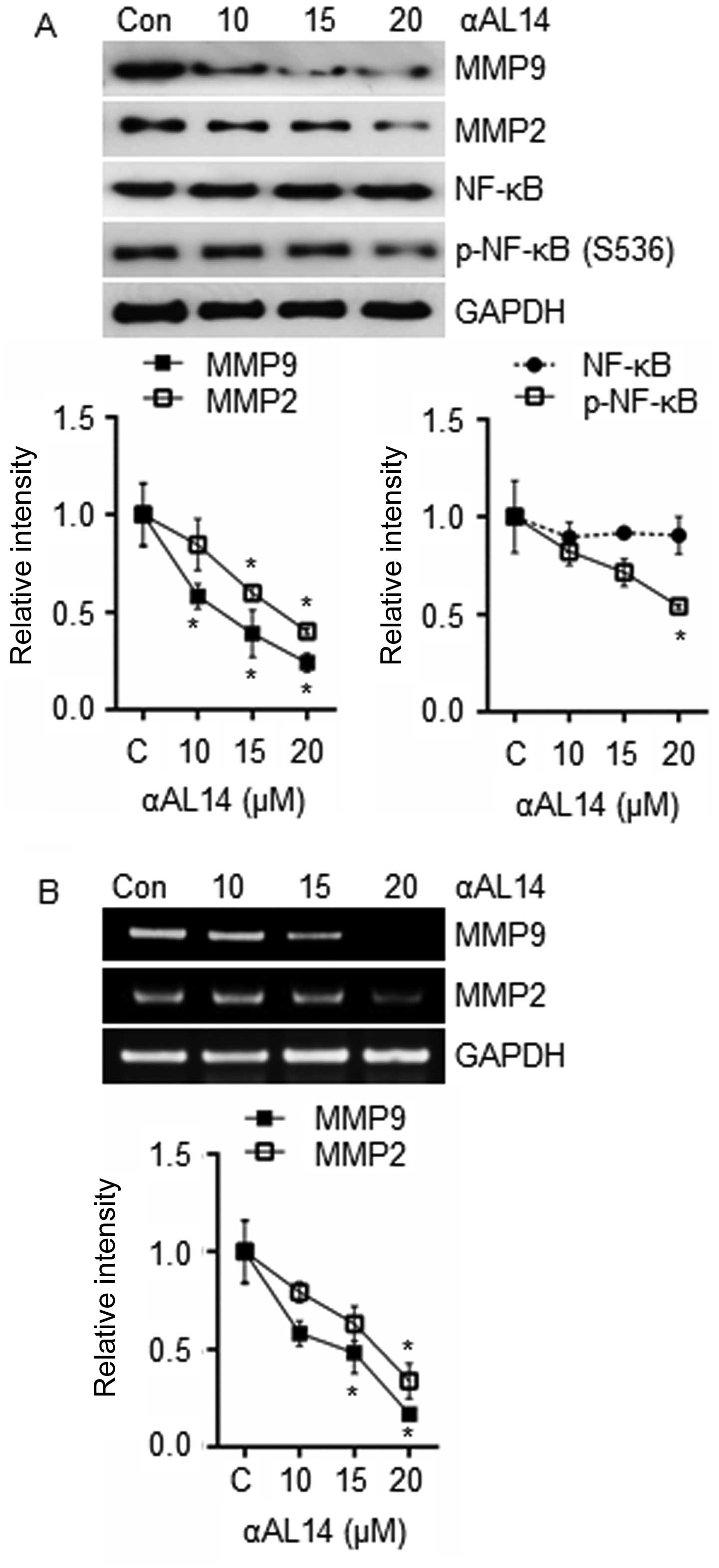
Effects of αAL14 on MMPs expression in
HUVECs
Because endothelial cells secret MMPs to gain
cellular motility by degrading surrounding ECM, expression level of
MMP9, MMP2 and NF-κB, one of the well-known transcription factors
for the transcription of MMP9 and MMP2, were examined. Expression
of MMP9 and MMP2 were suppressed by αAL14 treatment (Fig. 9A). Phosphorylated NF-κB were
reduced in comparison with control while total form of NF-κB was
not changed (Fig. 9A). As well as
the decrease in protein level of MMP9 and MMP2, decrease in mRNA
level was observed in response to αAL14 treatment (Fig. 9B). Therefore, we suggest that αAL14
can reduce the expression of MMP9 and MMP2 via suppressing the
activation of NF-κB.
Discussion
Amphipathic α-helical model peptides have been
considered as anti-bacterial agents due to their cationic net
charge and amphipathic structure (26). In a recent study, Raddum et
al demonstrated that seven different designed peptides based on
AnxA2 exhibited anti-angiogenic effects on capillary-like network
formation in co-culture system of HUVECs and smooth muscle cells.
