Introduction
In the last few years cancer treatments such as
chemotherapy, radiation therapy and surgery, as they are used in
human cancer treatment, became more important in veterinary
medicine. Conventional chemotherapeutic agents target dividing
cells, cancerous as well as non-neoplastic cells, causing several
side effects as myelosuppression, diarrhea, vomitus and anorexia
(1). Further, due to advanced
disease stage and resistance of prostate and bladder cancer,
treatment is difficult and often associated with poor prognosis
(2,3). Therefore, new alternatives which are
more effective have to be investigated.
Dichloroacetate (DCA), a small and cost-efficient
molecule, affects different metabolic pathways by inhibiting
pyruvate dehydrogenase kinase (PDK) (4,5).
This implicates that pyruvate dehydrogenase (PDH) is potentially
indirectly activated by DCA which yields a metabolic shift to favor
oxidation of pyruvate to acetyl-co-enzyme-A in mitochondria
(5). Despite this fact in the past
decades DCA has been used in the treatment of a multitude of
disorders like congenital lactate acidosis (6,7),
hypercholesterolemia (8),
hyperglycemia (9), congestive
heart failure (10) and only
recently in cancer research (11–16).
DCA was tested in different in vitro approaches in the field
of human oncology including colorectal cancer (17,18),
endometrial cancer (14), oral
squamous cell carcinoma (19) and
breast cancer (20). In a clinical
trial analyzing patients affected by glioblastoma and other solid
tumors, DCA decreased tumor growth and angiogenesis (15). With the exception of several
studies investigating pharmacokinetic effects of DCA in dogs
(21–23), there are currently no publications
on the effect of DCA in canine cancer. However, DCA was
successfully used in dogs with lactic acidosis (24) and is reported to be well tolerated
in dogs (22). Severe side effects
such as death and paralysis were observed only in a high-dose
long-term study (21).
Under aerobic conditions non-neoplastic cells refer
to glucose oxidation via mitochondria which oxidize pyruvate to
acetyl-co-enzyme-A (25). PDH
enables pyruvate to enter mitochondria. Energy production of cancer
cells is primarily shifted from glucose oxidation to aerobic
glycolysis which leads to increased cytosolic lactate production
despite the fact that enough oxygen is available (26). This behavior is referred to as
Warburg effect. The biochemist Otto Warburg first reported these
characteristics in 1926 and hypothesized that mitochondrial failure
could be the reason (26).
Carcinogenesis preferably sets on in hypoxic tissues where glucose
consumption is low. Accordingly, hypoxia inducible factor 1α is
activated and leads to an upregulation of glucose transporters and
PDK. Activation of PDK results in inhibition of PDH and thus in
glycolysis (27,28). Due to this metabolic change and the
decrease of mitochondrial depolarization, cancer cells have a
survival advantage and are not affected by intrinsic apoptosis
pathways (29,30).
For preclinical assessment of anticancer drugs in
vitro experiments with cell lines are important approaches in
human as well as in veterinary research. In vitro
investigations offer the possibility to receive more information on
efficaciousness and sensitivity of several tumor entities (31–33).
In this study several established (34) and new cell lines were used.
To our knowledge this is the first study in which
the effect of DCA on canine prostate adenocarcinoma and
transitional cell carcinoma (TCC) cells have been investigated. The
influence of DCA on cell counts, lactate levels, mitochondrial
activity, apoptosis and metabolic activity was determined. In
addition the effect of DCA on PDH and on apoptosis involved
proteins was evaluated. The effect of DCA on several microRNAs
(miR) has not been determined before. Further there is no
literature on the influence of DCA on bladder cancer in human or in
veterinary medicine.
Materials and methods
Cell lines and cell culture
Three canine prostate adenocarcinoma (DT08/46,
CT1258, DT15/08) and three canine TCC (DT08/40, DT15/06, DT15/09)
were used for experiments. Two TCC cell lines (DT08/40 and DT15/09)
derived from prostate tissue and one TCC (DT15/06) from female
bladder tissue. The cell lines were classified as prostate
adenocarcinoma or TCC after pathohistological examination of the
initial tissues. All cell lines were established in the Small
Animal Clinic, University of Veterinary Medicine Hannover, Germany.
The cell lines were cultured in 75 cm2 flask (TPP, Faust
Lab Science, Klettgau, Germany) with 10 ml medium 199 (Gibco™,
Thermo Fisher Scientific, Darmstadt, Germany), 10% fetal calf serum
(Hyclone®, Thermo Fisher Scientific), 2%
penicillin-streptomycin (Biochrom, Berlin, Germany), a medium
change was performed every 48 h. The cells were allowed to grow to
a density of 90% before splitting 1:3. The cells were incubated at
37°C and 5% CO2 in humidified air. For experiments the
cells were treated with 10 mM DCA (Sigma-Aldrich GmbH, Taufkirchen,
Germany) over 48 h. For cell splitting or after treatment period
the cells were trypsinized with TrypLE™ Express (Gibco™, Thermo
Fisher Scientific) and cell number was counted with an automated
Cellometer™ Auto T4 (Nexcelom Bioscience, Lawrence, MA, USA) and
compared with negative control. Cells were washed with PBS
(Biochrom) and stored at −80°C for further examinations
(quantitative RT-PCR and protein analysis). DCA was dissolved in
deionized water, filter-sterilized and pH was adjusted to 7.4 with
NaOH. The dosis of 10 mM was selected according to previous studies
with human HeLa cells (14). Even
if this concentration might not be safely reached in vivo,
this concentration was chosen to allow the comparability to other
human in vitro studies.
Lactate levels
For lactate level measurements supernatant from cell
culture was centrifuged at 1,000 rpm for 10 min to remove floating
cells and debris and 1.3 ml were transferred to a sodium fluoride
vessel (Sarstedt, Nümbrecht, Germany). To eliminate alterations due
to phenol red and lactate natively from fetal calf serum, medium
was used as negative control and deducted from measurements.
Colorimetric determination of lactate was performed with
Cobas® C311 (Hitachi, Tokyo, Japan). To relate the
assessed lactate levels to cell number and volume, the total
lactate content was normalized to intracellular protein
concentration which was determined with Pierce™ BCA assay (Thermo
Fisher Scientific) according to the manufacturer’s
instructions.
Metabolic activity
Cells were seeded in a 96-well plate (Falcon,
Corning, Amsterdam, The Netherlands) with 200 μl medium 199, 10%
fetal calf serum, 2% penicillin-streptomycin and incubated at 37°C
and 5% humidified CO2. Medium was changed every 24 h and
for measurement 20 μl MTT (CellTiter96® Aqueous One
Solution assay, Promega, Mannheim, Germany) was added into each
well. Absorbance was determined after 2 h with a Synergy2 plate
reader (BioTek, Bad Friedrichshall, Germany). Measurements were
performed every 24 h over a period of four days. Data was analyzed
with Gen5™ 1.11 Software (BioTek) and normalized to negative
control of medium.
Flow cytometry
For determination of apoptosis 105 cells
were cultured in a 6-well-plate (TPP, Faust Lab Science) with 4 ml
medium and treated with 10 mM DCA as described above for 48 h.
After this period, cells were trypsinized and centrifuged together
with medium containing non-adherent and dead cells at 1,000 rpm for
6 min. Supernatant was discarded and cells were resuspended in 500
μl assay buffer. Staining was performed with 5 μl Annexin-FITC and
1 μl Sytox (Annexin V-FITC Detection Kit Plus, PromoCell,
Heidelberg, Germany). After 5-min incubation at room temperature,
104 cells were analyzed with BD FACScalibur™ (BD
Biosciences, Heidelberg, Germany) and CellQuest™ Pro 6.0 software
(BD Biosciences). Annexin and Sytox were detected in FL-1. Data
analysis was performed with FlowJo Version 10.0.8r1 (FlowJo,
Ashland, OR, USA). Gates were set by mean of positive controls
(cells permeabilized with Saponin) and negative controls of each
cell line (non-treated viable cells).
RNA isolation and quantitative
RT-PCR
Total RNA was isolated from 106 cells
using the NucleoSpin Small RNA kit (Macherey Nagel, Düren, Germany)
as described in the manufacturer’s protocol. cDNA was prepared with
35 ng total RNA by reverse transcription using TaqMan®
MicroRNA Reverse Transkription kit (Applied Biosystems™, Thermo
Fisher Scientific) according to the manufacturer’s instructions.
