Introduction
Worldwide, >600,000 people are newly diagnosed
with head and neck cancer each year (1–3), and
head and neck squamous cell carcinoma (HNSCC) is one of the 10 most
common malignancies in the world and also known for clinical
progression and poor prognosis. The cancer-related death is mainly
caused by metastasis of tumors to regional lymph nodes and distant
organs. Many patients are diagnosed in advanced stages, in which
standard treatment is a combination of platinum-based chemotherapy,
radiation, and/or surgery (3–6). As
primary treatments for advanced HNSCC, recently there have been
advances in definitive chemoradiotherapy (CRT) instead of extended
surgery. Despite the recent progress of the treatment for improving
locoregional control in HNSCC patients, that for recurrence and
metastatic control remains insufficient (7,8),
indicating that there is a continued need for improved treatment
strategies. As an obvious risk factor of HNSCC, continuous
irritation by tobacco and alcohol abuse is well-known (3,7,9).
Furthermore, recent studies have shown that HNSCC is associated
with human papillomavirus (HPV) infection, especially at
oropharyngeal region (10–12). In contrast, there is no reliable
marker for the other sites of HNSCC. Therefore, the key molecules
that enhance chemo- and radiosensitivity in HNSCC and
identification of reliable markers for predicting the recurrence
and metastasis would be desirable to improve the prognosis of HNSCC
patients.
It has been reported that inflammation is an
important phase in tumor progression, and many cancers originate
from inflammation. In connection with digestive organs, many
studies have demonstrated that the human regenerating gene
(REG) expression is observed in chronic inflammation and in
tumors (13–20). Reg was first identified in
regenerating pancreatic islets in 1988 (21). Reg family belongs to the lectin
superfamily and encodes five small, secreted proteins (13,22–25).
Reg family proteins are classified into four subfamilies: type I,
II, III, and IV; the human REG family consists of five
members: REG Iα, REG Iβ, REG III,
hepatocarcinoma-intestine-pancreas/pancreatitis-associated
protein (HIP/PAP) and REG IV (13,20,22,26–29).
REG family protein has been shown to play roles, not only in normal
tissue regeneration (30,31), but also in the development of
various malignancies (32–43). Indeed, REG expression has
been reported to be associated with progression of cancers such as
esophageal, gastric, lung, and colorectal cancer (32–39,43).
Considering that oral and pharyngeal cavities are often exposed to
chronic inflammatory factors such as tobacco, alcohol and
mechanical stimuli, it is possible that REG expression is
associated with HNSCC. Recently we reported that REG III
expression was associated with prognosis of HNSCC patients
(44). In addition, we
demonstrated that REG III regulated cell proliferation and
chemo- and radiosensitivity in HNSCC in vitro. These results
suggested that enhancement of REG III expression increased
chemo- and radiosensitivity, and improved prognosis of HNSCC
patients. In the present study, we investigated the stimulator for
the enhancement of REG III expression in HNSCC cells.
Furthermore, we first demonstrate that the stimulator can effect
proliferation, invasion, and chemo- and radiosensitivity in HNSCC
cells.
Materials and methods
Cell culture and reagents
Human HNSCC cells, FaDu and HSC-4, were employed in
this study. FaDu cells were provided by the American Type Culture
Collection (Manassas, VA, USA). HSC-4 cells were obtained from the
Japanese Cancer Research Resources Bank (Tokyo, Japan). The cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA) and 100 U/ml
penicillin G and 100 μg/ml streptomycin and 250 ng/ml amphotericin
B (Gibco™ Antibiotic-Antimycotic; Gibco). The cells were maintained
in a 5% CO2/95% air, humidified atmosphere at 37°C.
Interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) were
purchased from Roche Applied Science (Indianapolis, IN, USA).
IL-1β, IL-8, IL-11, IL-13, IL-17, IL-22, hepatocyte growth factor
(HGF), basic fibroblast growth factor (bFGF), epidermal growth
factor (EGF), cardiotrophin, oncostatin M, interferon (IFN)-β,
quercetin, 3,4′,5-trihydroxy-trans-stilbene (resveratrol),
fisetin, genistein, chlorogenic acid, estradiol, bisphenol A,
sodium butyrate were obtained from Wako Pure Chemical Industries,
Ltd. (Osaka, Japan). Dexamethasone (Dx) and o-dianisidine were from
MP Biomedicals (Santa Ana, CA, USA). Daidzein was purchased from
Fujicco Co., Ltd. (Kobe, Japan), epigallocatechin gallate from Enzo
Life Sciences, Inc. (Framingdale, NY, USA), curcumin from Nacalai
Tesque, Inc. (Tokyo Japan), ciglitazone and troglitazone from
Cayman Chemical Co. (Ann Arbor, MI, USA), trichostatin A from Tokyo
Chemical Industry Co., Ltd. (Tokyo, Japan), and phorbol
12-myristate 13-acetate from Sigma-Aldrich (St. Louis, MO,
USA).
Isolation of stable transformants with
REG III promoter vector
The reporter constructs were prepared by inserting
the 5′-flanking regions of human REG III (−1,985 to +87 of
REG III gene) (28)
upstream of a luciferase reporter gene in pGL4.17 vector (luc2/Neo;
Promega Corp., Madison, WI, USA). The REG III promoter
vector was introduced into FaDu cells by electroporation using Gene
Pulser Xcell™ (Bio-Rad, Hercules, CA, USA) (44), after which the stable transformants
were selected in DMEM supplemented with 10% FBS and 500 μg/ml
Geneticin® (Invitrogen) for 3 weeks. Introduction of the
REG III promoter/luciferase construct in the
Geneticin®-resistant cells was confirmed by PCR.
Promoter assays
The REG III promoter vector was introduced
into FaDu cells as described above. The stable transformants were
seeded in 24-well plates at an initial density of 1×105
cells/well. After a 24-h incubation, the cells were treated with 20
ng/ml of IL-1β, 20 ng/ml of IL-6, 10 nM of IL-8, 100 ng/ml of
IL-11, 100 ng/ml of IL-13, 1 μg/ml of IL-17, 20 ng/ml of IL-22, 50
ng/ml of HGF, 10 nM of bFGF, 10 nM of EGF, 20 ng/ml of TNF-α, 20
ng/ml of cardiotrophin, 20 ng/ml of oncostatin M, 100 nM of Dx, 50
ng/ml of IFN-β, 50 μM of quercetin, 20 μM of resveratrol, 20 μM of
fisetin, 50 μM of genistein, 60 μM of chlorogenic acid, 200 μM of
o-dianisidine, 10 nM of okadaic acid, 10 μM of daidzein, 100 nM of
epigallocatechin gallate, 1 μM of estradiol, 10 μM of bisphenol A,
12.5 μM of curcumin, 50 μM of ciglitazone, 30 μM of troglitazone, 1
μM of trichostatin A, 1 mM of sodium butyrate, or 50 nM of phorbol
12-myristate 13-acetate. After a 24-h treatment, the medium was
aspirated from each well and washed with PBS, and the cells were
harvested with 100 μl of lysis buffer (0.1 M potassium phosphate,
pH 8.8, 0.2% Triton X-100). The cells were processed for luciferase
assay using a Pica Gene luminescence kit (TOYO B-Net Co., Ltd.,
Tokyo, Japan) as described (45–50).
