Introduction
Malignant pleural mesothelioma (MPM) from exposure
to asbestos is an aggressive tumor that arises from mesothelial
cells lining the intrathoracic cavities, and its worldwide
incidence continues to increase (1). The latency period between the time of
initial exposure and diagnosis is approximately 30 years although
it ranges from 20 to 50 years, and it will continue to be a health
concern globally in the next few decades with the continued use of
asbestos in developing countries (1,2).
Depending on different etiologies of the disease, MPM can be
divided into four types: epithelioid, sarcomatoid, desmoplastic and
biphasic, according to the World Health Organization (WHO)
classification of pleural tumors (3). MPM patients have poor prognosis and
current treatment strategies are limited. The median survival time
for the main types (epithelioid, sarcomatoid and biphasic) is only
18, 8 and 11 months, respectively (4–6).
Aggressive surgery, screening with proposed biomarkers, advances in
modern systemic chemotherapy using the combination of pemetrexed
and cisplatin, combination of radiotherapy and chemotherapy
regimens as well as combined treatment with preoperative radiation
followed by surgery are currently being tested, but their benefits
and long-term survival in patients with MPM is still rare (7–9). The
absence of major improvements in the survival rate has further
facilitated research into identifying new strategies aimed at
improving MPM survival. To improve these survival rates, more
targeted therapies with reduced toxicity are needed. Investigating
molecular analyses of MPM samples has led to novel targeted
strategies that inhibit specific key molecules in tumor growth and
progression.
We have previously screened for potential molecular
targets for diagnosis and/or treatment of advanced lung cancer and
MPM by analyzing gene expression using quantitative
reverse-transcriptase polymerase chain reaction (qRT-PCR) and RNA
interference (RNAi) techniques (10,11)
based on profiles of various databases, such as
NCBI-Gene® (http://www.ncbi.nlm.nih.gov/gene),
GeneCards® (http://www.genecards.org/), GenomeRNAi®
(http://genomernai.dkfz.de/GenomeRNAi//) and
CTDatabase® (http://www.cta.lncc.br/). Throughout these screenings,
we identified SORORIN [alias: cell division cycle associated
5 (CDCA5)] as a potential candidate target gene for the treatment
of MPM. Sororin, a cohesin-interacting protein essential for sister
chromatid cohesion, plays a novel role in the resolution of sister
chromatid arms by direct interaction with polo like kinase 1
(PLK1) (12).
The aims of the present study were to examine the
frequency of transcriptional expression of the SORORIN in addition
to PLK1, which is known for kinase of SORORIN, in MPM samples, to
investigate their functional roles in MPM cell proliferation by
using an RNAi technique, and to assess clinicopathological
relationships in MPM by immunohistochemical (IHC) study using
tissue microarray (TMA) and to explore the possibilities of these
target genes as potential molecular targets for therapeutic
agents.
Materials and methods
Malignant mesothelioma clinical samples
and tissue samples
Nine samples were taken from patients with MPMs,
including four cases of epithelioid, four biphasic and one
sarcomatoid subtypes, with written informed consent at Toronto
General Hospital (Toronto, Canada). For TMA analysis, a total of 53
MPM were obtained from patients who underwent surgery at Hokkaido
University Hospital and its affiliated hospitals between February
1990 and April 2012. Patient’s clinical information was extracted
from medical records. The protocol was approved by the appropriate
institutional review board of Hokkaido University (no. 012–0136).
Detailed information about demography and clinical characteristics
are summarized in Table I.
Postoperative pathological staging evaluation was demonstrated only
in curative operative cases (n=21), showing stage I disease in 1
case, stage II disease in 5 cases, and stage III disease in 15
cases. In all, one patient had T1 disease, 7 patients had T2
disease and 13 patients had T3 disease. A total of 9 patients had
N0 disease, 5 patients had N1 disease, and 7 patients had N2
disease (Table II). Histological
classification of tumors and stage were performed according to the
Union for International Cancer Control (UICC) pathological
tumor/node/metastasis (pTNM) classification criteria (13).
 | Table IThe clinicopathological
characteristics of patients with malignant pleural mesothelioma
(MPM). |
Table I
The clinicopathological
characteristics of patients with malignant pleural mesothelioma
(MPM).
| Variables | N (%) |
|---|
| Gender
(Male/female) | 49/4
(92.5/7.5) |
| Age, years
(mean) | 65.5 (range,
35–80) |
| Histology |
| Epithelioid | 34 (64.2) |
| Biphasic | 13 (24.5) |
| Sarcomatoid | 5 (9.4) |
| Desmoplastic | 1 (1.9) |
| Surgical
procedure |
| Extrapleural
pleuropneumonectomy (EPP) | 21 (39.6) |
| Pleural
biopsy | 28 (52.8) |
| Tumor resection of
recurrent tumors | 3 (5.7) |
| Radical
pleurectomy | 1 (1.9) |
|
| Total | 53 |
 | Table IIAssociations of SORORIN expression
and clinicopathological features in patients with
mesotheliomaa (n=21, curative
operative cases only). |
Table II
Associations of SORORIN expression
and clinicopathological features in patients with
mesotheliomaa (n=21, curative
operative cases only).