Relative quantification of microRNA expression of treated cells in
comparison to negative control was performed with Eppendorf
realplex4 Cycler (Eppendorf, Wesseling-Berzdorf,
Germany) using 1.33 μl cDNA in a total volume of 20 μl containing
TaqMan® Universal Master Mix NoAmpErase® UNG
(Applied Biosystems, Thermo Fisher Scientific) and
TaqMan® MicroRNA assays for Mir-141 (ID 245445_mat),
Mir-145 (ID 002278), Mir-375 (ID 000564) purchased from Thermo
Fisher Scientific. Procedure was maintained as described in the
manufacturer’s protocol. Data was normalized to the housekeeping
gene RNU6B (ID 001093) and analysis was performed with Rest2009
(Qiagen, Hilden, Germany).
Luminex magnetic bead analysis
Protein expression analysis was performed with
xMAP® Luminex Bead Technology using a Luminex 200™
instrument (Luminex Corp., Hertogenbosch, The Netherlands). Data
are shown as total amount (survivin pg/ml) or net MFI (other
targets) and were shown with xPONENT 3.1 software (Luminex Corp.).
Sample results with lower values as MFI < background MFI + 2×
standard deviation were excluded from analysis. Quantitation of
survivin was performed with ProcartaPlex Human Survivin Simplex kit
(eBioscience, Frankfurt am Main, Germany) using cell culture
supernatant as described in the manufacturer’s protocol. Survivin
quantity was normalized to protein concentration which was
established using Pierce BCA assay (Thermo Fisher Scientific). PDH
and apoptotic proteins (BAD and JNK) were detected with multiplex
assays from Merck Millipore (Multi-species PDH Complex Magnetic
Bead Panel and 7-Plex Early Apoptosis Magnetic Bead kit, Darmstadt,
Germany). Samples were processed as described in the manufacturer’s
instructions. Additionally, samples for PDH measurements were
filtered with centrifugal ultrafree filter units with a pore size
of 0.65 μm (Merck Millipore) at 7,000 rpm for 4 min.
Mitochondrial activity
Cells grown on 8-well μ-dishes (Ibidi, Martinsried,
Germany) treated with and without 10 mM DCA were fixed with 4%
paraformaldehyde, washed three times with HBSS containing calcium
and magnesium and stained with 4 μM MitoSox (Invitrogen, Thermo
Fisher Scientific) for 15 min. After staining, cells were washed
three times with HBSS (Gibco, Thermo Fisher Scientific) and cell
nucleus was counterstained with DAPI (dilution of 1:1,000,
Sigma-Aldrich GmbH) for 5 min. Fluorescence imaging was performed
with an inverted confocal laser scanning microscope (Eclipse
TE2000-E, Nikon, Düsseldorf, Germany) using a 60× water immersion
objective (Nikon). Images were taken with EZ-C1 1.80 software
(Nikon). The excitation occurred with a diode laser at 408 nm
(DAPI) and with a helium/neon laser at 543 nm (MitoSox). Total cell
fluorescence of MitoSox deducting background was analyzed with
ImageJ and normalized to cell counts.
Immunofluorescence staining of Ki67 and
TUNEL
Cells were seeded, fixed and washed as described
above. After washing with HBSS, cells were permeabilized with 0.2%
Triton X-100 for 20 min, washed and incubated overnight with a
canine specific rabbit-polyclonal Ki67 antibody with a dilution of
1:150 (Life Technologies, Thermo Fisher Scientific). For staining,
a monoclonal anti-rabbit Alexa Fluor® 555 antibody (Cell
Signaling Technology, Leiden, The Netherlands) was incubated for 1
h with a dilution of 1:250 and cells were counterstained with DAPI
(1:1,000) for 5 min. Fluorescence imaging protocol was the same as
described above. Total cell fluorescence was established as
described above. For TUNEL staining Apoptag Fluorescein Direct kit
(Merck Millipore) was used according to the manufacturer’s
instructions. The excitation occurred with an argonlaser at 488 nm
and imaging was performed as described above. The percentage of
TUNEL positive cells was evaluated.
Statistical analysis
Statistical analysis of data was performed with SAS
software 7.1 (SAS Institute Inc., Cary, NC, USA). For comparison of
two means, two-tailed t-test was used. The confidence value was set
to 5% (P<0.05) and was considered statistically significant.
Results
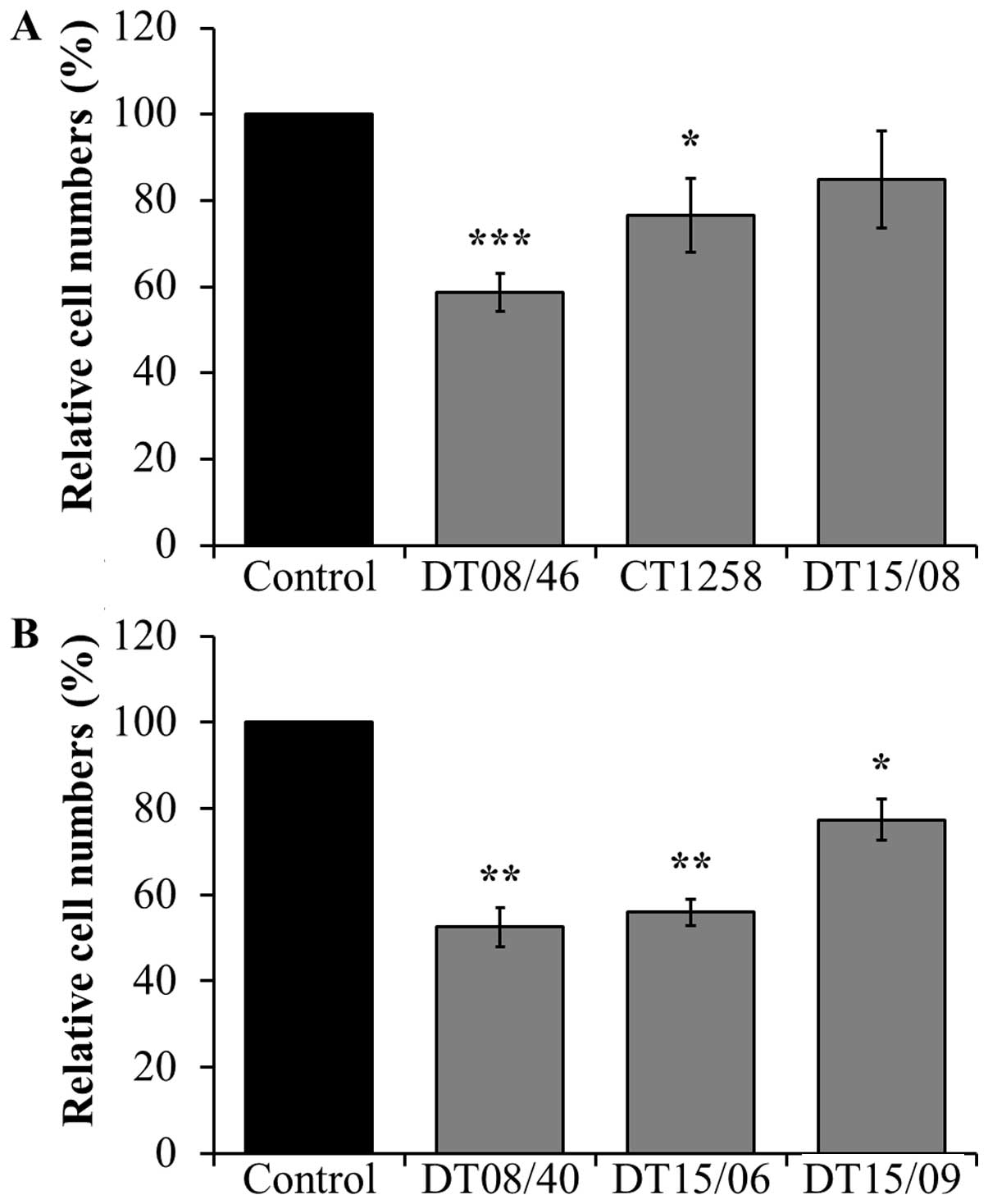
Cell counts after DCA treatment
In comparison to non-treated negative controls, the
prostate adenocarcinoma cell lines DT08/46 (P<0.0001) and CT1258
(P=0.0122) showed significantly lower cell numbers after treatment
with 10 mM DCA over 48 h (Fig.
