Introduction
Pancreatic neuroendocrine tumors (pNETs), also named
as islet cell tumors, are relatively rare, accounting for 1–2% of
all pancreatic neoplasms (1,2).
According to the hormonal symptoms, they are broadly divided into
functioning (F-pNETs) and non-functioning pNETs (NF-pNETs). An
estimated 66–91% of the pNETs were non-functioning and patients
with NF-pNETs have been reported to have poorer outcomes than those
with F-pNETs (3,4). With respect to recent studies and
consensus statements, in F-pNETs and symptomatic or large (>2
cm) NF-pNETs, radical pancreatic resection is the only treatment
capable of prolonging survival (5–7).
However, in patients with sporadic small (≤2 cm) NF-pNETs, there
are quite different views regarding the appropriate management
strategy to adopt (8).
Many recent studies suggested that there is an
increasing incidence of NF-pNETs, especially NF-pNETs (≤2 cm). An
epidemiological survey conducted in Japan shows that the number of
treated patients with pNETs in 2010 was ~1.2-times that in 2005 and
the number of the new incidences of non-functioning pNETs in 2010
was ~1.7-times that in 2005 (9).
Likewise, based on the first population-level analysis to
exclusively characterize NF-pNETs ≤2 cm in a surgical population,
the incidence of NF-pNETs ≤2 cm in the United States has increased
by 710.4% over the 22-year study period with annual percentage
change 12.8%; while the NF-pNETs ≤2 cm accounted for 20.2% of total
pNET diagnoses in 2009, in contrast to 12.3% of that in 1988, which
nearly doubled over the last 22 years (10). Whether this is the result of a true
increase in incidence of the disease or result of increased
detection is still speculative. However, we have reasons to believe
that the more frequent use of cross-sectional imaging, especially
the multiphasic computed tomography (CT) or magnetic resonance
imaging (MRI) and endoscopic ultrasound (EUS) have played some
important roles in this (2,3,10).
As for determining the appropriate management for
NF-pNETs ≤2 cm, surgery or surveillance, which one should be taken
into consideration remained controversial. In 2012, the European
Neuroendocrine Tumor Society (ENETS) Guideline suggested that a
non-operative approach could be advocated in selected cases for
NF-pNETs ≤2 cm that are discovered incidentally (6); for the reasons that most of NF-pNETs
≤2 cm are likely benign or intermediate-risk lesions and only 6% of
NF-pNETs ≤2 cm are malignant when incidentally discovered (11). However, in 2016, the latest ENETS
Guideline updated their views that patients with NF-pNETs ≤2 cm
have two options: i) It is recommended to have surveillance
approach for the patients with G1 or low G2, asymptomatic, mainly
with head lesion, no radiological signs suspicious for malignancy,
as well as patient factors such as personal wishes, age, or with
comorbidities; ii) while for the patients with G2, symptoms and
patient wishes, surgery is recommended (8). Moreover, during the surveillance
time, if the tumor size increase >0.5 cm or to a size of >2
cm, surgery is necessary (8).
However, according to the National Comprehensive Cancer Network
(NCCN) Guideline Version 2.2016, patients with NF-pNETs ≤2 cm
should undergo surgery (12).
Observation can be considered in cases as follows: tumors <1 cm,
incidentally discovered; while decision should be made based on
estimated surgical risk, site of tumor and patient comorbidities
(12). On the other hand, both in
the ENETS and NCCN guidelines, only retrospective cohort studies
and case series are included, due to the relative low incidence of
NF-pNETs. Therefore, before a large and prospective randomized
clinical trial with long-term followup come out, it still remains
controversial whether surgery or surveillance should be carried out
for the patients with NF-pNETs ≤2 cm.
To address the issue of whether surgery or
surveillance should be under taken for the patients with NF-pNETs
≤2 cm, an expert panel from high-volume centers in China
participated in a consensus conference hosted by the Chinese Study
Group for Neuroendocrine Tumors (CSNET) in June 2016 to review the
published literature and discuss the management strategies for the
patients with NF-pNETs ≤2 cm. This Chinese expert consensus from
the CSNET on pancreatic neuroendocrine tumors formulated a
personalized proposal on the management strategies for the patients
with NF-pNETs, which may add to the armamentarium available to
pancreatic surgeons and physicians.
Materials and methods
For the consensus statement, a comprehensive search
of medical literature was carried out using PubMed in April 2016.
The PubMed search terms included 'non-functioning',
'non-functional', 'pancreas', 'pancreatic', 'islet',
'neuroendocrine tumor', 'neuroendocrine neoplasm', 'endocrine
tumor', 'endocrine neoplasm', 'neuroendocrine', 'endocrine',
'tumor' and 'neoplasm' in various combinations. As the evidence
level suggested by the Oxford Centre for Evidence-based Medicine,
the reports were assessed and ranked. We excluded all case reports
and non-English papers. Literature reporting data on MEN-1 syndrome
were not included. A draft of the consensus statement was prepared
by the CSNET members (G.Y. and C.H.S.), and then it was discussed,
followed by an agreement by CSNET members at a conference held in
June 2016 in Guangzhou, China.
Results and Consensus statements
It is vital to make pathological
confirmation, before making the appropriate strategy for patients
with NF-pNETs ≤2 cm
Recent studies have suggested the results that the
incidence of NF-pNETs ≤2 cm have increased in the past few decades,
on account of widely-used cross-sectional imaging, especially the
multiphasic CT or MRI and nuclear medicine techniques (2,9,10).
