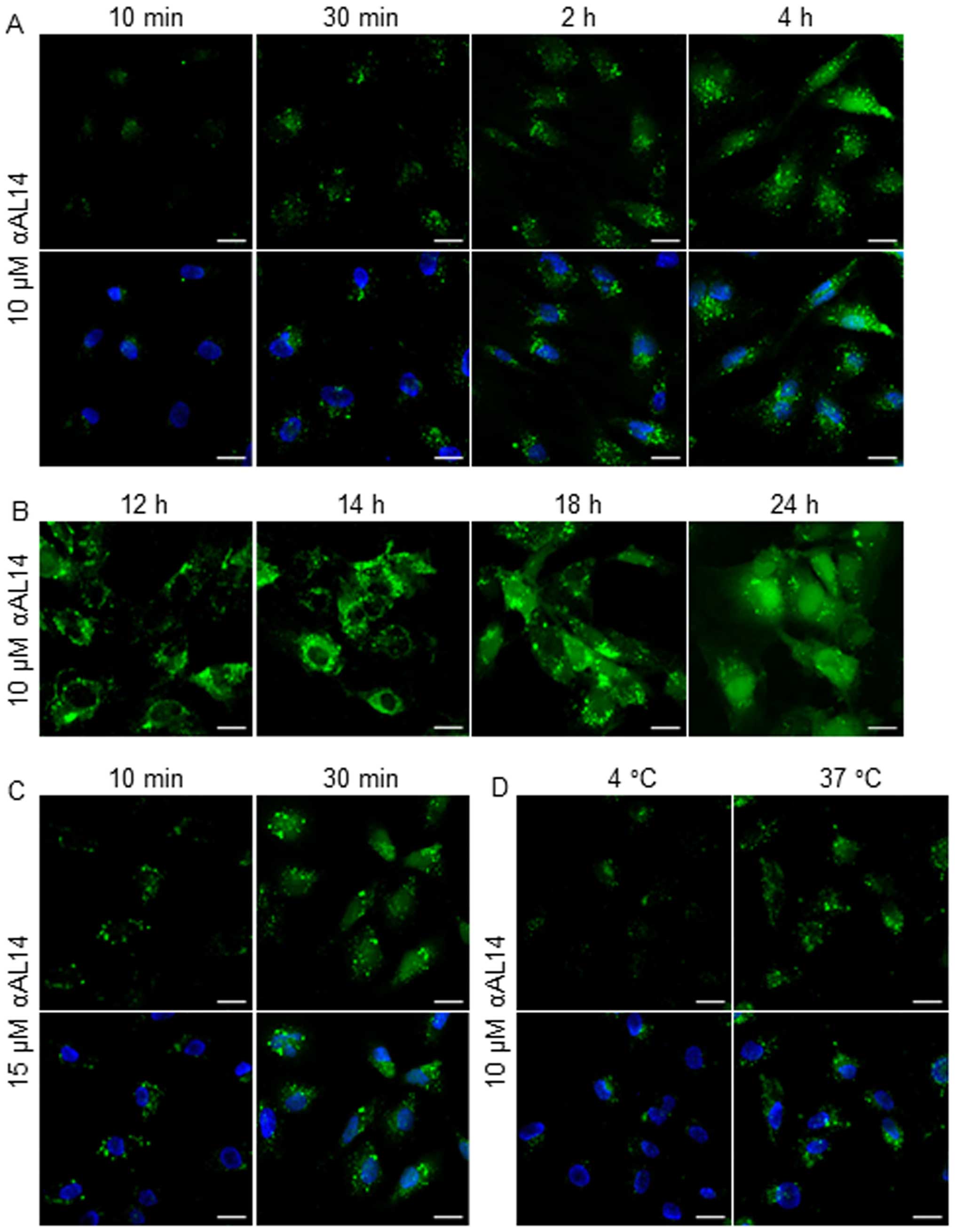
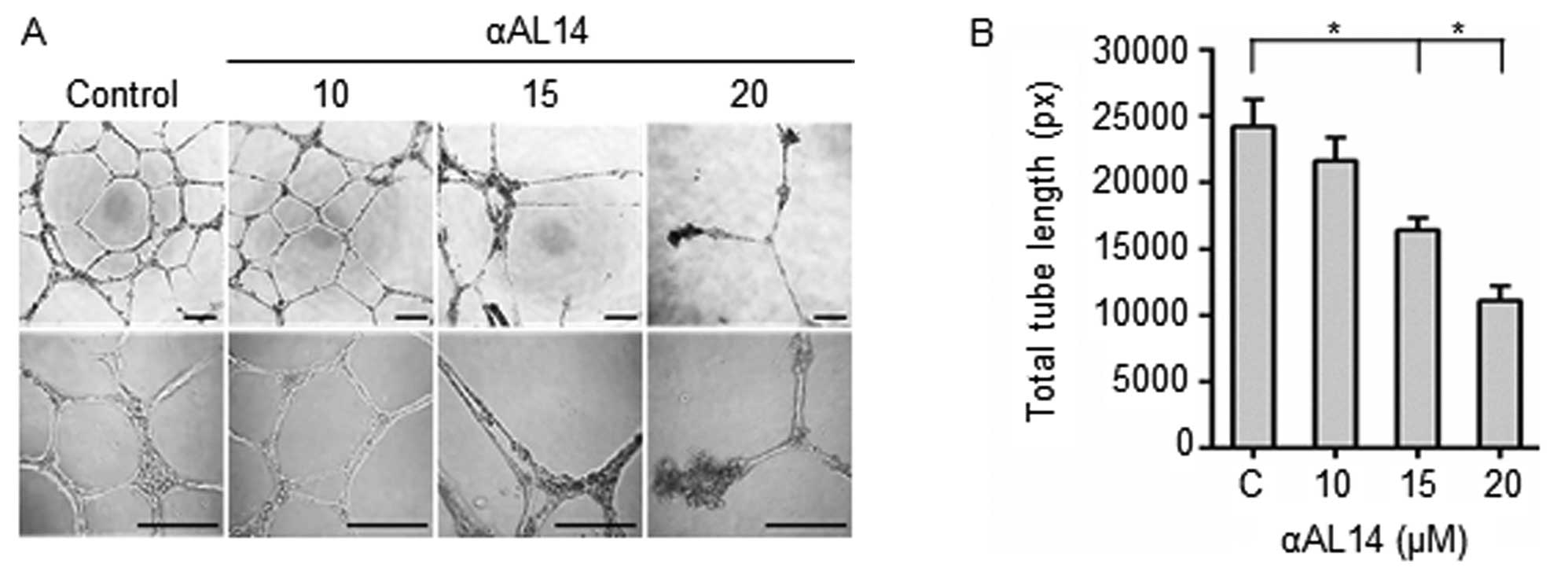
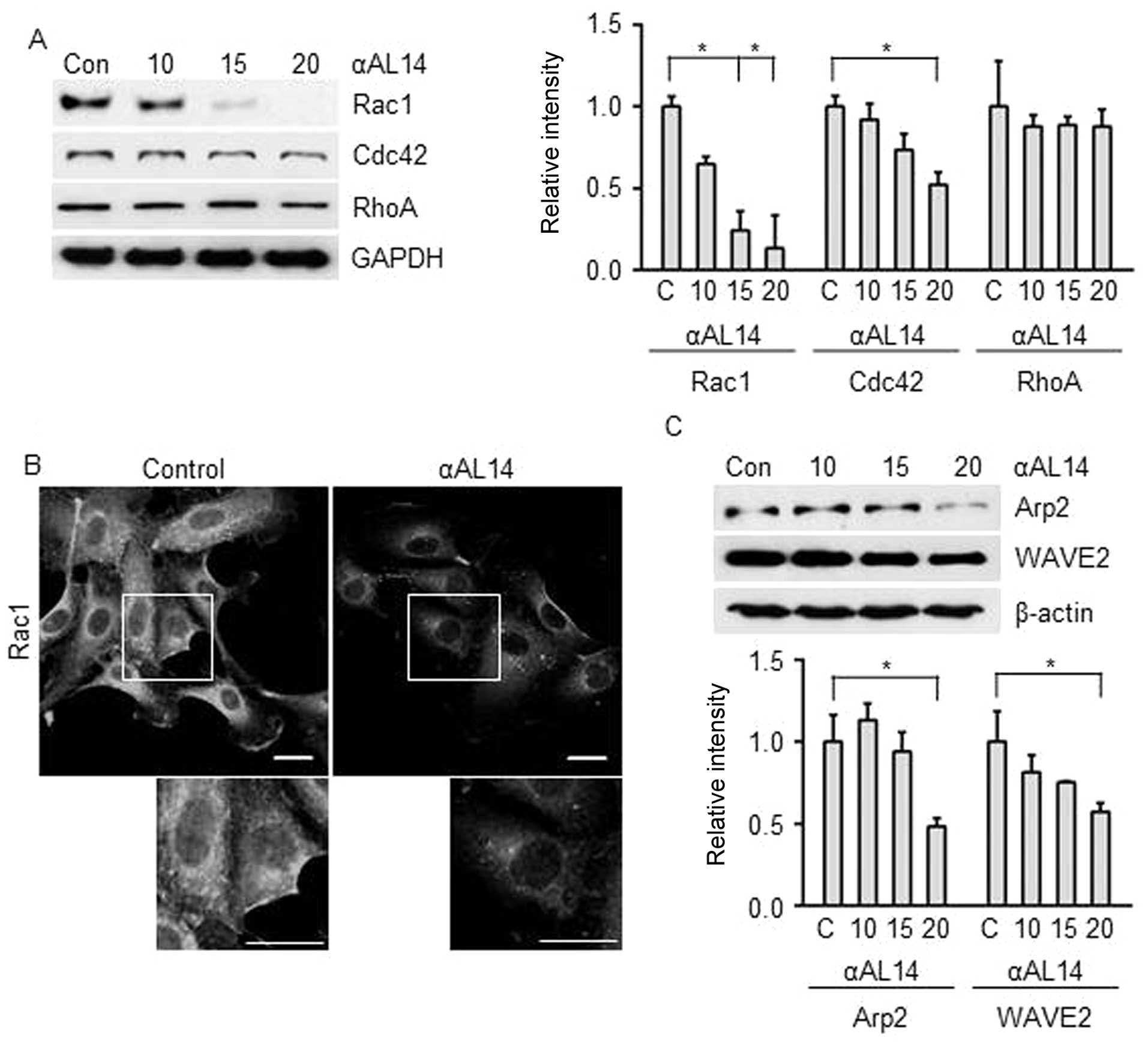
