Introduction
Each year an increasing number of patients is
diagnosed with esophageal cancer, which makes it the eight most
common cancer worldwide. With almost 500,000 new cases diagnosed
and 400,000 deaths in 2012, esophageal cancer is the sixth most
common cause of cancer-related death worldwide. The overall 5-year
survival rate for patients diagnosed with esophageal cancer is
<20% and the current treatment options are far from being
satisfactory (1–4). Therefore, innovative approaches are
urgently needed (5). In this
respect, photodynamic therapy (PDT) has emerged as an effective and
supporting modality for gastroenterological and dermatological
treatment and has already been shown to be a promising and
clinically relevant treatment modality for esophageal neoplasia
(6–8).
PDT involves the activation of a photosensitizing
drug by irradiation to locally generating reactive oxygen species
(e.g., singlet oxygen and superoxide anion radicals) which induces
tumor shrinkage by apoptosis and/or necrosis (9,10).
Recent developments in synthetic chemistry and optics created new
perspectives for the enhancement of the PDT as a promising
treatment option. Phthalocyanines are very interesting photoactive
substances, which are currently intensively investigated (11–13).
Here, we propose tetra-triethyleneoxysulfonyl
substituted zinc phthalocyanine (ZnPc) as a promising
photosensitizer for PDT of esophageal cancer. Results of a previous
in vitro study already demonstrated a long-lasting
elimination of cancer cells of different origin (e.g., pancreatic
carcinoid, colorectal carcinoma) (14). This study focused on the
photodynamic treatment of esophageal cancer and took both entities,
namely squamous cell carcinoma and adenocarcinoma into account to
evaluate the potential of this novel ZnPc as a candidate for future
PDT treatment of esophageal cancer.
Materials and methods
Photosensitizer
Tetra-triethyleneoxysulfonyl substituted zinc
phthalocyanine (ZnPc) was prepared by slightly modifying the
procedure described in the literature (15). The compound was dissolved in DMSO
of spectroscopic grade (Sigma-Aldrich, France). Concentration of
stock solutions was calculated by measuring their optical density
at 694 nm with UV/Vis spectrometer Ultraspec 2100 (Amersham
Biosciences, UK) and based on Lambert-Beer relationship with
ε694 nm = 2.04×105 M/cm (14).
Cell culture
The human esophageal squamous carcinoma cell line
Kyse-140 and the human esophageal adenocarcinoma cell line OE-33
were cultured in RPMI-1640 medium (Gibco, Thermo Fisher Scientific,
Germany) supplemented with 10% fetal bovine serum (FCS, Biochrom
AG, Germany), 100 U/ml penicillin and 100 μg/ml streptomycin
(Biochrom AG) (5,9). Medium of OE-33 cells was additionally
supplemented with 2 mM L-glutamine (Biochrom AG). All cells were
kept under standard conditions (37°C in a humidified atmosphere of
5% CO2). The culture medium was changed every second day
and once a week the cells were passaged using 1% trypsin/EDTA.
Light source and irradiation
Cancer cells were irradiated with a broad band light
source equipped with a 100 W halogen lamp (EFR 12 V/100 W GZ - 6.35
lamp, Omnilux, Germany). The spectral output of the lamp ranged
from 400 to 800 nm. To prevent infrared irradiation, a
heat-reflecting filter that cuts off transmission at 700 nm and
above was inserted into the optical path. The illuminated area
(5.5×4.5 cm) had an average power density of 80 W/m2.
The light energy dose was measured with a P-9710 radiometer
controlled by a silicon photocell (Optometer P 9710) from
Gigahertz-Optik (Munich, Germany). The total light energy dose was
calculated by integrating the energy signal over the entire period
of irradiation.
Photodynamic therapy
For PDT treatment cells were incubated with ZnPc
(1–10 μM) for 1–30 h in the dark at 37°C (5% CO2,
humidified atmosphere). Thereafter, the ZnPc-containing medium was
replaced by PBS, and cells were irradiated with a light dose of 10
J/cm2. During irradiation the temperature of the samples
never exceeded 37°C (temperature was measured with a digital
thermometer placed inside of irradiation system). After irradiation
PBS was removed and cells were maintained at 37°C in the incubator
(5% CO2, humidified atmosphere) in PS-free culture
medium. Cytotoxic effects of the PS in the absence of light (dark
controls) were determined by treating the samples in the same way
except for the irradiation.
Intracellular distribution of ZnPc
Cells grown on glass cover slips, were incubated
with increasing concentrations of non- photoactivated ZnPc (1–10
μM) for 24 h and then analyzed by measuring their
fluorescence with a confocal laser microscope (Leica, DMI 6000,
Germany) [excitation: HeNe laser (633 nm), detection: PMT (400–800
nm)], equipped with 63× glycerin immersion objective. Digital
images were processed using the Leica LAS AF Lite software.
Growth inhibitory effects of
ZnPc-PDT
Changes in the cell number were investigated by
performing crystal violet staining as described previously
(16). Briefly, the cells were
fixed with 1% glutaraldehyde and stained with 0.1% crystal violet.
The unbound dye was removed by washing the cells with water. Bound
crystal violet was solubilized with 0.2% Triton-X-100 in PBS. Light
extinction, which increases linearly with the cell number, was
analyzed at 570 nm using an ELISA-Reader.
Measurement of reactive oxygen species
(ROS)
Cells were incubated for 24 h with ZnPc, and then
illuminated with 10 J/cm2. ROS formation was determined
24 h later with CellROX Green and CellROX Orange (Thermo Fisher
Scientific, USA) according to the manufacturer's protocol. Control
cells underwent PDT without previous ZnPc loading. Cells were
analyzed by using a fluorescence microscope (Axioskop 40; objective
40×, NA 1.30, Zeiss, Germany) equipped with digital camera (Kappa,
DX4-285FW, Germany). Green fluorescence shows ROS formation in
nucleus and mitochondria (ex/em ~470/525 nm), orange fluorescence
displays ROS in the cytoplasm (ex/em ~546/575 nm). Separate sets of
cells were incubated with 1 mM of vitamin C (WEPA, Germany) for 1 h
before ZnPc-PDT to suppress ROS formation.
Detection of apoptosis
Changes in caspase-3 activity were calculated from
the cleavage of the fluorogenic substrate AC-DEVD-AMC
(Calbiochem-Novabiochem, Germany), as described previously
(17). Three and 6 h after
ZnPc-PDT, cells were lysed with lysis buffer and the lysates were
incubated for 1 h at 37°C with a substrate solution containing 20
μg/ml AC-DEVD-AMC, 20 mM HEPES, 10% glycerol, 2 mM DTT at pH
7.5. Substrate cleavage was measured fluorometrically using a
VersaFluor fluorometer (Bio-Rad, Germany; filter sets: ex 360/40
nm, em 460/10 nm).
Detection of apoptosis-specific changes in cellular
morphology were visualized by using cell viability/cytotoxicity
assay kit (Live/dead assay, Life Technologies, USA) as described
earlier (18). Six hours after
ZnPc-PDT, cells which were grown on cover slips, were incubated
with calcein- AM (160 nM) and EthD-1 (2 μM) for 30 min in
medium at 37°C and examined by fluorescence microscope from Zeiss
(Axioskop 40; objective 20×, 0.50 NA, Zeiss). Live cells were
identified by the presence of ubiquitous intracellular esterase
activity, leading to the conversion of non-fluorescent
cell-permeable calcein-AM to the green-fluorescent polyanionic dye
calcein (ex/em 495/510 nm), which is well retained in living cells.
Dead cells were determined by EthD-1 (ex/em 495/635 nm), which
becomes red-fluorescent upon binding to nucleic acids of cells with
damaged membranes.
Changes in Bcl-2 and Bax expression, and release of
cytochrome c from PDT-damaged mitochondria, indicating the
induction of mitochondria mediated apoptosis was analyzed by
western blotting as described previously (19). In brief, whole-cell extracts were
prepared by lysing cells with RIPA-buffer Cell lysates containing
30 μg protein were subjected to gel electrophoresis (12% gel
for Bcl-2 and Bax, 4–20% gel for cytochrome c, Bio-Rad,
USA). Proteins were transferred to PVDF membranes by
electroblotting for 1.5 h at 100 V. The blots were blocked in 5%
non-fat dry milk in TBS-Tween solution for 1 h at room temperature,
and then incubated at 4°C overnight with antibodies directed
against anti-human Bcl-2 (1:500) (Santa Cruz, USA), Bax (1:500)
(Cell Signaling, USA), cytochrome c (1:1,000) (Cell
Signaling) and anti-β-actin (1:2,000) (Sigma, USA) as loading
control. After incubation with horseradish peroxidase-coupled
anti-IgG antibodies (1:10,000, Amersham) for ≥1 h at room
temperature, the blots were developed and visualized by using
enhanced chemiluminescent detection kit (Amersham) and exposed to
ECL Hyperfilm.