| | SORORIN
expression | |
|---|
| |
| |
|---|
| Variables | No. of cases | SORORIN-H
(n=15) | SORORIN-L
(n=6) | P-valueb |
|---|
| Age (years) |
| <60 | 6 | 3 | 3 | 0.2906 |
| ≥60 | 15 | 12 | 3 | |
| Gender |
| Male | 20 | 14 | 6 | >0.9999 |
| Female | 1 | 1 | 0 | |
| pT status |
| pT1–2 | 8 | 7 | 1 | 0.3359 |
| pT3 | 13 | 8 | 5 | |
| pN status |
| pN0 | 9 | 7 | 2 | 0.6594 |
| pN1–2 | 12 | 8 | 4 | |
| p-stage |
| I–II | 6 | 5 | 1 | 0.6227 |
| III | 15 | 10 | 5 | |
| Histological
classification |
| Epithelioid | 13 | 11 | 2 | 0.1462 |
| Biphasic | 8 | 4 | 4 | |
| Sarcomatoid | 0 | 0 | 0 | |
| Desmoplastic | 0 | 0 | 0 | |
Malignant mesothelioma cell lines
The human malignant mesothelioma cell lines used
were as follows: NCI-H28, -H226, -H2052 and -H2452 were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). All cancer cells were grown in monolayers in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS). A human adult
normal mesothelial cell line (MES-F) was purchased from Zembio Inc.
(Research Triangle Park, NC, USA) and was grown in mesothelial cell
growth medium (Zembio). All cell lines were maintained at 37°C in
atmospheres of humidified air with 5% CO2.
cDNA sample preparation
Tumor samples from MPM patients with surgery were
excised and stored at −80°C. QIAzol Lysis reagent (Qiagen,
Valencia, CA, USA) and one 5-mm stainless steel bead (Qiagen) were
added before homogenizing with a TissueLyser Adapter Set (Qiagen)
for 2 min at 20 Hz. Total RNA was then purified using a miRNeasy
Mini kit (Qiagen). The amount and purity were measured using a
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Wilmington,
DE, USA).
Quantitative RT-PCR analysis
cDNA was synthesized from 2 μg total RNA using
QuantiTect® reverse transcription kit (Qiagen). The
primers were designed as follows: for PLK1, forward primer,
5′-cccctcacagtcctcaataa-3′ and reverse primer,
5′-tgtccgaatagtccaccc-3′; for SORORIN, forward primer, 5′-cg
ccagagacttggaaatgt-3′ and reverse primer, 5′-gtttctgtttctcgggt
ggt-3′; for actin, beta (ACTB), forward primer,
5′-gaaatcgtgcgt-gacattaa-3′ and reverse primer,
5′-aaggaaggctggaagagtg-3′. qRT-PCR analysis was performed using
LightCycler480® SYBR-Green I Master Ready-to-use hot
start reaction mix and LightCycler480® system (Roche,
South San Francisco, CA, USA). The thermal cycler conditions were
as follows: 5 min at 95°C to denature, 45 cycles at 95°C for 10
sec, 56°C for 20 sec, and 72°C for 10 sec for PCR amplification,
and 1 min at 65°C for melting. The threshold cycle value was
defined as the value obtained in the PCR cycle when the
fluorescence signal increased above the background threshold. PCR
reactions were carried out in duplicate.
RNA interference and cell viability
assay
All short interference RNA (siRNA) oligonucleotide
sequences for PLK1 and SORORIN siRNAs were purchased from Qiagen
for this study. AllStar Negative Control siRNA® (Qiagen)
were used as the negative control (NC-siRNA). The final
concentration of 10 nM of siRNAs was incubated with
HiPerFect® Transfection reagent (Qiagen) according to
the manufacturer’s instructions. The PLK1 inhibitor BI 6727 was
purchased from Selleck Chemicals (Houston, TX, USA). The
CellTiter96® AQueous One Solution Cell proliferation
assay (Promega, Madison, WI, USA) was used for the evaluation of
the number of viable cells according to the manufacturer’s
instructions, using a microplate spectrophotometer (μQuant; BioTek
Instruments, Inc., Winooski, VT, USA). Each experiment was
performed in triplicate.
Tissue microarray (TMA) construction and
immunohistochemistry (IHC)
Archival slides for all the cases were reviewed to
select three representative areas for each sample by an experienced
pathologist (K.H.). TMA blocks were then constructed using a manual
tissue microarrayer (JF-4; Sakura Finetek Japan Co., Ltd., Tokyo,
Japan) with a 1.0-mm diameter needle. The finalized array blocks
were sliced into 4-μm-thick sections and mounted on glass slides.