Quantitative real-time RT-PCR
Total RNA was isolated using RNeasy
Protect® Cell Mini Kit (Qiagen, Hiden, Germany) from
FaDu cells. cDNA was reverse transcribed from 0.5–2 μg samples of
total RNA using High-Capacity cDNA Reverse Transcription Kit
(Applied Biosystems, Foster City, CA, USA) as described (44,45,47–51).
cDNA was subjected to PCR with the following primers, synthesized
and prepared by Nihon Gene Research Laboratories, Inc. (NGRL;
Sendai, Japan): β-actin (NM_001101) sense, 5′-GCGAGAAGATGACCCAGA-3′
and antisense, 5′-CAGAGGCGTACAGGGATA-3′; REG III (AB161037)
sense 5′-GAATATTCTCCCCAAACTG-3′ and antisense,
5′-GAGAAAAGCCTGAAATGAAG-3′.
Real-time PCR was performed using KAPA SYBR FAST
qPCR Master Mix (Kapa Biosystems, Boston, MA, USA) and Thermal
Cycler Dice Real-Time System (Takara Bio, Inc., Otsu, Japan) as
described (43–45,47–51).
PCR was performed with an initial step of 3 min at 95°C followed by
40 cycles of 3 sec at 95°C, 20 sec at 60°C. The level of target
mRNA was normalized to the mRNA level of β-actin as an
internal standard.
Cell proliferation assay
Cell proliferation activity was assessed by using a
Cell Counting Kit-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt (WST-8) cleavage; Dojindo Laboratories, Kumamoto,
Japan] as described (43,44,47,50,52).
Cells were plated in 96-well plates and incubated for 24 h. Initial
density of FaDu and HSC-4 cells was 3×103 cells/well.
After 24 h, 30 μM of resveratrol was added to each well and
incubated for 24, 48 and 72 h, while dimethylsulfoxide (DMSO;
Sigma-Aldrich) was added as a control. At 24 h of incubation,
medium was changed and resveratrol was removed from each well.
After addition of 10 μl of WST-8 solution, the cells were incubated
for another 2 h. The absorbance of each well at 450 nm (reference
wavelength at 620 nm) was read by using a Multiskan™ FC Microplate
Photometer (Thermo Fisher Scientific, Waltham, MA, USA). Each
measurement was repeated at least four times on each cell line.
Chemo- and radiotherapy for cultured
cells
Cells were exposed to 0, 5 and 10 Gy radiation using
a MBR-1520R (Hitachi, Ltd., Tokyo, Japan) operating at 150 kV and
20 mA as described (44), which
delivered the dose at 0.8 Gy/min. Chemotherapy involved the
application of cisplatin (Nihon Kayaku Co., Tokyo, Japan) to the
cultures at a concentration of 0, 1.0, 3.0 or 10 μM as described
(44).
As for chemo- and radiosensitivity, the cell
viability was assessed using WST-8 cleavage. Cells were plated in
96-well plates and incubated for 24 h. Initial density of FaDu
cells was 3×103 cells/well, and of HSC-4 cells
1×103 cells/well. After 24 h, 30 μM of resveratrol were
added to each well, while DMSO was added as a control, and
incubated for 48 h. For radiotherapy, they were then irradiated at
0, 5, or 10 Gy respectively. For chemotherapy, cisplatin (0–10 μM)
was added to cultures. Following incubation for additional 72 h,
the absorbance of each well at 450 nm (reference wavelength at 620
nm) was read as described above. Each measurement was repeated at
least four times on each cell line.
Invasion assay
Invasion assays were performed using 24-well
Matrigel-coated Transwells (BD Biosciences, Bedford, MA, USA). A
total of 4×104 cells of FaDu and HSC-4 were suspended in
200 μl of serum-free DMEM and placed in the top chambers, and 700
μl of DMEM containing 10% FBS were added to the bottom chambers.
After 48 h of incubation at 37°C, non-invading cells were removed
from the top of the Matrigel with a cotton swab, while invading
cells on the bottom surface of the filter were fixed in 4%
paraformaldehyde and stained with Giemsa (Sigma-Aldrich) for 10
min. The invading cells were then visualized at ×200 magnification
and counted in five fields for each filter.
Data analysis
Data were expressed as means ± standard error (SE).
Statistical significant differences between groups were determined
by Student’s t-test using StatMate IV (Abacus Concepts, Berkeley,
CA, USA). P<0.05 was considered significant.
Results
Activation of REG III gene promoters in
FaDu cells
To investigate activators of REG III gene
expression, we tested various stimulative substances in two clones
of FaDu cells to which the REG III promoter vector was
stably introduced (Fig. 1). Among
these various stimulators, polyphenols such as resveratrol,
genistein, daidzein, and histone deacetylase inhibitors such as
sodium butyrate, significantly increased the REG III
promoter activity (Fig. 1). We
also tested the concentration-response relationship of resveratrol,
genistein, daidzein, sodium butyrate for REG III promoter
activation, and found that the optimum concentration was 10 μM
(resveratrol), 25 μM (genistein), 40 μM (daizein), and 2 mM (sodium
butyrate) (data not shown).
