Introduction
Mesenchymal stem cells (MSCs) have received great
interest due to multipotential differentiation, immunomodulation
abilities and great potential for clinical application (1). MSCs have been used for tissue
regeneration, immuno-disease treatment, and in most cases with
favorable results. In recent years, the potential of using MSCs for
cancer management were investigated. However, the results were not
consistent. For example, adipose tissue-derived mesenchymal stem
cells (ASCs) can suppress the growth of human breast cancer cell
line MCF-7 via secreting IFN-β (2)
and human immortalized myelogenous leukemia line K562 by secreting
DKK-1 (3). However, ASCs can
promote human melanoma cell growth (4). Song et al showed that bone
marrow-derived MSCs (BMSCs) could inhibit leukemia/lymphoma cell
proliferation (5). However, Huang
et al reported that MSCs derived from bone marrow could
promote the growth of human colorectal cancer cells (6).
Why are the above results regarding MSCs treatment
different? Our deduction is that the different tissue origin of
MSCs and tumor cells may cause the different effects of MSCs on
tumor cells. To test our hypothesis, we designed the experiments by
using both MSCs and tumor cell-derived from the same region, the
oral cavity. Gingival tissue is easy to obtain during tooth
extraction or periodontal treatments and normally the removed
gingival tissue is considered as biomedical waste (7,8).
Additionally, many reports have reported that MSCs can be isolated
from gingival tissue (hereafter called MSCs derived from normal
gingival tissue, GMSCs) (9).
Compared with ASCs and BMSCs, GMSCs have stable phenotype,
proliferate faster and are not tumorigenic (10,11).
Oral squamous cell carcinoma is a common cancer type in oral
cancer, which accounts for ~90% of oral cancer (12). Therefore, in this study, we used
two oral cancer cell lines (CAL27 and WSU-HN6) as our models to
investigate the effect of GMSCs on the proliferation of oral cancer
cells and the underlying mechanisms involved through the co-culture
of GMSCs/oral cancer cells, MTT [3-(4,
5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide], flow
cytometry, cytokine analysis, western blotting, pathway inhibition
assays and an animal study. Our data demonstrated that GMSCs could
suppress the growth of oral cancer cells in vitro and in
vivo, which indicated that GMSCs had a potential for the
management of oral epithelial dysplasia and oral cancer treatment
in the future.
Materials and methods
Cell lines
Human oral cancer cell lines CAL27 and WSU-HN6 were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco,
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco) and 1% penicillin-streptomycin in a humidified
incubator at 37°C with 5% CO2. Cells growing
exponentially (log phase) were used in the following
experiments.
Isolation of GMSCs
GMSCs were isolated and identified according to a
previous study (9,11). Briefly, normal gingival tissues
were obtained from three donors (no. 1: male, age 29; no. 2:
female, age 28; no. 3: female, age 30) with informed consent.
Samples were respectively cut to small size for tissue culture and
cultured in T25 flasks with α-minimum essential medium (α-MEM;
Gibco) containing 10% fetal bovine serum (FBS; Gibco) and 1%
penicillin-streptomycin (hereafter referred to as complete medium),
at 37°C in a humidified 5% CO2 atmosphere to generate
GMSCs. The cultured GMSCs at passage 3–7 were used for the
following experiments. The study was approved by the Ethics
Committee of the School of Stomatology, Peking University
(LA2014103).
Identification of GMSCs
GMSCs were incubated with either FITC-conjugated
human CD90, CD73, CD146, CD34, CD29 (Biolegend, San Diego, CA,
USA), or PE-conjugated human STRO-1, CD105 (BD Biosciences, San
Jose, CA, USA), CD45 antibody (Biolegend). Isotype-matched control
IgG or IgM (Southern Biotechnology Associates, Birmingham, AL, USA)
were used as controls. Flow cytometry was performed using Epics XL
(Beckman-Coulter Inc., Fullerton, CA, USA).
The osteogenic and adipogenic differentiations of
GMSCs were analyzed through the osteogenic and adipogenic induction
media described previously (13).
For osteogenic assay, GMSCs were cultured in α-MEM medium
supplemented with 10 nM dexamethasone, 10 mM β-glycerophosphate,
0.1 mM L-ascorbic acid-2-phosphate, and 2 mM gluta-mine
(Sigma-Aldrich, St. Louis, MO, USA) for 28 days. Mineralization
capacities of GMSCs were detected by alizarin red S (pH 4.2;
Sigma-Aldrich) staining. For adipogenic assay, GMSCs were cultured
in α-MEM supplemented with 1 μM dexamethasone, 0.5 mM
3-isobutyl-ethylxanthine, 10 mg/ml insulin, 60 mM indomethacin, and
2 mM glutamine for 21 days. Adipogenic abilities were detected by
lipid droplets by oil red O (Sigma-Aldrich).
GMSC/oral cancer cell direct contact
co-culture assay
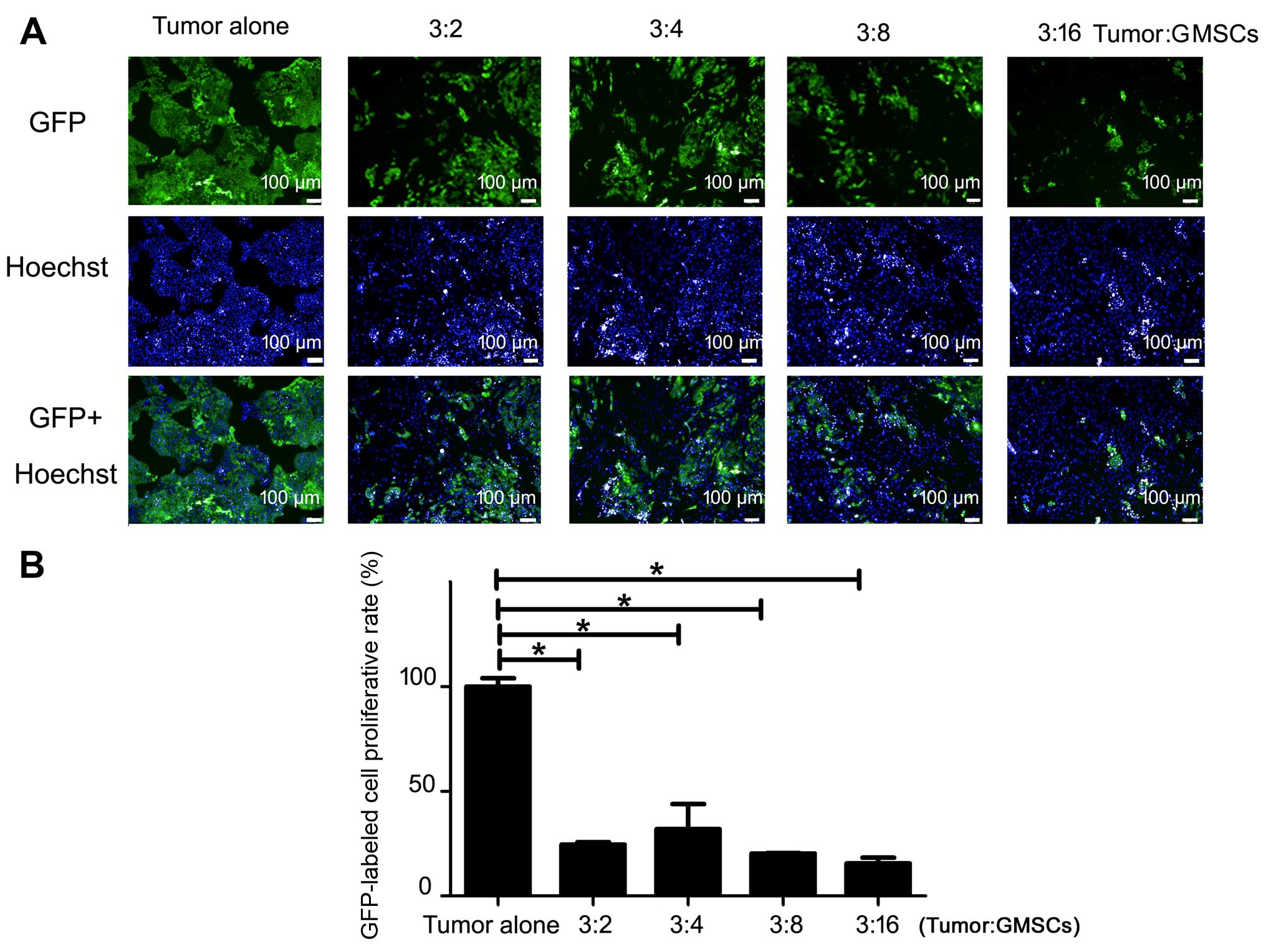
To investigate the effect of GMSCs on the growth of
oral cancer cell lines, we used green fluorescent protein
(GFP)-labeled oral cancer cells. To generate green fluorescent
protein (GFP)-labeled oral cancer cells, CAL27 and WSU-HN6 were
infected with retrovirus carrying GFP expression cassette for 2
days to ensure that GFP was expressed in all the cancer cells. Then
we co-cultured GMSCs-GFP-labeled oral cancer cells in a cell-cell
direct contact manner. At the indicated time points, the cell
morphology was photographed and cell proliferation was evaluated by
MTT assay at the indicated time points. Briefly, tumor cells
(1.5×103) were plated in triplicate in a 96-well plate
in a cell-cell direct contact manner with 0.1 ml complete medium
containing 2×103, 4×103, 8×103,
and 1.6×104 GMSCs (the ratio of tumor:GMSCs was 3:2,
3:4, 3:8 and 3:16 (here referred to as 3:2, 3:4, 3:8 and 3:16,
respectively), for the indicated times. The cell viability was
measured using MTT [3-(4,
5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.