Multiphasic CT and MRI are often used initially for
the screening and staging of these lesions (13). The classic appearance is of a
uniformly hypervascular, well-defined lesion that is most notably
prominent on arterial phases of contrast enhancement (14). While nuclear medicine techniques
can provide improved specificity and whole-body assessments for
distant disease. The primary nuclear medicine imaging tool for pNET
is somatostatin receptor scintigraphy (SRS) performed with a
radiolabeled somatostatin analogue (15). Its overall sensitivity for pNET is
~70–90% but varies with tumor type and diminishes particularly for
subcentimeter lesions (16,17).
18Fluorine-FDG PET/CT is used as a complementary
technique to SRS, which shows poor uptake in poorly differentiated
tumors (16). Moreover,
68Gallium PET/CT has been used extensively (18), other radioisotopes are under
investigation for imaging of neuroendocrine tumors (13).
However, neither cross-sectional imaging or nuclear
medicine techniques are able to obtain accurate preoperative
pathology results. The seminal article by Rosch et al
(19) in 1992 was the first to
describe the important role of Endoscopic Ultrasound-Guided
Fine-Needle Aspiration (EUS-FNA) in the detection of pNETs. Since
then, EUS-FNA has been increasingly used and has become an integral
part of the diagnosis of pNETs because of its high sensitivity for
detecting, localizing and diagnosing pNETs for >20 years
(20).
EUS-FNA is an excellent tool to establish the
correct diagnosis of pancreatic lesions. Similar to other
pancreatic lesions, NF-pNETs may be further evaluated by sampling
these tumors by FNA performed during the EUS examination to
optimize patient management (21).
Studies have reported sensitivities of 61–84% and overall accuracy
of up to 92.5% of EUS-FNA in establishing the diagnosis of pNETs
(22–24). Alternative methods for obtaining
tissue by EUS have been evaluated to overcome the limitations of
FNA. The Endoscopic Ultrasound-Guided Trucut needle biopsy
(EUS-FNTA) uses a 19-gauge needle to obtain core biopsies with the
benefit of procuring larger and substantive amounts of tissue with
conserved architecture that would enable histologic analysis
(25). Larghi et al
(26) reported that, among
patients with NF-pNETs ≤2 cm, retrieval of tissue specimens with
EUS-FNTA by using a 19-gauge needle is safe, feasible and highly
accurate for both diagnosis and Ki-67 determination. In addition,
the use of the Trucut needle has been limited by the technical
difficulties of using this device, particularly with the duodenal
approach (27).
In the updated ENETS guideline 2016, pathological
classification should be confirmed before the treatment strategy
can be decided among the patients with NF-pNETs ≤2 cm, because for
those patients with G1 or low G2, it is safe to have the
surveillance approach; while for those patients with G2, surgery is
the other approach (8). However,
due to the small tumor diameter (≤2 cm), a high-volume center and
an experienced sonographer are required to improve the accuracy and
reliability of the pathological confirmation.
Consensus statements
It is important for patients with suspected sporadic
small NF-pNETs to have pathological confirmation before the
appropriate treatment strategy is decided. While EUS-FNA has
limitations in the pathological confirmation, EUS-FNTA is an
excellent tool to establish the correct diagnosis. For the
small-volume centers, due to the low sensitivities and poor
accuracy, radiological signs suspicious for malignancy, such as CT,
MRI, SRS, 68Gallium or 18Fluorine-FDG PET/CT,
disease diagnosis can be made through serology results, such as
chromogranin A (CgA) and pancreatic polypeptide (PP).
The recommended strategy for patients
with NF-pNETs ≤2 cm
The appropriate treatment strategy for patients with
NF-pNETs ≤2 cm remains controversial. Based on several studies the
choice of radical surgery is advocated due to the risk of
malignancy of ~9–27% in NF-pNETs ≤2 cm (10,28).
