Introduction
Prostate cancer (PCa) is one of the most common
cancers in male urogenital system and is the main risk factor to
male health. Huggins et al (1) found that surgical castration and
estrogen treatment could delay the metastatic PCa progression and
first confirmed the reactivity of PCa to androgen deprivation in
1941 (1). Currently, androgen
deprivation therapy has become the main treatment method in PCa.
Unfortunately, most of PCa usually develop to an advanced stage
acquiring castration resistance (CRPC) after 18 months of
treatment. Androgen receptor (AR) signaling is a pivotal pathway
regulating prostate development and malignant transformation and is
therefore an anticancer drug target. Knockdown or suppression of AR
signaling has been shown to upregulate certain oncogenic candidates
including glucocorticoid receptor, which has been suspected to be a
mechanism of CRPC (2,3). Although targeted therapy has been the
focus of clinical research, several phase III clinical trials of
target drugs such as bevacizumab, sunitinib have been shown to fail
in significantly prolonging survival in patients with CRPC
(4,5). Thus, the novel molecular targets are
urgently needed to improve CRPC patient prognosis.
The structural maintenance of chromosome 1 alpha
(SMC1A) gene is located in Xp11.22-p11.21, consisting of 25
exons and 24 introns. SMC1A gene encodes a core subunit of
the cohesin complex, which is essential to sister chromatid
cohesion. SMC1, SMC3, SCC1 (also known as MDC1 and RAD21) and SCC3
(also known as SA2 and STAG2) subunits could interact with each
other and form a ring-shaped cohesin complex (6–8). As
is known, central components of the cohesin and condensin complexes
are required for conversion of inter-phase chromatin into
mitotic-like condense chromosomes (9). Structural maintenance of chromosome
(SMC) proteins are core component of the cohesin and condensin
complex and essential for chromosome condensation during DNA
replication and chromatid segregation of the genome in all
organisms. They are also involved in checkpoint responses and
epigenetic silencing of gene expression (10).
SMC1A gene plays a pivotal role in chromosome
function, gene regulation and double-stranded DNA repair. Mutations
of SMC1A gene may cause the Cornelia de Lange syndrome
(CdLS), which is dominantly a developmental disorder with
multisystem abnormalities including slow growth before and after
birth, characteristic facial features, upper extremity defects,
hirsutism, gastroesophageal dysfunction and cognitive retardation
(11,12). Eleven different SMC1A
mutations in 14 unrelated patients have been reported, in which all
patients had a mild to moderate CdLS phenotype (13–15).
Genes involving chromosome maintenance and DNA
repair have been found to be responsible for the malignant
transformation of tumors. Although the study of SMC1A
focuses mainly on the CdLS, it has been reported that upregulation
of SMC1A might be related to the development of
glioblastoma, colon and lung cancer (16–18).
However, the function of SMC1A in PCa and its correlation
with CRPC has not been studied yet. In the present study, based on
a lentiviral shRNA library screening, we identified SMC1A as
a novel oncogenic candidate. We further performed relevant research
to confirm the underlying roles of SMC1A in PCa cells, and
the expression levels and clinical significance of SMC1A in
PCa.
Materials and methods
Patient samples and immunohistochemical
staining
All of the patient samples for immunohistochemical
(IHC) analysis were obtained from the Department of Urinary
Surgery, Shanghai Changzheng Hospital, Shanghai, China. This study
was approved by the Clinical Research Ethics Committee of Shanghai
Changzheng Hospital, and written informed consents were obtained
from all the subjects.
For IHC analysis, tissue samples were
paraffin-embedded, cut into 5-μm-thick sections and pasted onto
glass slides. After deparaffinizing in xylene and dehydration with
graded ethanol washes, the specimens were sequentially incubated
with blocking solution for 10 min and 1:100 dilution of anti-SMC1A
antibody (SAB4300451; Sigma-Aldrich) at 4°C overnight, and stained
using UltraSensitive™ SP (mouse/rabbit) IHC kit (KIT9730; Fuzhou
Maixin Biotechnology, Co., Ltd., Fuzhou, China) according to the
user's manual.
Oncomine database analysis
The clinical significance of SMC1A expression
in prostate cancer were analyzed using the online Oncomine database
(www.onocomine.org) consisting of previously published
and publicly available microarray data. Welsh prostate dataset
(19) and Singh prostate dataset
(20), which have a total of 136
samples, were used to compare the differential expression of
SMC1A between normal (59 cases) and cancerous tissue (77
cases). Glinsky prostate dataset (21) (79 cancer cases) was used to analyze
the correlation of SMC1A expression level with cancer
biochemical recurrence. Moreover, three independent datasets
including Holzbeierlein prostate dataset (22), LaTulippe prostate dataset (23), and Chandran prostate dataset
(24), which have a total of 31
cancer tissues, were used to explore the relationship between
SMC1A expression level and distant metastasis.