The co-culture of GMSCs and non-GFP labeled CAL-27/WSU-HN6 was used
to confirm the co-culture results of GMSCs and GFP labeled
CAL-27/WSU-HN6. The effect of co-culture of GMSC-tumor cells was
determined by the ratio of OD570 (tumor cells and GMSCs
co-culture)/[OD570 (tumor cells alone) +
OD570 (GMSCs alone)]. Tumor cells alone served as
controls.
Transwell co-culture assay
Oral cancer cells and GMSCs were maintained at the
above ratio (the ratio of tumor:GMSCs referred to as 3:2, 3:4, 3:8
and 3:16) were plated in a 24-well plate at the same time. GMSCs
were seeded in the inserts with a pore size of 24-well Transwell
plate (Corning, Tewksbury, MA, USA). Tumor cells were plated in the
lower Transwell chambers. GMSCs and tumor cells were in the same
co-culture system via porous Transwell membrane. The cell viability
was measured using MTT assay at the indicated time points. All
experiments were in triplicate and repeated at least 2 times.
Generation of conditioned medium from
GMSCs
GMSCs were plated at the above ratio of GMSCs and
complete medium in a 10-cm plate for the indicated times to
generate conditioned media (equivalent to the ratio of tumor:GMSCs
3:2, 3:4, 3:8 and 3:16, respectively). The conditioned media were
passed through 0.2 μm filter and stored at −80°C until use.
Conditioned medium assay
Tumor cells were plated in triplicate in a 96-well
plate with the indicated conditioned medium derived from GMSCs. At
the indicated time points, MTT assay was performed to determine
cell viability. The growth rate was calculated with the following
formula: growth rate (%) = OD570 (Test)/OD570
(Ctrl) × 100%.
Serum exhausted exclusive assay
To eliminate the effect of consumption of serum in
conditioned medium, additional fresh serum (0, 2, 5 and 10%, was
added into the conditioned medium (the ratio of tumor:GMSCs was
3:16). The modified conditioned medium was used to treat tumor
cells for indicate times, and cell viability was carried out using
MTT assay.
Detection of apoptosis by flow
cytometry
Conditioned medium was harvested from GMSCs in a
10-cm plate cultured (equivalent to the ratio of tumor:GMSCs was
3:4) for 5 days. CAL27 and WSU-HN6 cells were treated for 48 h with
above conditioned medium. Then the cells were labeled with Annexin
V-FITC/PI staining according to the manufacturer's instructions
(Beyotime, Shanghai, China), and subjected to do apoptotic analysis
by flow cytometry (Beckman-Coulter Inc., Brea, CA, USA).
Determination of cytokine concentration
in conditioned medium by cytokine array
Conditioned medium was prepared as described above,
and then harvested from GMSCs (equivalent to the ratio of
tumor:GMSCs was 3:4) at day 5. Then the media were used to detect
multiple cytokine expression levels that allows for a detection of
9 different cytokines, respectively (Millipore Corp., Billerica,
MA, USA) according to the manufacturer's protocol. Measurements
were performed on Luminex 100 System (Luminex Corp., Austin, TX,
USA).
Effects of reconstruction of conditioned
medium on oral cancer cell growth
Based on the cytokine array results, the same
concentration of multiple cytokines were chosen to mix together in
various combinations to mimic the conditioned medium released from
GMSCs. Then the reconstructed conditioned media were used to treat
tumor cells, cell viability was detected by MTT assay as described
above.
Westernblotting
CAL27 and WSU-HN6 were treated with 5-day
conditioned media (equivalent to the ratio of tumor:GMSCs was 3:4)
for 24 or 48 h. The cells were harvested for protein extraction at
indicated time points. Protein concentration was tested using the
BCA protein assay (Thermo Fisher Scientific). Equal amount of
proteins were separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis, and then transferred to
polyvinylidene difluoride membranes. The membranes were blocked in
5% non-fat dry milk for 1 h and probed with antibodies against Bax,
Bcl-2, cleaved caspase-3, PARP, phosphorylated (p)-STAT3, total
(t)-STAT3, p-ERK1/2, t-ERK1/2, t-JNK, cyclin D1, CDK4, survivin,
PCNA (Cell Signaling Technology, Danvers, MA, USA), p-JNK (Abcam,
Cambridge, MA, USA), and GAPDH (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), respectively at 4°C, overnight. On the following
day, the protein blots were incubated with HRP-linked secondary
antibody. Immunoreactive proteins were visualized by enhanced
chemiluminescence (ECL) reagent (Applygen Technology, Beijing,
China).
Pathway inhibition assay
Tumor cells were pre-treated with JNK inhibitor
SP600125 (10 nM) (Selleck, Houston, TX, USA) for 1 h, then the
medium was replaced by conditioned medium with 10 nM JNK inhibitor
SP600125 at the indicated times for MTT and western blot
assays.
Animal experiment
This study was approved by Medical Ethics Committee
of the Peking University Health Center (LA2014-103). Six-week-old
male BALB/c nude mice (Vital River Laboratory Animal Technology
Co., Ltd., Beijing, China) were divided into two groups: control
group, 2×106 CAL27 in 100 μl PBS were injected
subcutaneously (s.c.) at the dorsal back of nude mice; treatment
group: 2×106 CAL27 and 1×106 GMSCs in 100 μl
PBS were co-injected s.c. at the dorsal of nude mice. The tumor
size was measured every 4 days. Tumor size was measured by caliper,
and tumor volume was calculated according to formula: volume =
length × width2/2.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). One-way ANOVA was used to evaluate the difference among
groups. Significance was defined as a p-value of <0.05.
Results
Identification of GMSCs
We successfully generated GMSCs from normal gingival
tissue. To identify the characteristics of GMSCs, we used FITC- and
PE-conjugated MSC biomarker antibodies to label GMSCs, and followed
by flow cytometry assay. The results showed that GMSCs were
positive for CD105, CD90, CD73, CD146, CD29 and STRO-1 and negative
for CD34 and CD45 (Fig. 1A), which
were consistent with previous studies (14,15).
To evaluate the multilineage differentiation abilities of normal
GMSCs, osteogenic and adipogenic capacities were detected. Using
osteoinductive condition, GMSCs formed mineralized nodules
(Fig. 1B). Under adiogenic
induction, GMSCs formed oil droplets (Fig. 1B). These properties of GMSCs are in
line with the characteristics of MSCs (14,15).