Antitumoral and antiangiogenic effects of
ZnPc-PDT
In vivo studies on the antitumoral and
antiangiogenic effects of ZnPc-PDT were performed by using chicken
chorioallantoic membrane assays (CAM) as described previously
(14,20).
Kyse-140 cells (1×106) were resuspended
in 10 μl cell culture medium and mixed with 10 μl
Matrigel (Corning, MA, USA). Cell suspensions were pipetted into
silicone ring (5 mm 0) that was placed on the CAM of fertilized
chicken eggs on day 7 of development. The tumor plaques were
allowed to adhere for 72 h before they were topically treated with
10 μl of ZnPc (10 μM) or NaCl 0.9% (as negative
control). Thereafter eggs were sealed with clear tape and incubated
in the dark at 37°C in a humidified incubator. Twenty-four hours
later tumors were illuminated with 10 J/cm2. Tumor
growth and viability of the developing embryo were controlled daily
by stereo microscopy (up to 72 h after treatment). At the end of
the experiment, tumors were resected, measured - (largest
diameter)2 × (perpendicular diameter) × 0.5 and
photographed with stereo-microscope equipped with camera (Di-Li,
digital microscope, Germany) (21).
For the antiangiogenic testing of ZnPc-PDT, 10
μl of ZnPc (10 μM) or 10 μl NaCl 0.9% (as
negative control) were topically applied inside of a silicone ring
(5 mm Ø) that was placed on the CAM of fertilized chicken eggs at
day 11 of the chicken embryo development. Thereafter eggs were
sealed with clear tape and incubated in the dark at 37°C in a
humidified incubator. Twenty-four hours later the treated areas
were illuminated with 10 J/cm2. Changes in the
microvasculature of the CAM were recorded 24 and 72 h after PDT by
using a stereo microscope (Axioplan; Zeiss) equipped with a digital
camera (MBF, Bioscience, USA).
Biotolerability of non-photoactivated
ZnPc in vivo
Safety of non-photoactivated ZnPc in vivo was
examined in male Wistar rats (n=15, weight 290±6 g; 11-week-old)
(22). Influence of the PS on the
immune system and organs such as liver or kidneys were examined to
monitor the potential dark toxicity of the compound. Rats were
divided into four treatment groups: group I, negative control, 0.9%
saline, n=3; groups II-IV, 0.5, 1 and 5 mg/kg, n=4/per group). ZnPc
or saline were injected intraperitoneally. Rodent cages were partly
covered with light-impermeable fabric. Body weight, behavior, food
and water consumption were controlled each day. After sacrificing
the animals on day 7, liver, kidney, and spleen were weighed to
calculate the organ coefficient [rat weight (g)/organ weight (g)]
(23). The amount of white blood
cells (WBC) in the blood was determined by using a hematology
analyzer (Cell-Dyn 3700, USA). Identification of leukocyte subsets
(lymphocytes, monocytes, neutrophils, eosinophils) was performed by
using Aerospray Slide Stainer 7120 (Wescor, USA). All animal
experiments were conducted under the authority of the Local Ethics
Committee for the Care and Use of Laboratory Animals of the Medical
University of Gdansk, Poland.
Results
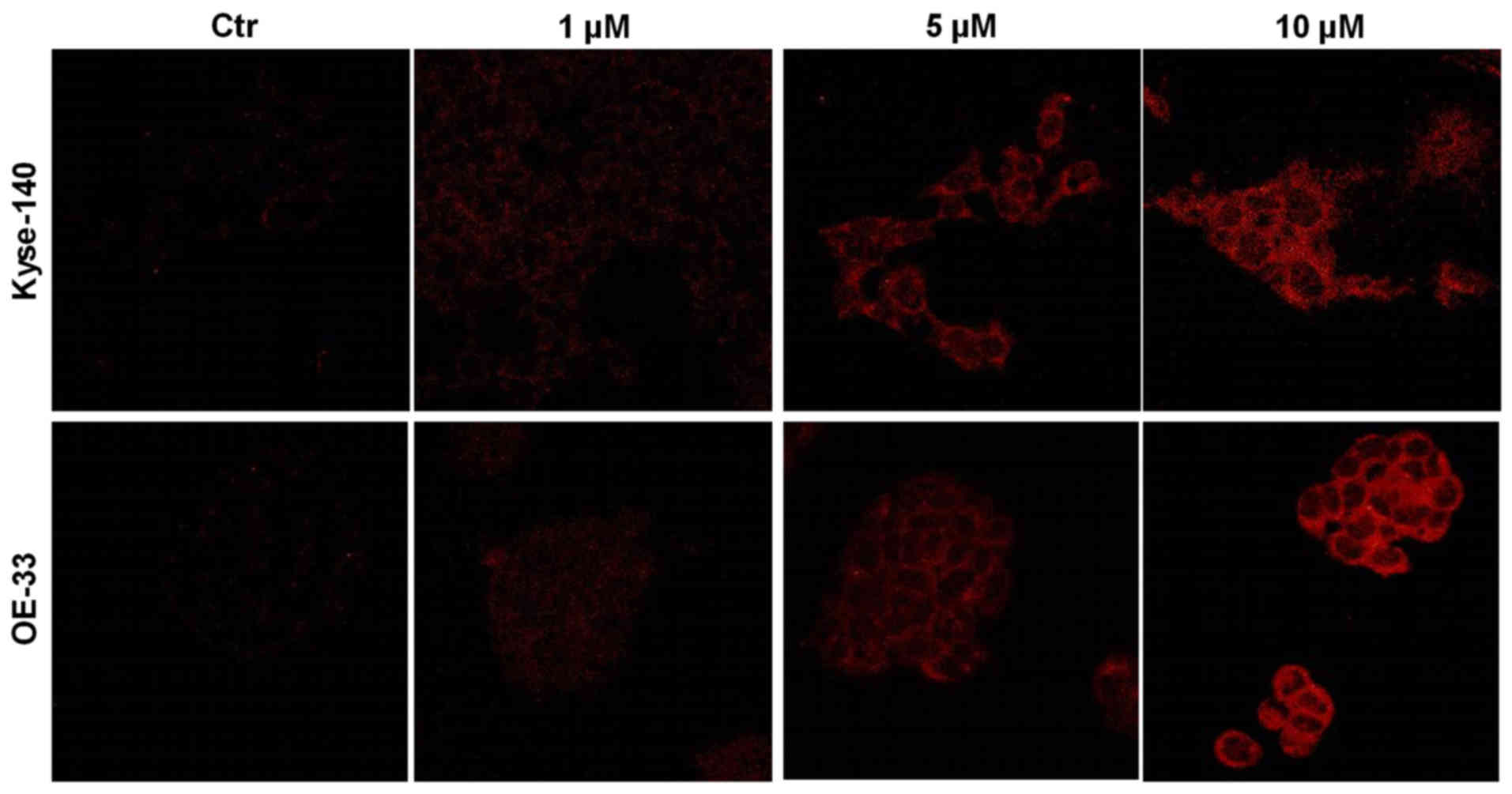
Uptake of ZnPc
Intracellular localization of increasing
concentrations of ZnPc in cancer cells was investigated using
confocal laser scanning microscopy. ZnPc-loaded Kyse-140 and OE-33
cells showed a dose-dependent uptake and homogenous cytoplasmic
distribution of non-photoactivated ZnPc after 24 h of incubation
(Fig. 1).
Determination of ZnPc-induced
phototoxicity
Changes in the cell number of Kyse-140 and OE-33
cells after treatment with non- and photoactivated ZnPc (1–10
μM) were analyzed by crystal violet staining.