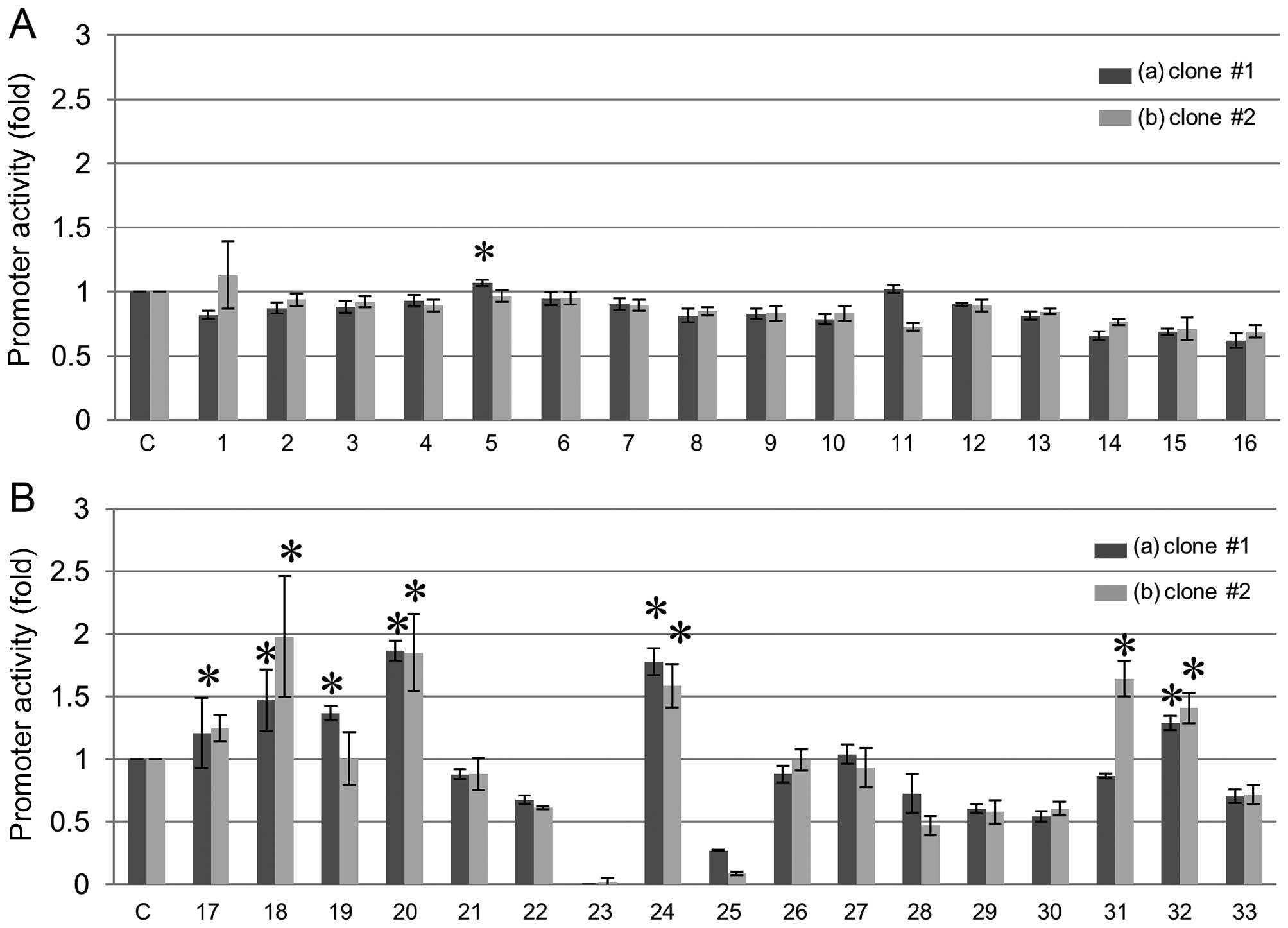 | Figure 1Screening of activator(s) for human
REG III transcription. Human REG III promoter (−1,985
to +87 of REG III gene) was introduced into FaDu human HNSCC
cells and stable transformants were selected (clone #1 and #2). (A)
Effects of cytokines and growth factors on activation of REG
III gene promoter of (a) clone #1, (b) clone #2 in FaDu cells.
Cells were transfected with the REG III gene reporter
plasmids and treated as follows: C, no addition as a control; 1,
IL-1β (20 ng/ml); 2, IL-6 (20 ng/ml); 3, IL-8 (10 nM); 4, IL-11
(100 ng/ml); 5, IL-13 (100 ng/ml); 6, IL-17 (1 μg/ml); 7, IL-22 (20
ng/ml); 8, HGF (50 ng/ml); 9, bFGF (10 nM); 10, EGF (10 nM); 11,
TNF-α (20 ng/ml); 12, cardiotrophin (20 ng/ml); 13, oncostatin M
(20 ng/ml); 14, Dx (100 nM); 15, IL-6 + Dx; 16, IFN-β (50 ng/ml).
The relative promoter activity was calculated by dividing the
promoter activity of unstimulated cells (column 1). Data are
expressed as means ± SE for each group. *P<0.05. (B)
Effects of polyphenols, epigallocatechin gallate, PPARγ activator
of thiazolidinediones, and histone deacetylase inhibitor on
activation of REG III gene promoter of (a) clone #1, (b)
clone #2 in FaDu cells. Cells were transfected with the REG
III gene reporter plasmids and treated as follows: C, no
addition as a control; 17, quercetin (50 μM); 18, resveratrol (20
μM); 19, fisetin (20 μM); 20, genistein (50 μM); 21, chlorogenic
acid (60 μM); 22, o-dianisidine (200 μM); 23, okadaic acid (10 nM);
24, daidzein (10 μM); 25, epigallocatechin gallate (100 nM); 26,
estradiol (1 μM); 27, bisphenol A (10 μM); 28, curcumin (12.5 μM);
29, ciglitazone (50 μM); 30, troglitazone (30 μM); 31, trichostatin
A (1 μM); 32, sodium butyrate (1 mM); 33, phorbol 12-myristate
13-acetate (50 nM). The promoter activity was calculated by
dividing the promoter activity of unstimulated cells (column 1).
Data are expressed as means ± SE for each group.
*P<0.05. REG, regenerating gene; HNSCC,
head and neck squamous cell carcinoma; IL, interleukin; HGF,
hepatocyte growth factor; bFGF, basic fibroblast growth factor;
EGF, epidermal growth factor; TNF-α, tumor necrosis factor-α; Dx,
dexamethasone; IFN, interferon; SE, standard error; resveratrol,
3,4′,5-trihydroxy-trans-stilbene. |
Induction of REG III mRNA by candidate
substances in FaDu cells
We examined mRNA levels of intrinsic REG III
in FaDu cells by using real-time RT-PCR, after treating cells with
10 μM resveratrol, 25 μM genistein, 40 μM daidzein, and 2 mM sodium
butyrate, respectively. Resveratrol significantly increased the
mRNA levels of REG III as compared with the others (Fig. 2). The combined addition of
resveratrol with other candidates did not enhance, rather
inhibited, the increment of REG III mRNA expression by
resveratrol alone. It might be due to over-stimulation for the
signaling pathway, and as a result, it might be higher
concentrations for the effect, as reported by Liu et al
(53). We also tested the
concentration (0–100 μM)-response relationship of REG III
mRNA expression by resveratrol and found that the optimum
concentration of resveratrol for REG III expression was 30
μM (data not shown).