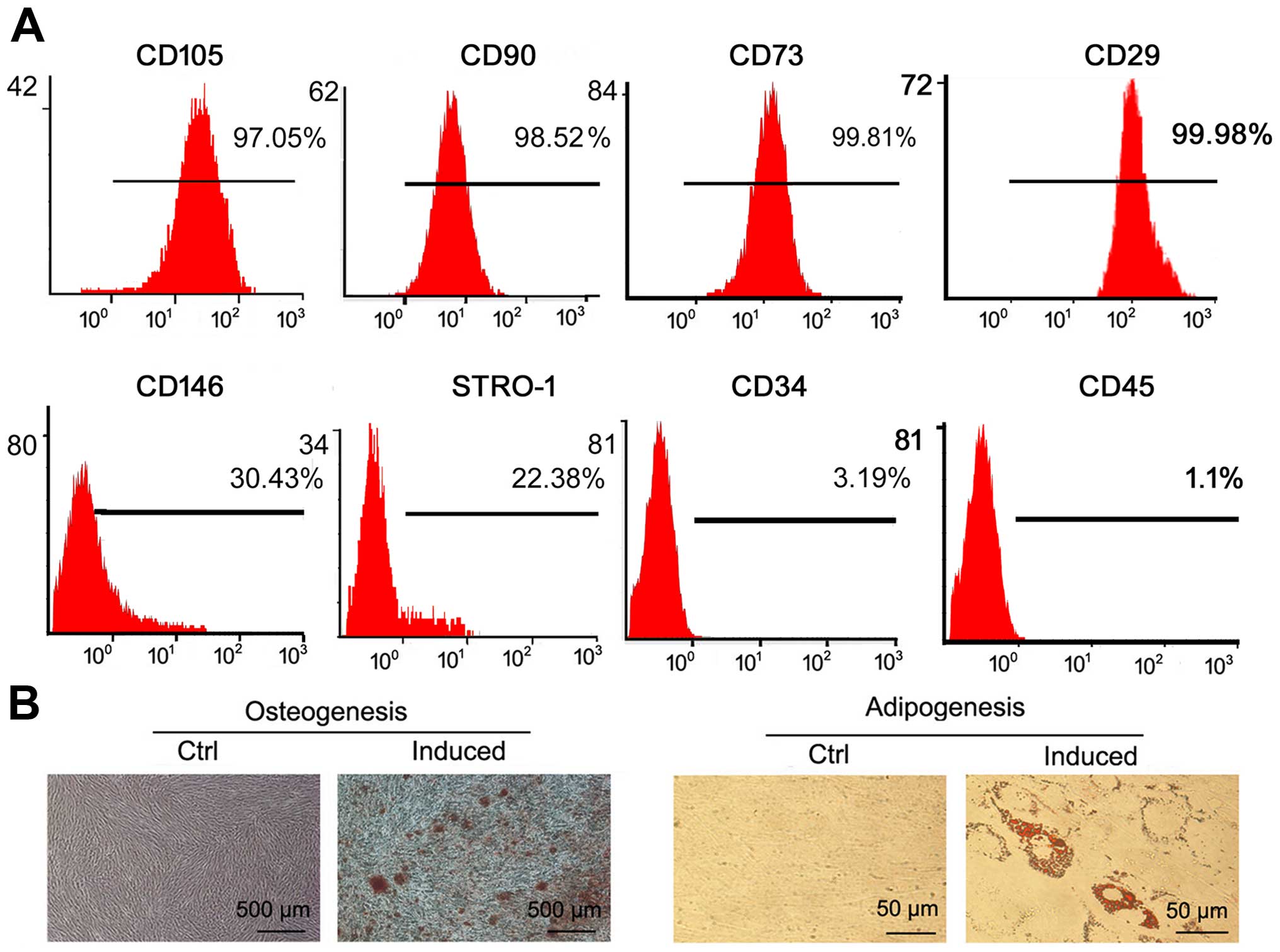 | Figure 1Identification of GMSCs. (A)
Detection of the MSC surface biomarkers (CD105, CD90, CD73, CD146,
STRO-1, CD29, CD34, CD45) in GMSCs by flow cytometry analysis. (B)
Osteogenesis (bar, 500 μm) and adipogenesis (bar, 50 μm) of GMSCs.
Under a microscope, no mineralized nodule or lipid droplet was
found in the corresponding control groups, respectively. |
GMSCs inhibit oral cancer cell
growth
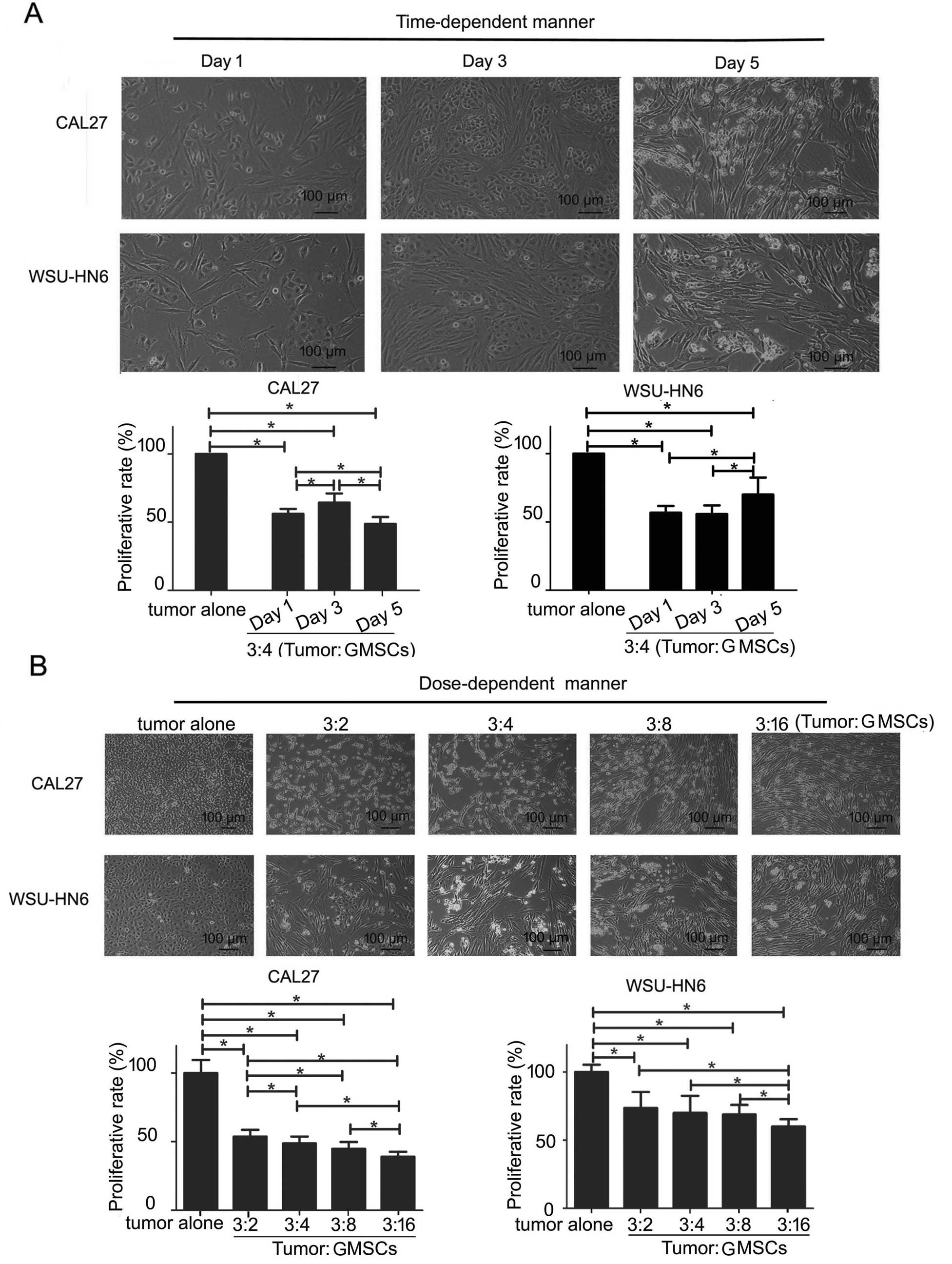
In a time-course assay, when CAL27 and WSU-HN6 were
treated with GMSCs for 5 days, the morphology of the two tumor
cells did not change obviously at days 1 and 3, with a huge changed
at day 5. Most tumor cells were suspended and shrank at day 5
(Fig. 2). MTT assay showed that
GMSCs could cause a decrease in a number of GMSCs-CAL27 (WSU-HN6)
co-culture at days 1, 3 and 5 (p<0.05, Fig. 2A). On day 5, the inhibitory effect
of GMSCs was in a dose-course (p<0.05, Fig. 2B). The total living cell number of
GMSCs-CAL27 and GMSCs-WSU-HN6 co-culture was even lower than of the
number in tumor alone group. Compared with tumor alone, at any dose
of GMSCs exerted inhibitory proliferative effect on CAL27 at day 5
(p<0.05, Fig. 3).