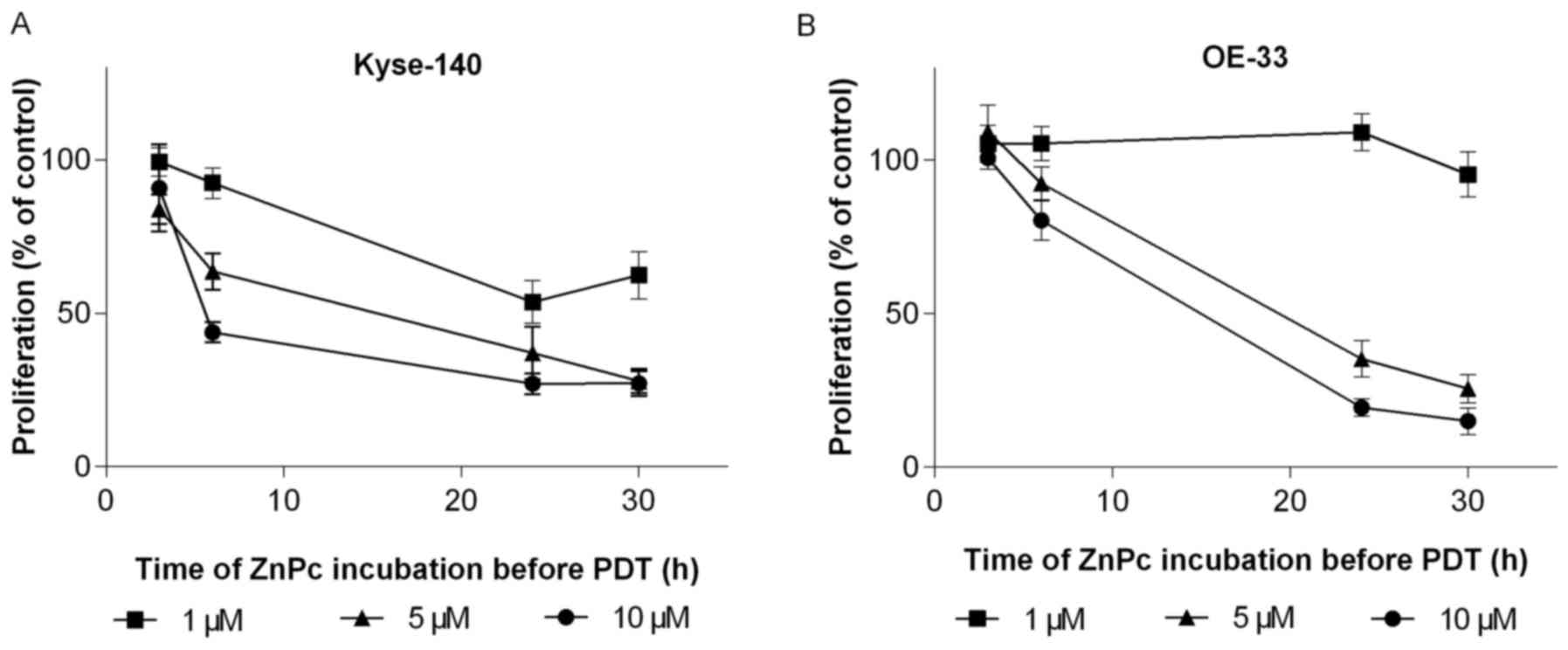
To establish the optimal loading time of ZnPc,
esophageal cancer cells were incubated for 1–30 h with rising
concentrations of ZnPc (1–10 μM). The subsequent PDT (10
J/cm2) showed a strong correlation between loading time
and growth rate of ZnPc-PDT treated cells. The longer the
incubation time, the more pronounced was the ZnPc-PDT induced
growth inhibition, reaching its maximum after 24 h of incubation.
No further increase was observed when the incubation time was
prolonged to 30 h. Squamous esophageal cancer cells (Kyse- 140)
were more sensitive (Fig. 2A) than
adenocarcinomatous OE-33 cells which required higher
ZnPc-concentrations for pronounced growth inhibitory effects
(Fig. 2B). Additional experiments
on Kyse-140 using the RTCA iCELLigence system (ACEA, Bioscences,
USA), confirmed the proliferation data (24). This system monitors the
proliferation of ZnPc-PDT treated cells in real-time. Photodynamic
treatment caused a 68% decrease in the cell index (value calculated
from changes in the electrical impedance, which correlates with
changes in cell numbers) of cancer cells preincubated with ZnPc (5
μM) at least 24 h before PDT (data not shown).
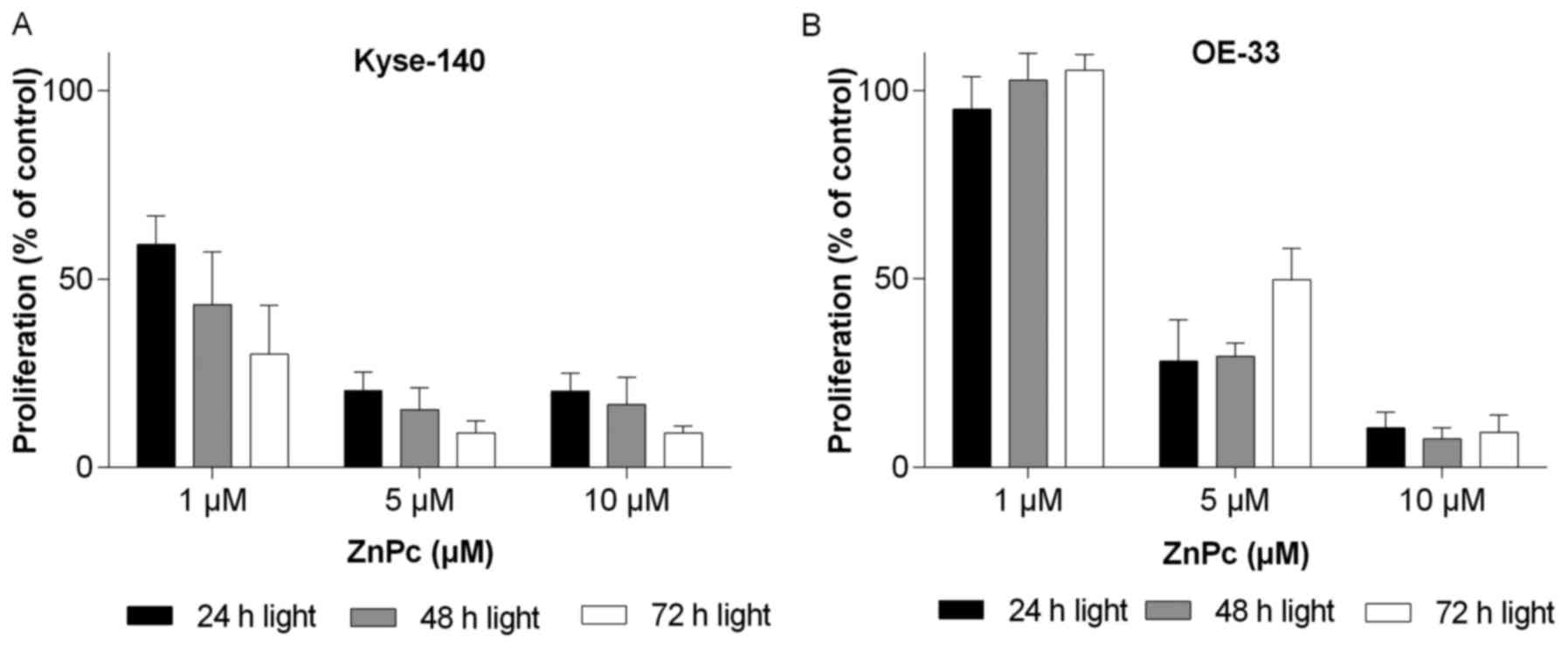
To measure the time course of growth inhibition of
ZnPc-PDT treated Kyse-140 and OE-33 cells (preincubated with ZnPc
for 24 h), cell proliferation was examined for up to 72 h after
photoactivation of ZnPc. Photoactivation of ZnPc (10 J/cm2)
resulted in a significant dose- and time-dependent decrease in cell
proliferation of Kyse-140 (Fig.
3A) and OE-33 (Fig. 3B) of
>90% as compared to untreated control cells. The phototoxic
effects of ZnPc-PDT was more pronounced in squamous Kyse-140 cancer
cells than in adenocarcinomatous OE-33 cells, which is reflected in
the respective IC50-values of 1.41±0.40 μM
(Kyse-140) and 3.35±0.79 μM (OE-33). Moreover, at least the
Kyse-140 cells that escaped from being killed by low dose ZnPc-PDT
(1 μM) did not regenerate (re-proliferate) in the following
days (Fig. 3A).