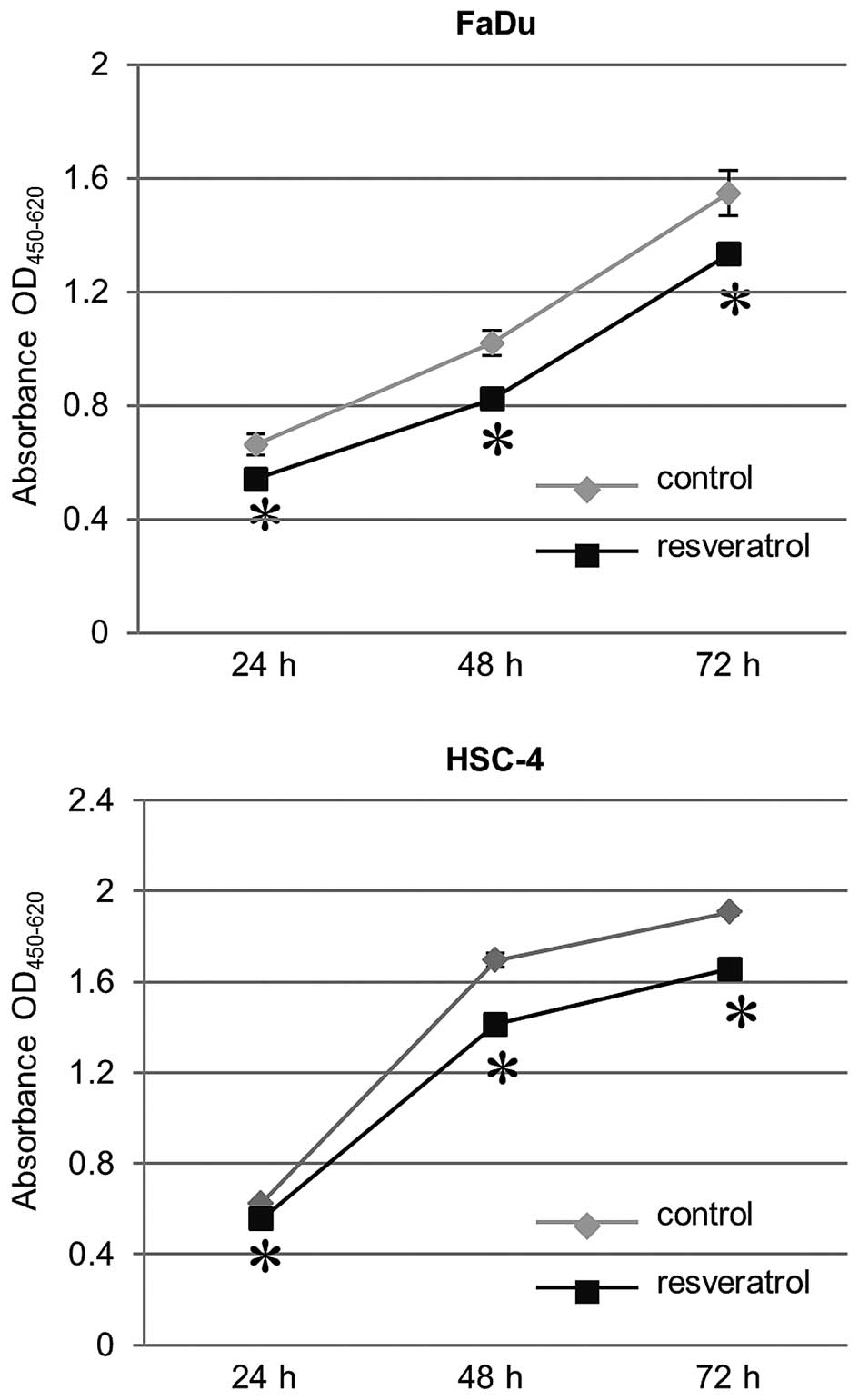
Effect of resveratrol on growth
inhibition in HNSCC cells
We sought to evaluate the effect of resveratrol on
cell proliferation of HNSCC cells, FaDu and HSC-4. In the
proliferation assay using WST-8 cleavage, HNSCC cells (both FaDu
and HSC-4 cells) treated with 30 μM resveratrol showed a
significant decrease in growth compared to untreated cells as a
control. The resveratrol-induced growth inhibitory effect in HNSCC
cells was time-dependent (Fig.
3).
Resveratrol enhances the chemo- and
radiosensitivity of HNSCC cells
We measured the chemo- and radiosensitivity by
addition of resveratrol to HNSCC cells using WST-8 cleavage. We
found that HNSCC cells treated with resveratrol showed a
significant increase in chemosensitivity (3.0 and 10 μM of
cisplatin) and radiosensitivity (5 and 10 Gy), as compared with
cells treated with DMSO as a control (Fig. 4). Therefore, these results
indicated that resveratrol enhances the chemo- and radiosensitivity
of HNSCC cells.
 | Figure 4Enhancement of chemo- and
radiosensitivity in HNSCC cells by resveratrol. Cells were treated
with (A and C) radiation at a dose of 0, 5 or 10 Gy, (B and D) 0,
1.0, 3.0, or 10 μM cisplatin. Thereafter each dish was incubated
for additional 72 h. Cell viability was assessed using WST-8 assay.