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM; cat.
no.12430-054), F-12 (cat. no. 21127022) and Roswell Park Memorial
Institute 1640 (RPMI-1640; cat. no. 11875-093) medium and fetal
bovine serum (FBS; cat. no. 10099-141) were purchased from Gibco
(Grand Island, NY, USA). TRIzol reagent was purchased from
Invitrogen (Carlsbad, CA, USA). Giemsa was from Chemicon
International (Temecula, CA, USA). M-MLV reverse (cat. no. M5301)
transcriptase was purchased from Promega (Madison, WI, USA).
Oligo-dT(18) was
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Terra™
qPCR Direct SYBR® Premix (638318) was from Takara Bio
(Shiga, Japan). Anti-SMC1A antibody (SAB4300451) was from
Sigma-Aldrich (Munich, Germany). Mouse anti-GAPDH (sc-32233), goat
anti-mouse IgG (sc-32233) and goat anti-rabbit IgG (sc-2030) were
from Santa Cruz Biotechnology (Dallas, TX, USA). All the other
chemicals were of analytical grade from Sangon Biotech.
Cell culture
Human embryonic kidney (HEK) 293T cells and human
prostate cancer cell lines PC-3, DU145, LNCap and 22RV1 were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Science (Shanghai, China). 293T cells were cultured in
DMEM containing 10% FBS. PC-3 and DU145 cells were maintained in
F-12 medium supplemented with 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin. 22RV1 and LNCap cells were incubated with
RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin. All cells were cultured in a humidified
incubator at 37°C with 5% CO2.
RNA interference and recombinant
lentivirus transduction
To silence the expression of SMC1A in PCa
cell lines, the short hairpin (shRNA) sequence identified to target
human SMC1A gene was 5′-TAGGAGGTTCTTCTGAGTACA-3′. The
sequence of the negative control shRNA was 5′-TTCTCC
GAACGTGTCACGT-3′. The oligos were annealed and ligated into pFH-L
vector (Holly Lab, Shanghai, China) through NheI/PacI
restriction sites to generate pFH-Lv-shSMC1A and pFH-Lv-shCon.
Finally, the sequencing was performed to confirm the results of
construction.
Lentiviruses were generated by triple transfection
with modified pFH-shRNA plasmid and pVSVG-I and pCMVΔR8.92 helper
plasmids into HEK-293T cells using Lipofectamine 2000 according to
the manufacturer's instructions. The lentiviral particles were then
harvested by centrifugation, filtered through a 0.45 μm filter and
then stored at −80°C.
PC-3 or DU145 cells were seeded at the concentration
of 5×104 cell/well in 6-well plates. After 24 h of
culture, lentivirus containing shRNA targeting SMC1A
(shSMC1A) or the negative control (shCon) were added at a
multiplicity of infection (MOI) of 50 into F-12 basic medium. After
6-h incubation, PC-3 and DU145 cells were cultured in complete
medium replacing the basic medium containing the lentivirus. Then,
after 5 days post-transfection, the green fluorescent protein (GFP)
expression was examined using fluorescent microscopy (Olympus; cat.
no.CKX41) to assess the infection efficiency.
Quantitative real-time RT-PCR
analysis
The total RNA was extracted using TRIzol reagent
according to the manufacturer's instruction and synthesized
complementary DNA (cDNA) by using M-MLV reverse transcriptase.
Real-time PCR reactions using Terra™ qPCR Direct SYBR®
Premix were run on Takara TP800 Thermal Cycler Dice™ real-time
system. The following primers were used: SMC1A:
5′-AGCGAAAGGCA GAGATAATGG-3′ (forward) and 5′-GGTAGTCAAGAGGC
AAGAAGG-3′ (reverse); β-actin: 5′-GTGGACATCCGCAA AGAC-3′ (forward)
and 5′-AAAGGGTGTAACGCAACTA-3′ (reverse). Thermal cycling conditions
were as follows: initial denaturation 1 min at 95°C, followed by 40
cycles of denaturation for 5 sec at 95°C, extension for 20 sec at
60°C and absorbance value was read at the extension stage. The data
were analyzed with Takara Thermal Dice Real-Time system software
ver3.0. SMC1A relative mRNA levels were calculated using the
2−ΔΔCt method with normalization to β-actin.
Western blot analysis
Cells were washed twice with ice-cold PBS and lysed
in 2X sodium dodecyl sulfate (SDS) sample buffer (2%
mercaptoethanol, 20% glycerol, 4% SDS in 100 mM Tris-HCl buffer, pH
6.8), and incubated for 15 min on ice. The supernatants were
collected by centrifugation at 12,000 × g for 15 min at 4°C, and a
BCA protein assay kit was used to measure the protein content.