To check the histopatho-logical diagnosis and adequacy of tissue
sampling, a section from each microarray was stained with regular
haematoxylin and eosin (H&E) and calretinin, and examined by a
pathologist (K.H.). SORORIN immunostaining were performed using an
automated IHC platform (Autostainer plus; Dako, Glostrup, Denmark).
Antigen retrieval was performed in the condition of pH 9.0 for 20
min. The detection kit used was the EnVision™+ Dual Link (K4063;
Dako), incubation with post primary for 60 min at RT. Anti-SORORIN
polyclonal antibody (bs-7717R CDCA5 Sororin antibody; Acris
Antibodies GmbH, Herford, Germany, 1/300) was diluted using mixed
antibody diluent (Dako:S2022 Antibody diluent). A polymer-based
detection system (EnVision™+ Dual Link #K4063) was used with
3′,3-Diaminobenzidine (DAB) as the chromogen. The positive controls
included a sample of testis, and normal lung and pleural samples
were used as negative controls. Slides were dehydrated and placed
on coverslips.
Evaluation of immunohistochemical
staining and statistical analysis
Digital images of IHC-stained TMA slides were
obtained using a whole slide scanner (ScanScope CS, Aperio
ePathology; Leica Microsystems, Inc., Richmond Hill, ON, Canada).
Annotation of tumor regions on whole slides was performed blinded
to clinical follow-up data using Aperio’s annotation software
(ImageScope Viewing Software: Positive Pixel Count v9.1; Aperio).
SORORIN were quantified with IHC scoring, which summated the
percentage of area stained at each intensity level multiplied by
the weighted intensity reported in other studies (14–16).
Initially, the weighted intensity of staining was graded as
follows; grade 0 (negative), 1+ (weak positive), 2+ (moderate
positive), and 3+ (strong positive) according to the Aperio’s
annotation software. According to the total amount of IHC scores,
SORORIN expression was then finally divided into two groups each
(the threshold leading to the lowest P-value in log-rank test):
low-level SORORIN expression (SORORIN-L, with an IHC score <1.2
and high-level SORORIN expression) (SORORIN-H, with an IHC score
≥1.2). We attempted to correlate clinicopathological variables such
as age, gender, pathological TNM stage and histological
classification with expression levels of SORORIN protein as
determined by TMA analysis. Immunoreactivity was assessed for
association with clinicopathological variables using the
χ2 test for variables. Kaplan-Meier method was used to
generate survival curves, and survival differences were analyzed
with the log-rank test, based on the status of SORORIN expression.
Values of P<0.05 were considered statistically significant. All
analyses were performed using the StatView version 5.0 software
(SAS Institute, Inc., Cary, NC, USA).
Results
Expression of PLK1 and SORORIN
transcripts in mesothelioma and normal human tissues
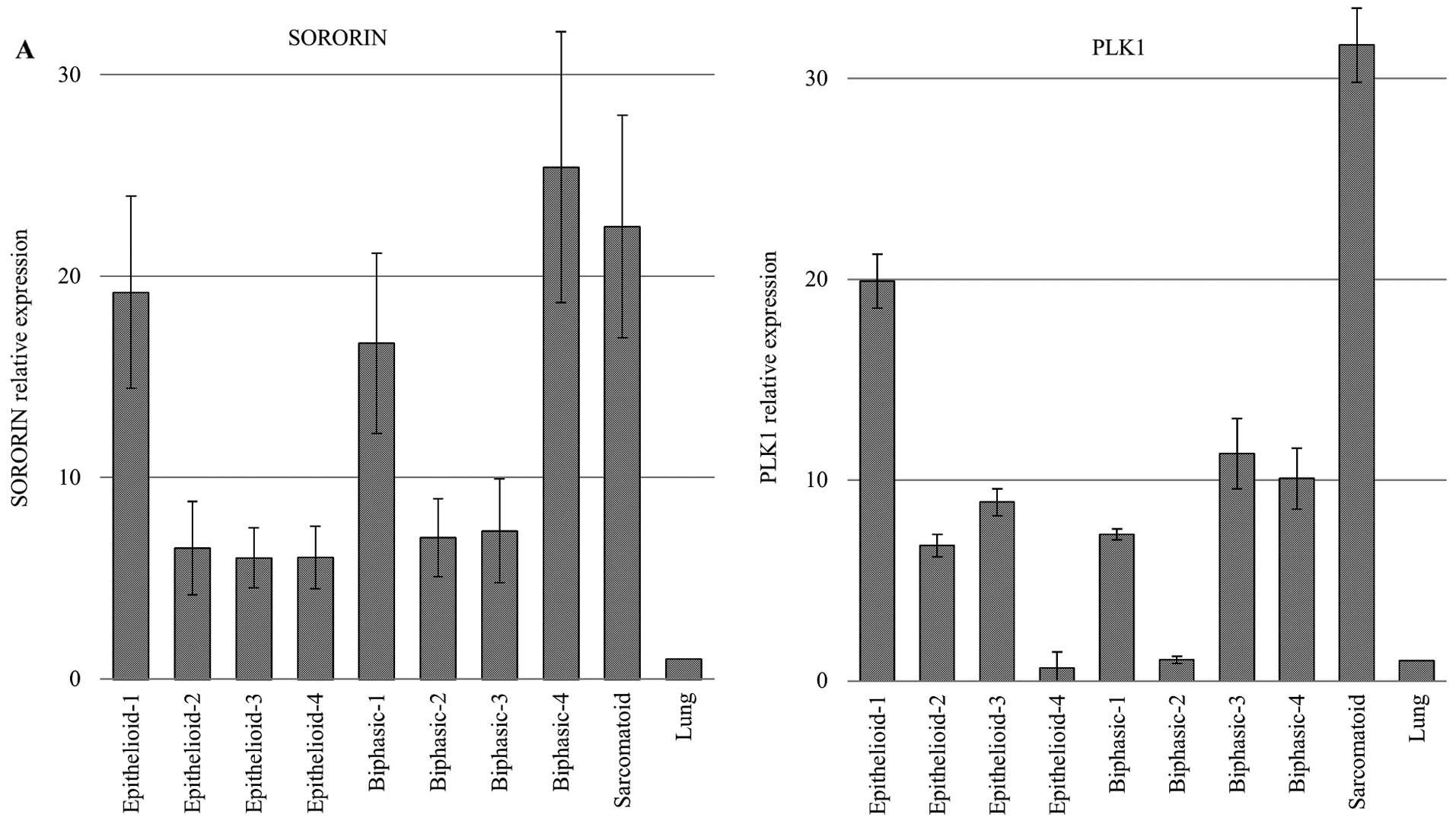
Through qRT-PCR screening for molecular targeted
genes in MPMs, we identified SORORIN and PLK1 overexpression in a
majority of MPM cases (Fig. 1A).
We also confirmed a high expression of these genes using four human
