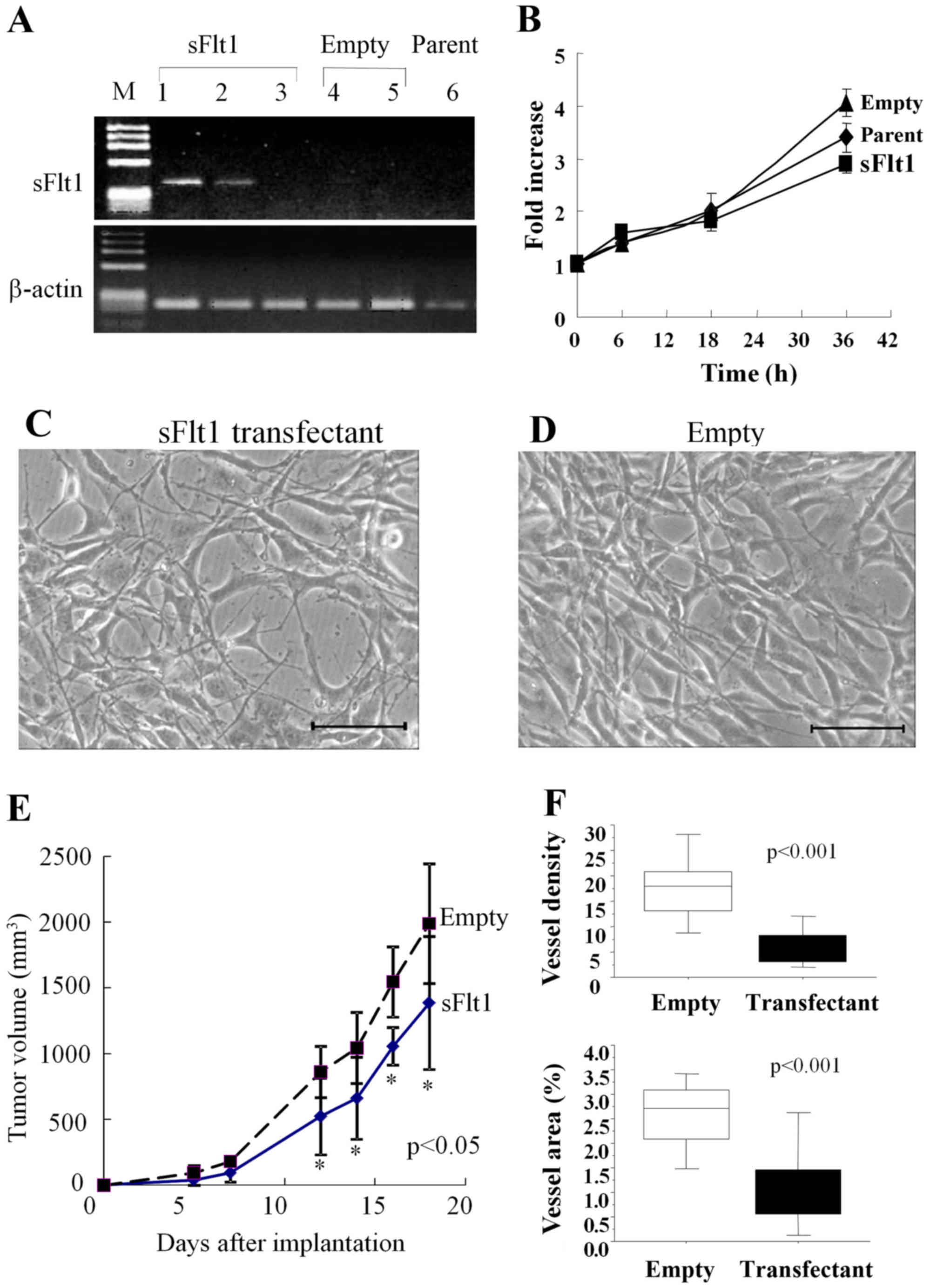
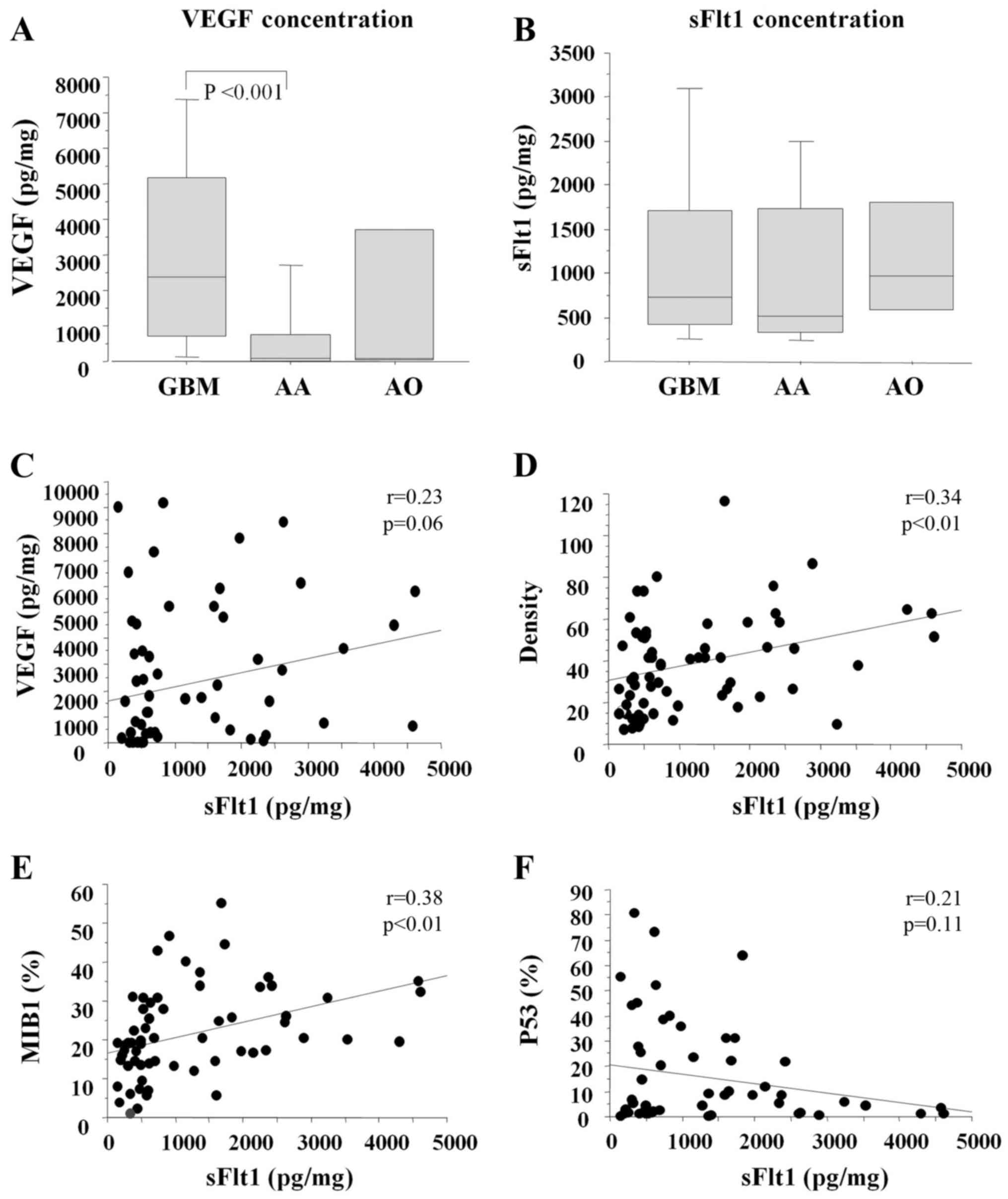
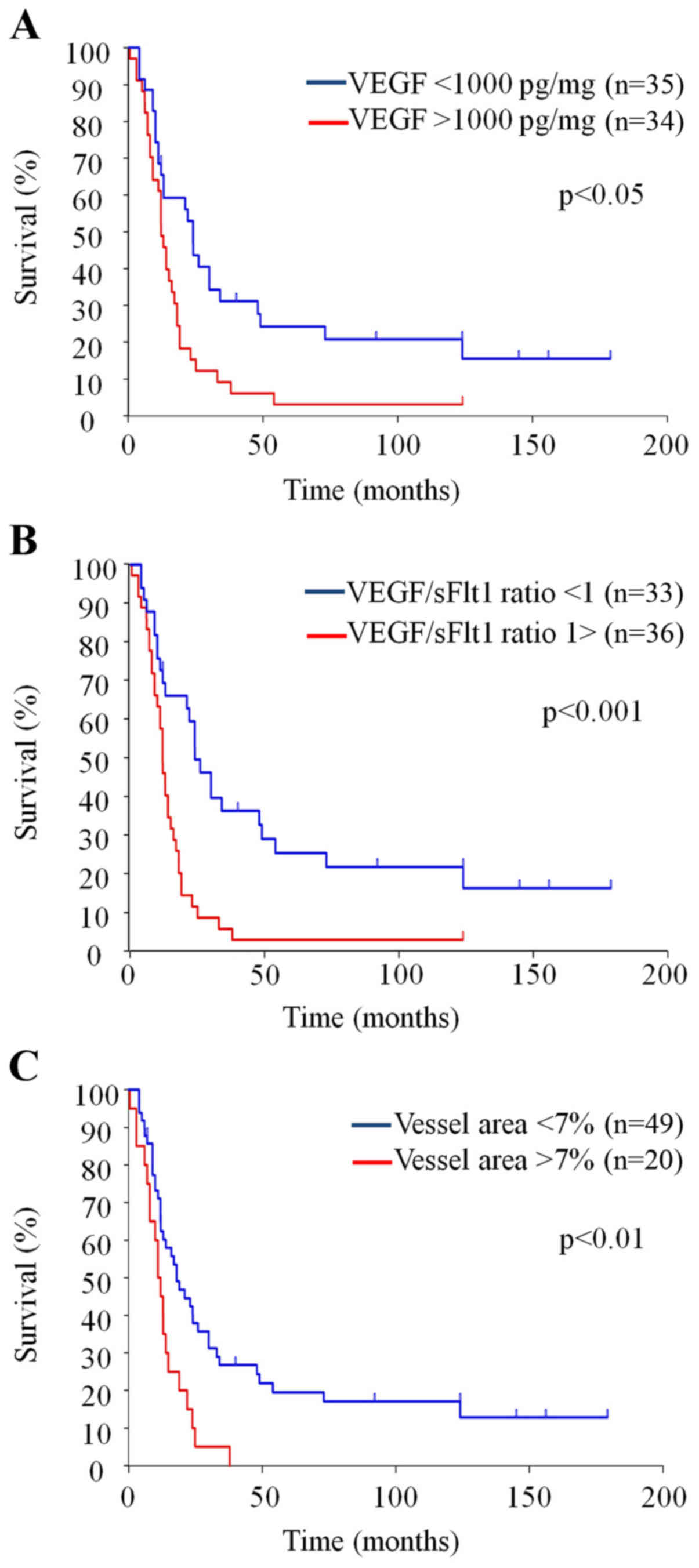
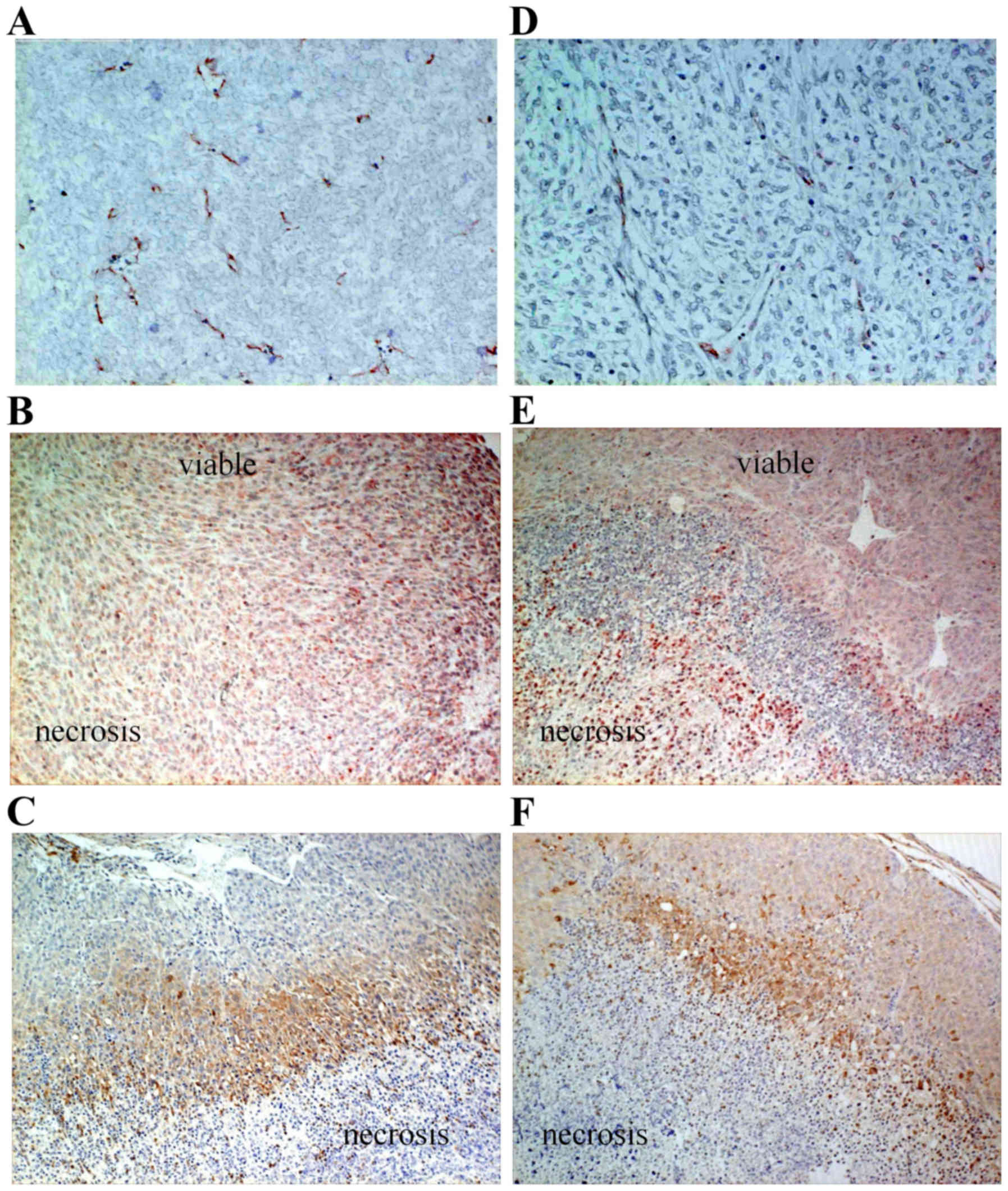
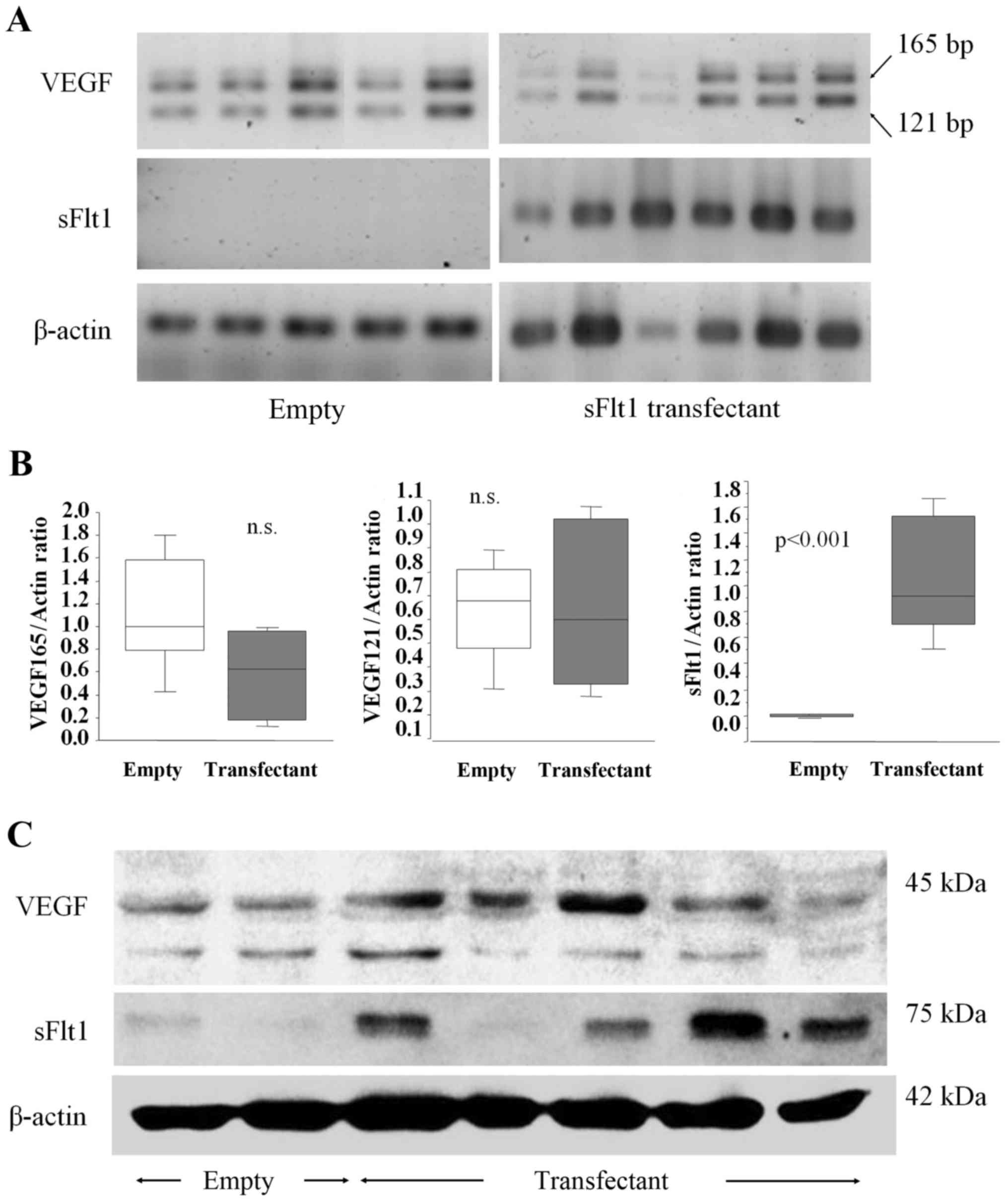
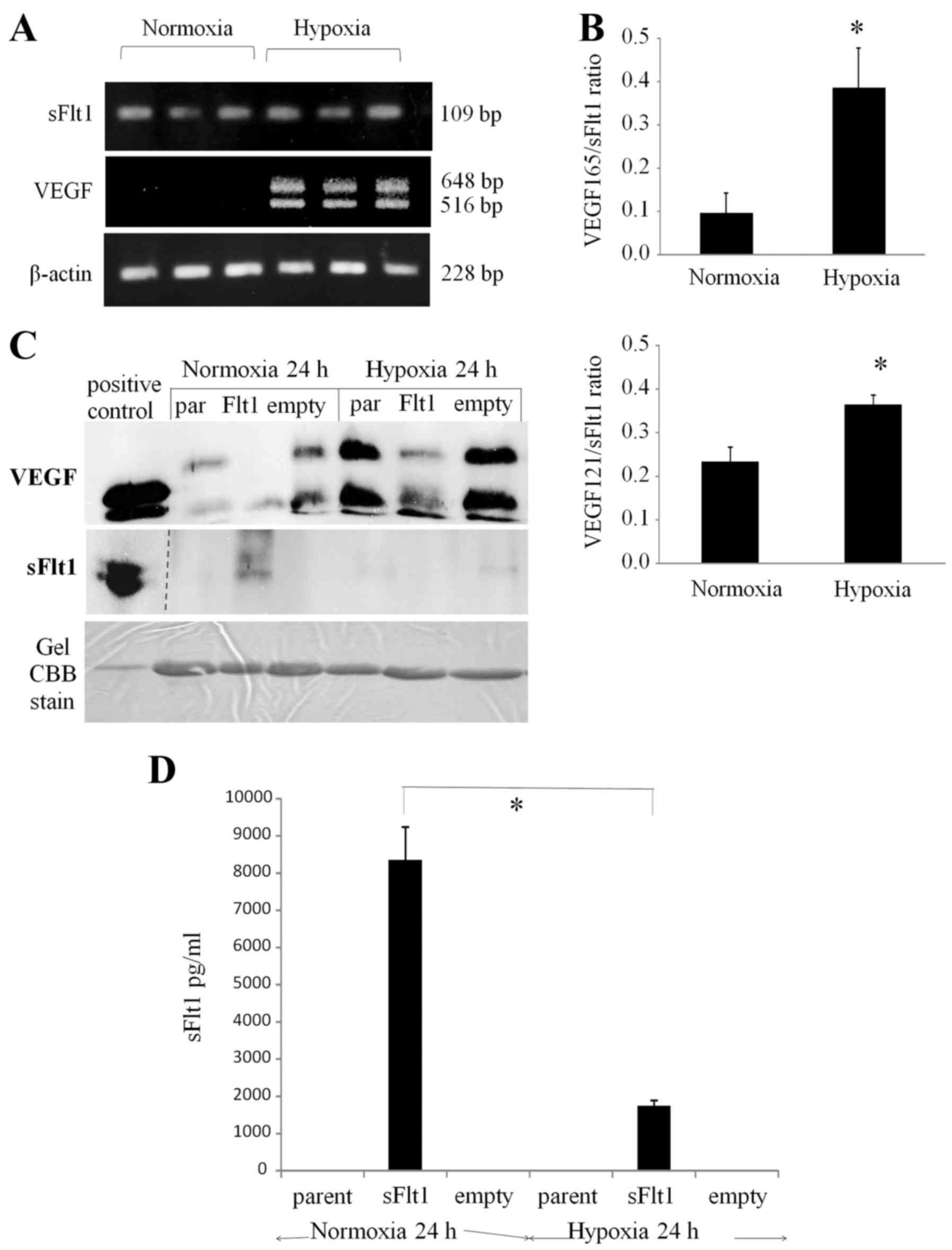
|
1
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu-Emerson C, Duda DG, Emblem KE, Taylor
JW, Gerstner ER, Loeffler JS, Batchelor TT and Jain RK: Lessons
from anti-vascular endothelial growth factor and anti-vascular