As D1-P2 peptide including α-helical conformational structures
tended to exert more significant inhibitory effect on formation of
capillary-like networks rather than others (6) we expected that αAL14 which is assumed
to contain α-helical structure has similar effects on endothelial
cells. Moreover, there have been efforts to develop endogenous
peptides that can penetrate into target cells across the cell
membrane because the peptides have lower toxicity without drawbacks
(3,5). Therefore, we focused on identifying
effects of the novel model peptide αAL14 on angiogenic processes
and its permeability in HUVECs. With negative effects of αAL14 on
tube formation in HUVECs, αAL14 was able to decrease endothelial
cell adhesive, migratory and invasive ability at the same range of
concentrations that affected tube formation but anti-proliferative
effects on HUVECs induced by apoptosis did not exist. It means that
αAL14 is more effective in controlling endothelial cell adhesion,
migration and invasion rather than its proliferation during
angiogenesis.
VEGFR2 has an important role in pathological
angiogenesis of which functions are allowed to become a molecular
target for treating various diseases. Phosphorylation of VEGFR2 is
a central event for activation of VEGF-mediated angiogenic pathways
including endothelial cell proliferation and migration (7,27).
In our study, as phosphorylation level of VEGFR2 was decreased by
αAL14 treatment, we expected that attenuated VEGFR2 can decrease
its downstream factors. As we expected, αAL14 inhibited the
subsequent downstream factors such as ERK, FAK, and Akt. Because
phosphorylated VEGFR2 at Tyr 1175 is directly connected with an
activation of MAPK/ERK and PI3K/Akt/mTOR signaling to promote
endothelial cell proliferation and migration via several downstream
signaling (28–31), our data suggest that, in
αAL14-treated HUVECs, angiogenesis can be regulated through the
decrease in phosphorylation of VEGFR2.
FAK is an important component in FAK signaling
relating to VEGF-induced endothelial cell migration, which can
activate itself through phosphorylation at Tyr 397 (32–35).
In addition, Jean et al have demonstrated that c-Src
activity is required for full activation of FAK by phosphorylating
Tyr 576/577 in response to VEGF treatment (36). However, our data show that
phosphorylation of FAK at Tyr 397 and 576/577 was diminished by
αAL14 treatment except total form of FAK. It demonstrates that
αAL14 can inhibit endothelial cell migration, by regulating
activation of FAK in HUVECs. In addition, based on the result that
the phosphorylation of FAK at 397 was much reduced compared to
576/577, it is suggested that auto-phosphorylation of FAK is
affected sensitively to exposure of αAL14 within 30 min.
In order to identify other molecular targets of
αAL14 in connection with endothelial cell migration, expression of
Rac1, Cdc42 and RhoA (members of Rho GTPAses) was evaluated because
they have critical roles in actin dynamics to extend cellular
protrusion (37–39). While RhoA expression was not
altered by αAL14 treatment, the expression of Rac1 and Cdc42 was
changed, and Rac1 was more affected than Cdc42. Because both Arp2
and WAVE2 cooperate with Rac1 for formation of lamellipodia
(40–42) cellular level of Arp2 and WAVE2 was
examined. From our data, we suggest that αAL14 may disorganize
formation of lamellipodia, which is caused by the weak signal of
Rac1 in HUVECs.
In terms of endothelial cell invasion, enzymatic
families of MMPs are involved in endothelial cell invasion
(12) and their transcription is
regulated by certain transcription factors such as NF-κB (13,43,44).
Therefore, our data suggest that the expression level of MMP2 and
MMP9 was inhibited by αAL14 through attenuating the activation of
their transcription factor NF-κB.
Although rapid entry of αAL14 was observed, its
internalization mechanism is not clear. However, we can suppose how
αAL14 is transferred through the cell membrane. The improvement on
internalization of αAL14 at higher temperature indicates that its
entry into cells might be mediated by endocytosis, interacting cell
membrane receptors. Because several studies have described that the
peptides which can easily penetrate into cells at higher
temperature rather than lower temperature are entered through
receptor-mediated endocytosis. Furthermore, α-helical structure in
peptides has an important role in recognition between peptides and
cell receptors (45–47). Therefore, we suggest that the
internalization of αAL14 containing α-helical structure is
connected with receptor-mediated endocytic process of VEGFR2, at
least in part, in HUVECs.
In conclusion, we confirmed that the novel model
peptide αAL14 penetrated into endothelial cells and regulated
various VEGFR2-mediated signaling such as MAPK/ERK, Akt and FAK
within relatively short time. αAL14 controlled not only Rac1 and
but also Arp2/3 and WAVE2, leading to decrease in cell migration.