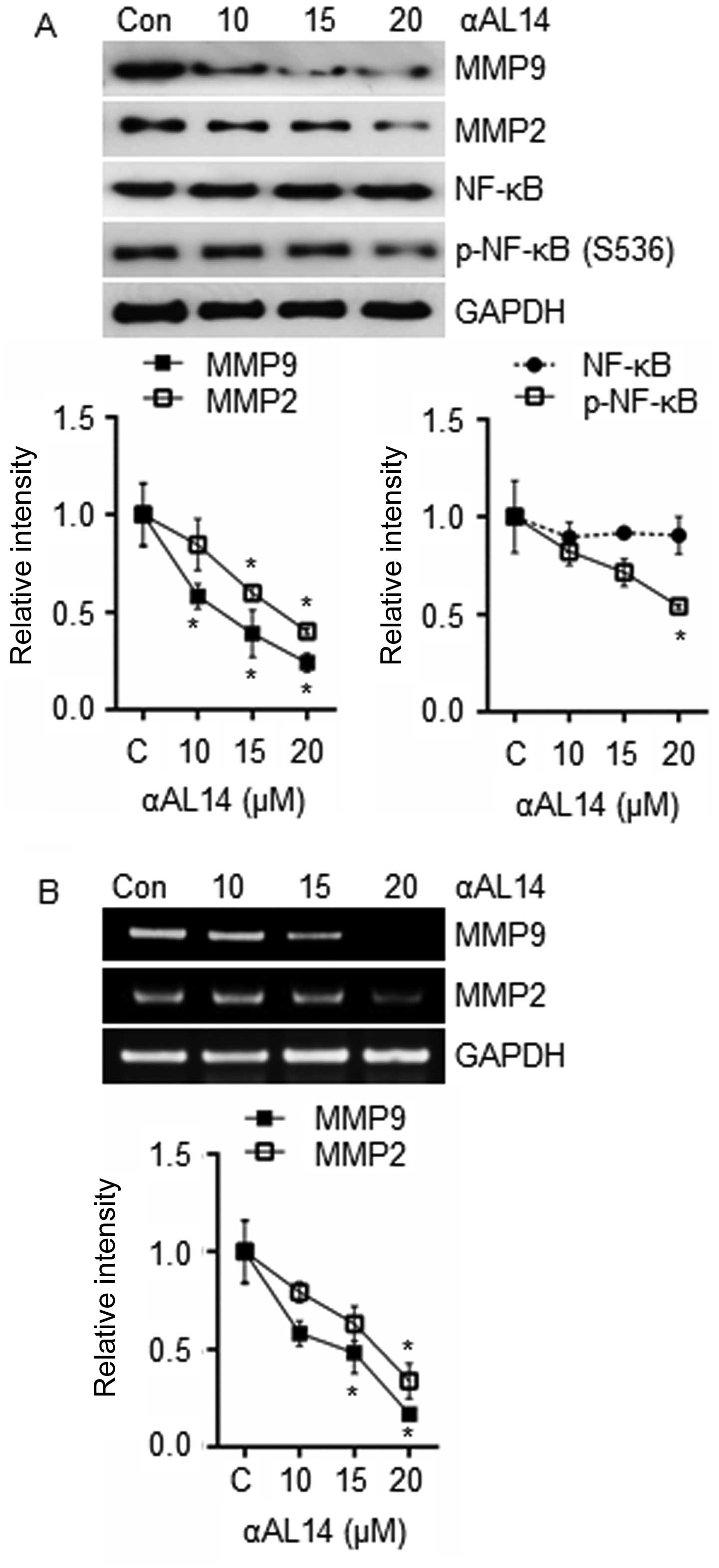
|
1
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulochana KN and Ge R: Developing
antiangiogenic peptide drugs for angiogenesis-related diseases.