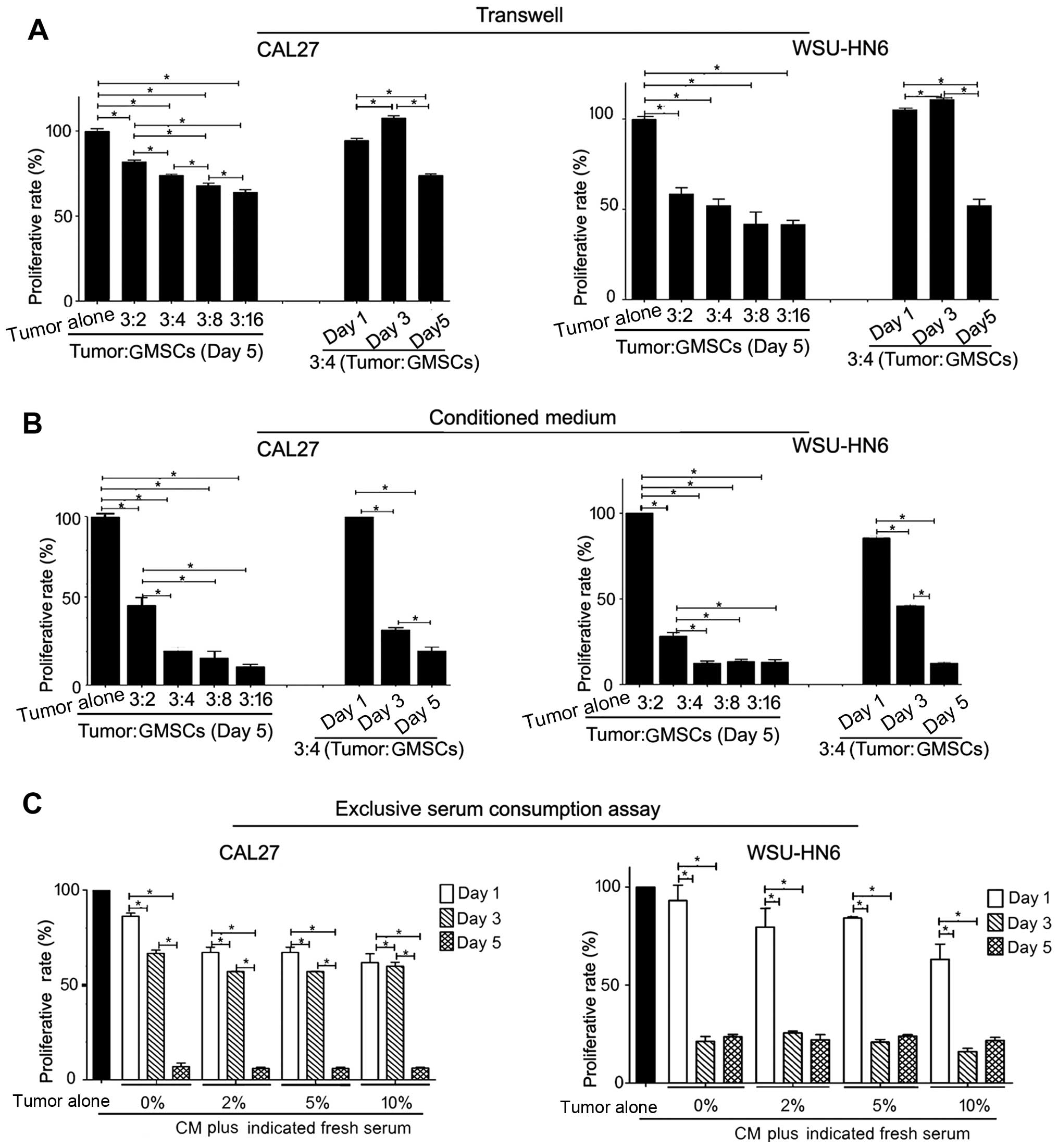
To understand whether cell-cell direct contact can
affect the growth inhibitory effect of GMSCs, we performed tumor
cell-GMSC indirect co-culture via Transwell system. Similar to the
results of 5-day direct co-culture, GMSCs dose-dependently showed
significant inhibitory effect compared with tumor alone (p<0.05,
Fig. 4A). CAL27 and WSU-HN6
exhibited a decrease in the number of cells (p<0.05, Fig. 4A). The results indicated that
soluble factors released from GMSCs played an important role in the
GMSC-mediated oral cancer cell proliferation.
To confirm this opinion, we generated conditioned
medium, and used it to treat tumor cells. Similar to the results of
direct co-culture and indirect co-culture via Transwell assay,
conditioned medium from GMSCs could significantly suppress the
growth of CAL27 and WSU-HN6 dose- and time-dependently (p<0.05).
Furthermore, conditioned medium of GMSCs showed greater inhibitory
effects on tumor cell lines than GMSCs in direct and indirect
co-culture systems (Fig. 4B). In
addition, we found an apparent discrepancy in the number of tumor
cells at day 3 between Transwell and conditioned medium (Fig. 4A and B).
To eliminate the effect of serum consumption during
generation of conditioned medium from GMSCs, we added extra fresh
serum (0, 2, 5 and 10%) to conditioned medium (equivalent to the
ratio of tumor:GMSCs was 3:16). The freshly-made conditioned media
was used to evaluate the growth inhibitory effect of GMSCs
(p<0.05). Similarly, serum concentration did not increase with
the growth of tumor cells (Fig.
4C). These results raised the possibility that the
anti-proliferation effect of GMSCs was not due to the serum
deprivation.
Conditioned medium from GMSCs induces
apoptosis of oral cancer cell lines
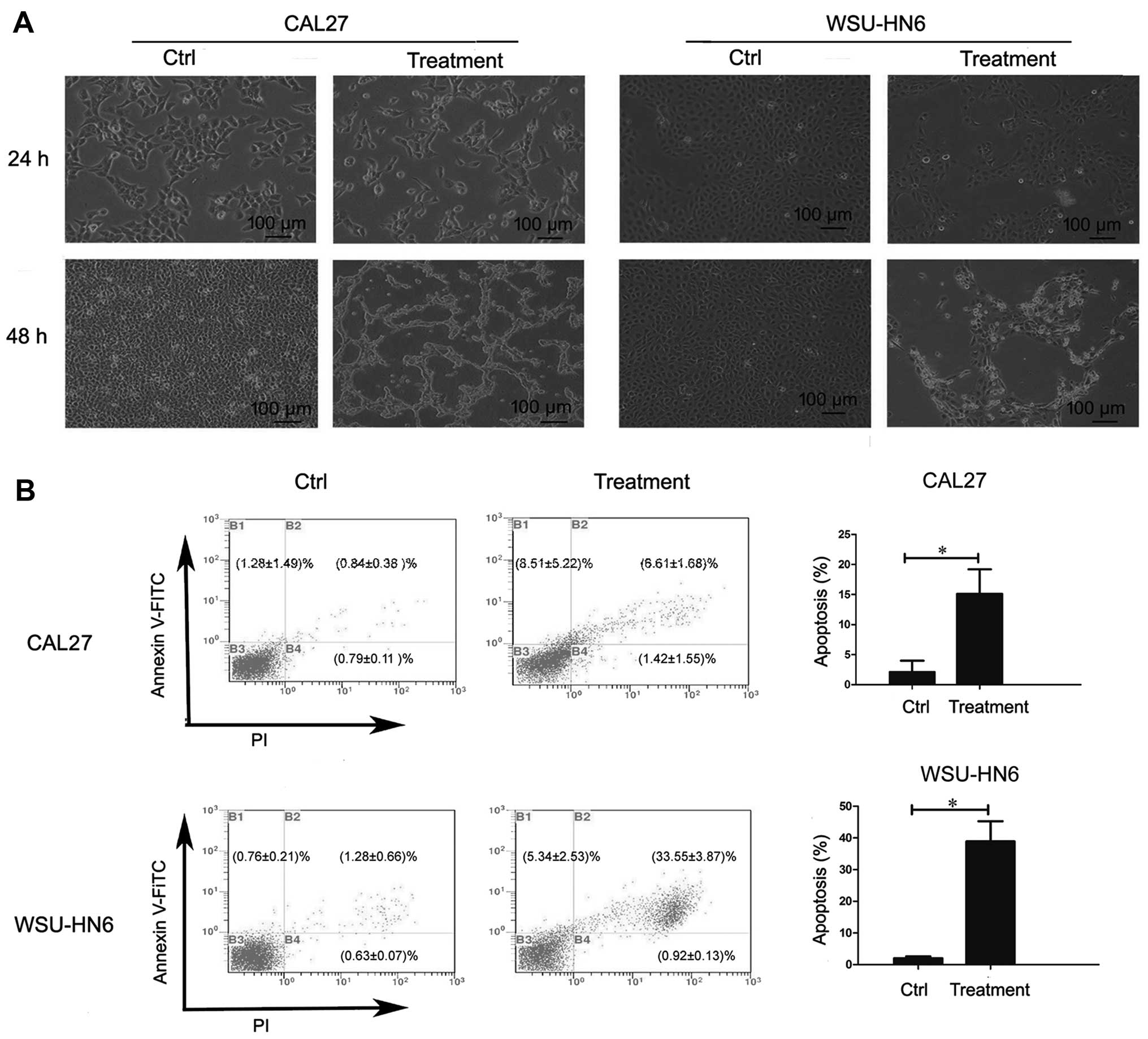
To investigate whether GMSCs inhibit the
proliferation of oral cancer cell lines through apoptosis, we
evaluated the effect of conditioned medium on oral cancer cells. At
24 and 48 h post-treatment by 5-day conditioned medium (equivalent
to the ratio of tumor:GMSCs was 3:4), CAL27 and WSU-HN6 were
harvested for morphology evaluation and apoptosis assay. As shown
in Fig. 5A, morphology checked by
a microscope displayed that the numbers of tumor cells were
partially suspended, and shrunken 48 h post-treatment. The results
indicated that tumor cells may have undergone apoptosis. Therefore,
apoptosis assay was performed, and the results showed that the
percentage of apoptotic cells [early apoptotic (Annexin
V-FITC+/PI−) + late apoptotic cells (Annexin
V-FITC+/PI+) cells)] increased from 2.12±1.87
(Ctrl) to 15.12±4.07% (treatment, p<0.05) in CAL27, and from
2.04±0.58 (Ctrl) to 38.90±6.53% (treatment, p<0.05) in WSU-HN6
(Fig. 5B).