Equal amounts of protein samples (30 μg) were loaded and separated
in 10% SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride (PVDF) membranes. Whereafter, the
membrane was blocked with TBST buffer containing 5% non-fat milk at
room temperature for 1 h, and incubated with the primary antibodies
in the blocking solution at 4°C overnight. After being washed three
times with TBST, the membrane was incubated with horseradish
peroxidase (HRP)-conjugated secondary antibody (1:5,000) at room
temperature for 1 h. The objective bands were detected by Pierce
ECL western blotting detection kit (Thermo Fisher Scientific,
Waltham, MA, USA). GAPDH was used as an internal control.
MTT assay
To evaluate the effect of SMC1A in the
proliferation of prostate cancer cells,
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed. Four days after lentivirus infection, both
PC-3 and DU145 cells were reseeded in 96-well plates at an ultimate
density of 2000 cells/well and the number of active cells was
measured for five consecutive days. Subsequently, MTT (10 μl, 5
mg/ml) was added to each well at a fixed time-point. After
incubation at 37°C for 4 h, the acidic isopropanol (150 μl/well)
was added and then incubated at 37°C. Optical density (OD) of each
well was measured at 570 nm using an ELx808 Absorbance Reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Colony formation assay
To examine the effect of SMC1A in the colony
formation of a single prostate cancer cell, the colony formation
assay was executed. Four days after lentivirus infection, both PC-3
and DU145 cells were reseeded in 6-well plates at a density of 200
cells/well and cultured for 14 days in the humidified incubator at
37°C with 5% CO2. Culture medium was replaced at 3-day
intervals. Then, cells were washed in PBS, fixed in 4%
paraformaldehyde for 30 min and stained with Giemsa for 15 min at
room temperature. The stained colonies were washed with
ddH2O and air-dried. Finally, the ability of colony
formation was observed through a light/fluorescence microscope and
the colonies (>50 cells/colony) were counted.
Flow cytometric analysis
Cell cycle distribution was analyzed by propidium
iodide (PI) staining. Briefly, both PC-3 and DU145 cells were
reseeded at a density of 1×105 cells/well in 6-cm dishes
after lentivirus infection. After the incubation period cells were
harvested and fixed in 70% ethanol overnight at 4°C. The next day,
cells were washed thrice and resuspended in PBS containing 100
μg/ml RNase A and 50 μg/ml PI, and then incubated in the dark at
room temperature for 30 min. Cells were analyzed by flow cytometry
using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA,
USA). The percentage of the cells in sub-G1, G0/G1, S and G2/M
phases were analyzed using ModFit software (Verity Software House,
Inc., Topsham, ME, USA).
Migration assay
To explore the effect of SMC1A in the
migration of prostate cancer cells, a 24-well Transwell chamber
with 8.0 μm pore polycarbonate filter inserts (Corning; cat. no.
#3422) was performed. After lentivirus infection, both PC-3 and
DU145 cells were reseeded at a density of 1×105
cells/well in serum-free F-12 containing 0.2% BSA in the upper
chamber of each Transwell. In addition, F-12 supplemented with 10%
FBS was added in the lower chamber. Then, the migration
installation was incubated at 37°C with 5% CO2 overnight
and the non-migrated cells on the upper surface of the filter were
lightly removed using cotton buds. The migrated cells on the lower
surface were fixed in 4% paraformaldehyde for 10 min, stained in
crystal violet for 2 min, and counted (five random fields per well)
under a bright-field microscope. Additionally, the migrated cells
were dissociated by 33% acetic acid and quantified at 570 nm using
the Epoch microplate spectrophotometer (BioTek Instruments).
Animal experiments
The impact of SMC1A silencing on the tumor
development of prostate cancer in vivo was examined. DU145
(Con group), DU145-Lv-shCon (shCon group) or DU145 Lv-shSMC1A
(shSMC1A group) at a density of 5×106 per mouse were
injected subcutaneously into 4-week-old BALB/c nude mice (n=10 per
group; Shanghai Laboratory Animal Center, Chinese Academy of
Sciences, Shanghai, China). The development and growth of solid
tumors were monitored by measuring tumor size using a vernier
caliper every three days for a 28-days period. The tumor volume was
calculated using a standard formula: tumor volume (mm3)
= width (mm)2 × length (mm) × 0.5. At the end of the
experiment, all mice were sacrificed and individual tumor weight
was measured using an electronic balance. All the animal
experiments were approved by the Animal Care Committee of the
Second Military Medical University.
Statistical analysis
GraphPad Prism 5.0 software was used to perform the
statistical analyses. Data are presented as mean ± SD from at least
three independent experiments. The Student's t-test was used to
compare the differences between the groups. P<0.05 was
considered to indicate a statistically significant result.