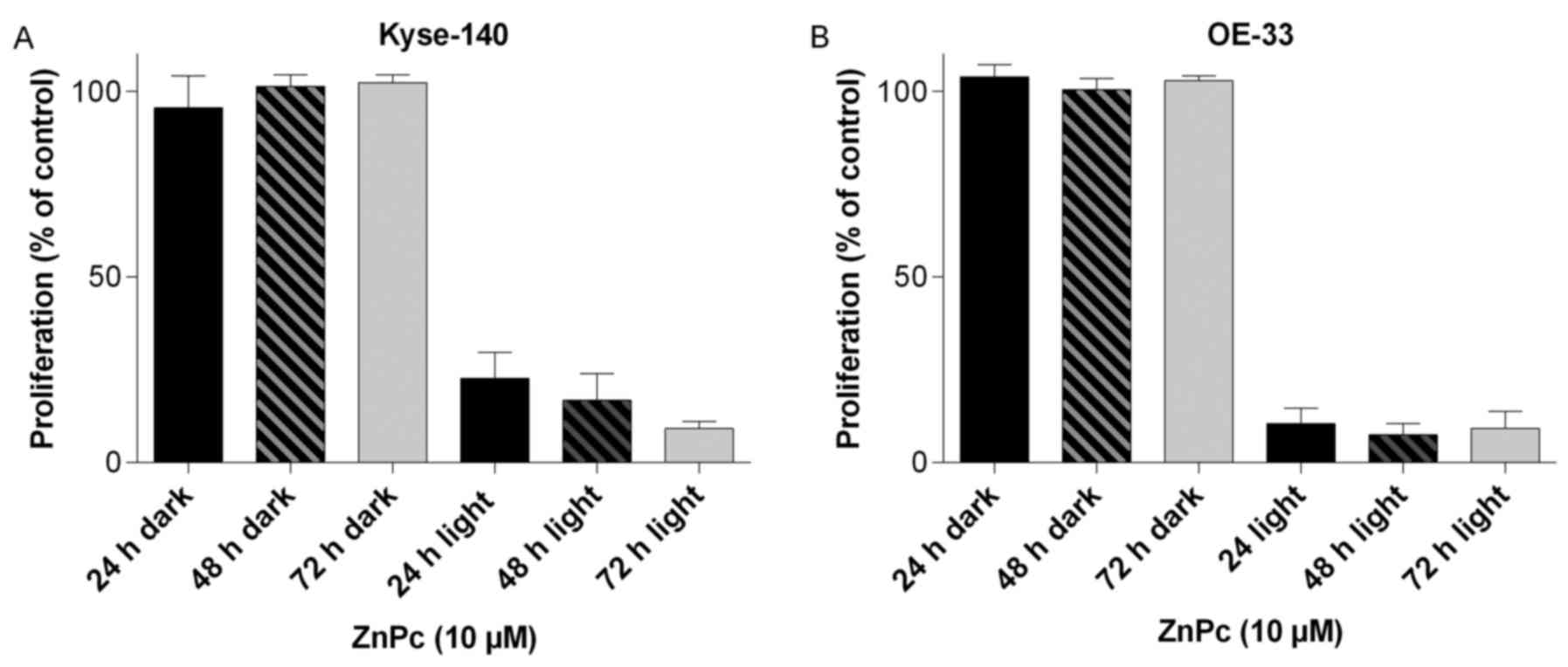
Checking for dark toxic effects of ZnPc, changes in
the cell number and morphology of Kyse-140 and OE-33 cells loaded
with ZnPc (1–10 μM) were determined for up to 72 h after the
incubation period. Even at concentrations as high as 10 μM,
no decrease in cell number (Fig.
4) nor changes in the morphology (data not shown) of either
esophageal cancer cell model could be observed, showing the
non-toxic nature of the non-illuminated compound.
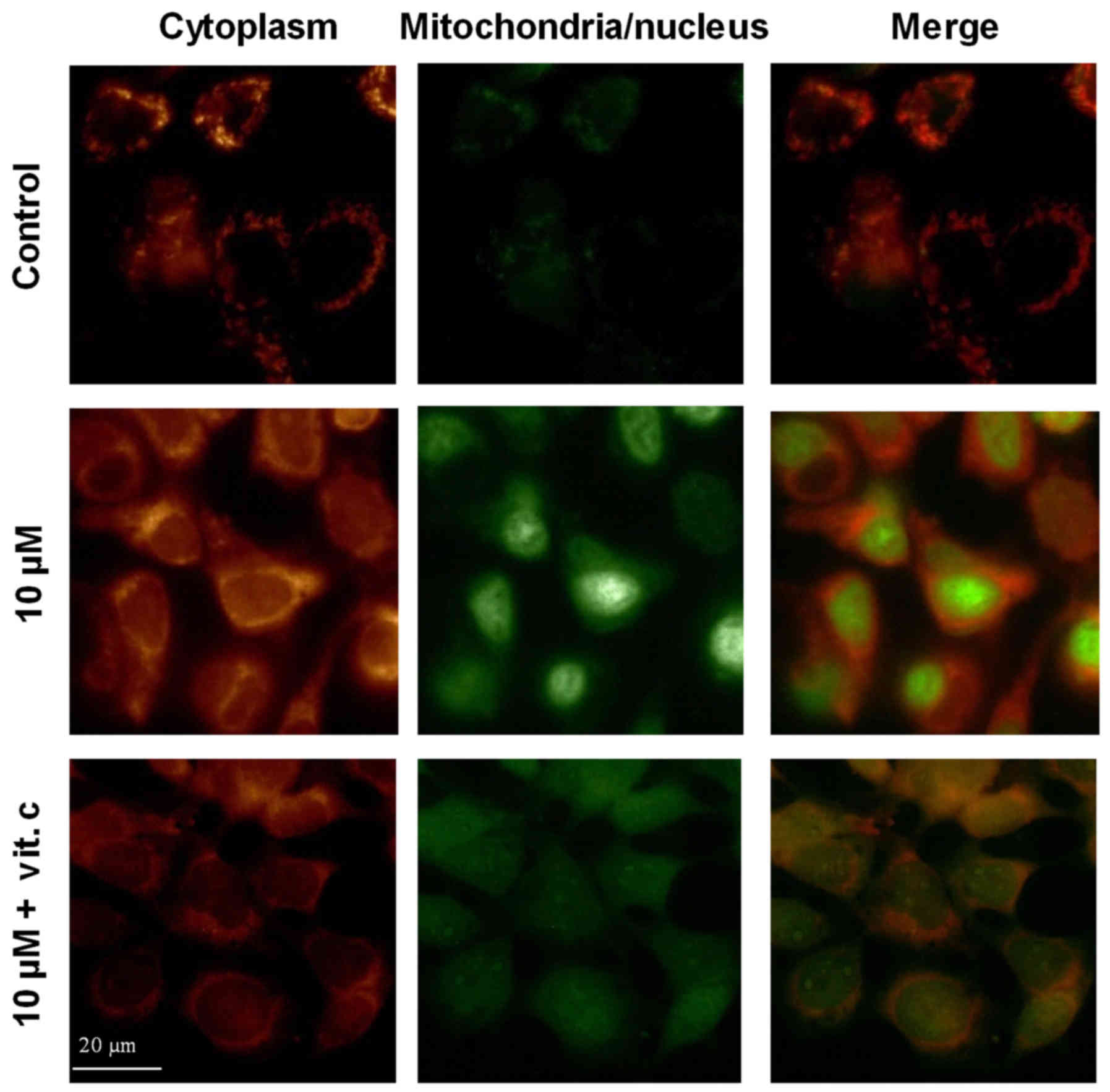
Induction of ROS formation by
ZnPc-PDT
One of the underlying mechanisms of photodynamic
therapy is the generation of highly reactive oxygen species (ROS)
and free radicals upon photoactivation of the PS, thereby damaging
the membranes of the cell and its organelles (e.g., mitochondria,
Golgi vesicles, ribosomes) which subsequently leads to severe cell
injury and death. ZnPc-PDT-induced formation of ROS was evaluated
by fluorescence microscopy employing dyes that monitor ROS
formation in the cytoplasm (orange fluorescence) or in the
nucleus/mitochondria (green fluorescence). Twenty-four hours after
ZnPc-PDT a robust increase in ROS formation was observed in either
Kyse-140 (Fig. 5) or OE-33 cells.