endothelial growth factor receptor trials in patients with
glioblastoma. J Clin Oncol. 33:1197–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shivakumar S, Prabhakar BT, Jayashree K,
Rajan MG and Salimath BP: Evaluation of serum vascular endothelial
growth factor (VEGF) and microvessel density (MVD) as prognostic
indicators in carcinoma breast. J Cancer Res Clin Oncol.
135:627–636. 2009. View Article : Google Scholar
|
|
5
|
Botelho F, Pina F and Lunet N: VEGF and
prostatic cancer: A systematic review. Eur J Cancer Prev.
19:385–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikawa R, Saran F, Mason W, Wick W,
Cloughesy TF, Henriksson R, Hilton M, Garcia J, Vogt T, Pallaud C,
et al: Biomarker (BM) evaluations in the phase III AVAglio study of
bevacizumab (Bv) plus standard radiotherapy (RT) and temozolomide
(T) for newly diagnosed glioblastoma (GBM). J Clin Oncol.
31:20232013.
|
|
7
|
Batchelor TT, Reardon DA, de Groot JF,
Wick W and Weller M: Antiangiogenic therapy for glioblastoma:
Current status and future prospects. Clin Cancer Res. 20:5612–5619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prea SM, Chan EC, Dusting GJ, Vingrys AJ,
Bui BV and Liu GS: Gene therapy with endogenous inhibitors of
angiogenesis for neovascular age-related macular degeneration:
Beyond anti-VEGF therapy. J Ophthalmol. 2015:2017262015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao N, Lee YF and Ge R: Novel endogenous
angiogenesis inhibitors and their therapeutic potential. Acta
Pharmacol Sin. 36:1177–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Volpert OV, Zaichuk T, Zhou W, Reiher F,
Ferguson TA, Stuart PM, Amin M and Bouck NP: Inducer-stimulated Fas
targets activated endothelium for destruction by anti-angiogenic
thrombospondin-1 and pigment epithelium-derived factor. Nat Med.
8:349–357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beerepoot LV, Witteveen EO, Groenewegen G,
Fogler WE, Sim BK, Sidor C, Zonnenberg BA, Schramel F, Gebbink MF
and Voest EE: Recombinant human angiostatin by twice-daily
subcutaneous injection in advanced cancer: A pharmacokinetic and
long-term safety study. Clin Cancer Res. 9:4025–4033.
2003.PubMed/NCBI
|
|
14
|
Kurup A, Lin C, Murry DJ, Dobrolecki L,
Estes D, Yiannoutsos CT, Mariano L, Sidor C, Hickey R and Hanna N:
Recombinant human angiostatin (rhAngiostatin) in combination with
paclitaxel and carboplatin in patients with advanced non-small-cell
lung cancer: A phase II study from Indiana University. Ann Oncol.