MPM cell lines and low expression in a normal human mesothelial
cell line MES-F (Fig. 1B). qRT-PCR
analysis using a cDNA panel containing normal human tissues also
showed that these genes are expressed only in the testis and thymus
among the variety of normal human organs (data not shown).
Growth inhibition of mesothelioma cells
by specific siRNA against PLK1 and SORORIN
To assess whether therapeutic candidate genes are
essential for growth and survival of MPM cells, we transfected each
specific siRNA against SORORIN (si-SORORIN-#4 and si-SORORIN-#6)
and PLK1 (si-PLK1-#2, si-PLK1-#6 and si-PLK1-#7), into human MPM
cell lines H28 and H2052 cells, using AllStar siRNA® as
the negative control (NC-siRNA). The mRNA level of transfected
cells with independent siRNAs targeting these genes was
significantly decreased in comparison to cells transfected with
control siRNAs 48 h after transfection (Fig. 2A). Next, to evaluate the
relationship between cell proliferation and gene knockdown, we
conducted a cell viability assay using the CellTiter 96®
AQueous One Solution Cell Proliferation assay. After siRNA
treatment, proliferation of H28 and H2052 cells were significantly
suppressed compared with control groups at 4 days after
transfection (Fig. 2B), suggesting
that upregulation of these candidate genes are related to growth or
survival of MPM cells. Typical microscopic images of the cells are
shown in Fig. 2C. Compared to
control cells or negative control siRNA-treated cells, SORORIN
siRNA-treated mesothelioma cells exhibited distended cell bodies
with enlarged nuclei, suggesting SORORIN-induced mitotic defects.
Next, we sought to determine how inhibition of SORORIN causes the
observed proliferation defect. The cell cycle distribution was
analyzed by flow cytometry. We found that the G2/M population was
remarkably elevated in siRNA against SORORIN-treated cells,
suggesting a potential increase in the number of mitotic cells
(Fig. 2D). These results
demonstrate that inhibition of SORORIN induced mitotic arrest,
resulting in a defect in cell cycle progression in mesothelioma
cells.
Pattern of PLK1 and SORORIN expression in
MPMs and correlation to clinicopathological parameters and
prognostic significance
We categorized SORORIN expression on the TMA
according to the IHC score described above. Positive staining of
tumor cells by SORORIN generally showed a nuclear and cytoplasmic
pattern in cancer tissue (Fig.
3A). No staining was observed in benign chronic or fibrous
pleuritis. Of the 53 MPM cases examined, SORORIN-H was observed in
27 cases (50.9%) (details are shown in Table III). Of those, 17 epithelioid
type (50.0% of 34 cases), 8 biphasic type (61.5% of 13 cases) and 2
sarcomatoid type (40.0% of 5 cases) showed SORORIN-H. We then tried
to correlate SORORIN expression with various clinicopathological
parameters. When we examined curative surgical cases, survival
analysis with Kaplan-Meier method indicated that MPM patients with
SORORIN-H had shorter overall 5-year survival tendency than those
with SORORIN-L (P=0.1664, by a log-rank test), although this was
not statistically significant (Fig.