ROS formation occurred ubiquitously and without a preferred
localization in the nucleus or mitochondria, as in both cell lines
there was a similar increase in ROS formation in the
nucleus/mitochondria as well as in the cytoplasm. To further
confirm ROS formation after ZnPc-PDT, Kyse-140 cells were incubated
with the antioxidant and free radical scavenger vitamin C (1 mM)
prior to PDT. One hour preincubation with vitamin C caused an
appreciable decrease of free radical formation in the cytoplasm as
well as in the nucleus/mitochondria (Fig. 5).
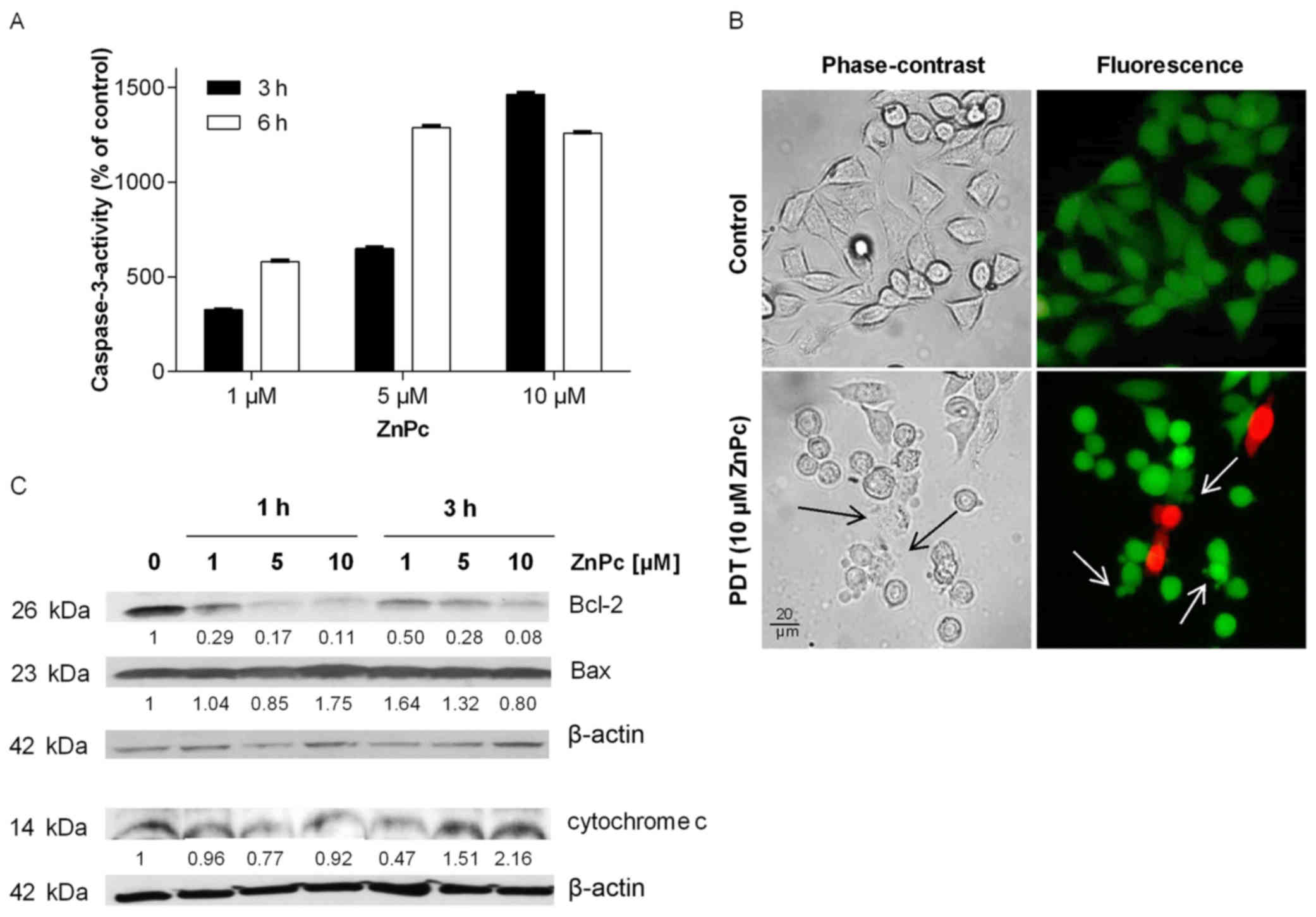
Apoptotic effect of ZnPc-PDT
Photodynamic treatment can lead to the induction of
apoptosis by ROS-mediated disturbance of mitochondrial integrity.
The molecular mechanism underlying the ZnPc-PDT induced apoptosis
and changes in cell morphology of Kyse-140 were analyzed for up to
6 h after PDT.
ZnPc-PDT caused time- and dose-dependent increase of
caspase-3-activity, which was measured by performing caspase-3
assays. ZnPc-PDT treated cells showed an increase in caspase-3
activity of up to 15-fold higher as compared to untreated, but
illuminated control cells (Fig.
6A). Employing fluorescence microscopy apoptosis-related
changes in cell morphology were determined 24 h after PDT. Cells
appeared shrunken and unstructured with simultaneous formation of
membrane-bound apoptotic bodies (Fig.
6B). As caspase-3 can be activated by either
mitochondria-dependent or -independent signaling pathways, we
checked the expression of Bax, Bcl-2 and cytochrome c to
confirm the hypothesis that PDT-induced apoptosis was mitochondria
driven because of ROS induced disturbance of mitochondrial
integrity. One hour after ZnPc-PDT the expression of antiapoptotic
Bcl-2 decreased dose-dependently with a simultaneous increase of
proapoptotic Bax. Three hours after ZnPc-PDT an increase in
cytochrome c expression was observed (Fig. 6C). Cytochrome c is released
from the mitochondrial intermembrane into the cytosol during
apoptosis further indicating mitochondria-dependent apoptosis.
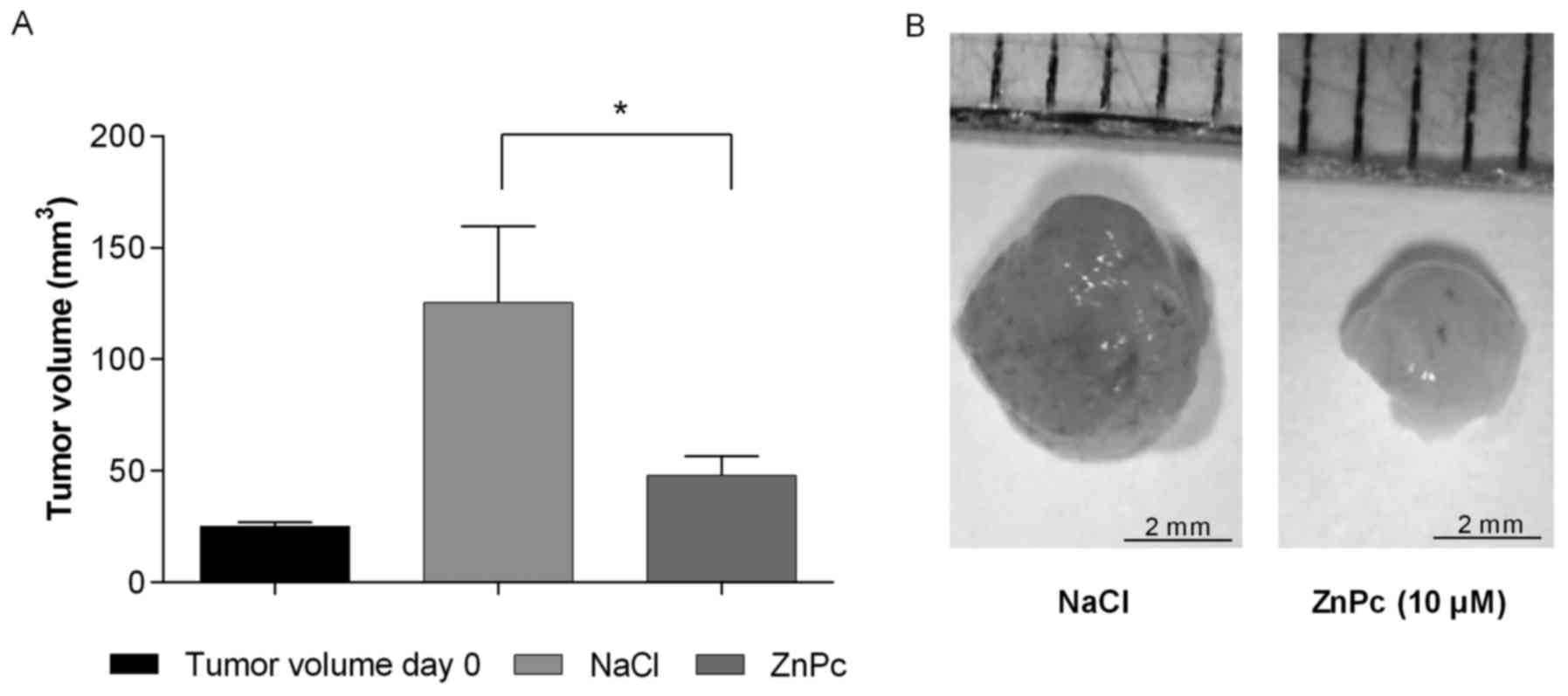
Antitumor and antiangiogenic activity of
ZnPc-PDT in vivo
Antineoplastic effects of the novel ZnPc-PDT was
studied in vivo by employing CAM-assays. Tumor plaques
(1×106 cancer cells) grown from squamous cell carcinoma
(Kyse-140) were inoculated onto the CAM of 7-day-old fertilized
chicken eggs. The plaques got attached to the microvessel network
of the CAM after 72 h and thereafter were topically treated with
ZnPc (10 μM) for 24 h and illuminated (10 J/cm2).