In addition, decreased expression of MMP2 and MMP9 resulted in
inhibition of cell invasion in HUVECs with αAL14 treatment for a
relative long time. Therefore, αAL14 may possess two features in
the regulation of angiogenesis. One is that αAL14 can inactivate
VEGFR2-mediated signaling with preferential internalization into
the cytoplasm in a short period of time. The other is maintaining
its anti-angiogenic effects by suppressing several factors involved
in endothelial cell migration and invasion with broad distribution
in cytoplasm and nucleus in HUVECs. Based on these data, we propose
that αAL14 has the potential for being developed as a new
angiogenesis inhibitor.
Acknowledgements
This study was supported by a grant from the Korea
Ministry of Environment (MOE) as ‘Eco-innovation Program
(201300030002)’.
Abbreviations:
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
VEGF
|
vascular endothelial growth factor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinases
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
FAK
|
focal adhesion kinase
|
|
MMPs
|
matrix metalloproteinases
|
|
NF-κB
|
nuclear factor-κB
|
|
ECM
|
extra-cellular matrix
|
References
|
1
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulochana KN and Ge R: Developing
antiangiogenic peptide drugs for angiogenesis-related diseases.
Curr Pharm Des. 13:2074–2086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura T and Matsumoto K: Angiogenesis
inhibitors: From laboratory to clinical application. Biochem
Biophys Res Commun. 333:289–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosca EV, Koskimaki JE, Rivera CG, Pandey
NB, Tamiz AP and Popel AS: Anti-angiogenic peptides for cancer
therapeutics. Curr Pharm Biotechnol. 12:1101–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raddum AM, Hollås H, Shumilin IA, Henklein
P, Kretsinger R, Fossen T and Vedeler A: The native structure of
annexin A2 peptides in hydrophilic environment determines their
anti-angiogenic effects. Biochem Pharmacol. 95:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahimi N: VEGFR-1 and VEGFR-2: Two
non-identical twins with a unique physiognomy. Front Biosci.
11:818–829. 2006. View
Article : Google Scholar :
|
|
9
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raftopoulou M and Hall A: Cell migration:
Rho GTPases lead the way. Dev Biol. 265:23–32. 2004. View Article : Google Scholar
|
|
11
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rundhaug JE: Matrix metalloproteinases and
angiogenesis. J Cell Mol Med. 9:267–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adya R, Tan BK, Chen J and Randeva HS:
Nuclear factor-kappaB induction by visfatin in human vascular
endothelial cells: Its role in MMP-2/9 production and activation.
Diabetes Care. 31:758–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam BH, Moon JY, Park EH, Kim YO, Kim DG,
Kong HJ, Kim WJ, Jee YJ, An CM, Park NG, et al: Antimicrobial
activity of peptides derived from olive flounder lipopolysaccharide
binding protein/bactericidal permeability-increasing protein
(LBP/BPI). Mar Drugs. 12:5240–5257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran GN and Sasisekharan V:
Conformation of polypeptides and proteins. Adv Protein Chem.
23:283–438. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim NH, Jung HI, Choi WS, Son BW, Seo YB,
Choi JS and Kim GD: Toluhydroquinone, the secondary metabolite of
marine algae symbiotic microorganism, inhibits angiogenesis in
HUVECs. Biomed Pharmacother. 70:129–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang CW, Kim NH, Jung HA, Choi HW, Kang
MJ, Choi JS and Kim GD: Desmethylanhydroicaritin isolated from
Sophora flavescens, shows antitumor activities in U87MG cells via
inhibiting the proliferation, migration and invasion. Environ
Toxicol Pharmacol. 43:140–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon JS, Kim HM, Yadunandam AK, Kim NH,
Jung HA, Choi JS, Kim CY and Kim GD: Neferine isolated from Nelumbo
nucifera enhances anti-cancer activities in Hep3B cells: Molecular
mechanisms of cell cycle arrest, ER stress induced apoptosis and
anti-angiogenic response. Phytomedicine. 20:1013–1022. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulet P, Stöcklin R and Menin L:
Anti-microbial peptides: From invertebrates to vertebrates. Immunol
Rev. 198:169–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vizioli J and Salzet M: Antimicrobial
peptides from animals: Focus on invertebrates. Trends Pharmacol
Sci. 23:494–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuzaki K: Why and how are peptide-lipid
interactions utilized for self-defense? Magainins and tachyplesins
as archetypes. Biochim Biophys Acta. 1462:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shai Y: Mechanism of the binding,
insertion and destabilization of phospholipid bilayer membranes by
α-helical antimicrobial and cell non-selective membrane-lytic
peptides. Biochim Biophys Acta. 1462:55–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takenawa T and Miki H: WASP and WAVE
family proteins: Key molecules for rapid rearrangement of cortical
actin filaments and cell movement. J Cell Sci. 114:1801–1809.