3B). No significant association was noted between SORORIN
expression and other clinicopathological variables in these cases
(Table II).
 | Table IIIImmunopositivity of SORORIN protein
in MPMs (n=53). |
Table III
Immunopositivity of SORORIN protein
in MPMs (n=53).
| SORORIN low
expression (n=26) | SORORIN high
expression (n=27) | | |
|---|
|
|
| | |
|---|
| Histology | Negative (IHC
score: −0.599) | Weak (IHC score:
0.600–1.199) | Moderate (IHC
score: 1.200–1.799) | Strong (IHC score:
1.800-) | Total (n) | % of high
expression cases |
|---|
| Epithelioid | 8 | 9 | 9 | 8 | 34 | 50.0 |
| Biphasic | 2 | 3 | 5 | 3 | 13 | 61.5 |
| Sarcomatoid | 2 | 1 | 1 | 1 | 5 | 40.0 |
| Desmoplastic | 1 | 0 | 0 | 0 | 1 | 0.0 |
| Total | 13 | 13 | 15 | 12 | 53 | 50.9 |
Combined treatment with PLK inhibitor and
siRNA against SORORIN shows a combinational growth suppressive
effect in cell proliferation in mesothelioma cells
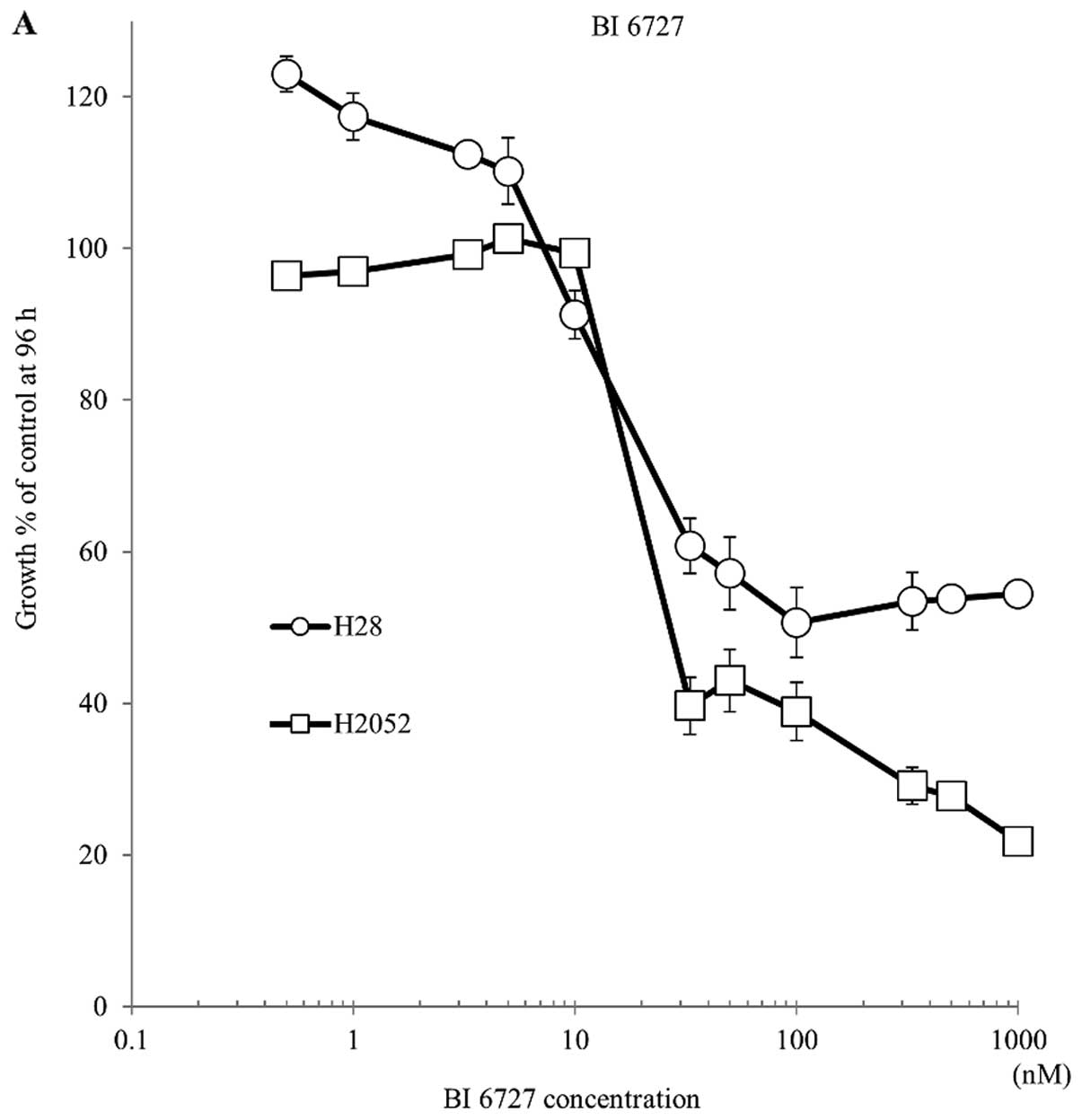
To investigate the effect of PLK inhibitor BI 6727
in mesothelioma cells, we monitored the cell viability of NCI-H28
and H2052 cells treated under various concentrations with BI 6727.