Seventy-two hours post PDT, tumors were excised, photographed and
changes in tumor volume was determined. Photodynamic treatment led
to a significant reduction of tumor growth of >70% after 3 days
when compared to control experiments, in which the tumors were also
illuminated with 10 J/cm2 but treated with NaCl instead of ZnPc
(Fig. 7).
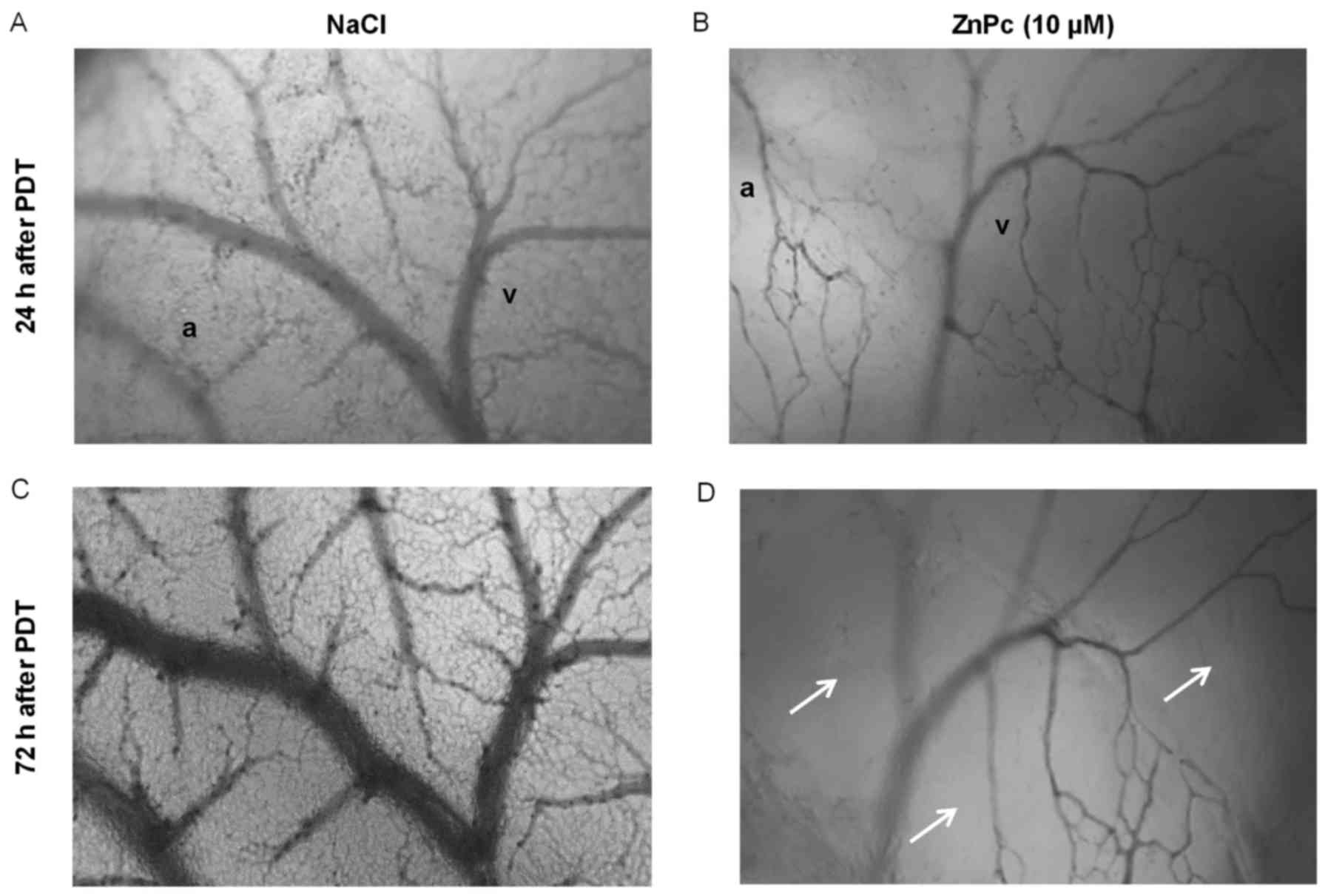
The antiangiogenic effects of ZnPc-PDT on the
microvessel formation were examined on the developing CAM for ≤ 72
h after treatment. An area of 5 mm Ø of 11-day-old CAMs was
incubated with 10 μM ZnPc for 24 h and then illuminated (10
J/cm2). ZnPc-PDT led to an increase of non-perfused
areas and to vasodegeneration of the vasculature and the capillary
plexus. NaCl treated and illuminated control CAMs showed no changes
in the vascular network, which still consisted of a continuously
perfused capillary plexus and larger vessels (arteries and veins).
The antiangiogenic changes of ZnPc-PDT were still evident after 72
h indicating a lasting antiangiogenic effect of the treatment in
vivo (Fig. 8).
Safety of non-illuminated ZnPc in
vivo
Prior to animal experiments with the novel ZnPc-PDT,
we performed a set of experiments evaluating the so-called 'dark
toxicity' of the novel photosensitizer in vivo to get
valuable information on its systemic effects in living organisms as
well on its expected clinical safety.
Injection of 10 μl ZnPc (10 μl of 10
μM) into an embryofeeding vein of the chorioallantoic
membrane at day 14 of embryonic development did not influence the
survival or further embryonic development. Twenty-four hours after
ZnPc injection, 100% of the 6 injected chicken embryos survived
(data not shown), further supporting the expected safety of the
novel photosensitizer in its non-photoactivated state.
