Introduction
Breast cancer is the most common malignant tumor in
women worldwide. Despite considerable progress in diagnosis and
therapy, it still constitutes an enormous health problem. In recent
years, many studies have sought new predictive and prognostic
markers.
Among the factors that have been investigated are
the metallothioneins (MTs), intracellular proteins of low molecular
weight (6–7 kDa). They are characterized by high levels of cysteine
residues and low aromatic amino acid content (1–3). MTs
have the ability to reversibly bind heavy metal ions, such as zinc,
copper, mercury, lead, and cadmium (1,4). Ten
functional metallothionein isoforms have been identified. On the
basis of small differences in their amino acid sequences, they are
categorized into four groups: MT-1, MT-2, MT-3, and MT-4 (4,5).
MT-1 and MT-2 are ubiquitous in all eukaryotic cells. MT-3 and MT-4
were originally described as tissue-specific proteins that occur,
respectively, in the neurons of the central nervous system and the
squamous epithelium of the skin and gastrointestinal tract.
However, recent studies of MT-3 isoform show its broader
distribution within an organism (5–10).
The MTs play an important role in maintaining metal
ion homeostasis and in protecting cells against heavy metal
toxicity and free radicals (4,11,12).
Through their interaction with zinc ions, the MTs affect the
activity of many zinc-dependent proteins, including enzymes and
transcription factors, thus regulating a number of basic
intracellular processes, such as proliferation, cell
differentiation, and apoptosis (3,12–14).
So far, studies have shown an increase of MT-1 and MT-2 in many
tumors of both epithelial and mesenchymal origin (15,16).
It has been demonstrated that increased expression of MT-1 and MT-2
is associated with greater proliferative potential of tumor cells
and is correlated with a more rapid course of the disease and
poorer prognosis (17–24). It has also been shown that MT-1 and
MT-2 are involved in the development of cancer cell resistance to
chemotherapy and radiotherapy (25–28).
MT-3 was first identified in neurons of the central
nervous system (6,29). It differs from the other MT
isoforms by the presence in the molecule of seven additional amino
acids, i.e., threonine in the N-terminal region and six amino acids
in the C-terminal region (30–32).
MT-3 has the ability to inhibit the growth of nerve cells and
neurite extension (6,33). In subsequent studies, MT-3 has also
been found outside the central nervous system, including the
kidney, prostate, and salivary glands (34–38).
Furthermore, it has been shown that the expression of this protein
is altered in certain cancers, including prostate, bladder, breast,
esophageal, stomach, and lung (36,39–43).
The aim of this study was to investigate the
expression of MT-3 in ductal breast cancers, as well as to
determine the relationship between the expression of MT-3 and
well-defined clinical and pathological factors in this type of
tumor.
Materials and methods
Patients and tissue samples
The study was conducted on two separate patient
cohorts. In the first group, immunohistochemical (IHC) studies were
performed on 134 archival paraffin blocks of invasive ductal breast
carcinoma (IDC) and 26 cases of mastopathy obtained from patients
treated in the Lower Silesian Oncology Centre in Wroclaw, Poland,
from 1999 to 2005. All the tissue specimens utilized for the study
were obtained prior to the beginning of treatment. The paraffin
sections, stained with hematoxylin and eosin (H&E), were used
to verify the diagnosis and the malignancy grade of the tumors (G),
according to WHO criteria (44).
The clinical data of patients were obtained from the hospital
archives and are summarized in Table
I. All patients were treated with mastectomy or conservative
quadrantectomy followed by axillary lymph node resection. Adjuvant
chemotherapy, appropriate to the stage of the disease, was applied
to 112 women (83.6%), whereas 88 patients (65.7%) were treated with
20 mg tamoxifen daily. Neoadjuvant chemotherapy was administered in
patients presenting stage III at diagnosis. Radiotherapy was
applied to 63 patients (47.0%). Patients were aged 57.89±12.26
years and were followed up for 51.08±37.61 months (ranging from 1
to 120 months). During this time 24 patients (17.9%) died of the
disease.
 | Table IPatient and tumor
characteristics. |
Table I
Patient and tumor
characteristics.
| IHC group | Real-time PCR
group |
|---|
|
|
|
|---|
| Parameters | No. | % | No. | % |
|---|
| Age |
| ≤50 years | 40 | 29.9 | 30 | 29.7 |
| >50 years | 94 | 70.1 | 71 | 70.3 |
| Menopausal
status |
| Pre | 42 | 31.3 | 29 | 28.7 |
| Post | 92 | 68.7 | 73 | 71.3 |
| Tumor size |
| pT1 | 72 | 53.7 | 26 | 25.7 |
| pT2 | 51 | 38.1 | 71 | 70.3 |
| pT3 | 7 | 5.2 | 3 | 3.0 |
| pT4 | 4 | 3.0 | 1 | 1.0 |
| Lymph nodes |
| Negative | 71 | 53.0 | 32 | 31.7 |
| Positive | 63 | 47.0 | 69 | 68.3 |
| pTNM |
| I | 43 | 32.1 | 10 | 9.9 |
| II | 70 | 52.2 | 54 | 53.5 |
| III | 18 | 13.4 | 37 | 36.6 |
| IV | 3 | 2.3 | 0 | 0.0 |
| Malignancy
grade |
| G1 | 14 | 10.5 | 2 | 2.0 |
| G2 | 68 | 50.7 | 41 | 40.6 |
| G3 | 52 | 38.8 | 58 | 57.4 |
| ER |
| Positive | 105 | 78.4 | 56 | 55.4 |
| Negative | 29 | 21.6 | 45 | 44.6 |
| PR |
| Positive | 90 | 67.2 | 50 | 49.5 |
| Negative | 44 | 42.8 | 51 | 50.5 |
| HER2 |
| Positive | 23 | 17.2 | 25 | 24.8 |
| Negative | 111 | 82.8 | 76 | 75.2 |
|
ER−/PR−/HER2− |
| Yes | 19 | 14.2 | 23 | 22.8 |
| No | 115 | 85.8 | 78 | 77.2 |
| Ki-67 |
| ≤25% | 85 | 63.4 | NA | NA |
| >25% | 49 | 36.6 | NA | NA |
The second patient cohort, the real-time PCR group,
comprised 101 IDC patients treated in the Centre of Oncology, Maria
Sklodowska-Curie Memorial Institute in Cracow, Poland, during the
years 2004–2005. The clinical data of these patients are summarized
in Table I. Additionally, in 42
cases non-malignant breast tissue (NMBT) adjacent to the tumor was
sampled as a control tissue. Experiments were performed on fresh
frozen tumor samples obtained prior to the initiation of systemic
treatment, during surgical biopsy, mastectomy, or conservative
quadrantectomy followed by axillary lymph node resection.
Eighty-five women (84.2%) were treated with systemic chemotherapy
and 57 patients (56.4%) received tamoxifen-based therapy.
Radiotherapy was applied to 68 patients (67.3%). The mean patient
age at diagnosis was 56.4±11.9 years, and patients were followed up
for 58.6±27.81 months (ranging from 1 to 12 months), during which
time 20 patients (19.8%) died of the disease. The study protocol
was approved by the Bioethical Committee of Wroclaw Medical
University.
Cell lines
Four human breast cancer cell lines, MCF-7, SKBR-3
(Cell Lines Collection of the Ludwik Hirszfeld Institute of
Immunology and Experimental Therapy, Wroclaw, Poland), MDA-MB-231
(ATCC, Washington, CO, USA), and BO2 (a derivative of MDA-MB-231,
provided by Dr Philippe Clezardin, INSERM U664, France), as well as
the normal human breast epithelial cell line hTERT-HME1 (ATCC),
were used in this study. All the breast cancer cells were cultured
in α-MEM medium supplemented with 10% fetal calf serum (FCS;
Invitrogen, Carlsbad, CA, USA), 2 mM L-glutamine and antibiotics.
The normal human breast epithelial cell line was cultured in MEGM
Bullet kit medium (Lonza, Basel, Switzerland).