1A). The third prostate cell line DT15/08 DCA did not show a
significant reduction (P=0.0748) but a tendency to a lower cell
amount, respectively a lower proliferation rate in native cell
culture was observed (Fig. 1A). As
shown in Fig. 1B the same
decreasing effect of DCA was noted in all examined TCC cell lines
DT08/40 (P=0.0031), DT15/06 (P=0.0016) and in DT15/09
(P=0.0143).
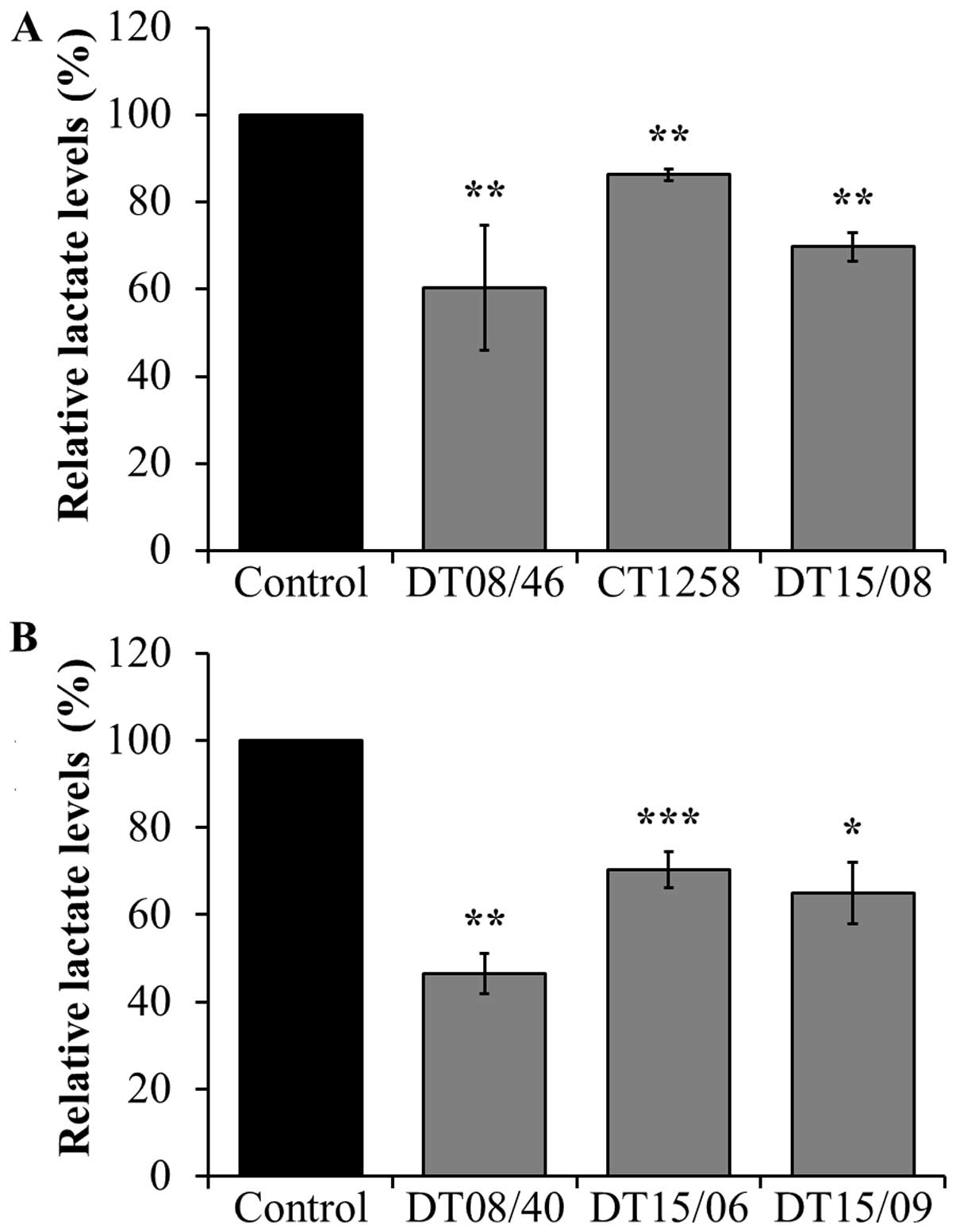
Lactate levels in supernatant of cell
culture after DCA exposure
To assess the lactate release lowering effect of DCA
treatment after 48 h, lactate amount in cell culture supernatant
was measured, respectively. In all cell lines of both canine cancer
entities, 10 mM DCA had a significant lactate lowering effect
(Fig. 2).
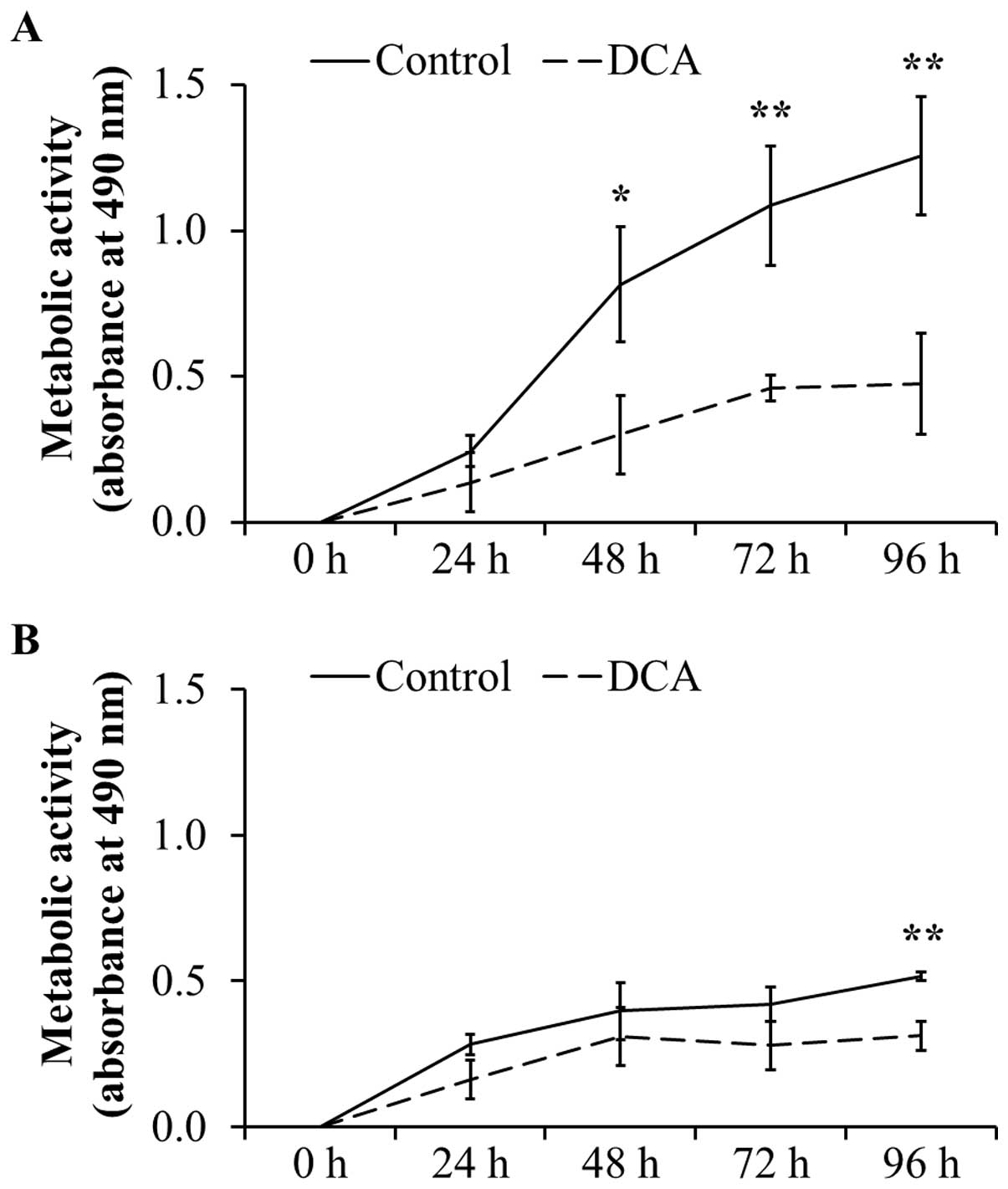
Metabolic activity after treatment with
DCA over a period of 96 h
To confirm the negative effect on cell
proliferation, the metabolic activity as indicator for
proliferation and cell viability was analyzed by MTT assays. As
shown in Fig. 3A, the metabolic
activity in DT15/08 was significantly reduced in comparison to the
respective negative control after 48 h of DCA treatment (P=0.0202).