Data are shown as means ± SE. *P<0.05. HNSCC, head
and neck squamous cell carcinoma; resveratrol,
3,4′,5-trihydroxy-trans-stilbene; SE, standard error. |
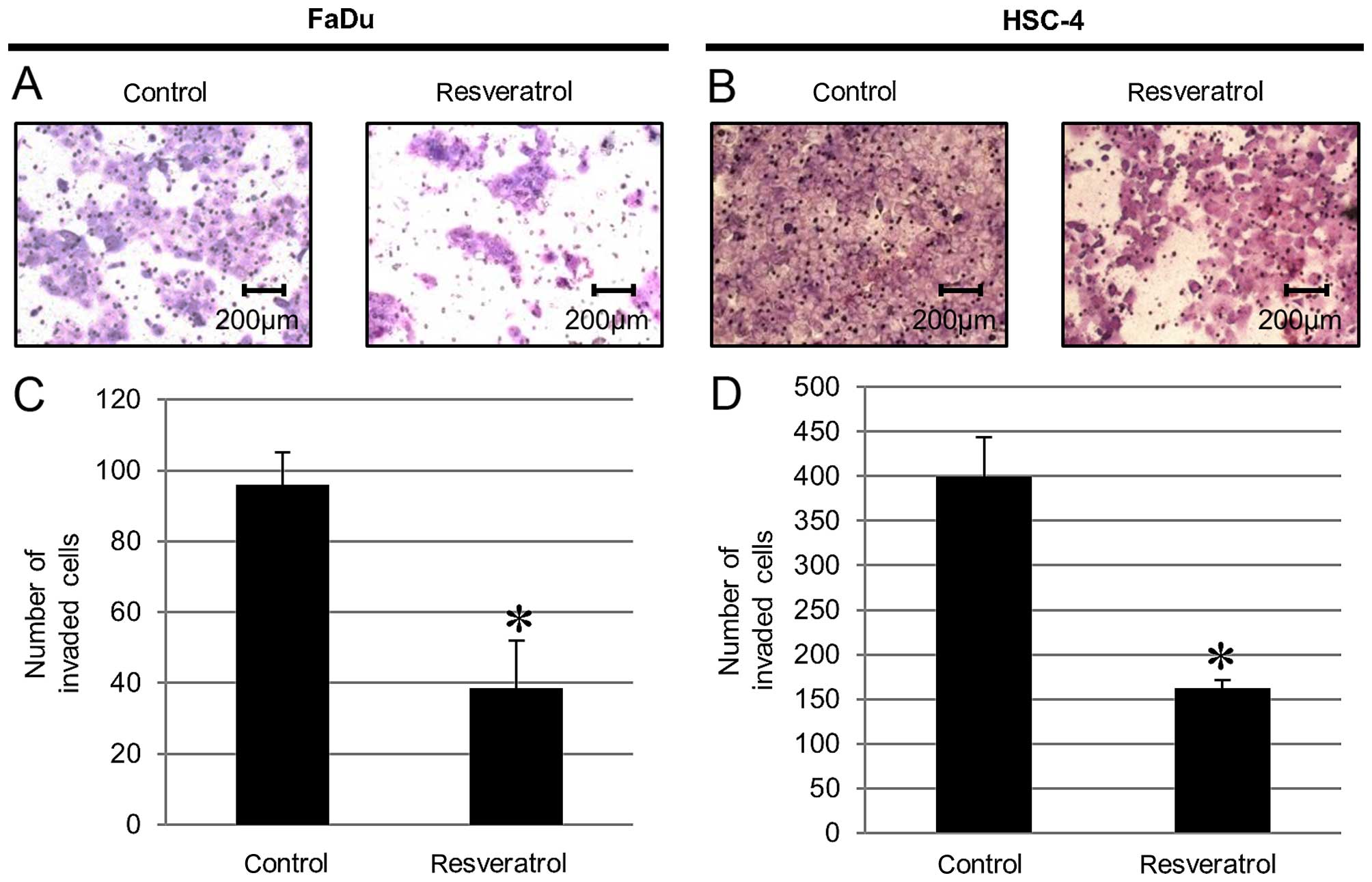
Resveratrol blocks cancer invasion of
HNSCC cells
We measured the invasive ability by addition of
resveratrol to HNSCC cells using an invasion assay. We found that
cells treated with resveratrol displayed significantly less
invasive ability compared to that of the untreated cells as a
control (Fig. 5).
Discussion
REG family proteins are believed to be associated
with human digestive diseases such as inflammation and cancer
(32–42,44,54,55).
Recently, among human REG gene family, we have reported that REG
III expression was associated with an improved survival rate
for HNSCC patients, and REG III regulated cell proliferation
and chemo- and radiosensitivity in HNSCC in vitro (44).
REG proteins are upregulated in many human cancers,
and play their roles through several signaling pathways. According
to previous reports, expression of REG was mainly induced by
various inflammatory cytokines and exogenous growth factors
(13). However, there is little
information on these pathways. Some studies have demonstrated that
cytokines such as IFN-γ, IL-6, IL-22, and TNF-α, enhance the
expression of REG I (46,49,56–58).
In addition, it is revealed that IL-8 regulates the expression of
REG protein in gastric cancer cells (59) and that REG I acts as an
anti-apoptotic factor through the STAT3 signaling pathway by
increasing the expression and phosphorylation of Akt and Bad in
gastric cancer cells (60).
Furthermore, some studies demonstrated that PPARγ activator of
thiazolidinediones such as ciglitazone and troglitazone inhibited
REG Iα gene transcription (61). However, the biological function and
cell signaling pathway of REG III have not been
elucidated.
In the present study, we investigated the
stimulators for the induction of the expression of REG III
in HNSCC in vitro. In comparison with other type of
REG family genes, expression of REG III was not
induced by inflammatory cytokines, growth factors, and PPARγ
activator of thiazolidinediones. We first revealed that
resveratrol, which is a naturally occurring polyphenol,
significantly increased the REG III promoter activity and
the mRNA levels of REG III in HNSCC cells.
Resveratrol is a natural compound present in fruits
including red grapes, vegetables and beverages including wine that
are part of the human diet (62–64).
In recent years, many studies indicated that resveratrol is
associated with antioxidant, anti-inflammatory, and
anticarcinogenic effects (53,65,66).
Concerning the anticarcinogenic potential of resveratrol, a number
of studies have suggested that resveratrol modulates multiple
cellular processes, including cell proliferation, apoptosis,
inflammation, and angiogenesis (63,67–71).
Several reports indicate that resveratrol inhibits proliferation of
cancer cells by inhibiting cell cycle progression (72–75).
Moreover, recent studies support the role of resveratrol in
inhibition of cancer invasion (76,77).
In the present study, we observed a reduction in cell growth rates
and the enhancement of chemo- and radiosensitivity in HNSCC cells
treated with resveratrol when compared with untreated cells as a
control. Furthermore, the present study demonstrated that
resveratrol blocked cancer invasion in HNSCC cells. These results
were compatible to the anticarcinogenic effect in cells transfected
with REG III (44). It can
be presumed that resveratrol could enhance the chemo- and
radiosensitivity and inhibit cancer progression through the REG
III expression pathway in HNSCC cells. However, the downstream
signaling pathway of REG III is still unresolved. The
signaling pathway of how REG III reduces cell proliferation,
blocks cancer invasion, and enhances the chemo- and
radiosensitivity in HNSCC need to be investigated in future
studies.