Based on the promising in vitro findings
(Fig. 4) and the aforementioned
in vivo results, we performed first animal studies on Wistar
rats. The rats were injected intraperitoneally with rising doses of
ZnPc (0.5–5 mg/kg) and kept in light-protected rodent cages for one
week. During the entire observation period the animals constantly
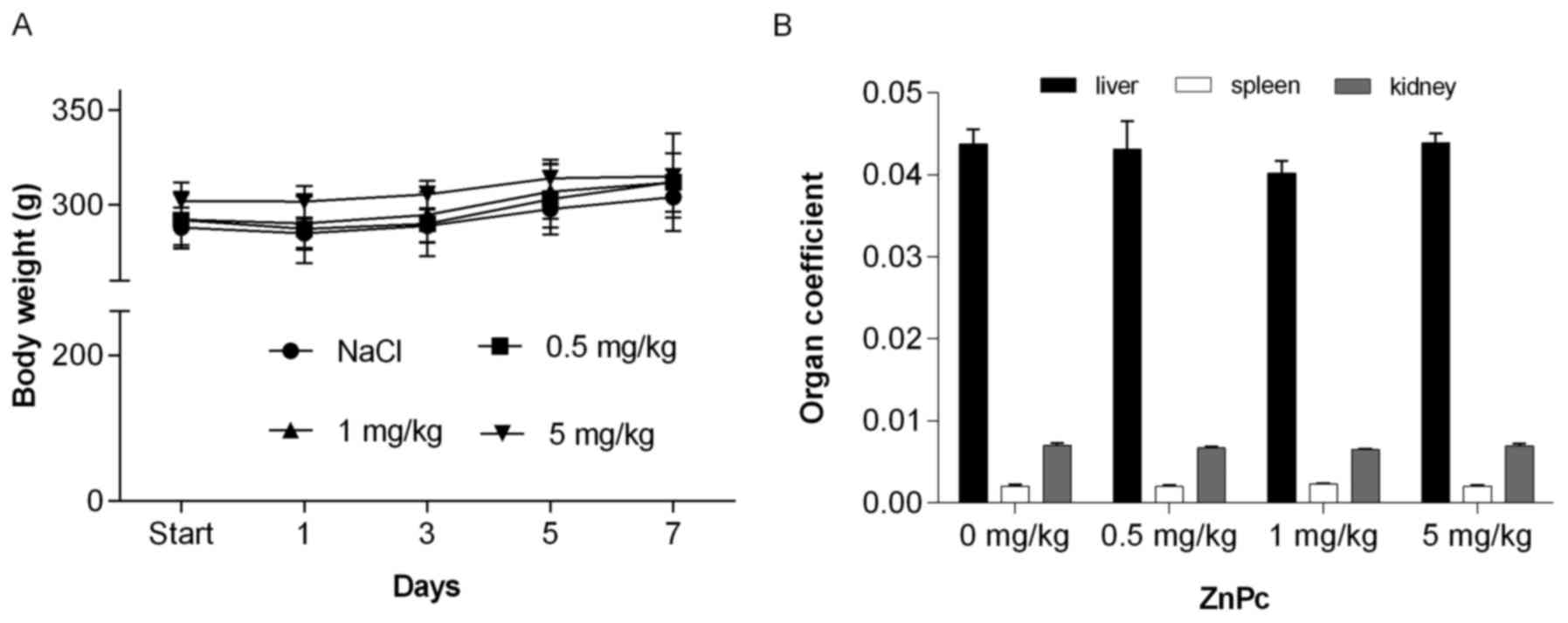
kept or even slightly gained weight (Fig. 9A), behaved normally, and did not
show any changes in food and water consumption, as compared to the
saline treated control animals. At the end of the experiment,
several organs (liver, spleen, and kidney) were removed and
weighed, also blood samples were taken for blood analysis. None of
the analyzed organs were swollen or shrunken which is reflected by
the constant organ coefficient (Fig.
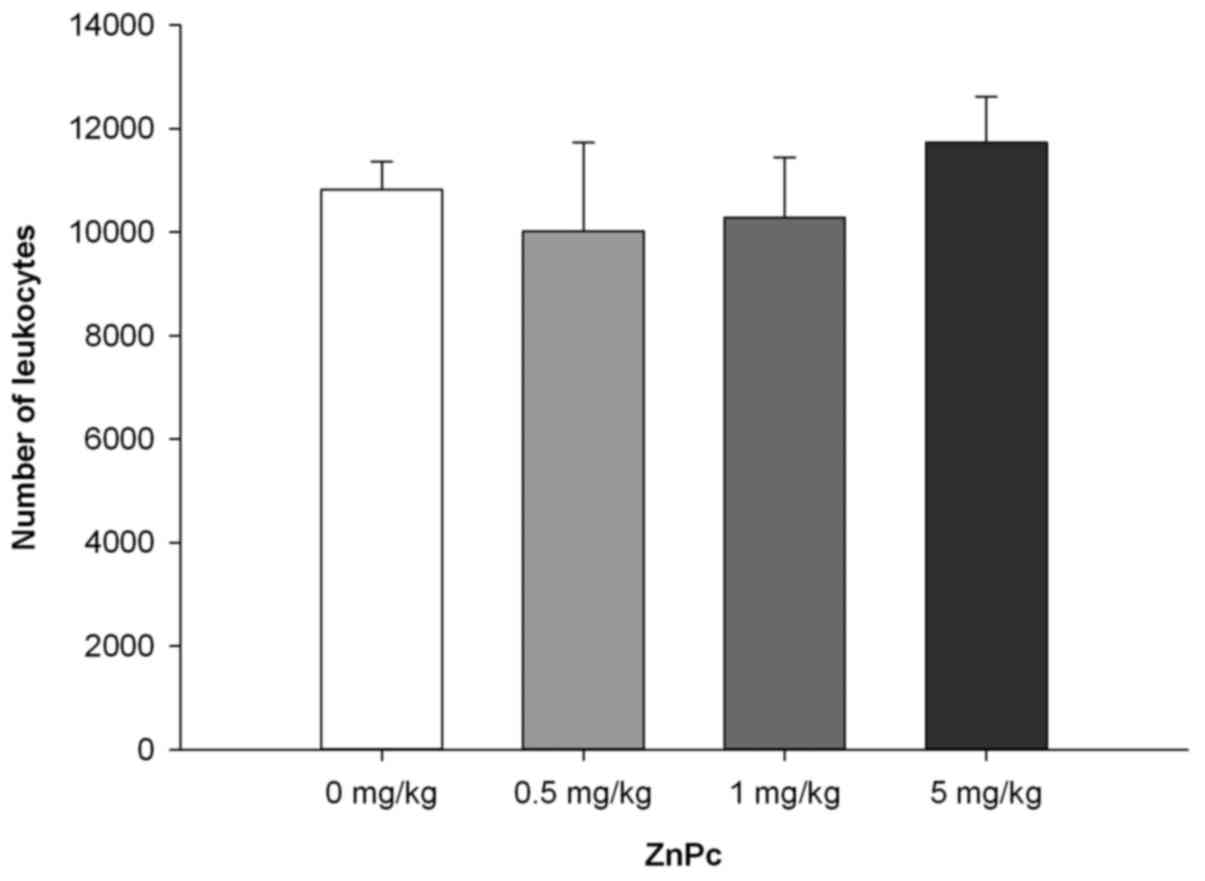
9B). No significant changes in leukocyte number (Fig. 10) or leukocyte subsets (data not
shown) of photosensitizer-treated animals were observed, indicating
that non-photoactivated ZnPc is generally well-tolerated and does
not induce systemic inflammation.
Discussion
Medical therapy of advanced esophageal cancer is
still unsatisfactory. Moreover, many patients with esophageal
cancer are in poor clinical condition precluding aggressive
chemotherapy (5). Thus, there is a
strong need for new, effective and well-tolerated treatment
approaches. An interesting alternative approach may be photodynamic
therapy (PDT) which is already in clinical use for the treatment of
Barret's dysplasia and esophageal cancer (25–28).
Besides elimination of the neoplastic tissue, PDT treatment may
also decrease the risk of esophageal stricture and perforation.
Moreover, technological improvements (e.g., light emitting fabrics
or optical fibers), enabled a rapid progress in the field of PDT
(29). However, there is still a
need for new and highly effective photosensitizers. In a previous
study we proposed tetra-triethyleneoxysulfonyl-substituted zinc
phthalocyanine as a promising photosensitizer for photodynamic
treatment of different types of gastrointestinal cancer (14). In this study, we focused on the
suitability and effectiveness of a PDT treatment with the novel
ZnPc in human esophageal cancer of either adenocarcinomatous or
squamous cell carcinomatous histology.
PDT efficiency is highly dependent on the
photophysical properties of the employed PS as well as on its
intracellular accumulation and distribution in the target
cells/tissue (30,31). For example, Shao et al and
Tynga et al showed that comparable ZnPc primarily
accumulates in mitochondria and lysosomes (32,33).
Employing confocal laser microscopy, we could demonstrate a time-
and dose-dependent uptake of non-photoactivated ZnPc in esophageal
cancer cells of both entities. However, in contrast to the
above-mentioned studies, we observed rather homogeneous cytoplasmic
distribution of PS in both cell lines. Nonetheless, photoactivation
of ZnPc caused ROS formation not only in the cytoplasm but also in
mitochondria and nucleus, pointing to its high phototoxic
potential.
The particular PDT protocols have a great impact on
the therapy outcome and differ from one cancer type to another
(34,35). The time interval between the
application of a photosensitizer and subsequent PDT is very
important due to the different intracellular uptake kinetics of
each particular photosensitizer (36). To estimate the optimal incubation
time of ZnPc in esophageal cancer cells, different pre-incubation
times (1–30 h) were evaluated. We could show that approximately 24
h were required to achieve pronounced antineoplastic PDT-effects
with ZnPc concentrations as low as 1 μM in EC cells.