On the other hand, a few studies suggested that such an aggressive
surgical indication could expose some patients, with low pancreatic
malignancies, to a high increased postoperative morbidity and
mortality, depending on factors including age, presence of
comorbidities, the sites of the tumor and the surgical approaches,
but would not benefit patients' survival in the long term (29,30)
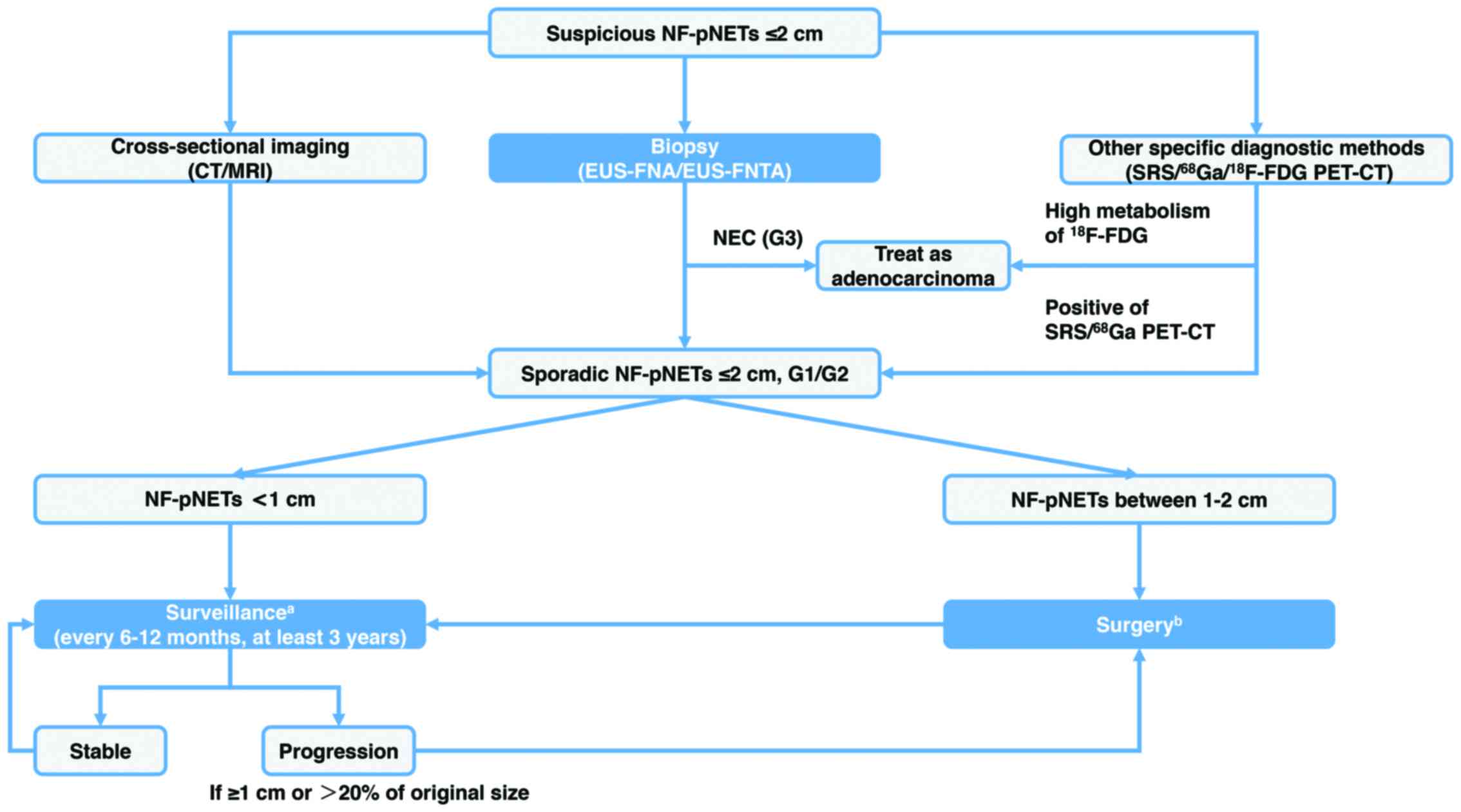
(Fig. 1).
Incidentally diagnosed, sporadic NF-pNETs can
display aggressive behavior, including extrapancreatic extension,
lymph nodal metastasis, distant-organ metastasis, recurrence which
may lead to disease-related death, even though the tumors are small
(≤2 cm). With respect to the first population-level analysis to
exclusively characterize NF-pNET ≤2 cm in a surgical population
from the United States, rates of extrapancreatic extension, lymph
nodal metastasis and distant metastasis in NF-pNETs ≤2 cm in the
SEER database were 17.9, 27.3 and 9.1%, respectively (10). Haynes et al (31) also reported a case series of 39
patients with NF-pNETs ≤2 cm, where 3 had late metastases or
recurrence after the resection. An Italian retrospective cohort
study showed that, among 23 patients with NF-pNETs ≤2 cm, 4 (17.4%)
had distant metastases before surgery; while in the other 19
patients without metastases before surgery, 4 had a local
malignancy (lymph node metastasis or local infiltration) (28). Likewise, a study from Finland
reported that among 24 patients with NF-pNETs ≤2 cm, 7 patients
with small symptomatic NF-pNETs showed signs of malignant behavior:
4 had lymph nodal metastases, 1 had liver metastases before
surgery, 3 developed liver metastases and 3 died of the disease
(32). A retrospective cohort
study from the United States showed that of the 56 patients with
NF-pNETs ≤2 cm, 3 developed distant metastasis after resection,
eventually resulting in 2 disease-related deaths (33).
On the contrary, several studies reported that the
patients with NF-pNETs ≤2 cm had less rate of malignant behavior
compared with larger ones (>2 cm); and the surgery approach may
lead to pancreatic postoperative complications. A multi-center
retrospective case series from Europe showed that among the 46
patients with NF-pNETs ≤2 cm followed up with serial imaging,
distant or nodal metastases appeared on the imaging in none of the
patients. Overall, 8 patients underwent surgery after a median time
from the initial evaluation of 41 months; all resected lesions were
ENETS T stage 1 (n=7) or 2 (n=1), grade 1, node negative, with
neither vascular nor peripancreatic fat invasion (34). Likewise, another matched designed
retrospective cohort study from the United States reported that,
during a median follow-up of 44 months of the observation group,
the median tumor size had not changed, and no patient had developed
evidence of metastases; and no patient died of the disease
(35). A recent study showed that
small NF-PNETs (<2 cm) in either the operative or non-operative
groups demonstrated no evidence of progression or metastasis; and
morbidity in the operative group was 35% with pancreatic pseudocyst
the most common (29). Lee et
al (30) described that the
surgery group patients had some type of complication, more than
half due to a clinically significant pancreatic leak, while no
recurrence or disease specific mortality was seen in the surgery
group, including 5 patients with positive lymph nodes.