Following 96 h of continuous DCA treatment the metabolic activity
was significant (P=0.007). Similar effect was observed in DT08/40
(Fig. 3B) after 96 h (P=0.0025)
and in DT15/06 (P=0.0419) after 96 h (data not shown) of DCA
treatment. The cell lines DT08/46, CT1258 and DT15/09 showed no
statistically significant effect of DCA on metabolic activity (data
not shown).
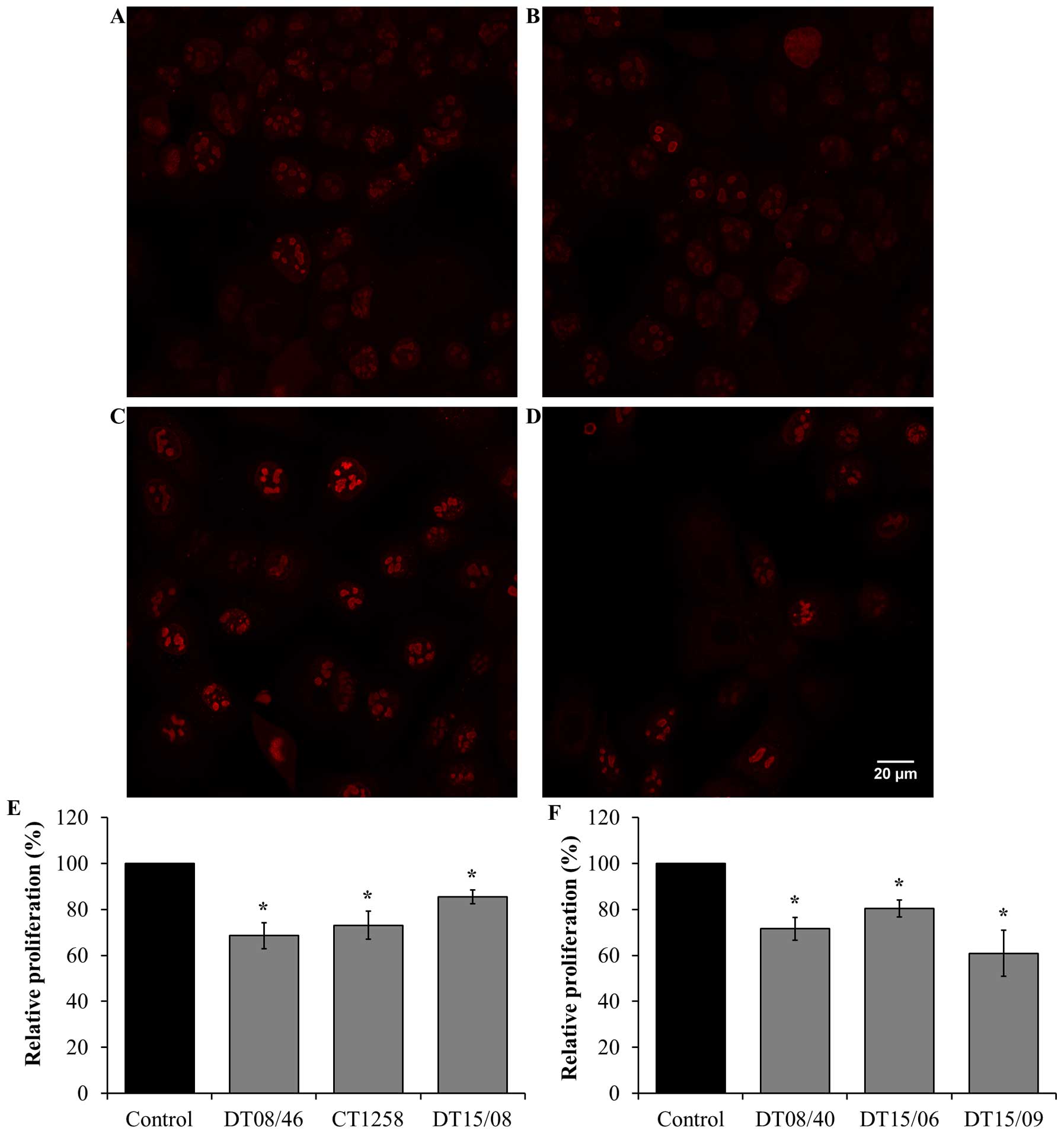
Proliferation after DCA treatment over 48
h
Cell proliferation in 10 mM DCA exposed cell lines
were analyzed by Ki67 staining and visualized by confocal
fluorescence microscopy. DCA decreased the amount of Ki67
indicating reduced cell proliferation after 48 h in all evaluated
cell lines with significance (P<0.05) (Fig. 4).
Effect of DCA on apoptosis
The effect of 10 mM DCA on apoptosis and viability
was assessed with Annexin and Sytox using flow cytometry. Regarding
apoptosis and dead cells no statistical significant effects were
noted in any of the cell lines (data not shown). Confirmation of
FACS apoptosis rates was done by TUNEL staining in order to
eliminate negative effects of trypsinization on cultured cells.
Imaging was performed using confocal fluorescence microscopy. The
results confirm the apoptosis ratio in all DCA treated cell lines
except DT15/06. Significant decreased apoptosis levels were
observed in DT15/08 (P=0.022) and DT15/09 (P=0.0265). DT15/06
showed significantly increased (P=0.041) apoptosis values (data not
shown). The other cell lines displayed no significant apoptosis
values.
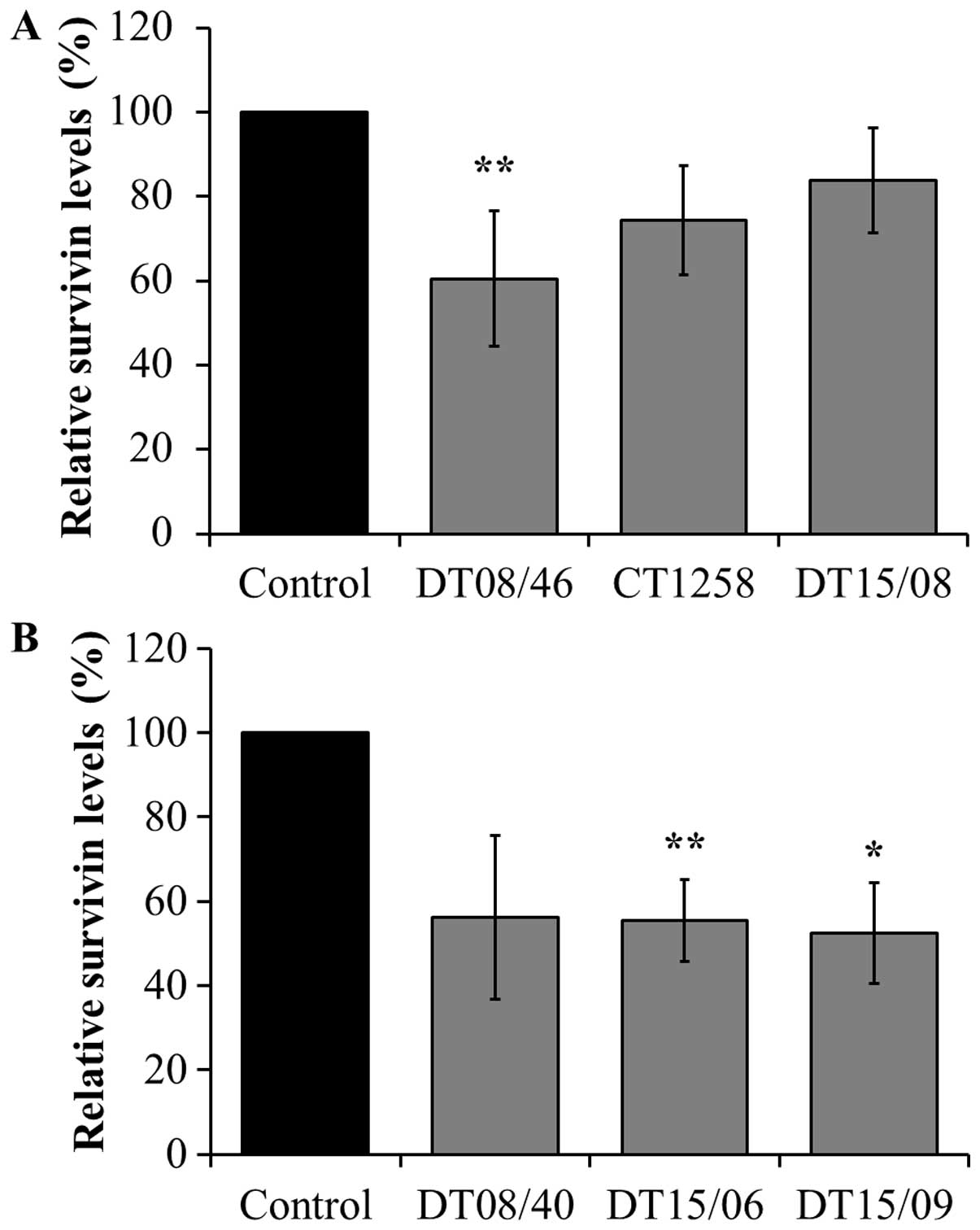
Survivin expression analyzed with
xMAP® Magnetic Bead Technology
DCA significantly decreased survivin production in
the prostate adenocarcinoma cell line DT08/46 (P=0.0053) and in the
TCC cell lines DT15/06 (P=0.0027) and DT15/09 (P=0.0206). The cell
lines CT1258 (P=0.0761), DT15/08 (P=0.1551) and DT08/40 (P=0.0598)
showed no significant effects in survivin production (Fig. 5).