Immunohistochemistry (IHC)
Resected tissue samples were fixed in 4% buffered
formalin, dehydrated and embedded in paraffin. For the IHC
reactions, paraffin blocks were cut into 4 μm-thick sections and
fixed on sialinized microscopic slides. Deparaffinization and
antigen retrieval were performed using Target Retrieval Solution,
pH 9.0 (Dako, Glostrup, Denmark) at 97°C for 20 min. Subsequently,
the sections were washed in Tris-buffered saline and incubated with
primary antibodies at room temperature for 20 min. All
immunohistochemical reactions were performed using Autostainer Link
48 (Dako). To detect MT-3 expression, a non-commercial rabbit
polyclonal antibody raised against GGEAAEAEAEKC peptide was used
(dilution 1:500; Invitrogen). The Ki-67 antigen was detected using
mouse monoclonal antibody, clone MIB-1 (prediluted and
ready-to-use, code no. IR626; Dako), estrogen receptor (ER) using
mouse monoclonal antibody, clone 1D5 (prediluted and ready-to-use,
code no. IR654; Dako) and progesterone receptor (PR) using mouse
monoclonal antibody, clone 636 (prediluted and ready-to-use, code
no. IR068; Dako). The sections were then visualized using EnVison
FLEX reagents (Dako). All slides were counterstained with
hematoxylin (Dako). HER2 expression status was determined using
HercepTest and HER2 FISH pharmDx kit (both from Dako), following
the procedure recommended by the manufacturer. All reactions were
conducted with negative controls.
Evaluation of IHC reactions
The IHC reactions were assessed under a BX-41 light
microscope (Olympus, Tokyo, Japan) in whole-tissue section. The
expression of MT-3 in cytoplasm (cMT-3) was evaluated using the
semiquantitative IRS scale, according to Remmele and Stegner
(45), which was used in our
previous study for MT-1/2 assessment in IDC (46). The scale is based on the percentage
of tumor cells showing a positive reaction (0 points, no cells with
positive reaction; 1 point, 1–10% cells with positive reaction; 2
points, 11–50%; 3 points, 51–80%; 4 points, >80% cells) as well
as on the intensity of the reaction color (0 points, no reaction; 1
point, low intensity; 2 points, moderate intensity; 3 points,
strong intensity reaction color). The final score represents the
product of the two values and falls in the range 0–12. The nuclear
expression of MT-3 (nMT-3) and of Ki-67 antigen were evaluated
using a semiquantitative five-grade scale based on the proportion
of cells with the reaction product: (0 points, no reaction; 1
point, 1–10%; 2 points, 11–25%; 3 points, 26–50%; 4 points, >50%
of cells have the reaction product). The status of ER and PR
receptors was scored from 0 to 3 points, depending on the
percentage of positive cells (0 points, no reactions; 1 point,
1–10%; 2 points, 11–50%; 3 points, 51–100% stained cells). The
expression of HER2 receptors was evaluated using a scale that takes
into account both the intensity of the membrane reaction and the
percentage of positive tumor cells (47).
RNA isolation, cDNA synthesis and
real-time PCR
Total RNA was isolated from the tissue samples and
the studied cell lines using RNeasy Mini kit (Qiagen, Hilden,
Germany) in line with the manufacturer’s protocol. To eliminate
genomic DNA contamination, on-column DNase digestion was performed
using RNase-Free DNase Set (Qiagen). The quantity and purity of RNA
samples were assessed by measuring the absorbance at 260 and 280 nm
with NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific,