Cell growth was significantly reduced in the presence of BI 6727 at
concentrations above 10 nM in both cell lines (Fig. 4A); however, cell viability did not
decrease at concentrations >50 nM, demonstrating the growth
inhibitory effect of BI 6727 in MPM cells. Next, to determine the
combinational effect with inhibition of SORORIN and PLK1, we
treated MPM cells with both BI 6727 and siRNAs against SORORIN at
the same time. We confirmed that suppression of SORORIN showed a
significantly growth suppressive effect in cell proliferation when
combined with BI 6727 in mesothelioma cells (Fig. 4B).
Discussion
Despite improvements in surgical techniques and
adjuvant chemoradiotherapy, MPM still has poor prognosis among
malignant tumors. To investigate therapeutic molecular target genes
for patients with advanced lung cancer and MPM, we have used
qRT-PCR and RNAi-based approach. Through these screenings, we
identified six therapeutic candidate genes (KIF11, KIF23, PLK1,
NUF2, NDC80 and SORORIN) for the treatment of MPM. PLK1’s
involvement as SORORIN’s interaction protein to regulate MPM cell
growth and survival has previously been demonstrated (17,18).
These observations support our findings from RNAi-based MPM
molecular target screening. We therefore focused on SORORIN and
PLK1 axis to further characterize their suitability as therapeutic
targets of MPM.
Sororin was initially identified as a
substrate of anaphase-promoting (APC) complex and as a regulator of
sister chromatid cohesion (19,20).
Sororin protein is degraded through APC complex-dependent
ubiquitination in the G1 phase, and is required for stable binding
of cohesion molecule to chromatid (20). The stabilization of these cohesin
complexes depends on Sororin, which prevents premature removal of
cohesion from chromatin (21).
Sororin also promotes cohesin release from sister chromatid arms in
prophase via interaction with Plk1 (12,22).
These sororin-containing cohesin rings maintain sister chromatids
together during mitosis until cohesin is removed from chromosome
arms during prophase and from the centromeric region during the
metaphase-to-anaphase transition (28). Physiological regulation of
spliceosome function can act as checkpoints to influence cell
division or genome stability (21). Overexpression of SORORIN is
associated with poor prognosis in NSCLC, and siRNA-mediated
knockdown against SORORIN has been shown to inhibit cell
proliferation in NSCLC cell lines (23). In this study, we found that SORORIN
was overexpressed in majority of MPM samples and inhibition of
SORORIN led to suppression of the MPM cell growth. Identification
of anticancer drugs that target components of the spliceosome, and
mutations or upregulating factors that affect the expression of
these components may be relevant in this context (24). Given the importance of splicing and
cohesin in cancer and other human diseases (25,26),
targeting the SORORIN genes deserve further exploration in future
studies.
PLK1 gene plays a crucial role in regulation of cell
division at several points during the mitotic phase of the cell
cycle, including mitotic entry, bipolar spindle formation,
chromosome alignment, segregation of chromosomes and cytokinesis
(27,28). PLK1 overexpression is a common
event seen in various tumors, such as colorectal cancers (CRCs)
(29), head and neck (30) and ovarian cancers (31). Overexpression of PLK1 is also
associated with poor prognosis in non-small cell lung cancers
(NSCLC) (32), and short
interference RNA (siRNA)-mediated knockdown against PLK1 has been
shown to inhibit cell proliferation in NSCLC cell lines (33) and also mesothelioma (18). PLK1 should thus be a good candidate
for targeted therapy in different types of cancer. In fact,
multiple PLK1 inhibitors, including BI 2536 and BI 6727
(volasertib), have been used as a promising target in clinical
trials (34–36). However, although several clinical
trials of BI 6727 in solid tumors have been performed recently and
some patients with solid tumors have responded well to single agent
BI 6727, drug-related adverse effects were observed including
dose-dependent hematologic toxicity (37,38).
Combination therapy is a potential option to improve efficacy, and
volasertib combined with platinum chemotherapy was investigated in
this context (39). Additional
studies in advanced solid tumors are investigating volasertib with
other agents combined, including trials involving the kinase
inhibitors afatinib and nintedanib with promising antitumor
activity (40,41). In this study, we demonstrated that
significant growth suppressive effect of the combinational use of
SORORIN and PLK1 inhibition. Although further investigation will be
needed, we can reduce the dose of PLK1 inhibitor and increase the
antitumor activity by combining it with the inhibition of
SORORIN.