Curr Pharm Des. 13:2074–2086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura T and Matsumoto K: Angiogenesis
inhibitors: From laboratory to clinical application. Biochem
Biophys Res Commun. 333:289–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosca EV, Koskimaki JE, Rivera CG, Pandey
NB, Tamiz AP and Popel AS: Anti-angiogenic peptides for cancer
therapeutics. Curr Pharm Biotechnol. 12:1101–1116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raddum AM, Hollås H, Shumilin IA, Henklein
P, Kretsinger R, Fossen T and Vedeler A: The native structure of
annexin A2 peptides in hydrophilic environment determines their
anti-angiogenic effects. Biochem Pharmacol. 95:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahimi N: VEGFR-1 and VEGFR-2: Two
non-identical twins with a unique physiognomy. Front Biosci.
11:818–829. 2006. View
Article : Google Scholar :
|
|
9
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raftopoulou M and Hall A: Cell migration:
Rho GTPases lead the way. Dev Biol. 265:23–32. 2004. View Article : Google Scholar
|
|
11
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rundhaug JE: Matrix metalloproteinases and
angiogenesis. J Cell Mol Med. 9:267–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adya R, Tan BK, Chen J and Randeva HS:
Nuclear factor-kappaB induction by visfatin in human vascular
endothelial cells: Its role in MMP-2/9 production and activation.
Diabetes Care. 31:758–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam BH, Moon JY, Park EH, Kim YO, Kim DG,
Kong HJ, Kim WJ, Jee YJ, An CM, Park NG, et al: Antimicrobial
activity of peptides derived from olive flounder lipopolysaccharide
binding protein/bactericidal permeability-increasing protein
(LBP/BPI). Mar Drugs. 12:5240–5257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran GN and Sasisekharan V:
Conformation of polypeptides and proteins. Adv Protein Chem.
23:283–438. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim NH, Jung HI, Choi WS, Son BW, Seo YB,
Choi JS and Kim GD: Toluhydroquinone, the secondary metabolite of
marine algae symbiotic microorganism, inhibits angiogenesis in
HUVECs. Biomed Pharmacother. 70:129–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang CW, Kim NH, Jung HA, Choi HW, Kang
MJ, Choi JS and Kim GD: Desmethylanhydroicaritin isolated from
Sophora flavescens, shows antitumor activities in U87MG cells via
inhibiting the proliferation, migration and invasion. Environ
Toxicol Pharmacol. 43:140–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon JS, Kim HM, Yadunandam AK, Kim NH,
Jung HA, Choi JS, Kim CY and Kim GD: Neferine isolated from Nelumbo
nucifera enhances anti-cancer activities in Hep3B cells: Molecular
mechanisms of cell cycle arrest, ER stress induced apoptosis and
anti-angiogenic response. Phytomedicine. 20:1013–1022. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulet P, Stöcklin R and Menin L:
Anti-microbial peptides: From invertebrates to vertebrates. Immunol
Rev. 198:169–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vizioli J and Salzet M: Antimicrobial
peptides from animals: Focus on invertebrates. Trends Pharmacol
Sci. 23:494–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuzaki K: Why and how are peptide-lipid
interactions utilized for self-defense? Magainins and tachyplesins
as archetypes. Biochim Biophys Acta. 1462:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shai Y: Mechanism of the binding,
insertion and destabilization of phospholipid bilayer membranes by
α-helical antimicrobial and cell non-selective membrane-lytic
peptides. Biochim Biophys Acta. 1462:55–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takenawa T and Miki H: WASP and WAVE
family proteins: Key molecules for rapid rearrangement of cortical
actin filaments and cell movement. J Cell Sci. 114:1801–1809.