Results
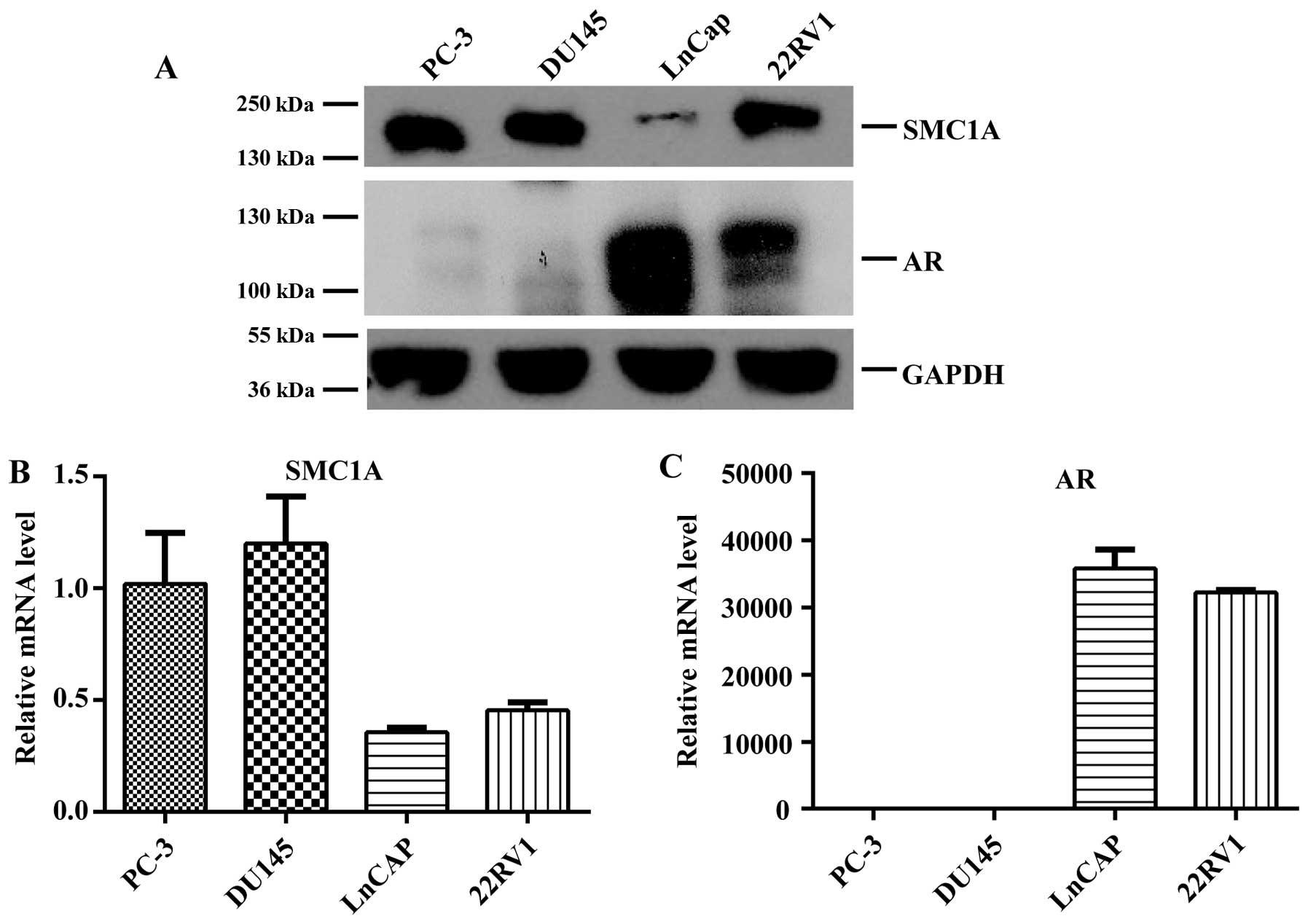
SMC1A is upregulated in
androgen-independent prostate cancer cells
Through a lentiviral shRNA library-based screening
on PC-3 cells, we identified SMC1A as a novel oncogenic
candidate. To validate and further explore the function of
SMC1A in prostate cancer, we first examined the expression
of SMC1A in different PCa cell lines. Notably, we found that
the expression of SMC1A was significantly increased in
androgen-independent prostate cancer cell lines PC-3 and DU145
compared with androgen-sensitive cell lines LNCap and 22RV1, showed
SMC1A expression was negatively correlated with the
expression status of androgen receptor (AR) in both the protein and
mRNA levels (Fig. 1A–C).
Considering that SMC1A expression was much higher in
androgen-independent prostate cancer cells PC-3 and DU145, they
were used for further investigation.