Wilmington, DE, USA). First-strand cDNA was synthesized using the
SuperScript III First-Strand Synthesis System for RT-PCR
(Invitrogen). The expression of MT-3 mRNA was determined by
real-time PCR with 7900HT Fast Real-Time PCR System and TaqMan Gene
Expression Master Mix (Applied Biosystems, Foster City, CA, USA)
according to the manufacturer’s protocol. β-actin was used as
reference gene. For the reactions, the following sets of primers
and TaqMan probes were used: Hs00359394_g1 for MT-3 and
Hs99999903_m1 for β-actin (Applied Biosystems). All reactions were
performed in triplicate under the following conditions: activation
of polymerase at 50°C for 2 min, initial denaturation at 94°C for
10 min and 40 cycles of denaturation at 94°C for 15 sec, followed
by annealing and elongation at 60°C for 1 min. The relative
expression of MT-3 mRNA (RQ) was calculated with the ΔΔCt method
(48).
Statistical analysis
Statistical analysis was performed using Prism 5.0
software (GraphPad, La Jolla, CA, USA). The Kolmogorov-Smirnov test
was applied to test for normality of the distribution. The
Mann-Whitney U test was used to compare the expression level of
cMT-3 and nMT-3 between the mastopathies and the IDC tissues, as
well as the MT-3 RQ between the NMBT and IDC samples. For paired
sample analysis, the Wilcoxon matched pairs test was used. The
Kruskal-Wallis and Dunn’s Multiple Comparison tests were utilized
to analyze data from more than two groups. The correlations between
the clinicopathological parameters and MT-3 expression were
analyzed using the Mann-Whitney U test, Fisher’s exact test and
Spearman’s correlation test. The Kaplan-Meier method and the
log-rank test were used to analyze patients’ overall survival. For
each variable, the hazard ratio (HR) and the 95% confidence
interval (95% CI) were estimated. The Unpaired t-test was used to
compare differences of MT-3 mRNA expression in studied cell lines.
The results were considered statistically significant in the
analyses when p<0.05.
Results
Immunohistochemical MT-3 expression and
its impact on the patient clinicopathological data
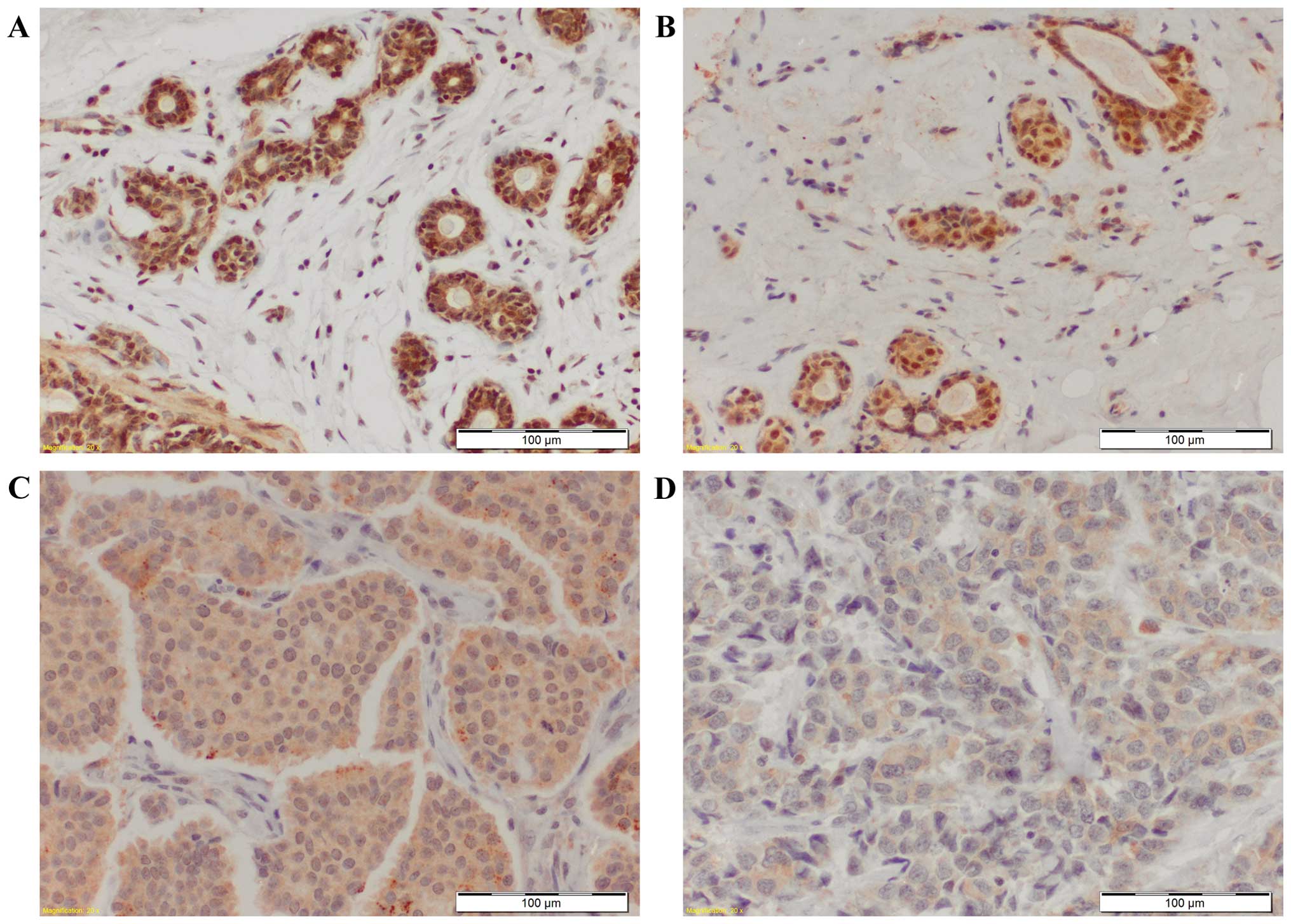
We noted nuclear as well as cytoplasmic MT-3
expression in the duct cells of mastopathies and in cancer cells of
IDC (Fig. 1). Significantly higher
nMT-3 and cMT-3 expression was found in mastopathies than in IDC
cancer cells (p<0.0001 and p<0.001, respectively; Fig. 2). When nMT-3 and cMT-3 expression
intensities were compared in IDC cases of particular malignancy
grades, no significant differences were found (Fig. 2). Statistical analysis revealed
that nMT-3 expression was significantly higher in mastopathies
(3.35±0.89) than in G1 (1.36±1.45; p<0.01), G2 (1.57±1.55;
p<0.001) and G3 (0.92±1.36; p<0.001) cases. Similar
relationships were noted for cMT-3 expression, which was
significantly higher in mastopathies (IRS 8.19±3.18) than in G2
(IRS 5.96±2.50; p<0.01) and G3 (IRS 6.37±1.81; p<0.05). nMT-3
expression was also significantly higher in G2 than in G3 cases
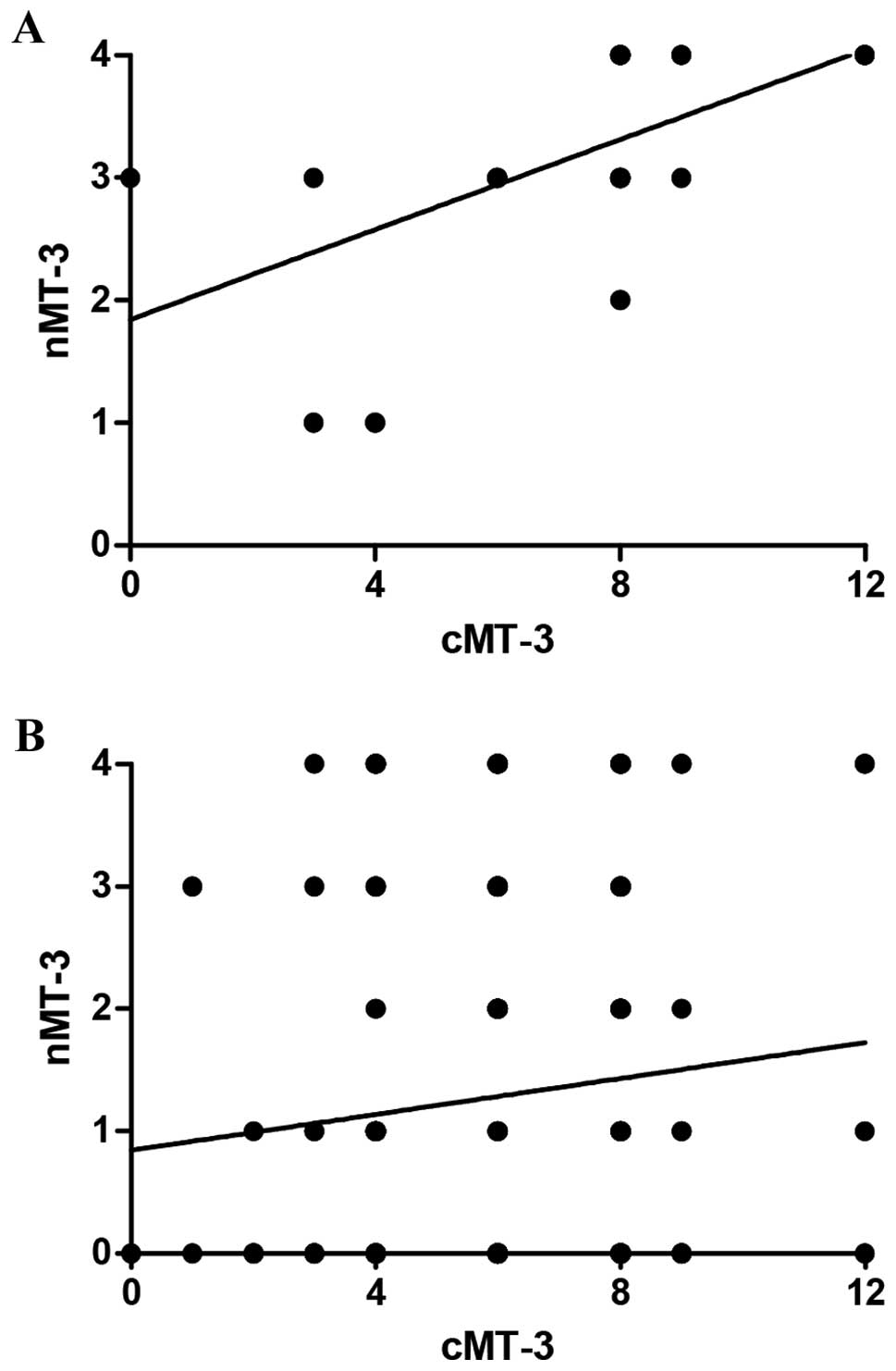
(p=0.017). Interestingly, the nMT-3 and cMT-3 expression correlated
in the analyzed mastopathy cases (r=0.71, p<0.0001), but not in
the IDC tissues (Fig. 3).
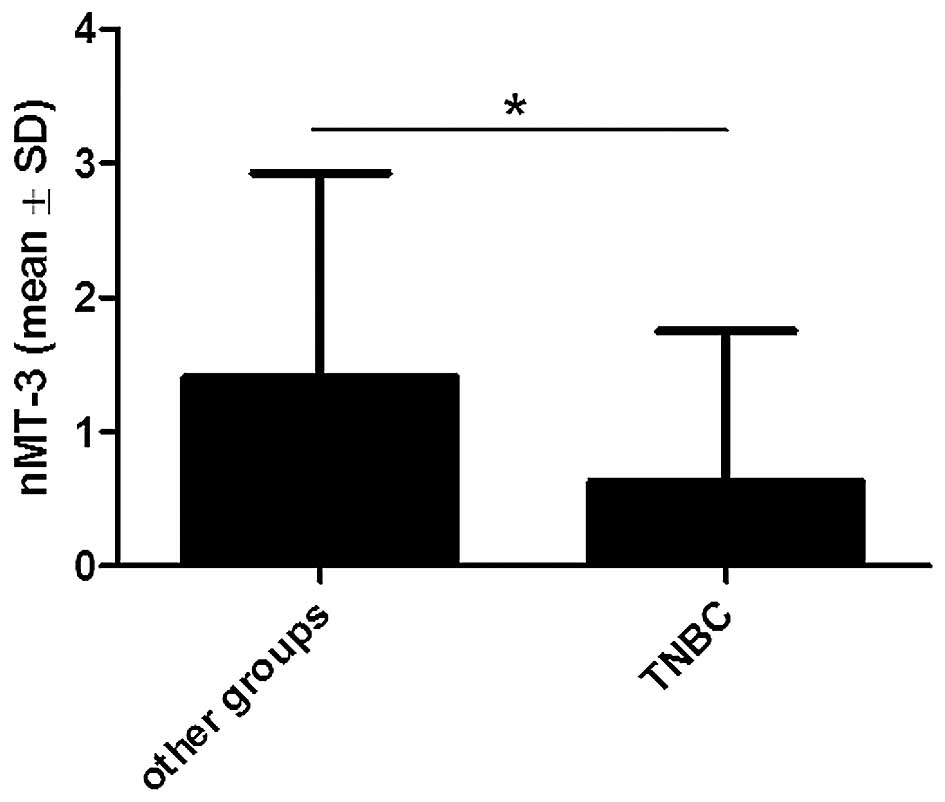
Moreover, it was found that nMT-3 expression was significantly
lower in triple-negative breast cancers (TNBC: ER−,
PR− and HER2−) than in the other IDC cases
(p<0.05; Fig. 4).