2001.PubMed/NCBI
|
|
26
|
Tossi A, Sandri L and Giangaspero A:
Amphipathic, α-helical antimicrobial peptides. Biopolymers.
55:4–30. 2000. View Article : Google Scholar
|
|
27
|
Cross MJ, Dixelius J, Matsumoto T and
Claesson-Welsh L: VEGF-receptor signal transduction. Trends Biochem
Sci. 28:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi T, Yamaguchi S, Chida K and
Shibuya M: A single autophosphorylation site on KDR/Flk-1 is
essential for VEGF-A-dependent activation of PLC-gamma and DNA
synthesis in vascular endothelial cells. EMBO J. 20:2768–2778.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dayanir V, Meyer RD, Lashkari K and Rahimi
N: Identification of tyrosine residues in vascular endothelial
growth factor receptor-2/FLK-1 involved in activation of
phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem.
276:17686–17692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida
N and Shibuya M: Essential role of Flk-1 (VEGF receptor 2) tyrosine
residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci USA.
102:1076–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi JH and Claesson-Welsh L: VEGF-induced
activation of phosphoinositide 3-kinase is dependent on focal
adhesion kinase. Exp Cell Res. 263:173–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holmqvist K, Cross MJ, Rolny C, Hägerkvist
R, Rahimi N, Matsumoto T, Claesson-Welsh L and Welsh M: The adaptor
protein shb binds to tyrosine 1175 in vascular endothelial growth
factor (VEGF) receptor-2 and regulates VEGF-dependent cellular
migration. J Biol Chem. 279:22267–22275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Braren R, Hu H, Kim YH, Beggs HE,
Reichardt LF and Wang R: Endothelial FAK is essential for vascular
network stability, cell survival, and lamellipodial formation. J
Cell Biol. 172:151–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calalb MB, Polte TR and Hanks SK: Tyrosine
phosphorylation of focal adhesion kinase at sites in the catalytic
domain regulates kinase activity: A role for Src family kinases.
Mol Cell Biol. 15:954–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jean C, Chen XL, Nam JO, Tancioni I, Uryu
S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, et al:
Inhibition of endothelial FAK activity prevents tumor metastasis by
enhancing barrier function. J Cell Biol. 204:247–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nobes CD and Hall A: Rho GTPases control
polarity, protrusion, and adhesion during cell movement. J Cell
Biol. 144:1235–1244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
40
|
Miki H, Yamaguchi H, Suetsugu S and
Takenawa T: IRSp53 is an essential intermediate between Rac and
WAVE in the regulation of membrane ruffling. Nature. 408:732–735.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oikawa T, Yamaguchi H, Itoh T, Kato M,
Ijuin T, Yamazaki D, Suetsugu S and Takenawa T: PtdIns(3,4,5)P3
binding is necessary for WAVE2-induced formation of lamellipodia.
Nat Cell Biol. 6:420–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blanchoin L, Amann KJ, Higgs HN, Marchand
JB, Kaiser DA and Pollard TD: Direct observation of dendritic actin
filament networks nucleated by Arp2/3 complex and WASP/Scar
proteins. Nature. 404:1007–1011. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai
KC, Ma CY, Wood WG and Chung JG: Berberine suppresses in vitro
migration and invasion of human SCC-4 tongue squamous cancer cells
through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and
-9. Cancer Lett. 279:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vayalil PK and Katiyar SK: Treatment of
epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and
-9 via inhibition of activation of mitogen-activated protein
kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145
cells. Prostate. 59:33–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamada T, Fialho AM, Punj V, Bratescu L,
Gupta TK and Chakrabarty AM: Internalization of bacterial redox
protein azurin in mammalian cells: Entry domain and specificity.
Cell Microbiol. 7:1418–1431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mehta RR, Yamada T, Taylor BN, Christov K,
King ML, Majumdar D, Lekmine F, Tiruppathi C, Shilkaitis A,
Bratescu L, et al: A cell penetrating peptide derived from azurin
inhibits angiogenesis and tumor growth by inhibiting
phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis. 14:355–369.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bang JY, Kim EY, Kang DK, Chang S-I, Han
M-H, Baek K-H and Kang I-C: Pharmacoproteomic analysis of a novel
cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol
Cell Proteomics. 10:M110.005264. 2011. View Article : Google Scholar : PubMed/NCBI
|