In conclusion, the present study demonstrates that
SORORIN and PLK1, which play crucial roles in tumor growth and cell
survival, are highly expressed in MPM. Suppression of SORORIN had
combinational growth inhibition of MPM cells when used with PLK1
inhibitor BI 6727, suggesting combination therapy targeting these
genes can be a potential new therapeutic strategy for patients with
MPMs. The urgent need for novel treatment approaches for MPM is
clear. Given our promising findings, further evaluation of these
approaches in xenograft models is recommended.
Acknowledgements
The authors thank Ms. Judy McConnell and Ms.
Alexandria Grindlay (Toronto General Hospital) for laboratory
management.
References
|
1
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options Oncol. 9:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carbone M, Kratzke RA and Testa JR: The
pathogenesis of mesothelioma. Semin Oncol. 29:2–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. 4th edition. 7. WHO Press; 2015
|
|
4
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becklake MR, Bagatin E and Neder JA:
Asbestos-related diseases of the lungs and pleura: Uses, trends and
management over the last century. Int J Tuberc Lung Dis.
11:356–369. 2007.PubMed/NCBI
|
|
7
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zucali PA, De Vincenzo F, Simonelli M and
Santoro A: Future developments in the management of malignant
pleural mesothelioma. Expert Rev Anticancer Ther. 9:453–467. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho BC, Feld R, Leighl N, Opitz I, Anraku
M, Tsao MS, Hwang DM, Hope A and de Perrot M: A feasibility study
evaluating Surgery for Mesothelioma After Radiation Therapy: The
‘SMART’ approach for resectable malignant pleural mesothelioma. J
Thorac Oncol. 9:397–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato T, Wada H, Patel P, Hu HP, Lee D,
Ujiie H, Hirohashi K, Nakajima T, Sato M, Kaji M, et al:
Overexpression of KIF23 predicts clinical outcome in primary lung
cancer patients. Lung Cancer. 92:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato T, Lee D, Wu L, Patel P, Young AJ,
Wada H, Hu HP, Ujiie H, Kaji M, Kano S, et al: Kinesin family
members KIF11 and KIF23 as potential therapeutic targets in
malignant pleural mesothelioma. Int J Oncol. 49:448–456.
2016.PubMed/NCBI
|
|
12
|
Zhang N, Panigrahi AK, Mao Q and Pati D:
Interaction of Sororin protein with polo-like kinase 1 mediates
resolution of chromosomal arm cohesion. J Biol Chem.
286:41826–41837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Union for International Cancer Control
(UICC). TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; 2009
|
|
14
|
Rizzardi AE, Johnson AT, Vogel RI,
Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ and Schmechel
SC: Quantitative comparison of immunohistochemical staining
measured by digital image analysis versus pathologist visual
scoring. Diagn Pathol. 7:422012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagashio R, Sato Y, Jiang SX, Ryuge S,
Kodera Y, Maeda T and Nakajima T: Detection of tumor-specific
autoantibodies in sera of patients with lung cancer. Lung Cancer.
62:364–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagashio R, Sato Y, Matsumoto T, Kageyama
T, Satoh Y, Shinichiro R, Masuda N, Goshima N, Jiang SX and Okayasu
I: Expression of RACK1 is a novel biomarker in pulmonary
adenocarcinomas. Lung Cancer. 69:54–59. 2010. View Article : Google Scholar
|
|
17
|
Kawata E, Ashihara E, Nakagawa Y, Kiuchi
T, Ogura M, Yao H, Sakai K, Tanaka R, Nagao R, Yokota A, et al: A
combination of a DNA-chimera siRNA against PLK-1 and zoledronic
acid suppresses the growth of malignant mesothelioma cells in
vitro. Cancer Lett. 294:245–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linton A, Cheng YY, Griggs K, Kirschner
MB, Gattani S, Srikaran S, Chuan-Hao Kao S, McCaughan BC, Klebe S,
van Zandwijk N, et al: An RNAi-based screen reveals PLK1, CDK1 and
NDC80 as potential therapeutic targets in malignant pleural
mesothelioma. Br J Cancer. 110:510–519. 2014. View Article : Google Scholar :
|
|
19
|
Rankin S, Ayad NG and Kirschner MW:
Sororin, a substrate of the anaphase-promoting complex, is required
for sister chromatid cohesion in vertebrates. Mol Cell. 18:185–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmitz J, Watrin E, Lénárt P, Mechtler K
and Peters JM: Sororin is required for stable binding of cohesin to
chromatin and for sister chromatid cohesion in interphase. Curr
Biol. 17:630–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valcárcel J and Malumbres M: Splicing
together sister chromatids. EMBO J. 33:2601–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N and Pati D: Sororin is a master
regulator of sister chromatid cohesion and separation. Cell Cycle.