Considering the survival prognosis, Massironi et
al (36) showed that the
4-year survival rate was 100% in the surveillance group, while it
was 90.5% among the surgery patients. On the other hand, a study
based on the National Cancer Data Base (NCDB) from the United
States showed that 380 patients with NF-pNETs ≤2 cm were divided
into the surgery group (81%) and the surveillance group (19%); the
5-year overall survival (OS) rate was 82.2% for the surgery group
and 34.3% for the surveillance group (37). Also, the SEER data presented that
the disease-specific survival at 5, 10 and 15 years for NF-pNETs ≤2
cm was 91.5, 84.0 and 76.8% (10).
Accordingly, based on later two relatively large population
studies, we can see that the patients with NF-pNETs ≤2 cm had an
overall survival advantage with resection compared to
observation.
Some studies have suggested a more rational tumor
size cut-off to distinguish the malignancy or not. Ende et
al (38) according to the
receiver operating characteristic (ROC) analysis, found that using
a cut-off point of 2.0 cm only led to a sensitivity of 85% in
screening for metastases, while lowering the cut-off point to 1.8
cm allowed for a sensitivity of 95%. Likewise, a multi-center
retrospective cohort study from France showed that on a ROC curve,
and tumor size had a significant impact on malignancy. A tumor size
cut-off was found on the ROC curve at 1.7 cm with a sensitivity of
92% and a specificity of 75% to predict malignancy (node or liver
metastasis) (39). Also, a
retrospective cohort study from the United States reported that an
operation became a significant predictor of overall survival for
tumors >1.5 cm but was not significant for tumors <1.5 cm,
controlling for age-adjusted Charlson comorbidity index (40). Similarly, two Asian studies from
Korea and Japan also take more aggressive approach to lower the
tumor size cut-off to 1.5 cm for surgery (41,42).
Moreover, a recent abstract from the CSNET has been
submitted and approved by the 13th Annual ENETS Conference Abstract
Reviewing Committee 2016. In the multi-institutional clinical
analysis in China, a total of 49 patients with NF-pNETs ≤2 cm who
undertook surgery were included; postoperative pathological
diagnosis showed that 14.3% were with positive regional lymph node
metastasis, 12.2% perineuronal invasion and 4.1% vascular invasion;
while at the last follow-up, 14.3% of the patients had recurrence
and metastasis and 6.1% died of tumor metastasis. Our own clinical
experience told us that, although NF-PNETs ≤2 cm usually exhibit
minimal or no growth over many years, the minority will recur and
metastasize. It suggests that these patients should receive
surgical management or other active treatment and long-term
follow-up (Ji M, Jin K and Zhang Y: 13th Annual ENETS Conference
for the Diagnosis and Treatment of Neuroendocrine Tumor Disease:
272, 2016).
However, surgery decision should be made based on
tumor location, the adjoin between the tumor and the vessels, and
the patients' condition. Most importantly, for patients with G1
NF-pNETs between 1–2 cm located in the head or the uncinate process
of pancreas, who need pancreaticoduodenectomy rather than
parenchyma-preserving resection, surveillance is more suitable.
Another interesting focus is the surgery management
for G3 neuroendocrine neoplasms (NEN). According to the ENETS
Guideline 2016, G3 NEN will probably be separated into G3 NET and
G3 NEC in the future (43). But as
for NF-pNETs ≤2 cm, the G3 NEN, including G3 NET and G3 NEC, are
very rare neoplasms representing 1–2% (42,43).
For the present, surgery with regional lymph node dissection is
recommended for those patients who are diagnosed as G3 NET or G3
NEC of NF-pNETs ≤2 cm. Considering the survival is poor in G3 NEC
(43), it should be treated as
pancreatic ductal adenocarcinoma. Moreover, due to the low
incidence of G3 NEN of NF-pNETs ≤2 cm, further studies are needed
to clarify this issue.
Consensus statements
According to the afore-mentioned facts, currently
the 2-cm cut-off is not suitable due to the wide use of
cross-sectional imaging and endoscopic ultrasound; not to mention
that this cut-off point is too high to omit some tumors with
malignant behavior. Therefore, more aggressive approach is
suggested to be taken, except for some selected patients with
NF-pNETs <1 cm, incidentally discovered and unacceptable
surgical risks, all others with NF-pNETs ≤2 cm should undergo tumor
resection and careful postoperative surveillance. The follow-up for
both surgery and surveillance patients should continue for at least
3 years, during which EUS, MRI/CT should be taken for every 6–12
months.
The surgery approaches for patients with
NF-pNETs ≤2 cm
The surgery management for patients with NF-pNETs ≤2
cm has two parts: i) the choice of surgical techniques, which
includes the typical and atypical techniques; and ii) the open or
minimally invasive procedures.
The typical surgical techniques contain
pancreaticoduodenectomy, distal pancreatectomy and total
pancreatectomy; while the atypical surgical techniques include
enucleation, middle pancreatectomy and middle-preserving
pancreatectomy (44). All these
surgical techniques can be performed by the open or minimally
invasive methods, which include the laparoscopic approach and the
robot-assisted surgical system. Therefore, the specific approach is
decided by the tumor site, the tumor size and whether with or
without lymphadenectomy.