17:97–103. 2006. View Article : Google Scholar
|
|
15
|
Rong B, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uronis HE, Cushman SM, Bendell JC, Blobe
GC, Morse MA, Nixon AB, Dellinger A, Starr MD, Li H, Meadows K, et
al: A phase I study of ABT-510 plus bevacizumab in advanced solid
tumors. Cancer Med. 2:316–324. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westphal JR: Technology evaluation:
ABT-510, Abbott. Curr Opin Mol Ther. 6:451–457. 2004.PubMed/NCBI
|
|
18
|
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong
Y, Li S, Lian B, Gu K, Tao M, et al: A phase II, randomized,
double-blind, placebo-controlled multicenter trial of Endostar in
patients with metastatic melanoma. Mol Ther. 21:1456–1463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davis-Smyth T, Chen H, Park J, Presta LG
and Ferrara N: The second immunoglobulin-like domain of the VEGF
tyrosine kinase receptor Flt-1 determines ligand binding and may
initiate a signal transduction cascade. EMBO J. 15:4919–4927.
1996.PubMed/NCBI
|
|
20
|
Kuo CJ, Farnebo F, Yu EY, Christofferson
R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn
E, et al: Comparative evaluation of the antitumor activity of
antiangiogenic proteins delivered by gene transfer. Proc Natl Acad
Sci USA. 98:4605–4610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takano S, Yoshii Y, Kondo S, Suzuki H,
Maruno T, Shirai S and Nose T: Concentration of vascular
endothelial growth factor in the serum and tumor tissue of brain
tumor patients. Cancer Res. 56:2185–2190. 1996.PubMed/NCBI
|
|
22
|
Takano S, Kamiyama H, Mashiko R, Osuka S,
Ishikawa E and Matsumura A: Metronomic treatment of malignant
glioma xenografts with irinotecan (CPT-11) inhibits angiogenesis
and tumor growth. J Neurooncol. 99:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levin VA, Uhm JH, Jaeckle KA, Choucair A,
Flynn PJ, Prados MD, Bruner JM, Chang SM, Kyritsis AP, Gleason MJ,
et al: Yung WKA: Phase III randomized study of postradiotherapy
chemotherapy with alpha-difluoromethylornithine-procarbazine,
N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine
(DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res.
6:3878–3884. 2000.PubMed/NCBI
|
|
24
|
Tsuboi K, Saijo K, Ishikawa E, Tsurushima
H, Takano S, Morishita Y and Ohno T: Effects of local injection of
ex vivo expanded autologous tumor-specific T lymphocytes in cases
with recurrent malignant gliomas. Clin Cancer Res. 9:3294–3302.
2003.PubMed/NCBI
|
|
25
|
Lamszus K, Ulbricht U, Matschke J,
Brockmann MA, Fillbrandt R and Westphal M: Levels of soluble
vascular endothelial growth factor (VEGF) receptor 1 in astrocytic
tumors and its relation to malignancy, vascularity, and VEGF-A.
Clin Cancer Res. 9:1399–1405. 2003.PubMed/NCBI
|
|
26
|
Sathornsumetee S, Cao Y, Marcello JE,
Herndon JE II, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW,
Vredenburgh JJ and Rich JN: Tumor angiogenic and hypoxic profiles
predict radiographic response and survival in malignant astrocytoma
patients treated with bevacizumab and irinotecan. J Clin Oncol.
26:271–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasselbalch B, Eriksen JG, Broholm H,
Christensen IJ, Grunnet K, Horsman MR, Poulsen HS, Stockhausen MT
and Lassen U: Prospective evaluation of angiogenic, hypoxic and
EGFR-related biomarkers in recurrent glioblastoma multiforme
treated with cetuximab, bevacizumab and irinotecan. APMIS.
118:585–594. 2010.PubMed/NCBI
|
|
28
|
Lu-Emerson C, Snuderl M, Kirkpatrick ND,
Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda
DG, et al: Increase in tumor-associated macrophages after
antiangiogenic therapy is associated with poor survival among
patients with recurrent glioblastoma. Neuro Oncol. 15:1079–1087.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duda DG, Willett CG, Ancukiewicz M, di
Tomaso E, Shah M, Czito BG, Bentley R, Poleski M, Lauwers GY,
Carroll M, et al: Plasma soluble VEGFR-1 is a potential dual
biomarker of response and toxicity for bevacizumab with
chemoradiation in locally advanced rectal cancer. Oncologist.
15:577–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batchelor TT, Duda DG, di Tomaso E,
Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J,
Hochberg FH, Benner T, et al: Phase II study of cediranib, an oral
pan-vascular endothelial growth factor receptor tyrosine kinase
inhibitor, in patients with recurrent glioblastoma. J Clin Oncol.