Lentivirus mediated SMC1A silencing in
prostate cancer cells
Both PC-3 and DU145 cells were untreated or
transfected with shCon or shSMC1A. The transfection efficiencies
were >90% in both cells confirmed by fluorescent microscope
(Fig. 2A). Western blot analysis
demonstrated that shSMC1A efficiently knocked down SMC1A
expression in protein levels in PC-3 and DU145 cells (Fig. 2B and C). The results of real-time
PCR indicated that SMC1A was downregulated >80 and 90% in
mRNA levels in PC-3 and DU145 cells, respectively (Fig. 2D and E; P<0.001, P<0.01).
Downregulation of SMC1A inhibits cell
proliferation and colony formation in prostate cancer cells
Effect of SMC1A silencing on prostate cancer
cell viability was assessed by MTT and colony formation assay. As
shown in Fig. 3A and B, the growth
rates of PC-3 and DU145 cells were significantly suppressed in
shSMC1A group in comparison to the Con or shCon group (P<0.001).
Furthermore, SMC1A depletion significantly inhibited colony
formation ability of PC-3 and DU145 cells in size and number
compared to the Con or shCon group (Fig. 3C–E; P<0.01, P<0.001). The
results suggested that both PC-3 and DU145 cells showed impaired
cell proliferation and colony formation abilities after
SMC1A knockdown, indicating a pivotal role of SMC1A
in regulation of prostate cancer cell vitality.
Knockdown of SMC1A modulates cell cycle
progression in prostate cancer cells
When culturing shSMC1A transfected PC-3 and DU145
cells, we noted that these cells showed more non-adherent cells
during passages. We decided to confirm how SMC1A affected
the proliferation of prostate cancer cells, and whether it
functioned through the cell cycle distribution. To verify this
hypothesis, PC-3 and DU145 cells were stained using PI and analyzed
with FACS. As expected, the results showed that knockdown of
SMC1A expression caused G0/G1 and G2/M-phase cell population
increase (P<0.05, P<0.001) while S-phase cell population
reduction (P<0.01) that indicated cell cycle arrest at
G2/M-phase in PC-3 cells (Fig.
4A). Moreover, SMC1A silencing presented G0/G1-phase
cell population increase (P<0.05) and S-phase cell population
reduction (P<0.001) that showed cell cycle arrest at S-phase in
DU145 cells (Fig. 4B). In
addition, the rate of cells in the Sub-G1 phase, representing
apoptotic cells indirectly, was remarkably increased in shSMC1A
group compared with Con and shCon groups in PC-3 and DU145 cells
(Fig. 4C and D; P<0.05,
P<0.01). These results indicated that SMC1A might be
involved in cell apoptotic and cell cycle progression.
Knockdown of SMC1A represses cell
migration ability of prostate cancer cells
PC-3 and DU145 cells were transfected with indicated
lentivirus for 96 h, and then subjected to Transwell assay for the
cell migration ability. As shown in Fig. 5, the cell numbers of PC-3 and DU145
cells in shSMC1A group which migrated to the lower chamber were
less than the Con and shCon groups (P<0.01, P<0.001). These
results indicated that knockdown of SMC1A significantly
inhibited the migration ability of PC-3 and DU145 cells.
Knockdown of SMC1A represses tumor growth
in a xenograft nude mouse model
To further study the function of SMC1A in
vivo, DU145 cells were untreated, or transfected with shCon or
shSMC1A, and subcutaneously injected into the nude mice to
investigate the impact of SMC1A on tumor growth. As shown in
Fig. 6A, knockdown of SMC1A
inhibited subcutaneous tumor growth of DU145 cells. At day 28, the
mice were euthanized and the tumors were removed. At the end of the
experiment, we found that the tumor volumes were significantly
reduced in time-dependent manner, while the tumor weighs were
markedly decreased by SMC1A silencing (Fig. 6B and C; P<0.001).
Upregulated SMC1A is related to
biochemical recurrence and distant metastasis in prostate cancer
patients
To illustrate the expression level of SMC1A
and the clinical significance of SMC1A in PCa patients, we
detected the specimens from patients and performed the data mining
of the publicly available Oncomine datasets. In the present study,
we found that the protein expression of SMC1A is
significantly upregulated in PCa tissues by IHC staining and
western blot assay (Fig. 7A and
B). Similar results were also observed that the mRNA expression
level of SMC1A was remarkably higher in PCa tissues than the
normal tissues using Singh prostate (Fig. 7C; n=102, P<0.001) and Welsh
rostate databases (Fig. 7D; n=34,
P=0.015). Notaby, we found that the expression of SMC1A was
obviously upregulated in the patients with postoperative
biochemical recurrence (BCR) at 3 years by analyzing Glinsky
prostate database (Fig. 7E; n=79,
P=0.0408). Furthermore, we also detected that SMC1A
expression was significantly and positively associated with distant
metastasis in Holzbeierlein prostate database (Fig. 7F; n=48, P=0.0307). This finding was
verified by other two independent databases including LaTulippe
prostate (n=32) and Chandran prostate (n=31) databases.