11:2073–2083. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen MH, Koinuma J, Ueda K, Ito T,
Tsuchiya E, Nakamura Y and Daigo Y: Phosphorylation and activation
of cell division cycle associated 5 by mitogen-activated protein
kinase play a crucial role in human lung carcinogenesis. Cancer
Res. 70:5337–5347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonnal S, Vigevani L and Valcárcel J: The
spliceosome as a target of novel antitumour drugs. Nat Rev Drug
Discov. 11:847–859. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper TA, Wan L and Dreyfuss G: RNA and
disease. Cell. 136:777–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Losada A: Cohesin in cancer: Chromosome
segregation and beyond. Nat Rev Cancer. 14:389–393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van de Weerdt BC and Medema RH: Polo-like
kinases: A team in control of the division. Cell Cycle. 5:853–864.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barr FA, Silljé HH and Nigg EA: Polo-like
kinases and the orchestration of cell division. Nat Rev Mol Cell
Biol. 5:429–440. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi T, Sano B, Nagata T, Kato H,
Sugiyama Y, Kunieda K, Kimura M, Okano Y and Saji S: Polo-like
kinase 1 (PLK1) is overexpressed in primary colorectal cancers.
Cancer Sci. 94:148–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knecht R, Elez R, Oechler M, Solbach C,
von Ilberg C and Strebhardt K: Prognostic significance of polo-like
kinase (PLK) expression in squamous cell carcinomas of the head and
neck. Cancer Res. 59:2794–2797. 1999.PubMed/NCBI
|
|
31
|
Weichert W, Denkert C, Schmidt M, Gekeler
V, Wolf G, Köbel M, Dietel M and Hauptmann S: Polo-like kinase
isoform expression is a prognostic factor in ovarian carcinoma. Br
J Cancer. 90:815–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolf G, Elez R, Doermer A, Holtrich U,
Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H and
Strebhardt K: Prognostic significance of polo-like kinase (PLK)
expression in non-small cell lung cancer. Oncogene. 14:543–549.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawata E, Ashihara E and Maekawa T: RNA
interference against polo-like kinase-1 in advanced non-small cell
lung cancers. J Clin Bioinforma. 1:62011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sebastian M, Reck M, Waller CF, Kortsik C,
Frickhofen N, Schuler M, Fritsch H, Gaschler-Markefski B, Hanft G,
Munzert G, et al: The efficacy and safety of BI 2536, a novel Plk-1
inhibitor, in patients with stage IIIB/IV non-small cell lung
cancer who had relapsed after, or failed, chemotherapy: Results
from an open-label, randomized phase II clinical trial. J Thorac
Oncol. 5:1060–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ellis PM, Chu QS, Leighl N, Laurie SA,
Fritsch H, Gaschler-Markefski B, Gyorffy S and Munzert G: A phase I
open-label dose-escalation study of intravenous BI 2536 together
with pemetrexed in previously treated patients with non-small-cell
lung cancer. Clin Lung Cancer. 14:19–27. 2013. View Article : Google Scholar
|
|
36
|
Liu X: Targeting Polo-Like Kinases: A
Promising Therapeutic approach for cancer treatment. Transl Oncol.
8:185–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schöffski P, Awada A, Dumez H, Gil T,
Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P and Munzert
G: A phase I, dose-escalation study of the novel Polo-like kinase
inhibitor volasertib (BI 6727) in patients with advanced solid
tumours. Eur J Cancer. 48:179–186. 2012. View Article : Google Scholar
|
|
38
|
Stadler WM, Vaughn DJ, Sonpavde G,
Vogelzang NJ, Tagawa ST, Petrylak DP, Rosen P, Lin CC, Mahoney J,
Modi S, et al: An open-label, single-arm, phase 2 trial of the
Polo-like kinase inhibitor volasertib (BI 6727) in patients with
locally advanced or metastatic urothelial cancer. Cancer.
120:976–982. 2014. View Article : Google Scholar
|
|
39
|
Awada A, Dumez H, Aftimos PG, Costermans
J, Bartholomeus S, Forceville K, Berghmans T, Meeus MA, Cescutti J,
Munzert G, et al: Phase I trial of volasertib, a Polo-like kinase
inhibitor, plus platinum agents in solid tumors: Safety,
pharmacokinetics and activity. Invest New Drugs. 33:611–620. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Machiels JP, Peeters M, Herremans C,
Surmont V, Specenier P, De Smet M, Pilz K, Strelkowa N, Liu D and
Rottey S: A phase I study of volasertib combined with afatinib, in
advanced solid tumors. Cancer Chemother Pharmacol. 76:843–851.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Braud F, Cascinu S, Spitaleri G, Pilz
K, Clementi L, Liu D, Sikken P and De Pas T: A phase I,
dose-escalation study of volasertib combined with nintedanib in
advanced solid tumors. Ann Oncol. 26:2341–2346. 2015. View Article : Google Scholar : PubMed/NCBI
|