For statistical analysis, nMT-3 and cMT-3 expression
was categorized, in order to enable comparison with the
clinicopathological data of the patient. Cases which showed nMT-3
expression level in cancer cells ≤10% were regarded as ‘low’, while
those showing nMT-3 expression >10% were regarded as ‘high’. In
cMT-3, cases with an IRS of 0–6 were classified as ‘low’, and those
with an IRS of 8–12 as ‘high’. Statistical analysis using Fisher’s
exact test did not reveal any significant associations in either
nMT-3 or cMT-3, with the clinicopathological data of the patients,
such as age at diagnosis, menopausal status, primary tumor size,
the presence of lymph node metastases, pTNM stage, malignancy grade
or expressions of ER, PR or HER2. nMT-3 and cMT-3 had no impact on
cancer cell proliferation measured by the expression of the Ki-67
antigen in cancer cells (data not shown).
MT-3 mRNA expression level in NMBT and
IDC samples
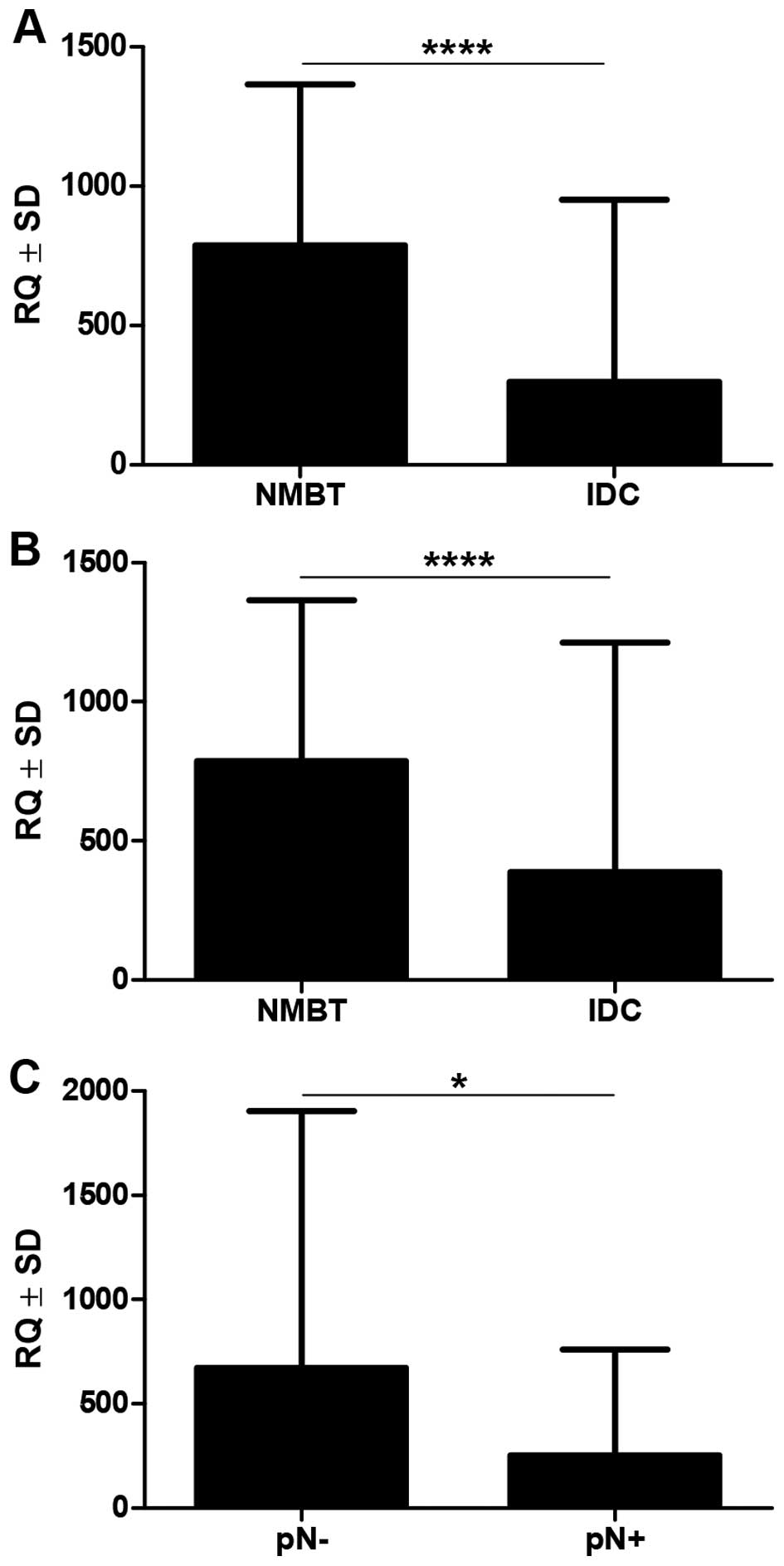
Real-time PCR revealed that MT-3 mRNA expression was
significantly upregulated in NMBT tissues (mean 786.5±579.3), as
compared to the paired (mean 296.8±653.6, p<0.0001, Wilcoxon
matched pair signed rank test) and all (mean 386.8±826.3,
p<0.0001, Mann-Whitney U test) analyzed IDC cases (Fig. 5). Cases characterized by lymph node
involvement had significantly lower MT-3 RQ levels than cases
without lymph node involvement (254.0±506.2 vs. 673.1±1232,
p=0.0199; Fig. 5). No associations
were noted between the expression level of MT-3 mRNA and patient
age, menopausal status, primary tumor size, malignancy grade, Ki-67
antigen expression or status of ER, PR or HER2 (Mann-Whitney U
test, data not shown).
Prognostic significance of MT-3
expression
Univariate survival analysis performed in all
studied IDC cases (n=134) revealed that the intensity of nMT-3 and
cMT-3 expression had no impact on the overall survival (OS) of the
patients. Of the factors investigated here, larger primary tumor
size (p=0.0038), presence of lymph node metastases (p=0.0011),
advanced disease stage (p<0.0001) and G3 malignancy grade
(p=0.0124) were associated with the patient poor OS (Table II and Fig. 6).
 | Table IIUnivariate analysis of overall
survival in invasive ductal breast carcinoma patients in the
immunohistochemical and real-time PCR groups. Significant p-values
are given in bold. |
Table II
Univariate analysis of overall
survival in invasive ductal breast carcinoma patients in the
immunohistochemical and real-time PCR groups. Significant p-values
are given in bold.
| Immunohistochemical
group | Real-time PCR
group |
|---|
|
|
|
|---|
| Clinicopathological
factor | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| nMT-3 (low vs.
high) | 1.231 | 0.5271–2.876 | 0.6309 | - | - | - |
| cMT-3 (low vs.
high) | 0.6957 | 0.3071–1.576 | 0.3845 | - | - | - |
| MT-3 RQ (low vs.
high) | - | - | - | 0.8775 | 0.3528–2.182 | 0.7787 |
| Age (≤50 vs. >50
years) | 1.692 | 0.7372–3.885 | 0.2146 | 0.7633 | 0.2882–2.022 | 0.5868 |
| Menopausal status
(pre vs. post) | 1.299 | 0.5711–2.953 | 0.5330 | 1.003 | 0.3802–2.648 | 0.9946 |
| Tumor size (pT1 vs.
pT2-pT4) | 3.399 | 1.483–7.789 | 0.0038 | 1.163 | 0.4324–3.128 | 0.7649 |
| Lymph nodes (pN0
vs. pN1-pN3) | 3.822 | 1.708–8.554 | 0.0011 | 1.158 | 0.4292–3.125 | 0.7720 |
| Stage (I, II vs.