Bcl-2-antagonist-of-cell-death (BAD) and
c-jun N-terminal kinases (JNK) expression analyzed with xMAP
Magnetic Bead Technology
After 48-h DCA incubation, the phosphorylated and
thus inactive form of BAD increased only significantly in DT08/46
(P=0.0285), CT1258 (P=0.0039) and DT15/06 (P=0.0235). Further no
effect of DCA could be observed on active phosphorylated JNK with
exception of DT08/46 (P=0.0044) which displayed decreased values
(data not shown).
Pyruvate-dehydrogenase (PDH) expression
analyzed with xMAP Magnetic Bead Technology
For confirmation of decreased lactate levels as
consequence of lower glycolysis, phosphorylated PDH (PDH-P)
quantity (Ser232, Ser293, Ser300) was measured with Luminex
Magnetic Bead Technology. Decreased levels of PDH-P and thus
increased active enzymes are associated with an increased pyruvate
oxidation and acetyl coenzyme A metabolism which leads to increased
TCA cycle activity (35). In PDH-P
(Ser232) a statistically significant decrease in comparison to
non-treated controls was observed in all cell lines except DT08/46.
This cell line showed a higher but non-significant PDH-P (Ser232)
level. The PDH-P at residue Ser293 was significantly affected by
DCA only in DT15/08 (P=0.0082) and DT15/06 (P=0.0122) cell lines.
In all other cell lines no effect could be observed after DCA
treatment. PDH-P at Ser300 was compromised by DCA significantly in
DT08/46 (P=0.0060), CT1258 (P=0.0215), DT15/08 (P=0.0002) and
DT15/06 (P=0.0097) (Table I).
 | Table IPDH-P expression after DCA exposure
with relative mean values ± SD (%). |
Table I
PDH-P expression after DCA exposure
with relative mean values ± SD (%).
| Cell line | PDH-P Ser232 | P-value | PDH-P Ser293 | P-value | PDH-P Ser300 | P-value |
|---|
| Control | 100 | | 100 | | 100 | |
| Prostate
adenocarcinoma |
| DT08/46 | 121.2±31.5 | 0.2715 | 127.7±27.8 | 0.0900 | 47.7±28.0 | 0.0060b |
| CT1258 | 11.0±5.1 | 0.0011b | 73.7±30.5 | 0.2740 | 24.3±19.5 | 0.0215a |
| DT15/08 | 18.0±17.6 | 0.0026b | 39.7±19.2 | 0.0082b | 18.4±6.8 | 0.0002c |
| Transitional cell
carcinoma |
| DT08/40 | 14.3±10.2 | 0.0047b | 84.5±22.8 | 0.3594 | 48.6±32.1 | 0.1090 |
| DT15/06 | 28.2±17.7 | 0.0039b | 72.3±5.4 | 0.0122a | 39.1±10.4 | 0.0097b |
| DT15/09 | 20.5±24.8 | 0.0308a | 99.8±71.3 | 0.9967 | 34.1±41.0 | 0.1085 |
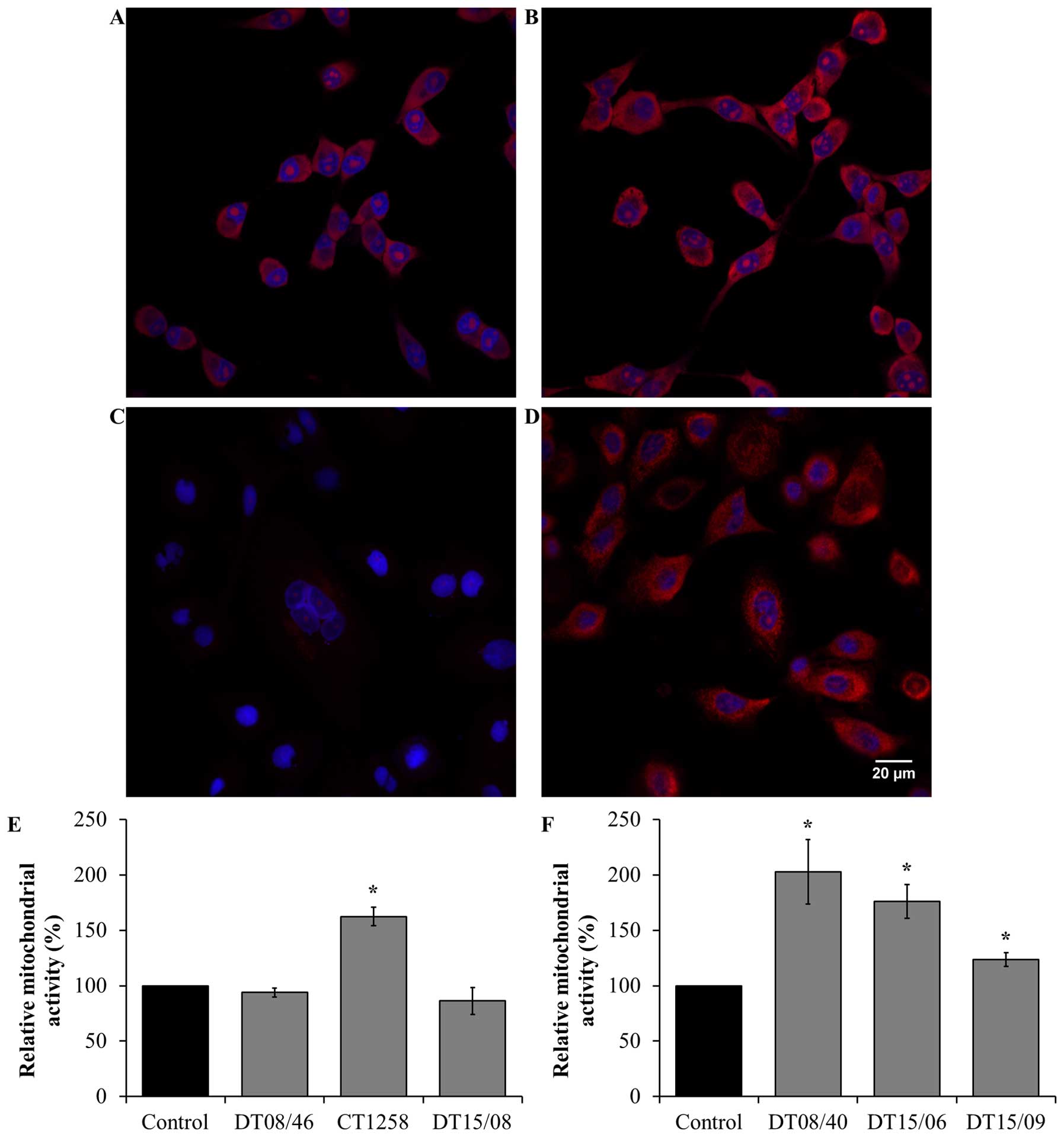
Mitochondrial activity after DCA
treatment
Verification of the metabolic alteration to glucose
oxidation mitochondrial activity was done by detection of
mitochondrial derived reactive oxygen species (ROS). In the
prostate adenocarcinoma cell lines (Fig. 6E) a significantly increased
mitochondrial activity was observed in CT1258 (P=0.0153). The cell
line DT08/46 (P=0.2829) and DT15/08 (P=0.3082) displayed no
decreasing effect after DCA treatment. In contrast DCA was able to
increase mitochondrial activity significantly (P<0.05) in all
TCC cell lines (Fig. 6F).