Several studies have reported that
parenchyma-preserving resections including enucleation and middle
pancreatectomy are generally safe and effective procedures for
treating small NF-pNETs (11,45,46).
These procedures may be associated with some acceptable
pancreas-related complications, mostly the pancreatic fistula, but
an excellent postoperative pancreatic function for patients
(45). Similarly, based on the
largest single-institution series on laparoscopic resection for
pNETs, it has been demonstrated that laparoscopic distal
pancreatectomy, with or without splenectomy, and laparoscopic
enucleation are safe and feasible in patients with small NF-pNETs
located in the body and tail of the pancreas (47). However, both the open and the
minimally invasive procedures, especially the enucleation, need to
be integrated with the use of intraoperative ultrasonography to
define correctly the surgical area with the main pancreatic duct to
reduce the pancreatic fistula as much as possible (44).
Consensus statements
The specific surgery approach is decided by the
tumor site, the tumor size, the age and the health condition. The
typical and atypical surgical techniques, which are taken in the
open or minimally invasive ways, are both suitable for NF-pNETs ≤2
cm, while the minimally invasive ways and parenchyma-preserving
resection are recommended. For patients with NF-pNETs ≤2 cm,
located on the head surface or in the body or tail of pancreas,
parenchyma-preserving resection, especially enucleation, is safe
and effective.
The lymphadenectomy strategy for patients
with NF-pNETs ≤2 cm
According to the afore-mentioned facts, individual
studies have shown a risk of lymph nodal metastases in NF-pNETs ≤2
cm between 12.9 and 27.3% (10,28,32,48).
Although debate exists regarding the value of lymphadenectomy with
surgery for small NF-pNETs (49),
several studies have suggested a correlation between the lymph node
metastases and the outcome. Another study based on the SEER data
from the United States showed that the lymph nodal metastases were
independently associated with the decreased disease-specific
survival (50). Likewise, a
retrospective cohort study from the United States reported that
positive lymph nodes rate of NF-pNETs <2 cm was 7.4%, but the
negative lymph nodes were correlated with better survival on
multivariate analysis (48).
Nevertheless, a Chinese study reported that
lymphadenectomy in small (≤2.5 cm) NF-PNETs is not routinely
necessary, for the reason that incidentally discovered NF-pNETs
≤2.5 cm were associated with a low-risk of lymph nodal metastases
(7.7%) and excellent survival (51). Another large population, including
total of 1854 patients with NF-pNETs ≤2 cm, based on NCDB data from
the United States showed that, among tumors ≤0.5 cm, 33% presented
with regional lymph nodal metastases and 11% with distant
metastases. In addition, the 5-year OS rate for patients not
undergoing surgery was 27.6 vs. 83.0% for partial pancreatectomy,
72.3% for pancreaticoduodenectomy and 86.0% for total
pancreatectomy. While the multivariate analysis demonstrated no
difference in OS based on the type of surgery or the addition of
regional lymphadenectomy (52).
Consensus statements
There is a relatively certain incidence of lymph
nodal metastases in the patients with NF-pNETs ≤2 cm, but the
correlation between the lymph node metastases and the overall
survival remain controversial. To avoid misunderstaging, here we
also recommend that lymph node dissection for patients with
NF-pNETs >1 cm, and lymph node sampling should be carried out
for tumors <1 cm. For experienced surgeons during minimally
invasive resections, we highly recommend lymph node dissection or
lymph node sampling.
Discussion
Pancreatic neuroendocrine tumors are relatively
rare, which mostly tend to be sporadic or non-functioning. Based on
the SEER and NCDB Databases, the incidence of NF-pNETs ≤2 cm has
increased by 2- or 3-fold in the last decades (10,52).
It is still speculative whether this is the result of a true
increase in incidence of disease or the result of increased
detection. However, we have reasons to believe that the more
frequent use of cross-sectional imaging, especially the multiphasic
CT or MRI and EUS has played an important role in this.
Although the involvement of aggressive behavior of
small NF-pNETs, such as extrapancreatic extension, lymph nodal
metastasis, distant metastasis, recurrence and even sometimes
disease-related death, are indicated by numerous individual studies
(10,28,31–33),
level I evidence or sizeable, multiple center, prospective,
controlled trials still appear insufficient. At the same time,
other series argued that, small NF-pNETs usually exhibit minimal or
no growth over many years, and nonoperative management may be
advocated when serial imaging demonstrates minimal or no growth
without suspicious features (29,30,34,35,53).
Considering this, a very interesting study from Italian scholars
have reported that they have developed a Markov model to
investigate whether the patients with NF-pNETs ≤2 cm should
directly undergo pancreatic surgery or should be followed
longitudinally to detect growth and malignancy. This model was
sensitive to diagnostic age and length of follow-up; in particular,
for patients >65 years of age, the two strategies provided
similar results but the surveillance strategy was more
cost-effective than the surgery strategy (54).
Also, several studies have shown that surgery is
safe and can be advocated for the patients with NF-pNETs ≤2 cm
(11,45,46).
Considering the risks of lymph nodal metastases involvement in the
patients with NF-pNETs ≤2 cm, lymph node dissection or at least
lymph node sampling is highly recommended for patients with small
NF-pNETs. Consequently, most patients with NF-pNETs ≤2 cm should
undergo tumor resection and careful postoperative surveillance,
except for some selected cases with NF-pNETs <1 cm, incidentally
discovered and unacceptable surgical risks.