In summary, these data suggested that resveratrol
can play an important role in the improvement of survival for HNSCC
through the REG III expression pathway and can be a
potential candidate for novel anticancer drugs for patients with
HNSCC.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pignon JP, le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group. Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozer E, Grecula JC, Agrawal A, Rhoades CA,
Young DC and Schuller DE: Long-term results of a multimodal
intensification regimen for previously untreated advanced
resectable squamous cell cancer of the oral cavity, oropharynx, or
hypopharynx. Laryngoscope. 116:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ang KK, Trotti A, Brown BW, Garden AS,
Foote RL, Morrison WH, Geara FB, Klotch DW, Goepfert H and Peters
LJ: Randomized trial addressing risk features and time factors of
surgery plus radiotherapy in advanced head-and-neck cancer. Int J
Radiat Oncol Biol Phys. 51:571–578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper JS, Zhang Q, Pajak TF, Forastiere
AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Long-term follow-up of the RTOG 9501/intergroup phase III
trial: Postoperative concurrent radiation therapy and chemotherapy
in high-risk squamous cell carcinoma of the head and neck. Int J
Radiat Oncol Biol Phys. 84:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adelstein DJ, Saxton JP, Rybicki LA,
Esclamado RM, Wood BG, Strome M, Lavertu P, Lorenz RR and Carroll
MA: Multiagent concurrent chemoradiotherapy for locoregionally
advanced squamous cell head and neck cancer: Mature results from a
single institution. J Clin Oncol. 24:1064–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldenberg D, Lee J, Koch WM, Kim MM,
Trink B, Sidransky D and Moon CS: Habitual risk factors for head
and neck cancer. Otolaryngol Head Neck Surg. 131:986–993. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar B, Cordell KG, Lee JS, Worden FP,
Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, et al:
EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as
indicators of response to therapy and survival in oropharyngeal
cancer. J Clin Oncol. 26:3128–3137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Worden FP, Kumar B, Lee JS, Wolf GT,
Cordell KG, Taylor JM, Urba SG, Eisbruch A, Teknos TN, Chepeha DB,
et al: Chemo-selection as a strategy for organ preservation in
advanced oropharynx cancer: Response and survival positively
associated with HPV16 copy number. J Clin Oncol. 26:3138–3146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiboski CH, Schmidt BL and Jordan RC:
Tongue and tonsil carcinoma: Increasing trends in the U.S.
population ages 20–44 years. Cancer. 103:1843–1849. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takasawa S: Regenerating gene (REG)
product and its potential clinical usage. Expert Opin Ther Targets.
20:541–550. 2016. View Article : Google Scholar
|
|
14
|
Watanabe T, Yonekura H, Terazono K,
Yamamoto H and Okamoto H: Complete nucleotide sequence of human reg
gene and its expression in normal and tumoral tissues. The reg
protein, pancreatic stone protein, and pancreatic thread protein
are one and the same product of the gene. J Biol Chem.
265:7432–7439. 1990.PubMed/NCBI
|
|
15
|
Fukui H, Kinoshita Y, Maekawa T, Okada A,
Waki S, Hassan S, Okamoto H and Chiba T: Regenerating gene protein
may mediate gastric mucosal proliferation induced by
hypergastrinemia in rats. Gastroenterology. 115:1483–1493. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higham AD, Bishop LA, Dimaline R,
Blackmore CG, Dobbins AC, Varro A, Thompson DG and Dockray GJ:
Mutations of RegIalpha are associated with enterochromaffin-like
cell tumor development in patients with hypergastrinemia.
Gastroenterology. 116:1310–1318. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shinozaki S, Nakamura T, Iimura M, Kato Y,
Iizuka B, Kobayashi M and Hayashi N: Upregulation of Reg 1α and
GW112 in the epithelium of inflamed colonic mucosa. Gut.
48:623–629. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ose T, Kadowaki Y, Fukuhara H, Kazumori H,
Ishihara S, Udagawa J, Otani H, Takasawa S, Okamoto H and Kinoshita
Y: Reg I-knockout mice reveal its role in regulation of cell growth
that is required in generation and maintenance of the villous
structure of small intestine. Oncogene. 26:349–359. 2007.
View Article : Google Scholar
|
|
19
|
Zhang YW, Ding LS and Lai MD: Reg gene
family and human diseases. World J Gastroenterol. 9:2635–2641.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Wang J, Wang H and Lai M: Reg
proteins and their roles in inflammation and cancer of the human
digestive system. Adv Clin Chem. 61:153–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Terazono K, Yamamoto H, Takasawa S, Shiga
K, Yonemura Y, Tochino Y and Okamoto H: A novel gene activated in
regenerating islets. J Biol Chem. 263:2111–2114. 1988.PubMed/NCBI
|
|
22
|
Moriizumi S, Watanabe T, Unno M,
Nakagawara K, Suzuki Y, Miyashita H, Yonekura H and Okamoto H:
Isolation, structural determination and expression of a novel reg
gene, human regI β. Biochim Biophys Acta. 1217:199–202. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanchez D, Figarella C, Marchand-Pinatel
S, Bruneau N and Guy-Crotte O: Preferential expression of reg I β
gene in human adult pancreas. Biochem Biophys Res Commun.
284:729–737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hervieu V, Christa L, Gouysse G, Bouvier
R, Chayvialle JA, Bréchot C and Scoazec JY: HIP/PAP, a member of
the reg family, is expressed in glucagon-producing enteropancreatic
endocrine cells and tumors. Hum Pathol. 37:1066–1075. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drickamer K: Two distinct classes of
carbohydrate-recognition domains in animal lectins. J Biol Chem.
263:9557–9560. 1988.PubMed/NCBI
|
|
26
|
Dusetti NJ, Frigerio JM, Fox MF, Swallow
DM, Dagorn JC and Iovanna JL: Molecular cloning, genomic
organization, and chromosomal localization of the human
pancreatitis-associated protein (PAP) gene. Genomics. 19:108–114.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lasserre C, Simon MT, Ishikawa H, Diriong
S, Nguyen VC, Christa L, Vernier P and Brechot C: Structural
organization and chromosomal localization of a human gene (HIP/PAP)
encoding a C-type lectin overexpressed in primary liver cancer. Eur
J Biochem. 224:29–38. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nata K, Liu Y, Xu L, Ikeda T, Akiyama T,
Noguchi N, Kawaguchi S, Yamauchi A, Takahashi I, Shervani NJ, et
al: Molecular cloning, expression and chromosomal localization of a
novel human REG family gene, REG III. Gene. 340:161–170. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hartupee JC, Zhang H, Bonaldo MF, Soares
MB and Dieckgraefe BK: Isolation and characterization of a cDNA
encoding a novel member of the human regenerating protein family:
Reg IV. Biochim Biophys Acta. 1518:287–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe T, Yonemura Y, Yonekura H, Suzuki
Y, Miyashita H, Sugiyama K, Moriizumi S, Unno M, Tanaka O and Kondo
H: Pancreatic beta-cell replication and amelioration of surgical
diabetes by Reg protein. Proc Natl Acad Sci USA. 91:3589–3592.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asahara M, Mushiake S, Shimada S, Fukui H,
Kinoshita Y, Kawanami C, Watanabe T, Tanaka S, Ichikawa A, Uchiyama
Y, et al: Reg gene expression is increased in rat gastric
enterochromaffin-like cells following water immersion stress.