Detection of the concentration of
cytokines in conditioned medium of GMSCs
To determine the concentration of cytokines in
conditioned medium of GMSCs, we carried out custom cytokine array.
Results showed that, among these cytokines, only IL-6, IL-8 and
GM-CSF's concentration was in the range of their biological
activities (Table I). Based on
previous studies, concentration of IFN-γ, which was out of the
range of biological activities, indicated that IFN-γ might not play
an important role in tumor cell proliferation or play a synergic
effect with other cytokines (4,16).
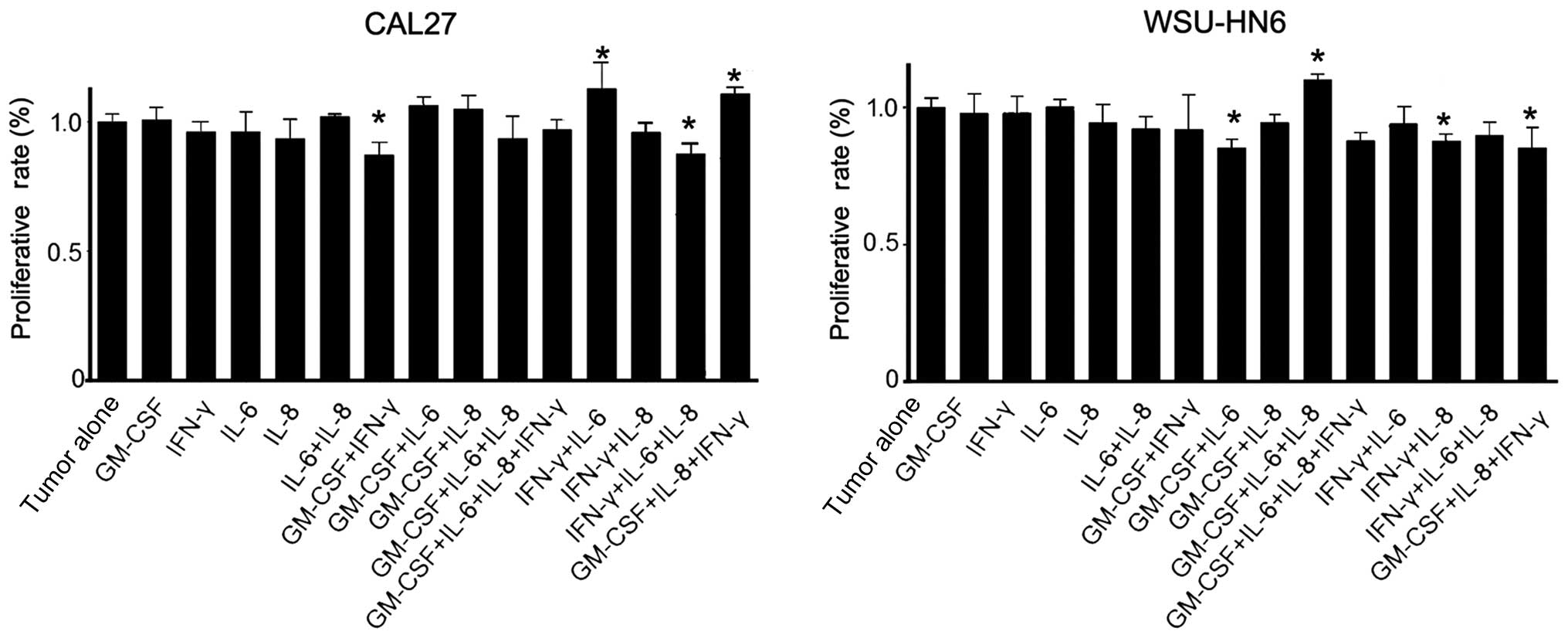
Therefore, we chose IL-6, IL-8, GM-CSF and IFN-γ to reconstruct the
artificial conditioned medium, and then treated the tumor cells
with the artificial conditioned medium. The results showed that
some cytokine combinations (GM-CSF+IFN-γ and IFN-γ+IL-6+IL-8 in
CAL27 groups and GM-CSF+IL-6, IFN-γ+IL-8 and GM-CSF+IL-8+IFN-γ in
WSU-HN6 groups) exhibited a significant anti-proliferative effect
on CAL27 or WSU-HN6 compared with corresponding tumor alone
(p<0.05). In addition, IFN-γ+IL-8+GM-CSF-, IFN-γ+IL-6-treated
CAL27 or GM-CSF+IL-6+IL-8-treated WSU-HN6 showed slightly
pro-proliferative effects (p<0.05, Fig. 6). However, compared with the
inhibitory effect of GMSCs or GMSCs-CM on the growth of the two
oral cancer cell lines, the constructed cytokine mixture only
exerted a very slight anti-proliferation effect on oral cancer
cells. The results indicated that there may be unknown soluble
factors, which inhibit the growth of oral cancer cell lines in the
conditioned medium from GMSCs.
 | Table ICytokine concentration in the
conditioned medium of GMSCs (pg/ml). |
Table I
Cytokine concentration in the
conditioned medium of GMSCs (pg/ml).
| Cytokine | Concentration |
|---|
| GM-CSF | 850.55 |
| IFN-γ | 11.56 |
| IL-10 | 11.9 |
| IL-2 | 9.72 |
| IL-4 | 15.2 |
| IL-6 | 11,275 |
| IL-8 | 3,491 |
| CCL-2 | 12,750 |
| TNF-α | 15.6 |
| VEGF-A | 178.89 |
GMSCs downregulate the
proliferation-related gene expression and upregulate
apoptosis-related gene expression
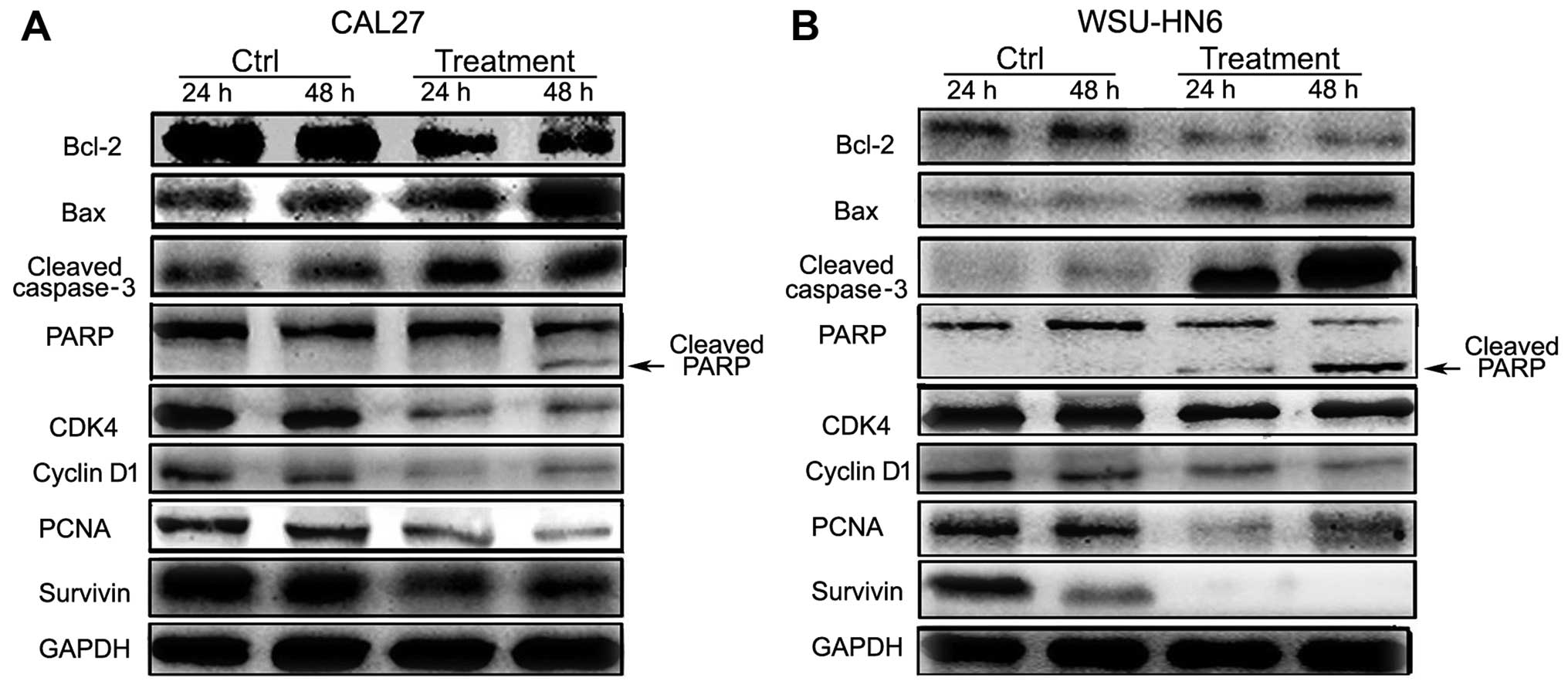
To assess the molecular changes regarding
proliferation and apoptosis during the process of GMSCs inhibiting
the growth of tumor cells, CAL27 and WSU-HN6 were treated with
conditioned medium (equivalent to the ratio of tumor:GMSCs referred
to as 3:4) for 24 and 48 h, the cells were examined by western
blotting for detection of Bcl-2, Bax, cleaved caspase-3, PARP,
CDK4, cyclin D1, PCNA and survivin. The results showed that a
decrease in Bcl-2 and a gradual increase in Bax, cleaved caspase-3
and cleaved PARP in CAL27 and WSU-HN6 from 24 to 48 h (Fig. 7). The expression of positive cell
proliferative markers, such as CDK4, cyclin D1, PCNA and survivin
were markedly downregulated compared to control groups (Fig. 7).
