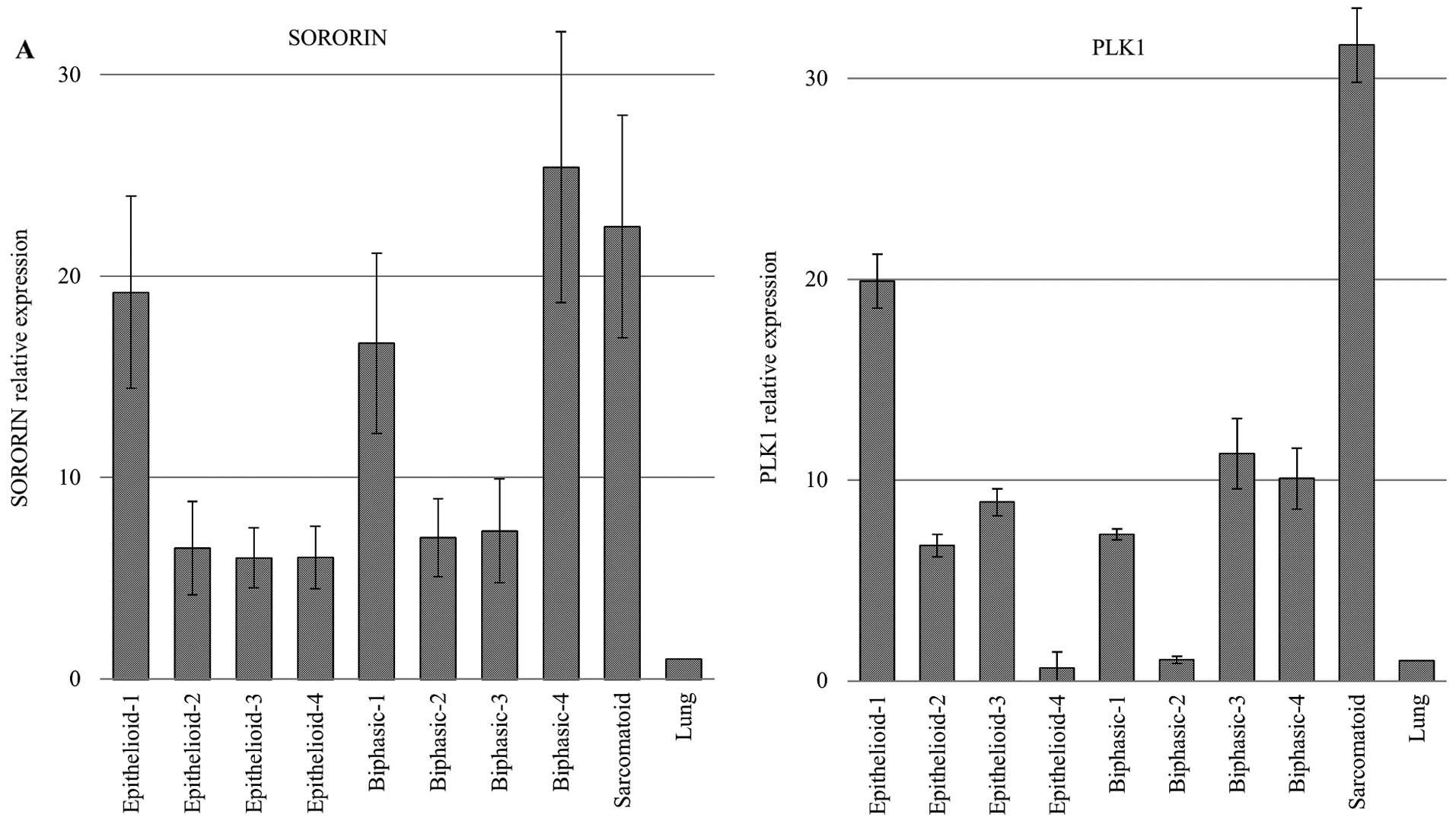
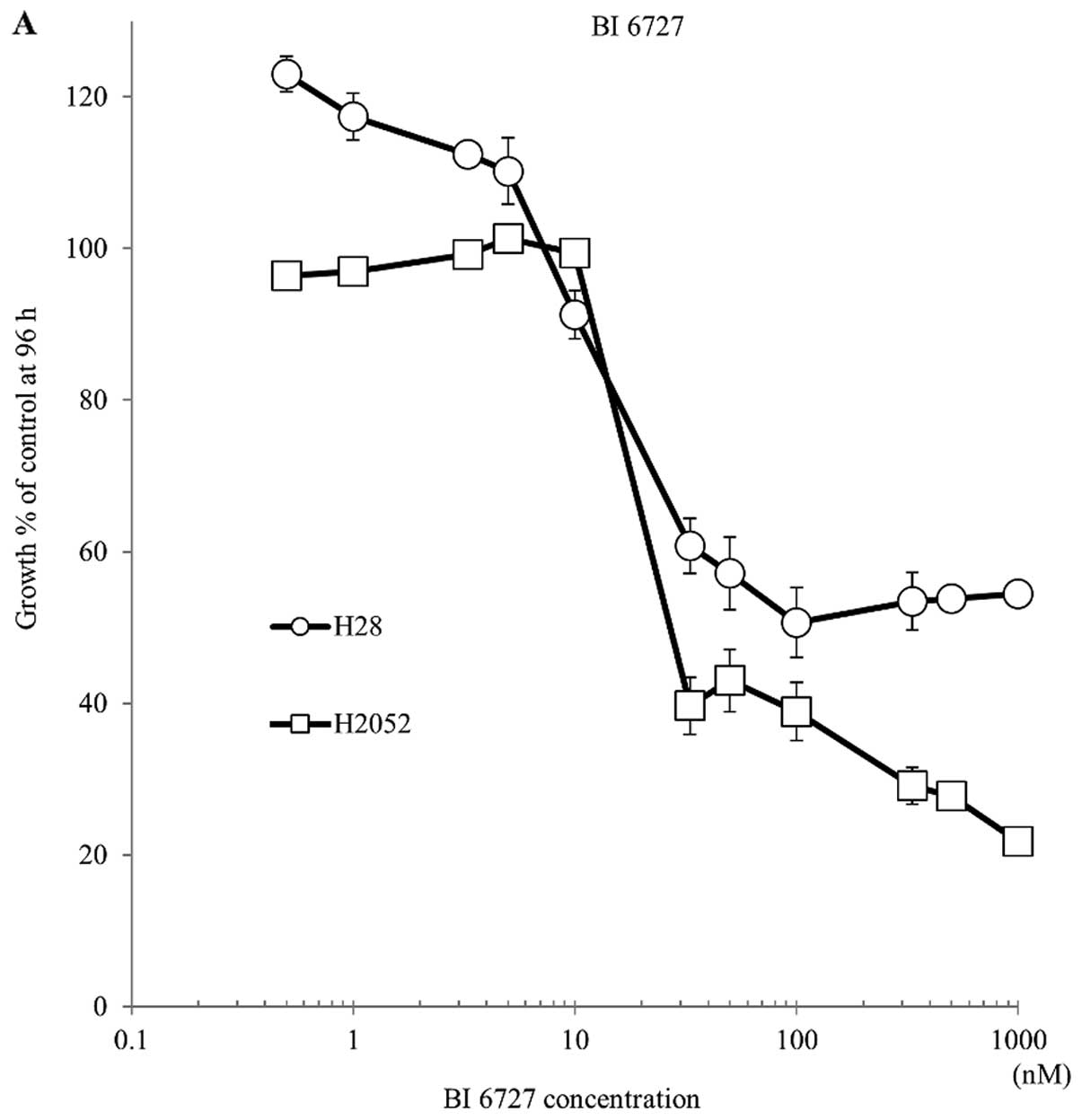
|
1
|
Yang H, Testa JR and Carbone M:
Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr
Treat Options Oncol. 9:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carbone M, Kratzke RA and Testa JR: The
pathogenesis of mesothelioma. Semin Oncol. 29:2–17. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. 4th edition. 7. WHO Press; 2015
|
|
4
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becklake MR, Bagatin E and Neder JA:
Asbestos-related diseases of the lungs and pleura: Uses, trends and
management over the last century. Int J Tuberc Lung Dis.