Gastroenterology. 111:45–55. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Macadam RC, Sarela AI, Farmery SM,
Robinson PA, Markham AF and Guillou PJ: Death from early colorectal
cancer is predicted by the presence of transcripts of the REG gene
family. Br J Cancer. 83:188–195. 2000.PubMed/NCBI
|
|
33
|
Violette S, Festor E, Pandrea-Vasile I,
Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M
and Lesuffleur T: Reg IV, a new member of the regenerating gene
family, is overexpressed in colorectal carcinomas. Int J Cancer.
103:185–193. 2003. View Article : Google Scholar
|
|
34
|
Oue N, Mitani Y, Aung PP, Sakakura C,
Takeshima Y, Kaneko M, Noguchi T, Nakayama H and Yasui W:
Expression and localization of Reg IV in human neoplastic and
non-neoplastic tissues: Reg IV expression is associated with
intestinal and neuroendocrine differentiation in gastric
adenocarcinoma. J Pathol. 207:185–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yonemura Y, Sakurai S, Yamamoto H, Endou
Y, Kawamura T, Bandou E, Elnemr A, Sugiyama K, Sasaki T, Akiyama T,
et al: REG gene expression is associated with the infiltrating
growth of gastric carcinoma. Cancer. 98:1394–1400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dhar DK, Udagawa J, Ishihara S, Otani H,
Kinoshita Y, Takasawa S, Okamoto H, Kubota H, Fujii T, Tachibana M,
et al: Expression of regenerating gene I in gastric
adenocarcinomas: Correlation with tumor differentiation status and
patient survival. Cancer. 100:1130–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bishnupuri KS, Luo Q, Murmu N, Houchen CW,
Anant S and Dieckgraefe BK: Reg IV activates the epidermal growth
factor receptor/Akt/AP-1 signaling pathway in colon
adenocarcinomas. Gastroenterology. 130:137–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayashi K, Motoyama S, Koyota S, Koizumi
Y, Wang J, Takasawa S, Itaya-Hironaka A, Sakuramoto-Tsuchida S,
Maruyama K, Saito H, et al: REG I enhances chemo- and
radiosensitivity in squamous cell esophageal cancer cells. Cancer
Sci. 99:2491–2495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou L, Zhang R, Wang L, Shen S, Okamoto
H, Sugawara A, Xia L, Wang X, Noguchi N, Yoshikawa T, et al:
Upregulation of REG Ialpha accelerates tumor progression in
pancreatic cancer with diabetes. Int J Cancer. 127:1795–1803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fukui H, Fujii S, Takeda J, Kayahara T,
Sekikawa A, Nanakin A, Suzuki K, Hisatsune H, Seno H, Sawada M, et
al: Expression of reg I α protein in human gastric cancers.
Digestion. 69:177–184. 2004. View Article : Google Scholar
|
|
41
|
Minamiya Y, Kawai H, Saito H, Ito M,
Hosono Y, Motoyama S, Katayose Y, Takahashi N and Ogawa J: REG1A
expression is an independent factor predictive of poor prognosis in
patients with non-small cell lung cancer. Lung Cancer. 60:98–104.
2008. View Article : Google Scholar
|
|
42
|
Mauro V, Carette D, Chevallier D, Michiels
JF, Segretain D, Pointis G and Sénégas-Balas F: Reg I protein in
healthy and seminoma human testis. Histol Histopathol.
23:1195–1203. 2008.PubMed/NCBI
|
|
43
|
Kimura M, Naito H, Tojo T, Itaya-Hironaka
A, Dohi Y, Yoshimura M, Nakagawara K, Takasawa S and Taniguchi S:
REG Iα gene expression is linked with the poor prognosis of lung
adenocarcinoma and squamous cell carcinoma patients via discrete
mechanisms. Oncol Rep. 30:2625–2631. 2013.PubMed/NCBI
|
|
44
|
Masui T, Ota I, Itaya-Hironaka A, Takeda
M, Kasai T, Yamauchi A, Sakuramoto-Tsuchida S, Mikami S, Yane K,
Takasawa S, et al: Expression of REG III and prognosis in head and
neck cancer. Oncol Rep. 30:573–578. 2013.PubMed/NCBI
|
|
45
|
Ota H, Tamaki S, Itaya-Hironaka A,
Yamauchi A, Sakuramoto-Tsuchida S, Morioka T, Takasawa S and Kimura
H: Attenuation of glucose-induced insulin secretion by intermittent
hypoxia via down-regulation of CD38. Life Sci. 90:206–211. 2012.
View Article : Google Scholar
|
|
46
|
Akiyama T, Takasawa S, Nata K, Kobayashi
S, Abe M, Shervani NJ, Ikeda T, Nakagawa K, Unno M, Matsuno S, et
al: Activation of Reg gene, a gene for insulin-producing β-cell
regeneration: Poly(ADP-ribose) polymerase binds Reg promoter and
regulates the transcription by autopoly(ADP-ribosyl)ation. Proc
Natl Acad Sci USA. 98:48–53. 2001.
|
|
47
|
Nakagawa K, Takasawa S, Nata K, Yamauchi
A, Itaya-Hironaka A, Ota H, Yoshimoto K, Sakuramoto-Tsuchida S,
Miyaoka T, Takeda M, et al: Prevention of Reg I-induced β-cell
apoptosis by IL-6/dexamethasone through activation of HGF gene
regulation. Biochim Biophys Acta. 1833:2988–2995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamauchi A, Itaya-Hironaka A,
Sakuramoto-Tsuchida S, Takeda M, Yoshimoto K, Miyaoka T, Fujimura
T, Tsujinaka H, Tsuchida C, Ota H, et al: Synergistic activations
of REG Iα and REG Iβ promoters by IL-6 and glucocorticoids through
JAK/STAT pathway in human pancreatic β cells. J Diabetes Res.
2015:1730582015. View Article : Google Scholar
|
|
49
|
Fujimura T, Fujimoto T, Itaya-Hironaka A,
Miyaoka T, Yoshimoto K, Yamauchi A, Sakuramoto-Tsuchida S, Kondo S,
Takeda M, Tsujinaka H, et al: Interleukin-6/STAT pathway is
responsible for the induction of gene expression of REG Iα, a new
auto-antigen in Sjögren’s syndrome patients, in salivary duct
epithelial cells. Biochem Biophys Rep. 2:69–74. 2015.
|
|
50
|
Tsujinaka H, Itaya-Hironaka A, Yamauchi A,
Sakuramoto-Tsuchida S, Ota H, Takeda M, Fujimura T, Takasawa S and
Ogata N: Human retinal pigment epithelial cell proliferation by the
combined stimulation of hydroquinone and advanced glycation
end-products via up-regulation of VEGF gene. Biochem Biophys Rep.