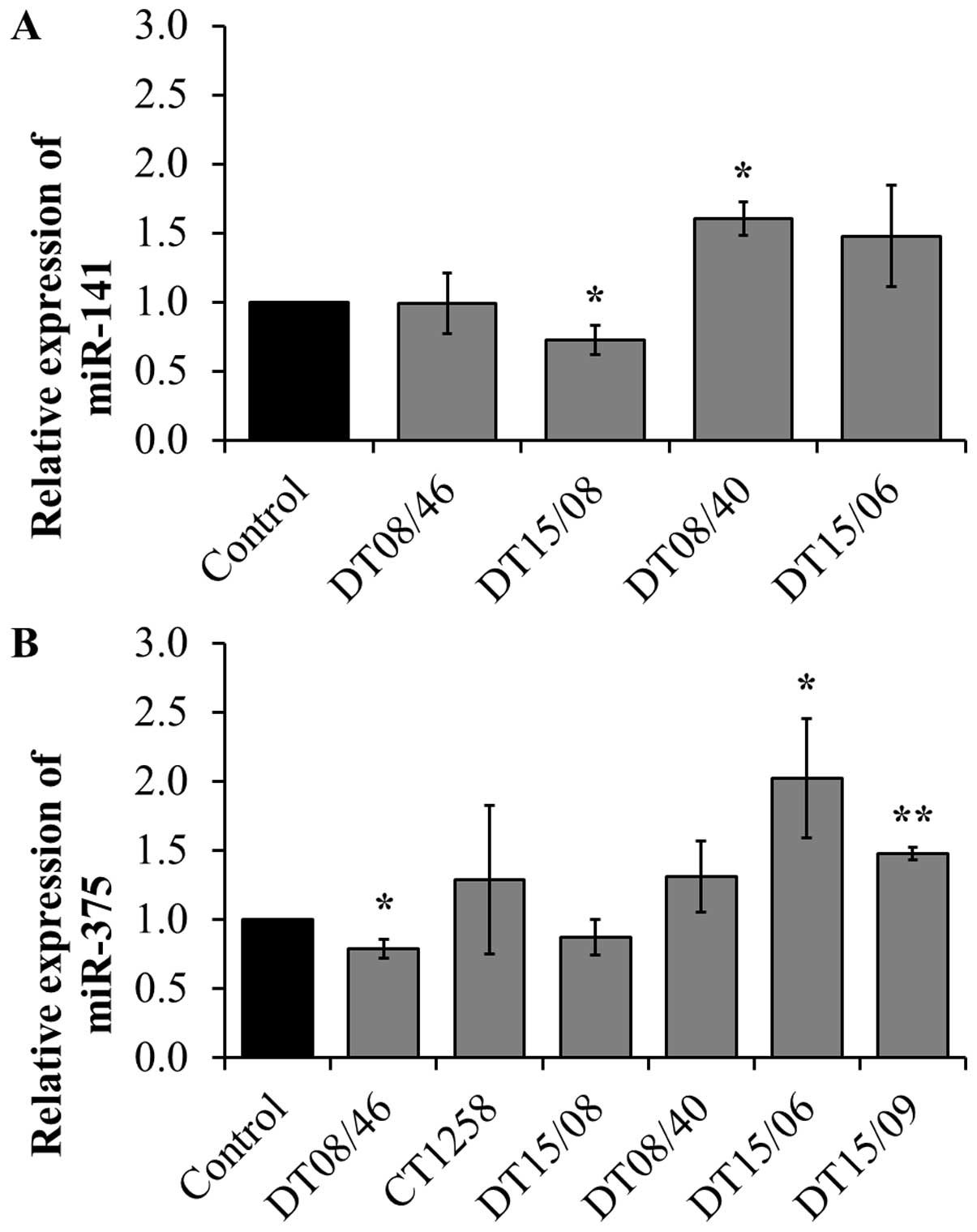
Quantitative miRNA RT-PCR
To assess if the observed lower proliferation rates
correlate with changes in micro-RNAs, three miR involved in
proliferation or apoptosis were evaluated. miR-141 expression
revealed significant changes with decreased levels in DT15/08
(P=0.0451) and increased levels in DT08/40 (P=0.0135) compared to
non-treated controls, whereas DT08/46 (P=0.9285) and DT15/06
(P=0.1520) showed no difference in comparison to the controls. In
CT1258 and DT15/09 miR-141 was downregulated and excluded from
analysis. DT08/46 (P=0.0322) showed a lower and DT15/06 (P=0.0267)
as well as DT15/09 (P=0.0031) a higher expression of miR-375. The
prostate adenocarcinoma cell line CT1258 displayed no significant
changes in all examined microRNAs, respectively. The expression of
miR-145 was not influenced by DCA treatment in any of the cell
lines (excluded from analysis due to Ct-values >30). In summary
prostate adenocarcinoma cell lines tend to show a downregulation of
miR-141 (DT15/08) and miR-375 (DT08/46, DT15/08), the TCC cell
lines showed upregulation of both (Fig. 7).
Discussion
DCA reduced the cell number in prostate
adenocarcinoma and in TCC cell lines. This can occur on the basis
of a reduced proliferation or increased apoptosis. In this study a
proliferation lowering effect was observed indicated by decreased
Ki67 in all cell lines and lower metabolic activities in DT15/08
and DT08/40. This finding is in accordance with previous results
presented by Bonnet et al in several human cell lines
(35) and Sun et al in
breast cancer (12). Further,
decreased cell proliferation after DCA exposure was also observed
in human prostate carcinoma (36),
colorectal (37), colon (11) and lung cancer (38). However, induction of mitochondria
dependent apoptosis by DCA, as previously reported by several
research groups (14,35,39)
could not be observed in this study. However, this phenomenon was
also observed by Feuerecker et al in murine and human
neuroblastoma cells (40).
Stockwin et al reported that high DCA concentrations are
required for apoptosis induction (38). The inconsistent results in one cell
line (DT15/06) between flow cytometry and TUNEL staining do not
allow an evaluation if DCA affects apoptosis. The results of
apoptotic protein expression JNK and BAD are consistent with the
effects observed in all other cell lines and support the hypothesis
that DCA showed no influence on apoptosis in canine prostate and
TCC cancer cells.
Survivin, an inhibitor of apoptosis and tumor
promoter (41,42), was significantly decreased in all
cell lines which displayed increased mitochondrial activity
(DT15/06, DT15/09). Decreased survivin levels are reported to
induce apoptosis via intrinsic pathways by activation of caspase-3
(41) and were observed in
endometrial cancer cell lines after DCA exposure (14). Unexpectedly, increased apoptosis
could not be observed in these cell lines with exception of DT15/06
(P=0.041) which showed a slight but inconsistent increase in cell
death. This leads to the conclusion that decreased survivin levels
possibly entail decreased proliferation. In addition, a
deregulation of further genes which are involved in apoptosis
induction is conceivable and would declare, why decreased survivin
levels did not result in apoptosis.
DCA is a PDK inhibitor which indirectly activates
PDH. Due to decreased values of PDH-P, pyruvate can be oxidized by
mitochondria (4,5). Decreased PDH phosphorylation was
observed in several human cancer cell lines (16,37,43).
In accordance with published results in human cell lines, this
study confirmed decreasing PDH phosphorylation in all DCA-treated
cells which are affirmed by reduced lactate release in all cell
lines. This indicates that DCA promotes glucose oxidation in canine
cancer cells as well as in human cancer cells. Furthermore,
increased levels of ROS in mitochondria of all cell lines except
two prostate adenocarcinoma cell lines were proved in this study.