The current consensus concerning the issue of the
management strategy for patients with sporadic small,
non-functioning pancreatic neuroendocrine tumors is supported and
unanimously approved by CSNET members from several high-volume
pancreas centers in China and suggests that surgery should be taken
for most patients with small NF-pNETs, except for some selected
ones with NF-pNETs <1 cm, incidentally discovered and
unacceptable surgical risks. The specific surgery management is
decided based on the tumor site, the tumor size, age and the health
condition. Although the dissection of lymph nodes is recommended by
the CSNET in most cases, multiple center, prospective and
controlled trials are still needed to confirm the correlation
between the lymph nodal metastases and the outcome.
Limitation and future directions
Due to the relative low incidence of NF-pNETs, all
the included studies are retrospective cohort studies or case
series. Therefore, the appropriate strategy for patients with
NF-pNETs ≤2 cm remains controversial before a large and prospective
randomized clinical trial with long-term follow-up is carried out.
At the same time, considering the yearly high incidence of
NF-pNETs, international cooperation is also recommended.
References
|
1
|
Yao JC, Eisner MP, Leary C, Dagohoy C,
Phan A, Rashid A, Hassan M and Evans DB: Population-based study of
islet cell carcinoma. Ann Surg Oncol. 14:3492–3500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al:
One hundred years after 'carcinoid': Epidemiology of and prognostic
factors for neuroendocrine tumors in 35,825 cases in the United
States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halfdanarson TR, Rabe KG, Rubin J and
Petersen GM: Pancreatic neuroendocrine tumors (PNETs): Incidence,
prognosis and recent trend toward improved survival. Ann Oncol.
19:1727–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito T, Igarashi H, Nakamura K, Sasano H,
Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, et
al: Epidemiological trends of pancreatic and gastrointestinal
neuroendocrine tumors in Japan: A nationwide survey analysis. J
Gastroenterol. 50:58–64. 2015. View Article : Google Scholar
|
|
5
|
Hill JS, McPhee JT, McDade TP, Zhou Z,
Sullivan ME, Whalen GF and Tseng JF: Pancreatic neuroendocrine
tumors: The impact of surgical resection on survival. Cancer.
115:741–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falconi M, Bartsch DK, Eriksson B, Klöppel
G, Lopes JM, O'Connor JM, Salazar R, Taal BG, Vullierme MP and
O'Toole D: Barcelona Consensus Conference participants: ENETS
Consensus Guidelines for the management of patients with digestive
neuroendocrine neoplasms of the digestive system:
Well-differentiated pancreatic non-functioning tumors.
Neuroendocrinology. 95:120–134. 2012. View Article : Google Scholar
|
|
7
|
Singh S, Dey C, Kennecke H, Kocha W,
Maroun J, Metrakos P, Mukhtar T, Pasieka J, Rayson D, Rowsell C, et
al: Consensus recommendations for the diagnosis and management of
pancreatic neuroendocrine tumors: Guidelines from a Canadian
National Expert Group. Ann Surg Oncol. 22:2685–2699. 2015.
View Article : Google Scholar
|
|
8
|
Falconi M, Eriksson B, Kaltsas G, Bartsch
DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G,
Klöppel G, et al: Vienna Consensus Conference participants: ENETS
Consensus Guidelines Update for the management of patients with
functional pancreatic neuroendocrine tumors and non-functional
pancreatic neuroendocrine tumors. Neuroendocrinology. 103:153–171.
2016. View Article : Google Scholar :
|
|
9
|
Ito T, Lee L, Hijioka M, Kawabe K, Kato M,
Nakamura K, Ueda K, Ohtsuka T and Igarashi H: The up-to-date review
of epidemiological pancreatic neuroendocrine tumors in Japan. J
Hepatobiliary Pancreat Sci. 22:574–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo EJ and Salem RR: Population-level
analysis of pancreatic neuroendocrine tumors 2 cm or less in size.
Ann Surg Oncol. 20:2815–2821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falconi M, Zerbi A, Crippa S, Balzano G,
Boninsegna L, Capitanio V, Bassi C, Di Carlo V and Pederzoli P:
Parenchyma-preserving resections for small nonfunctioning
pancreatic endocrine tumors. Ann Surg Oncol. 17:1621–1627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
NCCN: The NCCN Neuroendocrine Tumors
clinical practice guidelines in oncology (version 2.2016). National
Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf.
2016
|
|
13
|
Tamm EP, Bhosale P, Lee JH and Rohren EM:
State-of-the-art imaging of pancreatic neuroendocrine tumors. Surg
Oncol Clin N Am. 25:375–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sahani DV, Bonaffini PA, Fernández-Del
Castillo C and Blake MA: Gastroenteropancreatic neuroendocrine
tumors: Role of imaging in diagnosis and management. Radiology.
266:38–61. 2013. View Article : Google Scholar
|
|
15
|
Qiao Z, Zhang J, Jin X, Huo L, Zhu Z, Xing
H and Li F: 99mTc-HYNIC-TOC imaging in the evaluation of pancreatic
masses which are potential neuroendocrine tumors. Clin Nucl Med.