28:2817–2823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerstner ER, Eichler AF, Plotkin SR,
Drappatz J, Doyle CL, Xu L, Duda DG, Wen PY, Jain RK and Batchelor
TT: Phase I trial with biomarker studies of vatalanib (PTK787) in
patients with newly diagnosed glioblastoma treated with enzyme
inducing anti-epileptic drugs and standard radiation and
temozolomide. J Neurooncol. 103:325–332. 2011. View Article : Google Scholar
|
|
33
|
Quant EC, Batchelor T, Lassman AB, Schiff
D, Kaley TJ, Wong E, Mikkelsen T, Drappatz J, Norden AD, Beroukhim
R, et al: Preliminary results from a multicenter, phase II,
randomized, noncomparative clinical trial of radiation and
temozolomide with or without vandetanib in newly diagnosed
glioblastoma. J Clin Oncol. 29:20692011.
|
|
34
|
Shiose S, Sakamoto T, Yoshikawa H, Hata Y,
Kawano Y, Ishibashi T, Inomata H, Takayama K and Ueno H: Gene
transfer of a soluble receptor of VEGF inhibits the growth of
experimental eyelid malignant melanoma. Invest Ophthalmol Vis Sci.
41:2395–2403. 2000.PubMed/NCBI
|
|
35
|
Takayama K, Ueno H, Nakanishi Y, Sakamoto
T, Inoue K, Shimizu K, Oohashi H and Hara N: Suppression of tumor
angiogenesis and growth by gene transfer of a soluble form of
vascular endothelial growth factor receptor into a remote organ.
Cancer Res. 60:2169–2177. 2000.PubMed/NCBI
|
|
36
|
Ye C, Feng C, Wang S, Wang KZQ, Huang N,
Liu X, Lin Y and Li M: sFlt-1 gene therapy of follicular thyroid
carcinoma. Endocrinology. 145:817–822. 2004. View Article : Google Scholar
|
|
37
|
Mahasreshti PJ, Kataram M, Wang MH,
Stockard CR, Grizzle WE, Carey D, Siegal GP, Haisma HJ, Alvarez RD
and Curiel DT: Intravenous delivery of adenovirus-mediated soluble
FLT-1 results in liver toxicity. Clin Cancer Res. 9:2701–2710.
2003.PubMed/NCBI
|
|
38
|
Goldman CK, Kendall RL, Cabrera G,
Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ,
Huckle WR, et al: Paracrine expression of a native soluble vascular
endothelial growth factor receptor inhibits tumor growth,
metastasis, and mortality rate. Proc Natl Acad Sci USA.
95:8795–8800. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ikeda T, Sun L, Tsuruoka N, Ishigaki Y,
Yoshitomi Y, Yoshitake Y and Yonekura H: Hypoxia down-regulates
sFlt-1 (sVEGFR-1) expression in human microvascular endothelial
cells by a mechanism involving mRNA alternative processing. Biochem
J. 436:399–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mashiko R, Takano S, Ishikawa E, Yamamoto
T, Nakai K and Matsumura A: Hypoxia-inducible factor 1α expression
is a prognostic biomarker in patients with astrocytic tumors
associated with necrosis on MR image. J Neurooncol. 102:43–50.
2011. View Article : Google Scholar
|
|
42
|
Kung AL, Wang S, Klco JM, Kaelin WG and
Livingston DM: Suppression of tumor growth through disruption of
hypoxia-inducible transcription. Nat Med. 6:1335–1340. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW,
Wang JJ and Xu GX: Bifidobacterium longum as a delivery system of
TRAIL and endostatin cooperates with chemotherapeutic drugs to
inhibit hypoxic tumor growth. Cancer Gene Ther. 16:655–663. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu H, Li Z, Mao S, Ma B, Zhou S, Deng L,
Liu T, Cui D, Zhao Y, He J, et al: Antitumor effect of sFlt-1 gene
therapy system mediated by Bifidobacterium Infantis on Lewis lung
cancer in mice. Cancer Gene Ther. 18:884–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Biswal MR, Prentice HM, Dorey CK and
Blanks JC: A hypoxia-responsive glial cell-specific gene therapy
vector for targeting retinal neovascularization. Invest Ophthalmol
Vis Sci. 55:8044–8053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perche F, Biswas S, Patel NR and Torchilin
VP: Hypoxia-responsive copolymer for siRNA delivery. Methods Mol
Biol. 1372:139–162. 2016. View Article : Google Scholar
|