11:356–369. 2007.PubMed/NCBI
|
|
7
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zucali PA, De Vincenzo F, Simonelli M and
Santoro A: Future developments in the management of malignant
pleural mesothelioma. Expert Rev Anticancer Ther. 9:453–467. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho BC, Feld R, Leighl N, Opitz I, Anraku
M, Tsao MS, Hwang DM, Hope A and de Perrot M: A feasibility study
evaluating Surgery for Mesothelioma After Radiation Therapy: The
‘SMART’ approach for resectable malignant pleural mesothelioma. J
Thorac Oncol. 9:397–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato T, Wada H, Patel P, Hu HP, Lee D,
Ujiie H, Hirohashi K, Nakajima T, Sato M, Kaji M, et al:
Overexpression of KIF23 predicts clinical outcome in primary lung
cancer patients. Lung Cancer. 92:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato T, Lee D, Wu L, Patel P, Young AJ,
Wada H, Hu HP, Ujiie H, Kaji M, Kano S, et al: Kinesin family
members KIF11 and KIF23 as potential therapeutic targets in
malignant pleural mesothelioma. Int J Oncol. 49:448–456.
2016.PubMed/NCBI
|
|
12
|
Zhang N, Panigrahi AK, Mao Q and Pati D:
Interaction of Sororin protein with polo-like kinase 1 mediates
resolution of chromosomal arm cohesion. J Biol Chem.
286:41826–41837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Union for International Cancer Control
(UICC). TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; 2009
|
|
14
|
Rizzardi AE, Johnson AT, Vogel RI,
Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ and Schmechel
SC: Quantitative comparison of immunohistochemical staining
measured by digital image analysis versus pathologist visual
scoring. Diagn Pathol. 7:422012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagashio R, Sato Y, Jiang SX, Ryuge S,
Kodera Y, Maeda T and Nakajima T: Detection of tumor-specific
autoantibodies in sera of patients with lung cancer. Lung Cancer.
62:364–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagashio R, Sato Y, Matsumoto T, Kageyama
T, Satoh Y, Shinichiro R, Masuda N, Goshima N, Jiang SX and Okayasu
I: Expression of RACK1 is a novel biomarker in pulmonary
adenocarcinomas. Lung Cancer. 69:54–59. 2010. View Article : Google Scholar
|
|
17
|
Kawata E, Ashihara E, Nakagawa Y, Kiuchi
T, Ogura M, Yao H, Sakai K, Tanaka R, Nagao R, Yokota A, et al: A
combination of a DNA-chimera siRNA against PLK-1 and zoledronic
acid suppresses the growth of malignant mesothelioma cells in
vitro. Cancer Lett. 294:245–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linton A, Cheng YY, Griggs K, Kirschner
MB, Gattani S, Srikaran S, Chuan-Hao Kao S, McCaughan BC, Klebe S,
van Zandwijk N, et al: An RNAi-based screen reveals PLK1, CDK1 and
NDC80 as potential therapeutic targets in malignant pleural
mesothelioma. Br J Cancer. 110:510–519. 2014. View Article : Google Scholar :
|
|
19
|
Rankin S, Ayad NG and Kirschner MW:
Sororin, a substrate of the anaphase-promoting complex, is required
for sister chromatid cohesion in vertebrates. Mol Cell. 18:185–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmitz J, Watrin E, Lénárt P, Mechtler K
and Peters JM: Sororin is required for stable binding of cohesin to
chromatin and for sister chromatid cohesion in interphase. Curr
Biol. 17:630–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valcárcel J and Malumbres M: Splicing
together sister chromatids. EMBO J. 33:2601–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N and Pati D: Sororin is a master
regulator of sister chromatid cohesion and separation. Cell Cycle.
11:2073–2083. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen MH, Koinuma J, Ueda K, Ito T,
Tsuchiya E, Nakamura Y and Daigo Y: Phosphorylation and activation
of cell division cycle associated 5 by mitogen-activated protein
kinase play a crucial role in human lung carcinogenesis. Cancer
Res. 70:5337–5347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonnal S, Vigevani L and Valcárcel J: The
spliceosome as a target of novel antitumour drugs. Nat Rev Drug
Discov. 11:847–859. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper TA, Wan L and Dreyfuss G: RNA and
disease. Cell. 136:777–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Losada A: Cohesin in cancer: Chromosome
segregation and beyond. Nat Rev Cancer. 14:389–393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van de Weerdt BC and Medema RH: Polo-like
kinases: A team in control of the division. Cell Cycle. 5:853–864.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barr FA, Silljé HH and Nigg EA: Polo-like
kinases and the orchestration of cell division. Nat Rev Mol Cell
Biol. 5:429–440. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi T, Sano B, Nagata T, Kato H,
Sugiyama Y, Kunieda K, Kimura M, Okano Y and Saji S: Polo-like
kinase 1 (PLK1) is overexpressed in primary colorectal cancers.