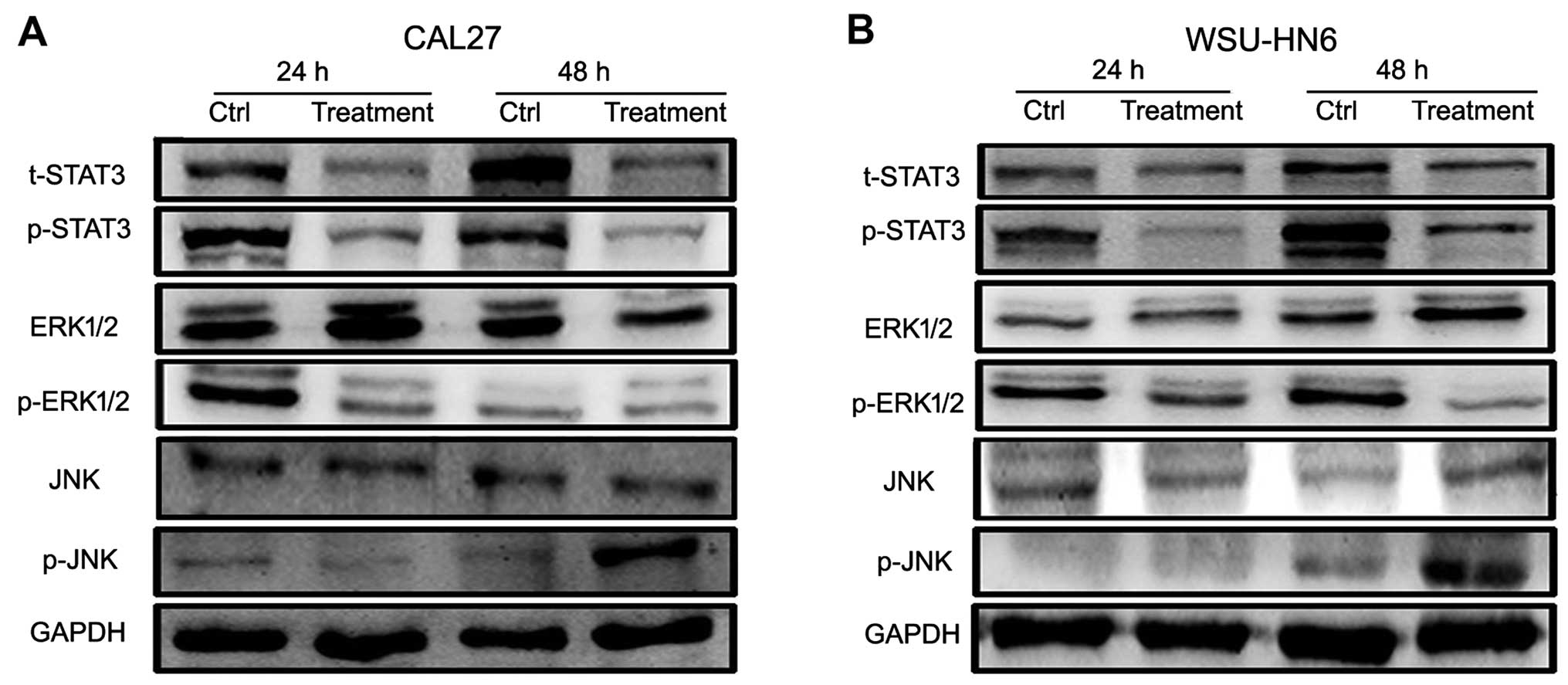
To determine which pathway is involved in the
process of conditioned medium of GMSC-induced oral cancer cell
growth inhibition, we detected proliferation-associated pathway
including STAT3, JNK, ERK pathways by western blotting. We found
that GMSCs-CM could activate the JNK pathway through
phosphorylation of JNK (p-JNK), and inactivate ERK and STAT3
pathways through a decrease in p-ERK1/2 and p-STAT3 (Fig. 8).
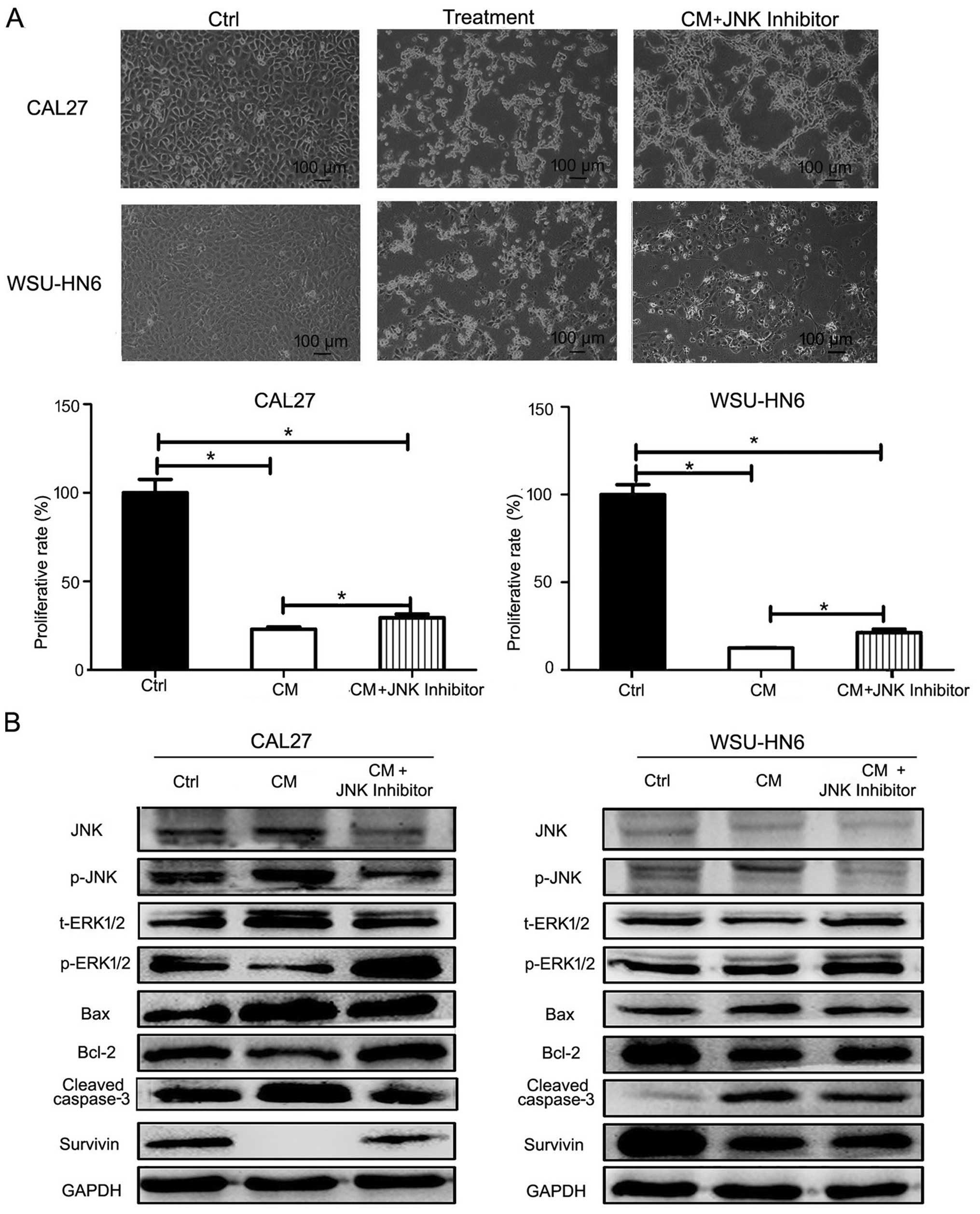
JNK inhibitor blocks the growth
inhibitory effect of GMSCs on oral cancer cells
To find whether pathway is involved in the process
of GMSCs inhibiting the growth of tumor cells, we performed pathway
inhibition assay. CAL27 or WSU-HN6 were pretreated with JNK
inhibitor SP600125 for 1 h, then treatment was continued with
conditioned medium containing SP600125. At 24 h post-treatment,
more apoptotic cells were found in conditioned medium group than
SP600125 plus conditioned medium group by microscopy (Fig. 9A). MTT results showed that less
viable cells were found in conditioned medium group than SP600125
plus conditioned medium group (Fig.