III, IV) | 19.28 | 5.497–67.66 |
>0.0001 | 1.460 | 0.5629–3.787 | 0.4364 |
| Malignancy grade
(G1, G2 vs. G3) | 2.948 | 1.264–6.878 | 0.0124 | 1.037 | 0.4207–2.557 | 0.9367 |
| Ki-67 expression
(≤25 vs. >25%) | 2.263 | 0.9319–5.495 | 0.0712 | - | - | - |
In addition, we examined impact of nMT-3 and cMT-3
expression on the patient overall survival in triple-negative
(TNBC; n=19) and non-triple-negative (other groups; n=115) IDC
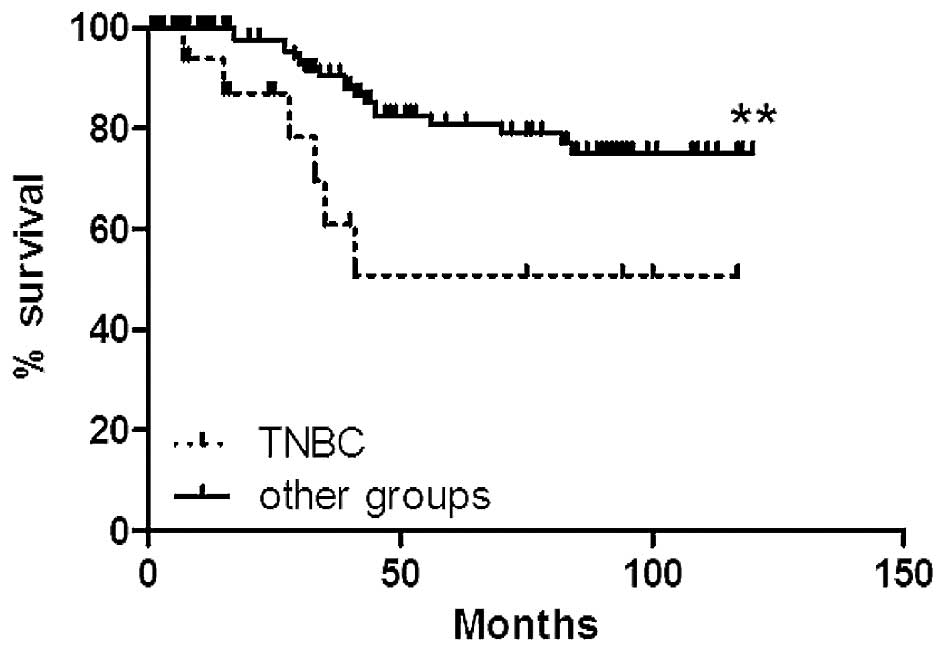
samples. TNBC cases were characterized by a significantly shorter
overall survival of the patients as compared to the other groups
(p<0.01; Fig. 7). In analyzed
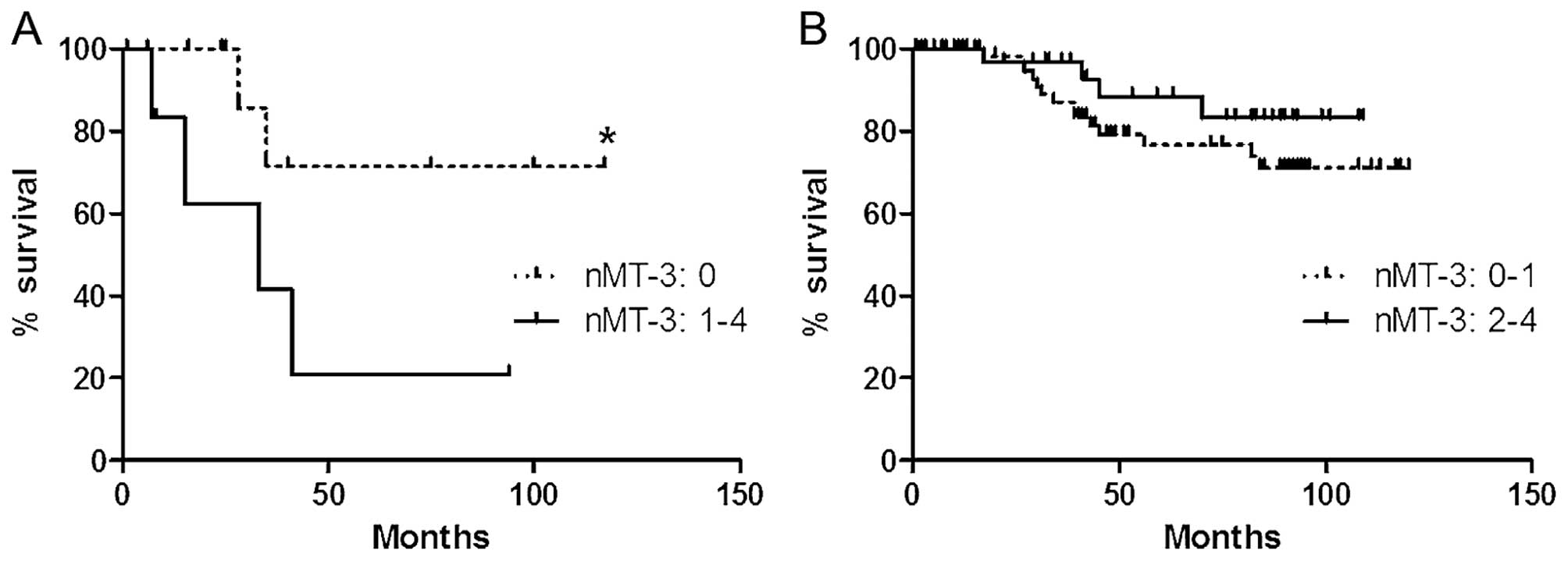
TNBC group, cases with nMT-3 expression manifested a significantly
shorter overall survival (p<0.05; Fig. 8).
In the real-time PCR patient cohort, none of the
analyzed parameters, including MT-3 mRNA expression level, had any
impact on the patient OS (Table
II).
MT-3 mRNA expression level in the
examined cell lines
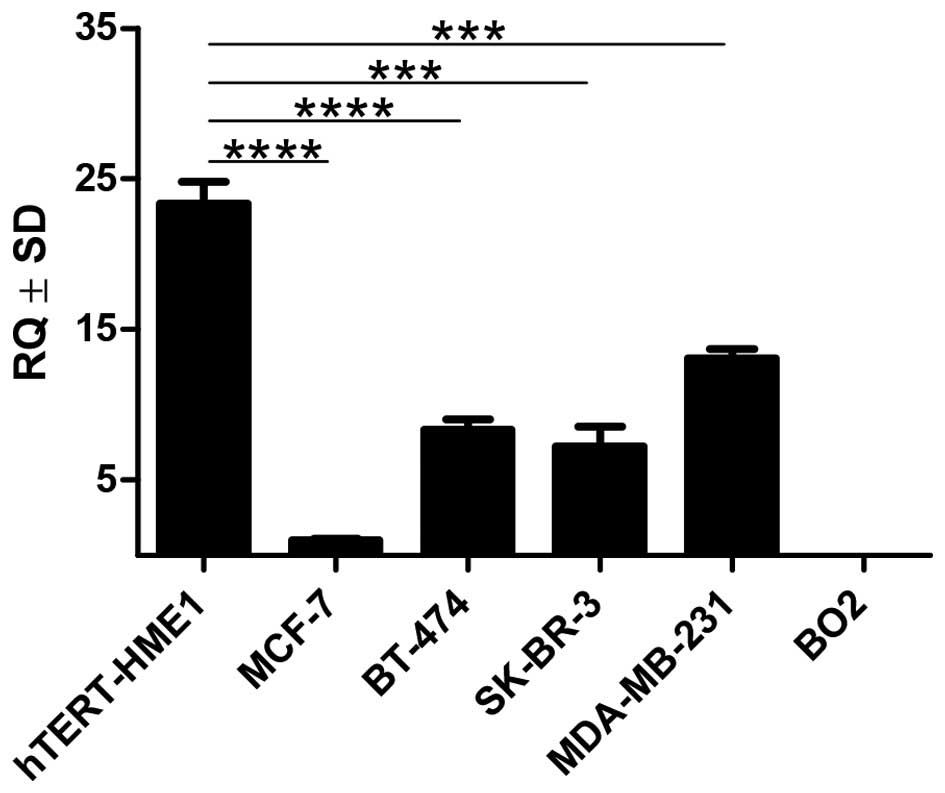
The level of MT-3 mRNA in the MCF-7, BT-474, SK-BR-3
and MDA-MB-231 breast cancer cell lines was markedly lower than in
the hTERT-HME1 line of normal human breast epithelial cells
(p<0.001). The BO2 cell line showed no expression of MT-3
(Fig. 9).
Discussion
MT-3 was discovered in the central nervous system as
a growth inhibitory factor (GIF) (6,29).
In vitro studies have shown that its presence inhibits the
growth of nerve cells and neurite formation (6,30,33).
A similar effect has been observed in glial cells (49). There is also evidence that MT-3
protects cells against the harmful effects of free radicals,
prevents DNA damage, and participates in repair processes (50–54).
The role of MT-3 outside the central nervous system has not yet
been clarified. Also unknown is the cause of the changes that are
seen in the expression of this protein in tumors.