40:397–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balachandran A, Bhosale PR, Charnsangavej
C and Tamm EP: Imaging of pancreatic neoplasms. Surg Oncol Clin N
Am. 23:751–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwekkeboom DJ and Krenning EP:
Somatostatin receptor imaging. Semin Nucl Med. 32:84–91. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, Pan Q, Yao S, Yu M, Wu W, Xue H,
Kiesewetter DO, Zhu Z, Li F, Zhao Y, et al: Glucagon-like peptide-1
receptor PET/CT with 68Ga-NOTA-Exendin-4 for detecting
localized insulinoma: A Prospective Cohort Study. J Nucl Med.
57:715–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rösch T, Lightdale CJ, Botet JF, Boyce GA,
Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H,
Schusdziarra V, et al: Localization of pancreatic endocrine tumors
by endoscopic ultrasonography. N Engl J Med. 326:1721–1726. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MK: Endoscopic ultrasound in
gastroenteropancreatic neuroendocrine tumors. Gut Liver. 6:405–410.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rustagi T and Farrell JJ: Endoscopic
diagnosis and treatment of pancreatic neuroendocrine tumors. J Clin
Gastroenterol. 48:837–844. 2014.PubMed/NCBI
|
|
22
|
Pais SA, Al-Haddad M, Mohamadnejad M,
Leblanc JK, Sherman S, McHenry L and DeWitt JM: EUS for pancreatic
neuroendocrine tumors: A single-center, 11-year experience.
Gastrointest Endosc. 71:1185–1193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atiq M, Bhutani MS, Bektas M, Lee JE, Gong
Y, Tamm EP, Shah CP, Ross WA, Yao J, Raju GS, et al: EUS-FNA for
pancreatic neuroendocrine tumors: A tertiary cancer center
experience. Dig Dis Sci. 57:791–800. 2012. View Article : Google Scholar
|
|
24
|
Jahan A, Yusuf MA and Loya A: Fine-needle
aspiration cytology in the diagnosis of pancreatic neuroendocrine
tumors: A single-center experience of 25 cases. Acta Cytol.
59:163–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wittmann J, Kocjan G, Sgouros SN,
Deheragoda M and Pereira SP: Endoscopic ultrasound-guided tissue
sampling by combined fine needle aspiration and trucut needle
biopsy: A prospective study. Cytopathology. 17:27–33. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larghi A, Capurso G, Carnuccio A, Ricci R,
Alfieri S, Galasso D, Lugli F, Bianchi A, Panzuto F, De Marinis L,
et al: Ki-67 grading of nonfunctioning pancreatic neuroendocrine
tumors on histologic samples obtained by EUS-guided fine-needle
tissue acquisition: A prospective study. Gastrointest Endosc.
76:570–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varadarajulu S, Fraig M, Schmulewitz N,
Roberts S, Wildi S, Hawes RH, Hoffman BJ and Wallace MB: Comparison
of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided
fine-needle aspiration. Endoscopy. 36:397–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lombardi M, De Lio N, Funel N, Sardella C,
Russo D, Urbani C, Rossi G, Campani D, Martino E, Marcocci C, et
al: Prognostic factors for pancreatic neuroendocrine neoplasms
(pNET) and the risk of small non-functioning pNET. J Endocrinol
Invest. 38:605–613. 2015. View Article : Google Scholar
|
|
29
|
Rosenberg AM, Friedmann P, Del Rivero J,
Libutti SK and Laird AM: Resection versus expectant management of
small incidentally discovered nonfunctional pancreatic
neuroendocrine tumors. Surgery. 159:302–309. 2016. View Article : Google Scholar
|
|
30
|
Lee LC, Grant CS, Salomao DR, Fletcher JG,
Takahashi N, Fidler JL, Levy MJ and Huebner M: Small,
nonfunctioning, asymptomatic pancreatic neuroendocrine tumors
(PNETs): Role for nonoperative management. Surgery. 152:965–974.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haynes AB, Deshpande V, Ingkakul T, Vagefi
PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL and
Fernández-del Castillo C: Implications of incidentally discovered,
nonfunctioning pancreatic endocrine tumors: Short-term and
long-term patient outcomes. Arch Surg. 146:534–538. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sallinen V, Haglund C and Seppänen H:
Outcomes of resected nonfunctional pancreatic neuroendocrine
tumors: Do size and symptoms matter? Surgery. 158:1556–1563. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cherenfant J, Stocker SJ, Gage MK, Du H,
Thurow TA, Odeleye M, Schimpke SW, Kaul KL, Hall CR, Lamzabi I, et
al: Predicting aggressive behavior in nonfunctioning pancreatic
neuroendocrine tumors. Surgery. 154:785–791; discussion 791-783.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaujoux S, Partelli S, Maire F, D'Onofrio
M, Larroque B, Tamburrino D, Sauvanet A, Falconi M and Ruszniewski
P: Observational study of natural history of small sporadic
nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol
Metab. 98:4784–4789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sadot E, Reidy-Lagunes DL, Tang LH, Do RK,
Gonen M, D'Angelica MI, DeMatteo RP, Kingham TP, Groot Koerkamp B,
Untch BR, et al: Observation versus resection for small
asymptomatic pancreatic neuroendocrine tumors: A matched
case-control study. Ann Surg Oncol. 23:1361–1370. 2016. View Article : Google Scholar
|
|
36
|
Massironi S, Rossi RE, Zilli A, Casazza G,
Ciafardini C and Conte D: A wait-and-watch approach to small
pancreatic neuroendocrine tumors: Prognosis and survival.