9A). Moreover, western blotting showed that SP600125 partly
abrogated the expression of p-JNK, increased p-ERK1/2, Bcl-2,
survivin expression and decreased Bax, cleaved caspase-3 expression
(Fig. 9B).
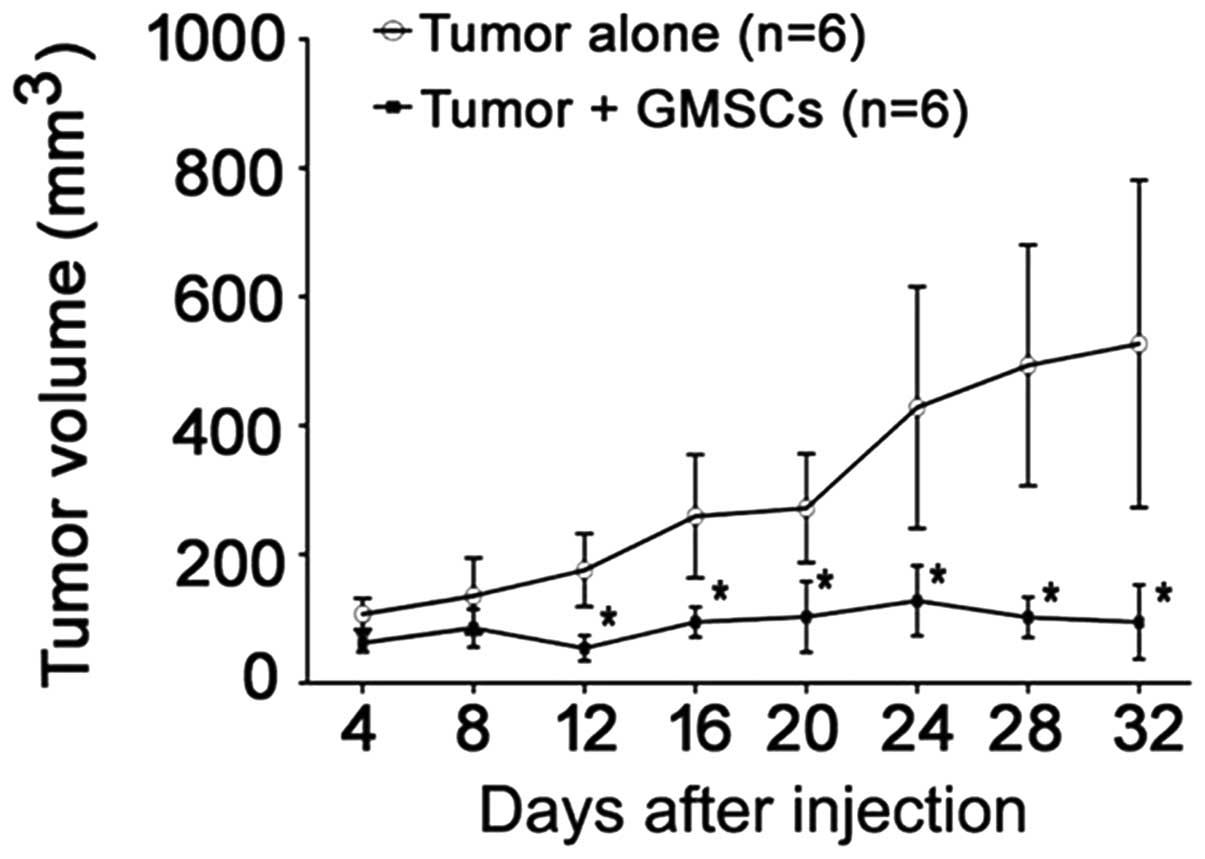
GMSCs inhibit oral cancer cell growth in
vivo
To investigate whether GMSCs have a similar growth
inhibitory effect on oral cancer cells in vivo, we carried
out an animal study. GMSCs and CAL27 were co-injected in nude mice.
The volume of tumor was recorded every 4 days. We found that the
volume of tumor in CAL27 plus GMSCs group was significantly smaller
that in CAL27 alone group (p<0.05, Fig. 10). It suggested that GMSCs were
able to inhibit the growth of CAL27 in vivo.
Discussion
In tumorigenesis, mutated epithelial cells interact
with surrounding stromal cells participating in the establishment
of tumor microenvironment, which is critically important for tumor
development (17). Actually,
unlike cancer cells, stromal cells in the tumor microenvironment
are genetically stable and may be an attractive therapeutic target
(18). As the precursor of most
stromal cells in tumor microenvironment, MSCs gained extensive
attention when evidence suggested tumor-associated MSCs present a
distinct phenotype compared to MSCs derived from normal tissue
(19). In adult tissue, the
microenvironment protects the slow-cycling, self-renewal potential
and undifferentiated state (11,20).
In tumor microenvironment, various inflammatory cytokines,
chemokines, and growth factors can be secreted by tumor-associated
MSCs, which can form the inflammatory environment and promote tumor
progression (21). Many studies
have shown that normal tissue-derived MSCs are capable of
remodeling tumor microenvironment, rather than only target cancer
cells. In our study, we firstly isolated MSCs from normal gingival
tissues, and identified that GMSCs have heterogeneity to
STRO-1+/CD90+/CD105+/CD146+/CD73+/CD29+
and CD34−/CD45− MSCs, and have osteogenic and
adipogenic capacities. The result is consistent with a previous
study (13).
Next, we investigated the effect of GMSCs on oral
cancer cell proliferation. To our surprise, by MTT and apoptosis
assays, we found that GMSCs had strong growth inhibition in the two
tumor cells not only by cell-cell direct contact, but also by
cell-cell indirect contact via Transwell system. Our findings are
consistent with some reports (22). Other contrary reports showed that
MSCs could enhance the growth of several different tumors. The
reason for this discrepancy is unknown, but it may be associated
with the tropism of MSCs and differences in the tumor model, the
dose or timing of MSCs applied, or other unknown reasons (23). So how to choose the source of MSCs
to be applied to different tumors still needs to be explored. Our
results indicate that using MSCs derived from the same tissue/organ
region as the tumor cells can be an appropriate choice for cancer
treatment. Our rationale is that normal MSCs can maintain the
homeostasis of epithelial tissue. Once tumor occurs, homeostasis is
broken, normal MSCs surrounding tumor may become tumor-associated
fibroblasts, which normal tissue microenvironment also changes to
tumor microenvironment. Therefore, using normal MSCs from the same
tissue/organ origin of tumor to treat tumor perhaps corrects the
altered microenvironment by tumor, and exerts an anticancer
effects.
In this study, we found that soluble factors
secreted by GMSCs play an important role in GMSC-induced oral
cancer growth inhibition. To confirm this result, we generated
conditioned medium from GMSCs and found that conditioned medium had
stronger growth inhibition than the above described cell-cell
co-culture. We suspect that the stronger inhibition growth of
conditioned medium may be associated with an insufficient amount of
inhibitory cytokines at days 1 and 3 in a cell-cell co-culture and
Transwell. Furthermore, this effect is dose- and time-dependent,
and regardless of serum concentration in the conditioned medium.
Our explanation is that at the beginning of cell-cell co-culture,
the quantity of the soluble factors released from GMSCs is very low
and not enough to inhibit oral cancer cell growth, even in turn
promotes oral cancer cell growth, just like the day 3 data in
co-culture system in this study. Along with the time extension,
GMSCs secrete more and more soluble factors and gradually exert
inhibitory effect on oral cancer cells (2,22,24).
To further explore which factors play the main role
in GMSC-induced oral cancer cell growth inhibition, we performed a
custom cytokine array and found that IL-6, IL-8, GM-SCF in
conditional medium of GMSCs was in the range of their biological
activities. Our results were in line with some reports. Motaln
et al (16) reported that
IL-6, IL-8, CCL2 are detected in hMSCs/glioblastoma multiforme
co-culture medium known to inhibit the growth of tumors. Kucerova
et al (4) showed that
inhibition of glioblastoma multiforme growth may be mediated by
high cytokine levels produced by adipose-derived MSCs, in
particular high levels of interleukins (IL-1b, IL-1ra, IL-2, IL-4,
IL-6, IL-8) combined with high levels of IFN-γ and G-CSF. By
construction of the artificial conditioned media with the same
quantity of the above differentially expressed factors in the
conditioned medium, we did not find similar anti-proliferation
effect as GMSCs or GMSCs-CM in this study. The results indicate
that there must be unknown soluble proteins, exosome or other
unknown factor released from GMSCs, which are the main
contributor(s) for GMSC-induced oral cancer cell growth
inhibition.