The increased expression of MT-3 was originally
described in cancers of the prostate and the bladder. It was found
that the level of this protein correlates with the tumor malignancy
grade (36,39). The results in bladder cancer appear
particularly promising: they suggest that MT-3 may be an effective
biomarker in this type of tumor (39). The increased expression of MT-3 has
also been observed in breast cancers, but its prognostic value
cannot be clearly described on the basis of these results. Sens
et al initially observed that increased expression of MT-3
is associated with poor prognosis (40). Although no significant relationship
was found between the expression of MT-3 and the
clinicopathological factors they investigated, the level of MT-3
protein was significantly higher in cases characterized by an
unfavorable course of the disease. The difference was even more
pronounced in the preinvasive ductal breast cancer DCIS (40). Later, the same group of researchers
published the results of a study on MT-3 expression in breast
cancer carried out on a larger group of cases. It was observed that
the lack of MT-3 expression is a rare but favorable prognostic
factor. However, the previous results were not confirmed, and no
association was found between the level of MT-3 and the prognosis
(55).
Our studies were carried out on a homogeneous and
well-characterized group of invasive ductal breast cancers (IDC).
To compare the level of expression of the MT-3 protein in cancerous
and non-cancerous tissue, we used cases of mastopathy. The presence
of the MT-3 protein was demonstrated by immunohistochemistry in
breast cancer cells as well as in normal mammary gland cells within
the mastopathies. The MT-3 expression, on both the protein and mRNA
levels, was significantly lower in cases of breast cancer than in
non-malignant tissue. This result differs fundamentally from those
of previous publications, which showed a lack of MT-3 expression in
normal breast tissue. As a consequence, changes in the expression
of MT-3 in cases of breast cancer were interpreted as
overexpression (40,55).
Furthermore, in our study we observed that
expression of MT-3 in cancer cells and normal breast cells occurs
in both the cytoplasm and the cell nuclei. For this reason, the
evaluation of the immunohistochemical reaction was carried out
separately in each cellular compartment. The importance of
subcellular localization of MTs has already been seen in the case
of MT-1 and MT-2. The translocation of these proteins from the
cytoplasm to the nucleus was observed, for instance, in the region
of hyperplasia in certain cancers as well as in vitro in
cells that were either proliferating or treated with high
concentrations of glucose and cytostatics (56–59).
It also seems that the separate assessment of the cytoplasmic and
nuclear fractions of MT-1 and MT-2 within the tumor cells may have
prognostic and predictive significance (26,60,61).
This has been confirmed by recent studies of MT-3 expression in
non-small cell lung cancers. It was shown that the nuclear
expression of MT-3 was significantly lower in cases of lung cancer
than in normal tissue, and decreased with the increasing malignancy
of cancer. In turn, the cytoplasmic expression of MT-3 in cases of
lung cancer was higher than in normal tissue. It was also found
that a lower cytoplasmic expression of MT-3 correlated positively
with the size of the primary tumor and is associated with a poor
prognosis (43). In our study, we
have shown that both the nuclear and cytoplasmic expression of the
MT-3 protein in cases of breast cancer is significantly lower than
in cases of non-cancerous tissue. However, we observed no
significant correlation between the level of MT-3 protein and the
clinicopathological factors and prognosis. The difference in the
expression of MT-3 in breast cancer cells and in non-cancerous
tissue suggests that MT-3 may play the role of suppressor of the
malignant transformation of breast epithelial cells.
It is also worth noting that we examined expression
of the MT-3 protein in triple-negative and non-triple-negative
breast cancer cases. It is well known that breast cancers of the
triple-negative phenotype, which do not express ER, PR and HER2,
are characterized by a more aggressive course, poor response to
standard treatment and significantly worse prognosis (62,63).
We have found that nuclear expression of MT-3 is significantly
lower in triple-negative breast cancer cases as compared to the
other studied breast cancer samples. Interestingly, in our study
nuclear expression of MT-3 in triple-negative breast cancers was
associated with significantly shorter overall survival of patients.
A similar result was obtained by Kmiecik et al, however this
phenomenon is now difficult to explain, and requires further
studies (64).
Our results are consistent with other authors who
showed that expression of MT-3 is decreased in gastric carcinoma,
esophageal squamous cell carcinoma, and adenocarcinoma of the
esophagus (41,42,65,66).
As in our study, it has not been shown that the reduced expression
of MT-3 has any relationship with clinicopathological factors or
survival. It has been found that underlying this phenomenon are
epigenetic processes such as DNA methylation within intron 1 and
the promoter of the MT-3 gene, as well as modifications of histones
(42,65,66).
Although the mechanism by which the expression of the MT-3 gene is
silenced in tumors of the gastrointestinal tract has been
determined by in vitro studies, the causes of this
phenomenon are unknown. Initially the relationship between the
degree of methylation of the MT-3 gene and prognostic factors was
unknown (42,65). However, detailed analysis of the
profile of epigenetic changes in cancers of the esophagus showed
that methylation of only a specific region within the promoter of
the MT-3 gene is responsible for the inhibition of transcription
and strongly correlates with the progression of cancer and
metastases in the lymph nodes (66). A decrease of MT-3 expression has
also been observed in bone marrow cells in the course of acute
lymphoblastic leukemia in children (67). As with the gastrointestinal tract
tumors, the inhibition of MT-3 synthesis was the result of
hypermethylation in the promoter of MT-3 gene.
The reduced expression of MT-3, seen in the cells of
some cancers, could suggest the suppressive role of the protein,
analogously to its function in the nervous system. In vitro
studies using stable transfection have demonstrated that MT-3 can
inhibit the growth not only of neurons, but also cells of
epithelial origin, such as PC-3 prostate cancer cells or MCF-7 and
Hs578T breast cancer cells (68,69).
However, the growth inhibitory activity of MT-3 was not confirmed
in all breast cancer cell lines, since the overexpression of this
protein had no effect on the growth of T-47D, MDA-MB-231, and BO2
cells (64,69). The inhibition of cell proliferation
associated with the overexpression of MT-3 has also been found in
the Eca-109 and TE13 esophageal cancer cells as well as in the
HL-60 and MV4–11 leukemia cells (67,70).
It is possible, that the decrease of MT-3 expression, associated
with malignant transformation, results in the loss of certain
suppressor mechanisms by cancer cells. This may aid cell
proliferation, promote tumor development and facilitate metastases.
Confirmation of this hypothesis may come from the results of the
gene expression profile in primary tumors and metastases. Studies
have shown that downregulation of MT-3 is one of the seventeen
changes in gene expression characteristic of metastases and clearly
correlates with a poor clinical outcome (71). A decrease of MT-3 expression was
also observed in metastases of pituitary gland adenocarcinoma to
the spinal cord (72). In the
present study we have shown that in cases of breast cancer with
metastases to lymph nodes, the MT-3 mRNA level was significantly
lower than in cases without lymph node involvement.
The results obtained in the in vitro model
also confirm our findings in the clinical specimens. We observed
that the level of MT-3 mRNA in the MCF-7, BT-474, SK-BR-3, and
MDA-MB-231 breast cancer cell lines were significantly lower than
in the hTERT-HME1 line of normal human breast epithelial cells.
Interestingly, the BO2 cell line, which is a metastasis of
MDA-MB-231 cells to the bone, showed no expression of MT-3.
In conclusion, we have shown that the expression of
MT-3 in cases of ductal breast cancer is decreased, compared to
non-malignant breast tissue. The results of this study suggest that
MT-3 may play a role in the pathogenesis and progression of breast
cancer.
Acknowledgements
This study was supported by research funds from the
Ministry of Science and Higher Education, research project no. N
N401 005437. Bartosz Pula was supported by the ‘START’ stipend
awarded by the Foundation for Polish Science.
References
|
1
|
Coyle P, Philcox JC, Carey LC and Rofe AM:
Metallothionein: the multipurpose protein. Cell Mol Life Sci.
59:627–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romero-Isart N and Vasák M: Advances in
the structure and chemistry of metallothioneins. J Inorg Biochem.