Increased ROS species in mitochondria which occur due to cellular
respiration, confirm the decreased PDH-P values and the lactate
reduction. It might be that increased mitochondrial activity does
not result in apoptosis, but in an increased cellular respiration
impeding the survival of cancerous cells with a changed metabolism.
This could clarify the increased viability of cells after DCA
exposure. Increased viability after DCA exposure was also observed
by McPherson et al who reported on increased pyruvate
oxidation and viability of embryos in an aged mouse model (44).
miR-375 upregulation has been reported to have
anti-proliferating effects in many cells such as gastric cancer
(45), pancreatic cancer (46), fetal cardiomyocyte-like cells
(47) and colon cancer (48). In prostate carcinogenesis
microRNA-375 has variable effects depending on tumor phenotype.
Costa-Pinheiro et al proved anti-proliferative effects in
PC-3 cells due to upregulation as well as increased apoptosis in
22Rv1 cells following miR-375 knockdown (49). Our results illustrate, the up- or
downregulation of miR-375 after DCA treatment is not consistent in
prostate adenocarcinoma cell lines, which is in accordance with the
findings described above. In comparison to prostate cancer cells,
all TCC cell lines showed increased miR-375 levels. There is no
literature describing miR-375 effects in bladder cancer. It could
be possible that these results are in accordance with
anti-proliferative effects which are described in many other cells.
The same findings were observed in microRNA-141. An upregulation of
miR-141 inhibited cancer proliferation and cell cycle progression
in neuroblastoma cells (50).
Furthermore miR-141 is downregulated in bladder cancer with muscle
invasion (51). In our study
miR-141 and miR-375 were found upregulated in all TCC cell lines
after DCA treatment indicating that these changes cause lower
proliferation rates. In prostate cancer miR-141 is reported to be
upregulated (52). In this study
the miR-141 levels decreased slightly, but we suppose that this
effect is too mild to affect cancer cells. However, an analysis
allowing to determine if a direct link between DCA and changes in
different microRNAs is causative for the observed biologic
responses would require a comprehensive transcriptomic
approach.
In conclusion, this study illustrates that canine
cancer cell lines are responding to DCA treatment and the effect
can be reduced to decreased proliferation rates, increased pyruvate
oxidation and mitochondrial activity. The results also show
differences between the examined cancer entities. Thus, TCC cell
lines seem to respond consistently and are more susceptible to DCA
treatment than prostate adenocarcinoma cell lines. In comparison to
most human cell lines DCA did not affect apoptosis which may
constitute that DCA might be useful for tumor growth restriction in
canine cancer, but not size reduction.
Moreover, DCA can be advantageous in sensitizing
canine cancer cells to other anticancer drugs and therefore it
might be appropriate for combination therapies (53). To ensure higher DCA concentrations
in cancerous tissues and to avoid severe generalized side effects
an intralesional therapy, comparable to intravesical chemotherapy
in human (54) and dogs with TCC
(55,56), could be a further possibility. The
concentration of 10 mM DCA was chosen to allow the comparability to
other human in vitro studies (14,18,37).
For clinical studies the DCA concentration has to be re-evaluated
with regard to compatibility and negative side effects. Therefore,
further studies with DCA concentrations in lower concentrations
should be examined.
Abbreviations:
|
DCA
|
dichloroacetate
|
|
PDH
|
pyruvate dehydrogenase
|
|
PDK
|
pyruvate dehydrogenase kinase
|
|
TCC
|
transitional cell carcinoma
|
|
RNA
|
ribonucleic acid
|
|
PCR
|
polymerase chain reaction
|
|
JNK
|
c-jun N-terminal kinases
|
|
BAD
|
Bcl-2-antagonist-of-cell-death
|
|
ROS
|
reactive oxygen species
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Vail DM: Supporting the veterinary cancer
patient on chemotherapy: Neutropenia and gastrointestinal toxicity.
Top Companion Anim Med. 24:122–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cornell KK, Bostwick DG, Cooley DM, Hall
G, Harvey HJ, Hendrick MJ, Pauli BU, Render JA, Stoica G, Sweet DC,
et al: Clinical and pathologic aspects of spontaneous canine
prostate carcinoma: A retrospective analysis of 76 cases. Prostate.
45:173–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mutsaers AJ, Widmer WR and Knapp DW:
Canine transitional cell carcinoma. J Vet Intern Med. 17:136–144.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sutendra G and Michelakis ED: Pyruvate
dehydrogenase kinase as a novel therapeutic target in oncology.
Front Oncol. 3:382013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stacpoole PW: The pharmacology of
dichloroacetate. Metabolism. 38:1124–1144. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stacpoole PW, Gilbert LR, Neiberger RE,
Carney PR, Valenstein E, Theriaque DW and Shuster JJ: Evaluation of
long-term treatment of children with congenital lactic acidosis
with dichloroacetate. Pediatrics. 121:e1223–e1228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stacpoole PW, Kerr DS, Barnes C, Bunch ST,
Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson
GN, et al: Controlled clinical trial of dichloroacetate for
treatment of congenital lactic acidosis in children. Pediatrics.
117:1519–1531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moore GW, Swift LL, Rabinowitz D, Crofford
OB, Oates JA and Stacpoole PW: Reduction of serum cholesterol in
two patients with homozygous familial hypercholesterolemia by
dichloroacetate. Atherosclerosis. 33:285–293. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stacpoole PW, Moore GW and Kornhauser DM:
Metabolic effects of dichloroacetate in patients with diabetes
mellitus and hyperlipoproteinemia. N Engl J Med. 298:526–530. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato T, Niizuma S, Inuzuka Y, Kawashima T,
Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, et al:
Analysis of metabolic remodeling in compensated left ventricular
hypertrophy and heart failure. Circ Heart Fail. 3:420–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sánchez-Aragó M, Chamorro M and Cuezva JM:
Selection of cancer cells with repressed mitochondria triggers
colon cancer progression. Carcinogenesis. 31:567–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun RC, Fadia M, Dahlstrom JE, Parish CR,
Board PG and Blackburn AC: Reversal of the glycolytic phenotype by
dichloroacetate inhibits metastatic breast cancer cell growth in
vitro and in vivo. Breast Cancer Res Treat. 120:253–260. 2010.
View Article : Google Scholar
|
|
13
|
Saed GM, Fletcher NM, Jiang ZL, Abu-Soud
HM and Diamond MP: Dichloroacetate induces apoptosis of epithelial
ovarian cancer cells through a mechanism involving modulation of
oxidative stress. Reprod Sci. 18:1253–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong JY, Huggins GS, Debidda M, Munshi NC
and De Vivo I: Dichloroacetate induces apoptosis in endometrial
cancer cells. Gynecol Oncol. 109:394–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michelakis ED, Sutendra G, Dromparis P,
Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR,
Fulton D, et al: Metabolic modulation of glioblastoma with
dichloroacetate. Sci Transl Med. 2:31ra342010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinnaird A, Dromparis P, Saleme B, Gurtu
V, Watson K, Paulin R, Zervopoulos S, Stenson T, Sutendra G, Pink
DB, et al: Metabolic modulation of clear-cell renal cell carcinoma
with dichloroacetate, an inhibitor of pyruvate dehydrogenase
kinase. Eur Urol. 69:734–744. 2016. View Article : Google Scholar
|
|
17
|
Delaney LM, Ho N, Morrison J, Farias NR,
Mosser DD and Coomber BL: Dichloroacetate affects proliferation but
not survival of human colorectal cancer cells. Apoptosis. 20:63–74.
2015. View Article : Google Scholar
|
|
18
|
Madhok BM, Yeluri S, Perry SL, Hughes TA
and Jayne DG: Dichloroacetate induces apoptosis and cell-cycle
arrest in colorectal cancer cells. Br J Cancer. 102:1746–1752.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruggieri V, Agriesti F, Scrima R,
Laurenzana I, Perrone D, Tataranni T, Mazzoccoli C, Lo Muzio L,
Capitanio N and Piccoli C: Dichloroacetate, a selective
mitochondria-targeting drug for oral squamous cell carcinoma: A
metabolic perspective of treatment. Oncotarget. 6:1217–1230. 2015.