Although we did not find the unknown main soluble
factors, we found the pathway by which GMSCs exert the inhibitory
growth effect of oral cancer cell lines. We carried out western
blotting to detect the proteins including Bcl-2 (25), p-STAT3 (26), survivin (27), proliferation (ERK1/2) (28) and apoptosis [Bax (25), cleaved caspase-3 (29), JNK (30)] which are associated with
proliferation, survival or apoptosis. The protein expression is in
line with the observed phenomena in this study as well as the
function of sthese proteins. Furthermore, by pathway inhibition
assay, we found that JNK is involved in GMSC-induced oral cancer
cell growth inhibition, JNK activity is important in the regulation
of tumor cell proliferation. JNK inhibitor reversed the
pro-apoptotic ability mediated by GMSCs through downregulation of
Bax, cleaved caspase-3 and p-JNK, and upregulation of survivin and
p-ERK1/2. It is suggested that modulation of JNK can promote the
key factors in inducing apoptosis via Bax and Bcl-2. Ryu et
al also reported that ASCs suppressed tumor growth via
JAK1/JAK2 pathway (2), so it can
be explained that GMSCs inhibit the growth of oral cancer cells not
only by JNK pathway, but also other pathways.
To further confirm the growth inhibitory effect of
GMSCs on oral cancer cells, we performed an animal study. Our
results clearly showed that GMSCs were able to inhibit the growth
of CAL27 in nude mice. Therefore, we deduce that GMSCs have a
promising clinical potential in the management of oral cancer or
oral epithelial dysplasia such as oral leukoplakia.
In conclusion, our results suggest that GMSCs can
suppress oral cancer cell growth by activation of JNK signaling
pathway. Unknown soluble factors released from GMSCs play a key
role in GMSC-induced oral cancer cell growth inhibition. Further
study is required to find which non-specified paracrine factor
inhibits the proliferation of oral cancer cells and how to apply
the potential of GMSC-mediated anticancer proliferation effect in
clinic.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81441034 and 81172556) and
Foundation of Capital Health Development (2014-2-4102 and
2011-4025-02).
References
|
1
|
Bernardo ME and Fibbe WE: Mesenchymal
stromal cells: Sensors and switchers of inflammation. Cell Stem
Cell. 13:392–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryu H, Oh JE, Rhee KJ, Baik SK, Kim J,
Kang SJ, Sohn JH, Choi E, Shin HC, Kim YM, et al: Adipose
tissue-derived mesenchymal stem cells cultured at high density
express IFN-β and suppress the growth of MCF-7 human breast cancer
cells. Cancer Lett. 352:220–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian
C, Li J, Yan X, Liu Y, Shao C, et al: Human mesenchymal stem cells
inhibit cancer cell proliferation by secreting DKK-1. Leukemia.
23:925–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kucerova L, Matuskova M, Hlubinova K,
Altanerova V and Altaner C: Tumor cell behaviour modulation by
mesenchymal stromal cells. Mol Cancer. 9:1292010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song N, Gao L, Qiu H, Huang C, Cheng H,
Zhou H, Lv S, Chen L and Wang J: Mouse bone marrow-derived
mesenchymal stem cells inhibit leukemia/lymphoma cell proliferation
in vitro and in a mouse model of allogeneic bone marrow transplant.
Int J Mol Med. 36:139–149. 2015.PubMed/NCBI
|
|
6
|
Huang WH, Chang MC, Tsai KS, Hung MC, Chen
HL and Hung SC: Mesenchymal stem cells promote growth and
angiogenesis of tumors in mice. Oncogene. 32:4343–4354. 2013.
View Article : Google Scholar
|
|
7
|
Egusa H, Okita K, Kayashima H, Yu G,
Fukuyasu S, Saeki M, Matsumoto T, Yamanaka S and Yatani H: Gingival
fibroblasts as a promising source of induced pluripotent stem
cells. PLoS One. 5:e127432010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang
J, Xu GT, Liang A and Liu S: Concise reviews: Characteristics and
potential applications of human dental tissue-derived mesenchymal
stem cells. Stem Cells. 33:627–638. 2015. View Article : Google Scholar
|
|
9
|
Xu X, Chen C, Akiyama K, Chai Y, Le AD,
Wang Z and Shi S: Gingivae contain neural-crest- and
mesoderm-derived mesenchymal stem cells. J Dent Res. 92:825–832.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomar GB, Srivastava RK, Gupta N,
Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC and Wani MR: Human
gingiva-derived mesenchymal stem cells are superior to bone
marrow-derived mesenchymal stem cells for cell therapy in
regenerative medicine. Biochem Biophys Res Commun. 393:377–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W,
Yang P and Pei X: Gingiva-derived mesenchymal stem cell-mediated
therapeutic approach for bone tissue regeneration. Stem Cells Dev.
20:2093–2102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petersen PE: Oral cancer prevention and
control - the approach of the World Health Organization. Oral
Oncol. 45:454–460. 2009. View Article : Google Scholar
|
|
13
|
Zhang Z, Han Y, Song J, Luo R, Jin X, Mu
D, Su S, Ji X, Ren YF and Liu H: Interferon-γ regulates the
function of mesenchymal stem cells from oral lichen planus via
indoleamine 2,3-dioxy-genase activity. J Oral Pathol Med. 44:15–27.
2015. View Article : Google Scholar
|
|
14
|
Hung BP, Hutton DL, Kozielski KL, Bishop
CJ, Naved B, Green JJ, Caplan AI, Gimble JM, Dorafshar AH and
Grayson WL: Platelet-derived growth factor BB enhances osteogenesis
of adipose-derived but not bone marrow-derived mesenchymal
stromal/stem cells. Stem Cells. 33:2773–2784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei M, Li K, Li B, Gao LN, Chen FM and Jin
Y: Mesenchymal stem cell characteristics of dental pulp and
periodontal ligament stem cells after in vivo transplantation.
Biomaterials. 35:6332–6343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motaln H, Gruden K, Hren M, Schichor C,
Primon M, Rotter A and Lah TT: Human mesenchymal stem cells exploit
the immune response mediating chemokines to impact the phenotype of
glioblastoma. Cell Transplant. 21:1529–1545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu R, Wei S, Chen J and Xu S: Mesenchymal
stem cells in lung cancer tumor microenvironment: Their biological
properties, influence on tumor growth and therapeutic implications.
Cancer Lett. 353:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urbanek K, Cesselli D, Rota M, Nascimbene
A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J,
et al: Stem cell niches in the adult mouse heart. Proc Natl Acad
Sci USA. 103:9226–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao L, Xu ZL, Zhao TJ, Ye LH and Zhang
XD: Dkk-1 secreted by mesenchymal stem cells inhibits growth of
breast cancer cells via depression of Wnt signalling. Cancer Lett.
269:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III : Concise review: Dissecting a discrepancy in the
literature: do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao
RC, Ye L and Zhang X: Suppression of tumorigenesis by human
mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Um HD: Bcl-2 family proteins as regulators
of cancer cell invasion and metastasis: A review focusing on
mitochondrial respiration and reactive oxygen species. Oncotarget.
7:5193–5203. 2016.
|
|
26
|
Su TH, Shiau CW, Jao P, Liu CH, Liu CJ,
Tai WT, Jeng YM, Yang HC, Tseng TC, Huang HP, et al: Sorafenib and
its derivative SC-1 exhibit antifibrotic effects through signal
transducer and activator of transcription 3 inhibition. Proc Natl
Acad Sci USA. 112:7243–7248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Liu D, Zhou Y, Li Y, Xie J, Lee RJ,
Cai Y and Teng L: Silencing of survivin expression leads to reduced
proliferation and cell cycle arrest in cancer cells. J Cancer.
6:1187–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheridan C, Brumatti G, Elgendy M, Brunet
M and Martin SJ: An ERK-dependent pathway to Noxa expression
regulates apoptosis by platinum-based chemotherapeutic drugs.
Oncogene. 29:6428–6441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wier EM, Fu K, Hodgson A, Sun X and Wan F:
Caspase-3 cleaved p65 fragment dampens NF-κB-mediated
anti-apoptotic transcription by interfering with the p65/RPS3
interaction. FEBS Lett. 589:3581–3587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bogoyevitch MA and Kobe B: Uses for JNK:
The many and varied substrates of the c-Jun N-terminal kinases.
Microbiol Mol Biol Rev. 70:1061–1095. 2006. View Article : Google Scholar : PubMed/NCBI
|