Specifically, comparing with primary site, SMC1A was visibly
upregulated in three metastasis tissues (Fig. 7G, lymph node, n=5; bone, n=2,
P=0.0067; soft tissues, n=2, P=0.0267; total metastasis, P=0.0087)
in LaTulippe prostate database and also obviously elevated in lymph
node (n=13, P<0.0001), adrenal gland (n=2, P=0.0303), liver
(n=5, P=0.0047) and lung (n=1) metastasis as revealed in Chandran
prostate database (Fig. 7H; total
metastasis, P=0.0047).
Discussion
The present study focused on SMC1A, whose
clinical significance and potential biological functions in PCa are
still unknown. We found that SMC1A was significantly
upregulated in both PCa clinical tissues and CRPC cell lines. By
silencing SMC1A expression and database mining, we confirmed
that the expression SMC1A was closely related to the
progression, metastasis and recurrence of human PCa.
Cohesin factors are involved in DNA repair and
genome stability. Defects in cohesin-associated genes are emerging
as potential drivers of genomic instability and carcinogenic
progression. In several tumor types, mutations of cohesin genes
have been identified (25). Many
tumors are either over-expressed or lowly expressed with cohesin
gene (26–29). It has been shown that the loss of
cohesion subunits would induce genomic instability in human cancers
and the associated aneuploidy, as is observed in many cell lines
which mutated in cohesin, resulting in further genomic instability
(30–32). The above suggest that cohesin
dysfunction may contribute to tumor development and
progression.
SMC1A gene encoding a core component of the
cohesin complex and cohesin-associated genes have been considered
as potential drivers of tumor development and progression in many
studies (17,18,26,28,33,34).
Mannini et al (35) found
that SMC1A mutations were associated with the canonical role
of cohesion. In fact, mutations affecting correct chromosome
segregation lead to chromosome instability. Additionally, some
studies demonstrated that SMC1A mutations may contribute to
tumorigenesis by regulating the expression of oncogenes or
suppressor genes.
The present study is the first revealing the
potential role of SMC1A in PCa. In this study, we studied
the expression of SMC1A in both PCa cell lines and clinical
tissues. We found that the SMC1A expression was much higher
in the androgen-independent cells PC-3, and DU145 than in the
androgen-sensitive cells LNCap and 22RV1 and was negatively
correlated with AR status. In clinical samples, we found that the
protein and mRNA expressions of SMC1A were markedly
upregulated in PCa tissues using IHC staining, western blot assay
and Oncomine database mining. In addition, through analysis of
clinical significance of SMC1A in PCa, we found that the
expression level of SMC1A was correlated to biochemical
recurrence and distant metastasis, suggesting that SMC1A could be a
potential prognostic indicator.
Detection of the proliferation is one of the widely
used methods to evaluate and measure the tumor responses to a new
oncogene. Herein, we examined the proliferation-inducing effects of
SMC1A silencing on prostate cancer cells in vitro. We
found that knockdown of SMC1A by small interfering RNA could
inhibit the growth and proliferation of CRPC cells PC-3, and DU145
by MTT and colony formation assay. Moreover, SMC1A silencing
led to cell cycle arrest at G2/M phase in PC-3 cells while at S
phase in DU145 cells. The difference of cell cycle arrest in PC-3
and DU145 cells may be due to the different cell sources that PC-3
cell is from a human prostatic adenocarcinoma metastatic to bone
(36), while DU145 cell is from
metastasis to the brain (37).
Moreover, when SMC1A was silenced, the number of cells in
the sub-G1 phase was increased significantly in both PC-3 and DU145
cells, indicating that knockdown of SMC1A could give rise to
PCa cell apoptosis. In addition, the migration of tumor cells is
one of the main risk factors for tumor progression. In this study,
Transwell assay showed that depletion of SMC1A could reduce
the migration of PC-3 and DU145 cells. However, the underlying
molecular mechanism needs further investigation.
To further confirm the efficacy of the antitumor
growth of SMC1A silencing in vivo, the xenograft nude
mouse models were established by subcutaneous injecting the
different treatments of DU145 cells. The results indicated that
SMC1A silencing could significantly reduce tumor growth in
xenograft models, which suggested that SMC1A may be a
potential anti-tumor target for drug development.
In conclusion, the results have suggested that
overexpression of SMC1A is a crucial molecule associated
with PCa. It is involved in proliferation, cell cycle regulation,
apoptosis and migration process of CRPC cells. The potential
application of SMC1A targeted therapy will need further
investigation in pre-clinical and clinical studies.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China for Youth (nos.