View Article : Google Scholar :
|
|
20
|
Xintaropoulou C, Ward C, Wise A, Marston
H, Turnbull A and Langdon SP: A comparative analysis of inhibitors
of the glycolysis pathway in breast and ovarian cancer cell line
models. Oncotarget. 6:25677–25695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cicmanec JL, Condie LW, Olson GR and Wang
SR: 90-Day toxicity study of dichloroacetate in dogs. Fundam Appl
Toxicol. 17:376–389. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maisenbacher HW III, Shroads AL III, Zhong
G, Daigle AD, Abdelmalak MM, Samper IS, Mincey BD, James MO and
Stacpoole PW: Pharmacokinetics of oral dichloroacetate in dogs. J
Biochem Mol Toxicol. 27:522–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lukas G, Vyas KH, Brindle SD, Le Sher AR
and Wagner WE Jr: Biological disposition of sodium dichloroacetate
in animals and humans after intravenous administration. J Pharm
Sci. 69:419–421. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park R, Arieff AI, Leach W and Lazarowitz
VC: Treatment of lactic acidosis with dichloroacetate in dogs. J
Clin Invest. 70:853–862. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Racker E: History of the Pasteur effect
and its pathobiology. Mol Cell Biochem. 5:17–23. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Warburg O, Wind F and Negelein E: Über den
Stoffwechsel von Tumoren im Körper. Klin Wochenschr. 5:829–832.
1926.(In German). View Article : Google Scholar
|
|
27
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lum JJ, Bui T, Gruber M, Gordan JD,
DeBerardinis RJ, Covello KL, Simon MC and Thompson CB: The
transcription factor HIF-1alpha plays a critical role in the growth
factor-dependent regulation of both aerobic and anaerobic
glycolysis. Genes Dev. 21:1037–1049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plas DR and Thompson CB: Cell metabolism
in the regulation of programmed cell death. Trends Endocrinol
Metab. 13:75–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simon D, Knebel JW, Baumgartner W,
Aufderheide M, Meyer-Lindenberg A and Nolte I: In vitro efficacy of
chemotherapeutics as determined by 50% inhibitory concentrations in
cell cultures of mammary gland tumors obtained from dogs. Am J Vet
Res. 62:1825–1830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knapp DW, Chan TC, Kuczek T, Reagan WJ and
Park B: Evaluation of in vitro cytotoxicity of nonsteroidal
anti-inflammatory drugs against canine tumor cells. Am J Vet Res.
56:801–805. 1995.PubMed/NCBI
|
|
33
|
Sartin EA, Barnes S, Toivio-Kinnucan M,
Wright JC and Wolfe LG: Heterogenic properties of clonal cell lines
derived from canine mammary carcinomas and sensitivity to tamoxifen
and doxorubicin. Anticancer Res. 13:229–236. 1993.PubMed/NCBI
|
|
34
|
Winkler S, Murua Escobar H, Eberle N,
Reimann-Berg N, Nolte I and Bullerdiek J: Establishment of a cell
line derived from a canine prostate carcinoma with a highly
rearranged karyotype. J Hered. 96:782–785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonnet S, Archer SL, Allalunis-Turner J,
Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta
L, Bonnet S, et al: A mitochondria-K+ channel axis is
suppressed in cancer and its normalization promotes apoptosis and
inhibits cancer growth. Cancer Cell. 11:37–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao W, Yacoub S, Shiverick KT, Namiki K,
Sakai Y, Porvasnik S, Urbanek C and Rosser CJ: Dichloroacetate
(DCA) sensitizes both wild-type and over expressing Bcl-2 prostate
cancer cells in vitro to radiation. Prostate. 68:1223–1231. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ho N and Coomber BL: Pyruvate
dehydrogenase kinase expression and metabolic changes following
dichloroacetate exposure in anoxic human colorectal cancer cells.
Exp Cell Res. 331:73–81. 2015. View Article : Google Scholar
|
|
38
|
Stockwin LH, Yu SX, Borgel S, Hancock C,
Wolfe TL, Phillips LR, Hollingshead MG and Newton DL: Sodium
dichloroacetate selectively targets cells with defects in the
mitochondrial ETC. Int J Cancer. 127:2510–2519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie J, Wang BS, Yu DH, Lu Q, Ma J, Qi H,
Fang C and Chen HZ: Dichloroacetate shifts the metabolism from
glycolysis to glucose oxidation and exhibits synergistic growth
inhibition with cisplatin in HeLa cells. Int J Oncol. 38:409–417.
2011.
|
|
40
|
Feuerecker B, Seidl C, Pirsig S, Bruchelt
G and Senekowitsch-Schmidtke R: DCA promotes progression of
neuroblastoma tumors in nude mice. Am J Cancer Res. 5:812–820.
2015.PubMed/NCBI
|
|
41
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abemayor E, Kovachich GB and Haugaard N:
Effects of dichloroacetate on brain pyruvate dehydrogenase. J
Neurochem. 42:38–42. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McPherson NO, Zander-Fox D and Lane M:
Stimulation of mitochondrial embryo metabolism by dichloroacetic
acid in an aged mouse model improves embryo development and
viability. Fertil Steril. 101:1458–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou N, Qu Y, Xu C and Tang Y:
Upregulation of microRNA-375 increases the cisplatin-sensitivity of
human gastric cancer cells by regulating ERBB2. Exp Ther Med.
11:625–630. 2016.PubMed/NCBI
|
|
46
|
Zhou J, Song S, He S, Zhu X, Zhang Y, Yi
B, Zhang B, Qin G and Li D: MicroRNA-375 targets PDK1 in pancreatic
carcinoma and suppresses cell growth through the Akt signaling
pathway. Int J Mol Med. 33:950–956. 2014.PubMed/NCBI
|
|
47
|
Wang L, Song G, Liu M, Chen B, Chen Y,
Shen Y, Zhu J and Zhou X: MicroRNA-375 overexpression influences
P19 cell proliferation, apoptosis and differentiation through the
Notch signaling pathway. Int J Mol Med. 37:47–55. 2016.
|
|
48
|
Zaharie F, Muresan MS, Petrushev B, Berce
C, Gafencu GA, Selicean S, Jurj A, Cojocneanu-Petric R, Lisencu CI,
Pop LA, et al: Exosome-carried microRNA-375 inhibits cell
progression and dissemination via Bcl-2 blocking in colon cancer. J
Gastrointestin Liver Dis. 24:435–443. 2015.PubMed/NCBI
|
|
49
|
Costa-Pinheiro P, Ramalho-Carvalho J,
Vieira FQ, Torres-Ferreira J, Oliveira J, Gonçalves CS, Costa BM,
Henrique R and Jerónimo C: MicroRNA-375 plays a dual role in
prostate carcinogenesis. Clin Epigenetics. 7:422015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Z, Lei H and Sun Q: MicroRNA-141 and
its associated gene FUS modulate proliferation, migration and
cisplatin chemosensitivity in neuroblastoma cell lines. Oncol Rep.
35:2943–2951. 2016.PubMed/NCBI
|
|
51
|
Mahdavinezhad A, Mousavi-Bahar SH,
Poorolajal J, Yadegarazari R, Jafari M, Shabab N and Saidijam M:
Evaluation of miR-141, miR-200c, miR-30b Expression and
Clinicopathological features of bladder cancer. Int J Mol Cell Med.
4:32–39. 2015.PubMed/NCBI
|
|
52
|
Brase JC, Johannes M, Schlomm T, Fälth M,
Haese A, Steuber T, Beissbarth T, Kuner R and Sültmann H:
Circulating miRNAs are correlated with tumor progression in
prostate cancer. Int J Cancer. 128:608–616. 2011. View Article : Google Scholar
|
|
53
|
Xue X, You S, Zhang Q, Wu Y, Zou GZ, Wang
PC, Zhao YL, Xu Y, Jia L, Zhang X, et al: Mitaplatin increases
sensitivity of tumor cells to cisplatin by inducing mitochondrial
dysfunction. Mol Pharm. 9:634–644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Porten SP, Leapman MS and Greene KL:
Intravesical chemotherapy in non-muscle-invasive bladder cancer.
Indian J Urol. 31:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Song D, Wientjes MG, Gan Y and Au JL:
Bladder tissue pharmacokinetics and antitumor effect of
intravesical 5-fluorouridine. Clin Cancer Res. 3:901–909.
1997.PubMed/NCBI
|
|
56
|
Abbo AH, Jones DR, Masters AR, Stewart JC,
Fourez L and Knapp DW: Phase I clinical trial and pharmacokinetics
of intravesical mitomycin C in dogs with localized transitional
cell carcinoma of the urinary bladder. J Vet Intern Med.
24:1124–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|