2:123–131. 2015.
|
|
51
|
Takasawa S, Kuroki M, Nata K, Noguchi N,
Ikeda T, Yamauchi A, Ota H, Itaya-Hironaka A, Sakuramoto-Tsuchida
S, Takahashi I, et al: A novel ryanodine receptor expressed in
pancreatic islets by alternative splicing from type 2 ryanodine
receptor gene. Biochem Biophys Res Commun. 397:140–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ota H, Itaya-Hironaka A, Yamauchi A,
Sakuramoto-Tsuchida S, Miyaoka T, Fujimura T, Tsujinaka H,
Yoshimoto K, Nakagawara K, Tamaki S, et al: Pancreatic β cell
proliferation by intermittent hypoxia via up-regulation of Reg
family genes and HGF gene. Life Sci. 93:664–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu XJ, Bao HR, Zeng XL and Wei JM:
Effects of resveratrol and genistein on nuclear factor κB, tumor
necrosis factor α and matrix metalloproteinase 9 in patients with
chronic obstructive pulmonary disease. Mol Med Rep. 13:4266–4272.
2016.PubMed/NCBI
|
|
54
|
Ogawa H, Fukushima K, Naito H, Funayama Y,
Unno M, Takahashi K, Kitayama T, Matsuno S, Ohtani H, Takasawa S,
et al: Increased expression of HIP/PAP and regenerating gene III in
human inflammatory bowel disease and a murine bacterial
reconstitution model. Inflamm Bowel Dis. 9:162–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vives-Pi M, Takasawa S, Pujol-Autonell I,
Planas R, Cabre E, Ojanguren I, Montraveta M, Santos AL and
Ruiz-Ortiz E: Biomarkers for diagnosis and monitoring of celiac
disease. J Clin Gastroenterol. 47:308–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dusetti NJ, Mallo GV, Ortiz EM, Keim V,
Dagorn JC and Iovanna JL: Induction of lithostathine/reg mRNA
expression by serum from rats with acute pancreatitis and cytokines
in pancreatic acinar AR-42J cells. Arch Biochem Biophys.
330:129–132. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kinoshita Y, Ishihara S, Kadowaki Y, Fukui
H and Chiba T: Reg protein is a unique growth factor of gastric
mucosal cells. J Gastroenterol. 39:507–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Usami S, Motoyama S, Koyota S, Wang J,
Hayashi-Shibuya K, Maruyama K, Takahashi N, Saito H, Minamiya Y,
Takasawa S, et al: Regenerating gene I regulates interleukin-6
production in squamous esophageal cancer cells. Biochem Biophys Res
Commun. 392:4–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yoshino N, Ishihara S, Rumi MA,
Ortega-Cava CF, Yuki T, Kazumori H, Takazawa S, Okamoto H, Kadowaki
Y and Kinoshita Y: Interleukin-8 regulates expression of Reg
protein in Helicobacter pylori-infected gastric mucosa. Am J
Gastroenterol. 100:2157–2166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sekikawa A, Fukui H, Fujii S, Ichikawa K,
Tomita S, Imura J, Chiba T and Fujimori T: REG Ialpha protein
mediates an anti-apoptotic effect of STAT3 signaling in gastric
cancer cells. Carcinogenesis. 29:76–83. 2008. View Article : Google Scholar
|
|
61
|
Yamauchi A, Takahashi I, Takasawa S, Nata
K, Noguchi N, Ikeda T, Yoshikawa T, Shervani NJ, Suzuki I, Uruno A,
et al: Thiazolidinediones inhibit REG Iα gene transcription in
gastrointestinal cancer cells. Biochem Biophys Res Commun.
379:743–748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cal C, Garban H, Jazirehi A, Yeh C,
Mizutani Y and Bonavida B: Resveratrol and cancer: Chemoprevention,
apoptosis, and chemo-immunosensitizing activities. Curr Med Chem
Anticancer Agents. 3:77–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rubiolo JA, López-Alonso H, Martín-Vázquez
V, Fol-Rodríguéz NM, Vieytes MR, Botana LM and Vega FV: Resveratrol
inhibits proliferation of primary rat hepatocytes in G0/G1 by
inhibiting DNA synthesis. Folia Biol (Praha). 58:166–172. 2012.
|
|
64
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
Mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kovacic P and Somanathan R: Multifaceted
approach to resveratrol bioactivity: Focus on antioxidant action,
cell signaling and safety. Oxid Med Cell Longev. 3:86–100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1β-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liang YC, Tsai SH, Chen L, Lin-Shiau SY
and Lin JK: Resveratrol-induced G2 arrest through the inhibition of
CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells.
Biochem Pharmacol. 65:1053–1060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
ElAttar TM and Virji AS: Modulating effect
of resveratrol and quercetin on oral cancer cell growth and
proliferation. Anticancer Drugs. 10:187–193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yeh CB, Hsieh MJ, Lin CW, Chiou HL, Lin
PY, Chen TY and Yang SF: The antimetastatic effects of resveratrol
on hepatocellular carcinoma through the downregulation of a
metastasis-associated protease by SP-1 modulation. PLoS One.
8:e566612013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: Molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hong YB, Kang HJ, Kim HJ, Rosen EM,
Dakshanamurthy S, Rondanin R, Baruchello R, Grisolia G, Daniele S
and Bae I: Inhibition of cell proliferation by a resveratrol analog
in human pancreatic and breast cancer cells. Exp Mol Med.
41:151–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing G1/S
phase cell cycle arrest and apoptosis through caspase/cyclin CDK
pathways. Mol Med Rep. 10:1697–1702. 2014.PubMed/NCBI
|
|
74
|
Wang G, Guo X, Chen H, Lin T, Xu Y, Chen
Q, Liu J, Zeng J, Zhang XK and Yao X: A resveratrol analog,
phoyunbene B, induces G2/M cell cycle arrest and apoptosis in HepG2
liver cancer cells. Bioorg Med Chem Lett. 22:2114–2118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar
|
|
76
|
Baribeau S, Chaudhry P, Parent S and
Asselin É: Resveratrol inhibits cisplatin-induced
epithelial-to-mesenchymal transition in ovarian cancer cell lines.
PLoS One. 9:e869872014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lin FY, Hsieh YH, Yang SF, Chen CT, Tang
CH, Chou MY, Chuang YT, Lin CW and Chen MK: Resveratrol suppresses
TPA-induced matrix metalloproteinase-9 expression through the
inhibition of MAPK pathways in oral cancer cells. J Oral Pathol
Med. 44:699–706. 2015. View Article : Google Scholar
|