81001136 and 81202020), the National Natural Science Foundation of
China (nos. 30973006, 81170637 and 81572525), the Shanghai
Committee of Science and Technology General Program for Medicine
(no. 11JC1402302), the Key Project of Science and Innovation
Foundation of Shanghai Ministry of Education (no. 14zz084) and the
Military Fund for Health Care (no. CWS13BJ09).
References
|
1
|
Lytton B: Prostate cancer: A brief history
and the discovery of hormonal ablation treatment. J Urol.
165:1859–1862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arora VK, Schenkein E, Murali R, Subudhi
SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis
C, et al: Glucocorticoid receptor confers resistance to
antiandrogens by bypassing androgen receptor blockade. Cell.
155:1309–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie N, Cheng H, Lin D, Liu L, Yang O, Jia
L, Fazli L, Gleave ME, Wang Y, Rennie P, et al: The expression of
glucocorticoid receptor is negatively regulated by active androgen
receptor signaling in prostate tumors. Int J Cancer. 136:E27–E38.
2015. View Article : Google Scholar
|
|
4
|
Michaelson MD, Oudard S, Ou YC, Sengeløv
L, Saad F, Houede N, Ostler P, Stenzl A, Daugaard G, Jones R, et
al: Randomized, placebo-controlled, phase III trial of sunitinib
plus prednisone versus prednisone alone in progressive, metastatic,
castration-resistant prostate cancer. J Clin Oncol. 32:76–82. 2014.
View Article : Google Scholar
|
|
5
|
Kelly WK, Halabi S, Carducci M, George D,
Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, et
al: Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guacci V, Koshland D and Strunnikov A: A
direct link between sister chromatid cohesion and chromosome
condensation revealed through the analysis of MCD1 in S.
cerevisiae. Cell. 91:47–57. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Losada A, Hirano M and Hirano T:
Identification of Xenopus SMC protein complexes required for sister
chromatid cohesion. Genes Dev. 12:1986–1997. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaelis C, Ciosk R and Nasmyth K:
Cohesins: Chromosomal proteins that prevent premature separation of
sister chromatids. Cell. 91:35–45. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura K, Cuvier O and Hirano T:
Chromosome condensation by a human condensin complex in Xenopus egg
extracts. J Biol Chem. 276:5417–5420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harvey SH, Krien MJ and O'Connell MJ:
Structural maintenance of chromosomes (smc) proteins, a family of
conserved atpases. Genome Biol. 3:Reviews3003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krantz ID, McCallum J, DeScipio C, Kaur M,
Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA,
et al: Cornelia de Lange syndrome is caused by mutations in NIPBL,
the human homolog of Drosophila melanogaster Nipped-B. Nat Genet.
36:631–635. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ
and Strachan T: NIPBL, encoding a homolog of fungal Scc2-type
sister chromatid cohesion proteins and fly Nipped-B, is mutated in
Cornelia de Lange syndrome. Nat Genet. 36:636–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borck G, Zarhrate M, Bonnefont JP, Munnich
A, Cormier-Daire V and Colleaux L: Incidence and clinical features
of X-linked Cornelia de Lange syndrome due to SMC1L1 mutations. Hum
Mutat. 28:205–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deardorff MA, Kaur M, Yaeger D, Rampuria
A, Korolev S, Pie J, Gil-Rodríguez C, Arnedo M, Loeys B, Kline AD,
et al: Mutations in cohesin complex members SMC3 and SMC1A cause a
mild variant of cornelia de Lange syndrome with predominant mental
retardation. Am J Hum Genet. 80:485–494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musio A, Selicorni A, Focarelli ML,
Gervasini C, Milani D, Russo S, Vezzoni P and Larizza L: X-linked
Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet.
38:528–530. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Z, Lin M, Li K, Fu Y, Liu X, Yang D,
Zhao Y, Zheng J and Sun B: Knocking down SMC1A inhibits growth and
leads to G2/M arrest in human glioma cells. Int J Clin Exp Pathol.
6:862–869. 2013.PubMed/NCBI
|
|
17
|
Yang Y, Zhang Z, Wang R, Ma W, Wei J and
Li G: siRNA-mediated knockdown of SMC1A expression suppresses the
proliferation of glioblastoma cells. Mol Cell Biochem. 381:209–215.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Yu S, Cui L, Wang W, Li J, Wang K
and Lao X: Role of SMC1A overexpression as a predictor of poor
prognosis in late stage colorectal cancer. BMC Cancer. 15:902015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Welsh JB, Sapinoso LM, Su AI, Kern SG,
Wang-Rodriguez J, Moskaluk CA, Frierson HF Jr and Hampton GM:
Analysis of gene expression identifies candidate markers and
pharmacological targets in prostate cancer. Cancer Res.