88:388–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dziegiel P, Pula B, Kobierzycki C,
Stasiolek M and Podhorska-Okolow M: Metallothioneins: structure and
functions. Adv Anat Embryol Cell Biol. 218:3–20. 2016. View Article : Google Scholar
|
|
4
|
Vasák M: Advances in metallothionein
structure and functions. J Trace Elem Med Biol. 19:13–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thirumoorthy N, Manisenthil Kumar KT,
Shyam Sundar A, Panayappan L and Chatterjee M: Metallothionein: an
overview. World J Gastroenterol. 13:993–996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchida Y, Takio K, Titani K, Ihara Y and
Tomonaga M: The growth inhibitory factor that is deficient in the
Alzheimer’s disease brain is a 68 amino acid metallothionein-like
protein. Neuron. 7:337–347. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quaife CJ, Findley SD, Erickson JC,
Froelick GJ, Kelly EJ, Zambrowicz BP and Palmiter RD: Induction of
a new metallothionein isoform (MT-IV) occurs during differentiation
of stratified squamous epithelia. Biochemistry. 33:7250–7259. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moffatt P and Séguin C: Expression of the
gene encoding metallothionein-3 in organs of the reproductive
system. DNA Cell Biol. 17:501–510. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haq F, Mahoney M and Koropatnick J:
Signaling events for metallothionein induction. Mutat Res.
533:211–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hozumi I, Suzuki JS, Kanazawa H, Hara A,
Saio M, Inuzuka T, Miyairi S, Naganuma A and Tohyama C:
Metallothionein-3 is expressed in the brain and various peripheral
organs of the rat. Neurosci Lett. 438:54–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato M and Kondoh M: Recent studies on
metallothionein: protection against toxicity of heavy metals and
oxygen free radicals. Tohoku J Exp Med. 196:9–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Theocharis SE, Margeli AP and Koutselinis
A: Metallothionein: a multifunctional protein from toxicity to
cancer. Int J Biol Markers. 18:162–169. 2003.PubMed/NCBI
|
|
13
|
Cherian MG, Jayasurya A and Bay BH:
Metallothioneins in human tumors and potential roles in
carcinogenesis. Mutat Res. 533:201–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nielsen AE, Bohr A and Penkowa M: The
Balance between life and death of cells: roles of metallothioneins.
Biomark Insights. 7:99–111. 2007.
|
|
15
|
Theocharis SE, Margeli AP, Klijanienko JT
and Kouraklis GP: Metallothionein expression in human neoplasia.
Histopathology. 45:103–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pedersen MØ, Larsen A, Stoltenberg M and
Penkowa M: The role of metallothionein in oncogenesis and cancer
prognosis. Prog Histochem Cytochem. 44:29–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dziegiel P, Forgacz J, Suder E, Surowiak
P, Kornafel J and Zabel M: Prognostic significance of
metallothionein expression in correlation with Ki-67 expression in
adenocarcinomas of large intestine. Histol Histopathol. 18:401–407.
2003.PubMed/NCBI
|
|
18
|
Tai SK, Tan OJ, Chow VT, Jin R, Jones JL,
Tan PH, Jayasurya A and Bay BH: Differential expression of
metallothionein 1 and 2 isoforms in breast cancer lines with
different invasive potential: identification of a novel nonsilent
metallothionein-1H mutant variant. Am J Pathol. 163:2009–2019.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dziegiel P, Salwa-Zurawska W, Zurawski J,
Wojnar A and Zabel M: Prognostic significance of augmented
metallothionein (MT) expression correlated with Ki-67 antigen
expression in selected soft tissue sarcomas. Histol Histopathol.
20:83–89. 2005.
|
|
20
|
Gallicchio LM, Flaws JA, Fowler BA and
Ioffe OB: Metallothionein expression in invasive and in situ breast
carcinomas. Cancer Detect Prev. 29:332–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szajerka A, Dziegiel P, Szajerka T, Zabel
M, Winowski J and Grzebieniak Z: Immunohistochemical evaluation of
metallothionein, MCM-2 and Ki-67 antigen expression in tumors of
the adrenal cortex. Anticancer Res. 28:2959–2965. 2008.PubMed/NCBI
|
|
22
|
Cardoso SV, Silveira-Júnior JB, De
Carvalho Machado V, De-Paula AM, Loyola AM and De Aguiar MC:
Expression of metallothionein and p53 antigens are correlated in
oral squamous cell carcinoma. Anticancer Res. 29:1189–1193.
2009.PubMed/NCBI
|
|
23
|
Wojnar A, Pula B, Piotrowska A, Jethon A,
Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M and Dziegiel P:
Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2
proteins in invasive ductal breast carcinoma. Anticancer Res.
31:3027–3033. 2011.PubMed/NCBI
|
|
24
|
Wang N, Dong CR, Jiang R, Tang C, Yang L,
Jiang QF, Chen GG and Liu ZM: Overexpression of HIF-1α,
metallothionein and SLUG is associated with high TNM stage and
lymph node metastasis in papillary thyroid carcinoma. Int J Clin
Exp Pathol. 15:322–330. 2013.
|
|
25
|
Surowiak P, Matkowski R, Materna V,
Györffy B, Wojnar A, Pudelko M, Dziegiel P, Kornafel J and Zabel M:
Elevated metallothionein (MT) expression in invasive ductal breast
cancers predicts tamoxifen resistance. Histol Histopathol.
20:1037–1044. 2005.PubMed/NCBI
|
|
26
|
Surowiak P, Materna V, Maciejczyk A,
Pudeko M, Markwitz E, Spaczyski M, Dietel M, Zabel M and Lage H:
Nuclear metallothionein expression correlates with cisplatin
resistance of ovarian cancer cells and poor clinical outcome.
Virchows Arch. 450:279–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yap X, Tan HY, Huang J, Lai Y, Yip GW, Tan
PH and Bay BH: Over-expression of metallothionein predicts
chemoresistance in breast cancer. J Pathol. 217:563–570. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dziegiel P, Pula B, Kobierzycki C,
Stasiolek M and Podhorska-Okolow M: The role of metallothioneins in
carcinogenesis. Adv Anat Embryol Cell Biol. 218:29–63. 2016.
View Article : Google Scholar
|
|
29
|
Palmiter RD, Findley SD, Whitmore TE and
Durnam DM: MT-III, a brain-specific member of the metallothionein
gene family. Proc Natl Acad Sci USA. 89:6333–6337. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuji S, Kobayashi H, Uchida Y, Ihara Y
and Miyataka T: Molecular cloning of human growth inhibitory factor
cDNA and its downregulationin Alzheimer’s disease. EMBO J.
11:4843–4850. 1992.PubMed/NCBI
|
|
31
|
Sogawa CA, Asanuma M, Sogawa N, Miyazaki
I, Nakanishi T, Furuta H and Ogawa N: Localization, regulation, and
function of metallothionein-III/growth inhibitory factor in the
brain. Acta Med Okayama. 55:1–9. 2001.PubMed/NCBI
|
|
32
|
Dziegiel P, Pula B, Kobierzycki C,
Stasiolek M and Podhorska-Okolow M: Metallothionein-3. Adv Anat
Embryol Cell Biol. 218:21–27. 2016. View Article : Google Scholar
|
|
33
|
Sewell AK, Jensen LT, Erickson JC,
Palmiter RD and Winge DR: Bioactivity of metallothionein-3
correlates with its novel beta domain sequence rather than metal
binding properties. Biochemistry. 34:4740–4747. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoey JG, Garrett SH, Sens MA, Todd JH and
Sens DA: Expression of MT-3 mRNA in human kidney, proximal tubule
cell cultures, and renal cell carcinoma. Toxicol Lett. 92:149–160.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garrett SH, Sens MA, Todd JH, Somji S and
Sens DA: Expression of MT-3 protein in the human kidney. Toxicol
Lett. 105:207–214. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garrett SH, Sens MA, Shukla D, Nestor S,
Somji S, Todd JH and Sens DA: Metallothionein isoform 3 expression
in the human prostate and cancer-derived cell lines. Prostate.
41:196–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim D, Garrett SH, Sens MA, Somji S and
Sens DA: Metallothionein isoform 3 and proximal tubule vectorial
active transport. Kidney Int. 61:464–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Irie Y, Mori F, Keung WM, Mizushima Y and
Wakabayashi K: Expression of neuronal growth inhibitory factor
(metallothionein-III) in the salivary gland. Physiol Res.
53:719–723. 2004.PubMed/NCBI
|
|
39
|
Sens MA, Somji S, Lamm DL, Garrett SH,
Slovinsky F, Todd JH and Sens DA: Metallothionein isoform 3 as a
potential biomarker for human bladder cancer. Environ Health
Perspect. 108:413–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sens MA, Somji S, Garrett SH, Beall CL and
Sens DA: Metallothionein isoform 3 overexpression is associated
with breast cancers having a poor prognosis. Am J Pathol.