2001.PubMed/NCBI
|
|
26
|
Tossi A, Sandri L and Giangaspero A:
Amphipathic, α-helical antimicrobial peptides. Biopolymers.
55:4–30. 2000. View Article : Google Scholar
|
|
27
|
Cross MJ, Dixelius J, Matsumoto T and
Claesson-Welsh L: VEGF-receptor signal transduction. Trends Biochem
Sci. 28:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi T, Yamaguchi S, Chida K and
Shibuya M: A single autophosphorylation site on KDR/Flk-1 is
essential for VEGF-A-dependent activation of PLC-gamma and DNA
synthesis in vascular endothelial cells. EMBO J. 20:2768–2778.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dayanir V, Meyer RD, Lashkari K and Rahimi
N: Identification of tyrosine residues in vascular endothelial
growth factor receptor-2/FLK-1 involved in activation of
phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem.
276:17686–17692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida
N and Shibuya M: Essential role of Flk-1 (VEGF receptor 2) tyrosine
residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci USA.
102:1076–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi JH and Claesson-Welsh L: VEGF-induced
activation of phosphoinositide 3-kinase is dependent on focal
adhesion kinase. Exp Cell Res. 263:173–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holmqvist K, Cross MJ, Rolny C, Hägerkvist
R, Rahimi N, Matsumoto T, Claesson-Welsh L and Welsh M: The adaptor
protein shb binds to tyrosine 1175 in vascular endothelial growth
factor (VEGF) receptor-2 and regulates VEGF-dependent cellular
migration. J Biol Chem. 279:22267–22275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Braren R, Hu H, Kim YH, Beggs HE,
Reichardt LF and Wang R: Endothelial FAK is essential for vascular
network stability, cell survival, and lamellipodial formation. J
Cell Biol. 172:151–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calalb MB, Polte TR and Hanks SK: Tyrosine
phosphorylation of focal adhesion kinase at sites in the catalytic
domain regulates kinase activity: A role for Src family kinases.
Mol Cell Biol. 15:954–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jean C, Chen XL, Nam JO, Tancioni I, Uryu
S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, et al:
Inhibition of endothelial FAK activity prevents tumor metastasis by
enhancing barrier function. J Cell Biol. 204:247–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nobes CD and Hall A: Rho GTPases control
polarity, protrusion, and adhesion during cell movement. J Cell
Biol. 144:1235–1244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
40
|
Miki H, Yamaguchi H, Suetsugu S and
Takenawa T: IRSp53 is an essential intermediate between Rac and
WAVE in the regulation of membrane ruffling. Nature. 408:732–735.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oikawa T, Yamaguchi H, Itoh T, Kato M,
Ijuin T, Yamazaki D, Suetsugu S and Takenawa T: PtdIns(3,4,5)P3
binding is necessary for WAVE2-induced formation of lamellipodia.
Nat Cell Biol. 6:420–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blanchoin L, Amann KJ, Higgs HN, Marchand
JB, Kaiser DA and Pollard TD: Direct observation of dendritic actin
filament networks nucleated by Arp2/3 complex and WASP/Scar
proteins. Nature. 404:1007–1011. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai
KC, Ma CY, Wood WG and Chung JG: Berberine suppresses in vitro
migration and invasion of human SCC-4 tongue squamous cancer cells
through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and
-9. Cancer Lett. 279:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vayalil PK and Katiyar SK: Treatment of
epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and
-9 via inhibition of activation of mitogen-activated protein
kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145
cells. Prostate. 59:33–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamada T, Fialho AM, Punj V, Bratescu L,
Gupta TK and Chakrabarty AM: Internalization of bacterial redox
protein azurin in mammalian cells: Entry domain and specificity.
Cell Microbiol. 7:1418–1431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mehta RR, Yamada T, Taylor BN, Christov K,
King ML, Majumdar D, Lekmine F, Tiruppathi C, Shilkaitis A,
Bratescu L, et al: A cell penetrating peptide derived from azurin
inhibits angiogenesis and tumor growth by inhibiting
phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis. 14:355–369.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bang JY, Kim EY, Kang DK, Chang S-I, Han
M-H, Baek K-H and Kang I-C: Pharmacoproteomic analysis of a novel
cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol
Cell Proteomics. 10:M110.005264. 2011. View Article : Google Scholar : PubMed/NCBI
|