61:5974–5978. 2001.PubMed/NCBI
|
|
20
|
Singh D, Febbo PG, Ross K, Jackson DG,
Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, et
al: Gene expression correlates of clinical prostate cancer
behavior. Cancer Cell. 1:203–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glinsky GV, Glinskii AB, Stephenson AJ,
Hoffman RM and Gerald WL: Gene expression profiling predicts
clinical outcome of prostate cancer. J Clin Invest. 113:913–923.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holzbeierlein J, Lal P, LaTulippe E, Smith
A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, et
al: Gene expression analysis of human prostate carcinoma during
hormonal therapy identifies androgen-responsive genes and
mechanisms of therapy resistance. Am J Pathol. 164:217–227. 2004.
View Article : Google Scholar
|
|
23
|
LaTulippe E, Satagopan J, Smith A, Scher
H, Scardino P, Reuter V and Gerald WL: Comprehensive gene
expression analysis of prostate cancer reveals distinct
transcriptional programs associated with metastatic disease. Cancer
Res. 62:4499–4506. 2002.PubMed/NCBI
|
|
24
|
Chandran UR, Ma C, Dhir R, Bisceglia M,
Lyons-Weiler M, Liang W, Michalopoulos G, Becich M and Monzon FA:
Gene expression profiles of prostate cancer reveal involvement of
multiple molecular pathways in the metastatic process. BMC Cancer.
7:642007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Yan M, Patra J, Natrajan R, Yan Y,
Swagemakers S, Tomaszewski JM, Verschoor S, Millar EK, van der Spek
P, et al: Enhanced RAD21 cohesin expression confers poor prognosis
and resistance to chemotherapy in high grade luminal, basal and
HER2 breast cancers. Breast Cancer Res. 13:R92011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghiselli G and Iozzo RV: Overexpression of
bamacan/SMC3 causes transformation. J Biol Chem. 275:20235–20238.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hagemann C, Weigelin B, Schommer S,
Schulze M, Al-Jomah N, Anacker J, Gerngras S, Kühnel S, Kessler AF,
Polat B, et al: The cohesin-interacting protein, precocious
dissociation of sisters 5A/sister chromatid cohesion protein 112,
is up-regulated in human astrocytic tumors. Int J Mol Med.
27:39–51. 2011.
|
|
28
|
Oikawa K, Ohbayashi T, Kiyono T, Nishi H,
Isaka K, Umezawa A, Kuroda M and Mukai K: Expression of a novel
human gene, human wings apart-like (hWAPL), is associated with
cervical carcinogenesis and tumor progression. Cancer Res.
64:3545–3549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang N, Ge G, Meyer R, Sethi S, Basu D,
Pradhan S, Zhao YJ, Li XN, Cai WW, El-Naggar AK, et al:
Overexpression of Separase induces aneuploidy and mammary
tumorigenesis. Proc Natl Acad Sci USA. 105:13033–13038. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barber TD, McManus K, Yuen KW, Reis M,
Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S,
et al: Chromatid cohesion defects may underlie chromosome
instability in human colorectal cancers. Proc Natl Acad Sci USA.
105:3443–3448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheltzer JM, Blank HM, Pfau SJ, Tange Y,
George BM, Humpton TJ, Brito IL, Hiraoka Y, Niwa O and Amon A:
Aneuploidy drives genomic instability in yeast. Science.
333:1026–1030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Solomon DA, Kim T, Diaz-Martinez LA, Fair
J, Elkahloun AG, Harris BT, Toretsky JA, Rosenberg SA, Shukla N,
Ladanyi M, et al: Mutational inactivation of STAG2 causes
aneuploidy in human cancer. Science. 333:1039–1043. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YF, Jiang R, Li JD, Zhang XY, Zhao
P, He M, Zhang HZ, Sun LP, Shi DL, Zhang GX, et al: SMC1A knockdown
induces growth suppression of human lung adenocarcinoma cells
through G1/S cell cycle phase arrest and apoptosis pathways in
vitro. Oncol Lett. 5:749–755. 2013.PubMed/NCBI
|
|
34
|
Hömme C, Krug U, Tidow N, Schulte B,
Kühler G, Serve H, Bürger H, Berdel WE, Dugas M, Heinecke A, et al:
Low SMC1A protein expression predicts poor survival in acute
myeloid leukemia. Oncol Rep. 24:47–56. 2010.PubMed/NCBI
|
|
35
|
Mannini L, Liu J, Krantz ID and Musio A:
Spectrum and consequences of SMC1A mutations: The unexpected
involvement of a core component of cohesin in human disease. Hum
Mutat. 31:5–10. 2010. View Article : Google Scholar
|
|
36
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
37
|
Stone KR, Mickey DD, Wunderli H, Mickey GH
and Paulson DF: Isolation of a human prostate carcinoma cell line
(DU 145). Int J Cancer. 21:274–281. 1978. View Article : Google Scholar : PubMed/NCBI
|