159:21–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
El-Rifai W, Frierson HF Jr, Harper JC,
Powell SM and Knuutila S: Expression profiling of gastric
adenocarcinoma using cDNA array. Int J Cancer. 92:832–838. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith E, Drew PA, Tian ZQ, De Young NJ,
Liu JF, Mayne GC, Ruszkiewicz AR, Watson DI and Jamieson GG:
Metallothionien 3 expression is frequently down-regulated in
oesophageal squamous cell carcinoma by DNA methylation. Mol Cancer.
13:422005. View Article : Google Scholar
|
|
43
|
Werynska B, Pula B, Muszczynska-Bernhard
B, Gomulkiewicz A, Jethon A, Podhorska-Okolow M, Jankowska R and
Dziegiel P: Expression of metallothionein-III in patients with
non-small cell lung cancer. Anticancer Res. 33:965–974.
2013.PubMed/NCBI
|
|
44
|
Ellis IO, Schnitt SJ, Sastre-Garau X,
Bussolati G, Tavassoli FA, Eusebi V, Peterse JL, Mukai K, Tabár L,
Jacquemier J, et al: Invasive breast carcinoma. Pathology and
Genetics of Tumours of the Breast and Female Genital Organs. World
Health Organization Classification of Tumours. Tavassoli FA and
Devilee P: IARC Press; Lyon: pp. 13–59. 2003
|
|
45
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
46
|
Gomulkiewicz A, Podhorska-Okolow M, Szulc
R, Smorag Z, Wojnar A, Zabel M and Dziegiel P: Correlation between
metallothionein (MT) expression and selected prognostic factors in
ductal breast cancers. Folia Histochem Cytobiol. 48:242–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mueller-Holzner E, Fink V, Frede T and
Marth C: Immunohistochemical determination of HER2 expression in
breast cancer from core biopsy specimens: a reliable predictor of
HER2 status of the whole tumor. Breast Cancer Res Treat. 69:13–19.
2001. View Article : Google Scholar
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
49
|
Amoureux MC, Wurch T and Pauwels PJ:
Modulation of metallothionein-III mRNA content and growth rate of
rat C6-glial cells by transfection with human 5-HT1D receptor
genes. Biochem Biophys Res Commun. 214:639–645. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
You HJ, Oh DH, Choi CY, Lee DG, Hahm KS,
Moon AR and Jeong HG: Protective effect of metallothionein-III on
DNA damage in response to reactive oxygen species. Biochim Biophys
Acta. 1573:33–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jeong HG, Youn CK, Cho HJ, Kim SH, Kim MH,
Kim HB, Chang IY, Lee YS, Chung MH and You HJ: Metallothionein-III
prevents gamma-ray-induced 8-oxoguanine accumulation in normal and
hOGG1-depleted cells. J Biol Chem. 279:34138–34149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hozumi I, Uchida Y, Watabe K, Sakamoto T
and Inuzuka T: Growth inhibitory factor (GIF) can protect from
brain damage due to stab wounds in rat brain. Neurosci Lett.
395:220–223. 2006. View Article : Google Scholar
|
|
53
|
Kim HG, Hwang YP and Jeong HG:
Metallothionein-III induces HIF-1 alpha-mediated VEGF expression in
brain endothelial cells. Biochem Biophys Res Commun. 369:666–671.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luo Y, Xu Y, Bao Q, Ding Z, Zhu C, Huang
ZX and Tan X: The molecular mechanism for human metallothionein-3
to protect against the neuronal cytotoxicity of Aβ(1–42) with Cu
ions. J Biol Inorg Chem. 18:39–47. 2013. View Article : Google Scholar
|
|
55
|
Somji S, Garrett SH, Zhou XD, Zheng Y,
Sens DA and Sens MA: Absence of metallothionein 3 expression in
breast cancer is a rare, but favorable marker of outcome that is
under epigenetic control. Toxicol Environ Chem. 92:1673–1695. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cherian MG and Apostolova MD: Nuclear
localization of metallothionein during cell proliferation and
differentiation. Cell Mol Biol (Noisy-le-grand). 46:347–356.
2000.
|
|
57
|
Apostolova MD, Chen S, Chakrabarti S and
Cherian MG: High-glucose-induced metallothionein expression in
endothelial cells: an endothelin-mediated mechanism. Am J Physiol
Cell Physiol. 281:C899–C907. 2001.PubMed/NCBI
|
|
58
|
Bay BH, Jin R and Jayasurya A: Analysis of
metallothionein expression in human cancers. Acta Histochem
Cytochem. 34:171–176. 2001. View Article : Google Scholar
|
|
59
|
Takahashi Y, Ogra Y and Suzuki KT: Nuclear
trafficking of metallothionein requires oxidation of a cytosolic
partner. J Cell Physiol. 202:563–569. 2005. View Article : Google Scholar
|
|
60
|
Szelachowska J, Dziegiel P,
Jelen-Krzeszewska J, Jelen M, Tarkowski R, Wlodarska I, Spytkowska
B, Gisterek I, Matkowski R and Kornafel J: Prognostic significance
of nuclear and cytoplasmic expression of metallothioneins as
related to proliferative activity in squamous cell carcinomas of
oral cavity. Histol Histopathol. 23:843–851. 2008.PubMed/NCBI
|
|
61
|
Kobierzycki C, Pula B, Skiba M, Jablonska
K, Latkowski K, Zabel M, Nowak-Markwitz E, Spaczynski M, Kedzia W,
Podhorska-Okolow M, et al: Comparison of minichromosome maintenance
proteins (MCM-3, MCM-7) and metallothioneins (MT-I/II, MT-III)
expression in relation to clinicopathological data in ovarian
cancer. Anticancer Res. 33:5375–5383. 2013.PubMed/NCBI
|
|
62
|
Elias AD: Triple-negative breast cancer: a
short review. Am J Clin Oncol. 33:637–645. 2010. View Article : Google Scholar
|
|
63
|
Lin NU, Vanderplas A, Hughes ME, Theriault
RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP and Weeks JC:
Clinicopathologic features, patterns of recurrence, and survival
among women with triple-negative breast cancer in the National
Comprehensive Cancer Network. Cancer. 118:5463–5472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kmiecik AM, Pula B, Suchanski J, Olbromski
M, Gomulkiewicz A, Owczarek T, Kruczak A, Ambicka A, Rys J,
Ugorski, et al: Metallothionein-3 increases triple-negative breast
cancer cell invasiveness via induction of metalloproteinase
expression. PLoS One. 10:e01248652015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Deng D, El-Rifai W, Ji J, Zhu B, Trampont
P, Li J, Smith MF and Powel SM: Hypermethylation of
metallothionein-3 CpG island in gastric carcinoma. Carcinogenesis.
24:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng D, Hu TL, Jiang A, Washington MK,
Moskaluk CA, Schneider-Stock R and El-Rifai W: Location-specific
epigenetic regulation of the metallothionein 3 gene in esophageal
adenocarcinomas. PLoS One. 6:e220092011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tao YF, Xu LX, Lu J, Cao L, Li ZH, Hu SY,
Wang NN, Du XJ, Sun LC, Zhao WL, et al: Metallothionein III (MT3)
is a putative tumor suppressor gene that is frequently inactivated
in pediatric acute myeloid leukemia by promoter hypermethylation. J
Transl Med. 12:1822014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dutta R, Sens DA, Somji S, Sens MA and
Garrett SH: Metallothionein isoform 3 expression inhibits cell
growth and increases drug resistance of PC-3 prostate cancer cells.
Prostate. 52:89–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gurel V, Sens DA, Somji S, Garrett SH,
Nath J and Sens MA: Stable transfection and overexpression of
metallothionein isoform 3 inhibits the growth of MCF-7 and Hs578T
cells but not that of T-47D or MDA-MB-231 cells. Breast Cancer Res
Treat. 80:181–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tian ZQ, Xu YZ, Zhang YF, Ma GF, He M and
Wang GY: Effects of metallothionein-3 and metallothionein-1E gene
transfection on proliferation, cell cycle, and apoptosis of
esophageal cancer cells. Genet Mol Res. 12:4595–4603. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar
|
|
72
|
Giorgi RR, Correa-Giannella ML, Casarini
AP, Machado MC, Bronstein MD, Cescato VA and Giannella-Neto D:
Metallothionein isoform 3 gene is differentially expressed in
corticotropin-producing pituitary adenomas. Neuroendocrinology.
82:208–214. 2005. View Article : Google Scholar
|