Oncotarget. 7:18978–18983. 2016.PubMed/NCBI
|
|
37
|
Sharpe SM, In H, Winchester DJ, Talamonti
MS and Baker MS: Surgical resection provides an overall survival
benefit for patients with small pancreatic neuroendocrine tumors. J
Gastrointest Surg. 19:117–123; discussion 123. 2015. View Article : Google Scholar
|
|
38
|
Ende AR, Sedarat A, Shah P, Jhala N,
Fraker DL, Drebin JA, Metz DC and Kochman ML: Risk factors for
aggressive nonfunctional pancreatic neuroendocrine tumors and the
role of endoscopic ultrasound guided fine-needle aspiration. Endosc
Ultrasound. 5:49–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Regenet N, Carrere N, Boulanger G, de
Calan L, Humeau M, Arnault V, Kraimps JL, Mathonnet M, Pessaux P,
Donatini G, et al: Is the 2-cm size cutoff relevant for small
nonfunctioning pancreatic neuroendocrine tumors: A French
multicenter study. Surgery. 159:901–907. 2016. View Article : Google Scholar
|
|
40
|
Zhang IY, Zhao J, Fernandez-Del Castillo
C, Braun Y, Razmdjou S, Warshaw AL, Lillemoe KD and Ferrone CR:
Operative versus nonoperative management of nonfunctioning
pancreatic neuroendocrine tumors. J Gastrointest Surg. 20:277–283.
2016. View Article : Google Scholar
|
|
41
|
Kishi Y, Shimada K, Nara S, Esaki M,
Hiraoka N and Kosuge T: Basing treatment strategy for
non-functional pancreatic neuroendocrine tumors on tumor size. Ann
Surg Oncol. 21:2882–2888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jung JG, Lee KT, Woo YS, Lee JK, Lee KH,
Jang KT and Rhee JC: Behavior of small, asymptomatic,
nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs).
Medicine (Baltimore). 94:e9832015. View Article : Google Scholar
|
|
43
|
Garcia-Carbonero R, Sorbye H, Baudin E,
Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C,
Anlauf M, Cwikla JB, et al: Vienna Consensus Conference
participants: ENETS Consensus Guidelines for high-grade
gastroenteropancreatic neuroendocrine tumors and neuroendocrine
carcinomas. Neuroendocrinology. 103:186–194. 2016. View Article : Google Scholar
|
|
44
|
Maurizi A, Partelli S and Falconi M:
Pancreatic Surgery. Front Horm Res. 44:139–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cherif R, Gaujoux S, Couvelard A, Dokmak
S, Vuillerme MP, Ruszniewski P, Belghiti J and Sauvanet A:
Parenchyma-sparing resections for pancreatic neuroendocrine tumors.
J Gastrointest Surg. 16:2045–2055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Faitot F, Gaujoux S, Barbier L, Novaes M,
Dokmak S, Aussilhou B, Couvelard A, Rebours V, Ruszniewski P,
Belghiti J, et al: Reappraisal of pancreatic enucleations: A
single-center experience of 126 procedures. Surgery. 158:201–210.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fernández-Cruz L, Blanco L, Cosa R and
Rendón H: Is laparoscopic resection adequate in patients with
neuroendocrine pancreatic tumors? World J Surg. 32:904–917. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Toste PA, Kadera BE, Tatishchev SF, Dawson
DW, Clerkin BM, Muthusamy R, Watson R, Tomlinson JS, Hines OJ,
Reber HA, et al: Nonfunctional pancreatic neuroendocrine tumors
<2 cm on preoperative imaging are associated with a low
incidence of nodal metastasis and an excellent overall survival. J
Gastrointest Surg. 17:2105–2113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Clancy TE: Surgical management of
pancreatic neuroendocrine tumors. Hematol Oncol Clin North Am.
30:103–118. 2016. View Article : Google Scholar
|
|
50
|
Curran T, Pockaj BA, Gray RJ, Halfdanarson
TR and Wasif N: Importance of lymph node involvement in pancreatic
neuroendocrine tumors: Impact on survival and implications for
surgical resection. J Gastrointest Surg. 19:152–160; discussion
160. 2015. View Article : Google Scholar
|
|
51
|
Jiang Y, Jin JB, Zhan Q, Deng XX and Shen
BY: Impact and clinical predictors of Lymph node metastases in
nonfunctional pancreatic neuroendocrine tumors. Chin Med J (Engl).
128:3335–3344. 2015. View Article : Google Scholar
|
|
52
|
Gratian L, Pura J, Dinan M, Roman S, Reed
S and Sosa JA: Impact of extent of surgery on survival in patients
with small nonfunctional pancreatic neuroendocrine tumors in the
United States. Ann Surg Oncol. 21:3515–3521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bettini R, Partelli S, Boninsegna L,
Capelli P, Crippa S, Pederzoli P, Scarpa A and Falconi M: Tumor
size correlates with malignancy in nonfunctioning pancreatic
endocrine tumor. Surgery. 150:75–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cucchetti A, Ricci C, Ercolani G, Campana
D, Cescon M, D'Ambra M, Pinna AD, Minni F and Casadei R: Efficacy
and cost-effectiveness of immediate surgery versus a wait-and-see
strategy for sporadic nonfunctioning T1 pancreatic endocrine
neoplasms. Neuroendocrinology. 101:25–34. 2015. View Article : Google Scholar
|