Cancer Sci. 94:148–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knecht R, Elez R, Oechler M, Solbach C,
von Ilberg C and Strebhardt K: Prognostic significance of polo-like
kinase (PLK) expression in squamous cell carcinomas of the head and
neck. Cancer Res. 59:2794–2797. 1999.PubMed/NCBI
|
|
31
|
Weichert W, Denkert C, Schmidt M, Gekeler
V, Wolf G, Köbel M, Dietel M and Hauptmann S: Polo-like kinase
isoform expression is a prognostic factor in ovarian carcinoma. Br
J Cancer. 90:815–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolf G, Elez R, Doermer A, Holtrich U,
Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H and
Strebhardt K: Prognostic significance of polo-like kinase (PLK)
expression in non-small cell lung cancer. Oncogene. 14:543–549.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawata E, Ashihara E and Maekawa T: RNA
interference against polo-like kinase-1 in advanced non-small cell
lung cancers. J Clin Bioinforma. 1:62011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sebastian M, Reck M, Waller CF, Kortsik C,
Frickhofen N, Schuler M, Fritsch H, Gaschler-Markefski B, Hanft G,
Munzert G, et al: The efficacy and safety of BI 2536, a novel Plk-1
inhibitor, in patients with stage IIIB/IV non-small cell lung
cancer who had relapsed after, or failed, chemotherapy: Results
from an open-label, randomized phase II clinical trial. J Thorac
Oncol. 5:1060–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ellis PM, Chu QS, Leighl N, Laurie SA,
Fritsch H, Gaschler-Markefski B, Gyorffy S and Munzert G: A phase I
open-label dose-escalation study of intravenous BI 2536 together
with pemetrexed in previously treated patients with non-small-cell
lung cancer. Clin Lung Cancer. 14:19–27. 2013. View Article : Google Scholar
|
|
36
|
Liu X: Targeting Polo-Like Kinases: A
Promising Therapeutic approach for cancer treatment. Transl Oncol.
8:185–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schöffski P, Awada A, Dumez H, Gil T,
Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P and Munzert
G: A phase I, dose-escalation study of the novel Polo-like kinase
inhibitor volasertib (BI 6727) in patients with advanced solid
tumours. Eur J Cancer. 48:179–186. 2012. View Article : Google Scholar
|
|
38
|
Stadler WM, Vaughn DJ, Sonpavde G,
Vogelzang NJ, Tagawa ST, Petrylak DP, Rosen P, Lin CC, Mahoney J,
Modi S, et al: An open-label, single-arm, phase 2 trial of the
Polo-like kinase inhibitor volasertib (BI 6727) in patients with
locally advanced or metastatic urothelial cancer. Cancer.
120:976–982. 2014. View Article : Google Scholar
|
|
39
|
Awada A, Dumez H, Aftimos PG, Costermans
J, Bartholomeus S, Forceville K, Berghmans T, Meeus MA, Cescutti J,
Munzert G, et al: Phase I trial of volasertib, a Polo-like kinase
inhibitor, plus platinum agents in solid tumors: Safety,
pharmacokinetics and activity. Invest New Drugs. 33:611–620. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Machiels JP, Peeters M, Herremans C,
Surmont V, Specenier P, De Smet M, Pilz K, Strelkowa N, Liu D and
Rottey S: A phase I study of volasertib combined with afatinib, in
advanced solid tumors. Cancer Chemother Pharmacol. 76:843–851.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Braud F, Cascinu S, Spitaleri G, Pilz
K, Clementi L, Liu D, Sikken P and De Pas T: A phase I,
dose-escalation study of volasertib combined with nintedanib in
advanced solid tumors. Ann Oncol. 26:2341–2346. 2015. View Article : Google Scholar : PubMed/NCBI
|