Prolonging the incubation time to >24 h did not further enhance
the PDT outcome at this concentration, but the data also showed
that at higher concentrations of the PS (5 μM) comparable
results were achieved with much shorter pre-incubation times of
only 10 h. Thus for the determination of optimal treatment
protocols for patients, it will be interesting to figure out the
reasonable compromise between a preferably short preincubation time
and a well-tolerated but still highly effective PS
concentration.
To estimate the cytotoxic potential of ZnPc-PDT,
proliferation studies were performed. We observed a significant
decrease in the cell number of esophageal adeno- and squamous cell
carcinoma cells of ≥90% after ZnPc-PDT, showing that the treatment
is working well in both histologies. Kyse- 140 cells seemed to be
even more susceptible to the treatment, as shown by the lower
IC50-value of ZnPc of 1.41 μM, as compared to
3.35 μM in OE-33 cells. Another difference between squamous
and adenocarcinoma cells became apparent when looking at the
longevity of the PDT-induced growth inhibition. While squamous
Kyse-140 cells which escaped from being immediately killed by the
initial PDT treatment did not seem to be able to (re-)proliferate
in the following days, PDT-surviving adenocarcinomatous OE-33 cells
started to re-grow 48 h after treatment. This effect could be
compensated by using higher concentrations of PS for the specific
cell line. Further studies are required to reveal if specific
cellular responses e.g., cell cycle regulation might play a role in
the different behavior of the two cell types and the respective
mode of action.
Generation and localization of free radicals and
singlet oxygen species have a strong influence on the efficiency of
PDT (37). Here, we demonstrated
ROS formation in esophageal cancer cells by ZnPc-PDT for the first
time. By employing green and orange fluorescent dyes, which
distinguish between free radical formation and localization in the
cytoplasm (orange) or cell organelles such as the mitochondria or
the nucleus (green), we observed a dose-dependent production of ROS
after ZnPc-PDT in both esophageal carcinoma entities. Prominent
increases in the ROS production of Kyse-140 and OE-33 cells
resulted in cell death rates of 70–90% already 24 h after PDT. The
increase in green fluorescence indicates ROS formation near or in
the nucleus and mitochondria. This notion may be of importance when
deciphering the underlying signaling events and pathways of
ZnPc-PDT in terms of apoptosis (38). Nucleus and mitochondria specific
ROS formation was partially blocked by pre-incubation with the
antioxidant vitamin C, supporting the idea that ROS formation may
be of particular importance for the antiproliferative effects of
the novel photosensitizer. This is consistent with the findings of
Grimm et al who investigated the influence of different
exogenous antioxidants on melanoma cell proliferation after PDT
with 5-aminolevulinic acid (5-ALA) (39). After exposure to 5-ALA, human
melanoma cells were incubated with vitamin C at different doses and
then irradiated with light followed by a significant decrease of
the cell death rate.
In the last decade, a significant progress has been
made in the development of apoptosis-modulating cancer therapeutics
which are currently examined in several human clinical trials
(40). Most importantly, the
induction of apoptosis, unlike necrosis, does not cause
inflammation and is thus preferred over cytotoxic and necrotic
treatment strategies (30,41). It has been shown that PDT can
induce apoptosis and in a previous study we demonstrated that
ZnPc-PDT induced apoptotic cell death of gastrointestinal cancer
cells. However, it was unclear whether extrinsic or intrinsic
apoptotic pathways were involved (14). In this study we investigated the
changes in the expression of proteins associated with
mitochondria-driven apoptosis. Photoactivated ZnPc caused a
downregulation of antiapoptotic Bcl-2 and a concomitant
upregulation of proapoptotic Bax. Importantly, an increase in
cytochrome c was detected. Together with the marked increase
in caspase-3 activity, the data indicated that ZnPc-PDT induces a
mitochondria-dependent, intrinsic induction of apoptosis in
esophageal cancer cells (38).
The main goal of PDT treatment is the elimination of
the neoplastic tissue. Numerous studies on new photosensitizers
showed their efficiency to eliminate cancer cells in vitro
(33,42,43).
However, the therapeutic potential and suitability of a new PS can
only be assessed in a combination of in vitro and respective
in vivo studies. In our in vivo model (CAM-assay), we
showed strong antitumor effects of photoactivated ZnPc on
esophageal tumor plaques, which were significantly smaller
(>70%) than ZnPc-untreated control tumors (44). Based on the encouraging data, it is
at least conceivable that ZnPc-PDT will show equivalent
effectiveness in animal models.
Destructive effects of PDT on the microvasculature
are well known and have been successfully used for the treatment of
choroidal neovascularization or age-related macular degeneration
(45,46). In oncology, damage of tumor
vascularity and blood flow stasis are significant aspects of PDT
that contribute to tumor regression (47). However, it has been reported by
Weiss et al that PDT-damaged vasculature can regenerate
within 48 h after PDT (21,45).
Here, we also demonstrated that ZnPc-PDT exerts a destructive
influence on the blood vessel formation and the microvasculature,
but in contrast to the findings by Weiss et al these effects
were comparably long-lasting, as even 72 h after treatment no
regeneration of the vascular network of the CAM could be observed.
Long-lasting antiangiogenic effects of enhanced PDT-treatment with
ZnPc may be of particular importance if looking for suitable
combination treatments in which the PDT is combined with
chemotherapeutics that are antiangiogenic or whose antineoplastic
potency depends on an antiangiogenic environment (48).
Last but not least, clinical safety, good
tolerability and absence of toxicity in its non-photoactivated
state (dark toxicity) are important features of appropriate
photosensitizers. Checking for dark toxic effects of the novel ZnPc
in vitro and in vivo, we showed that
non-photoactivated ZnPc does not affect cell survival or
proliferation nor does it exhibit any sign of toxicity in in
vivo experiments. Testing embryonic survival of fertilized
chicken eggs after intravenous application of ZnPc did not lead to
signs of toxicity either. Embryonic development as well as survival
was not even affected by the highest doses of ZnPc. In line with
comparable results of Zhang et al, who investigated dark
toxic effects of phthalocyanines we felt encouraged to perform
first animal tests (22). Young
Wistar rats injected with ZnPc (0.5–5 mg/kg bodyweight) did not
show signs of dark toxicity or incompatibility towards the
non-photoactivated compound. All animals behaved normally, did not
show changes in water or food consumption and did not lose but even
slightly gained weight during the experiment. Even more important,
the white blood cell count of ZnPc-treated animals did not differ
significantly from control animals. Moreover, no changes in the
organ coefficients were observed, indicating that no inflammatory
processes or organ specific disturbances occurred. Taken together,
the novel photosensitizer ZnPc appears to have an excellent
biotolerability thus rendering it an interesting photosensitizer
for further in vivo studies.
In conclusion, in this study we showed the
extraordinary potential of ZnPc for PDT-treatment of esophageal
cancer. Further in vivo investigations are warranted to
estimate antitumor activity of photoactivated ZnPc in animal
models.
Acknowledgments
Weronika Kuzyniak was funded by a scholarship of the
Studienstiftung des deutschen Volkes. Gustav Steinemann was
supported by a scholarship of the FAZIT-Stiftung, Frankfurt a. M.,
Germany. Jacob Schmidt was funded by a scholarship of the Berliner
Krebsgesellschaft e.V.
References
|
1
|
Thrift AP: The epidemic of oesophageal
carcinoma: Where are we now? Cancer Epidemiol. 41:88–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ireland CJ, Thompson SK, Laws TA and
Esterman A: Risk factors for Barrett's esophagus: A scoping review.
Cancer Causes Control. 27:301–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meves V, Behrens A and Pohl J: Diagnostics
and early diagnosis of esophageal cancer. Viszeralmedizin.
31:315–318. 2015. View Article : Google Scholar
|
|
4
|
Chang TL, Tsai YF, Chao YK and Wu MY:
Quality-of-life measures as predictors of post-esophagectomy
survival of patients with esophageal cancer. Qual Life Res.
25:465–475. 2016. View Article : Google Scholar
|
|
5
|
Sutter AP, Höpfner M, Huether A, Maaser K
and Scherübl H: Targeting the epidermal growth factor receptor by
erlotinib (Tarceva) for the treatment of esophageal cancer. Int J
Cancer. 118:1814–1822. 2006. View Article : Google Scholar
|
|
6
|
Triesscheijn M, Baas P, Schellens JHM and
Stewart FA: Photodynamic therapy in oncology. Oncologist.
11:1034–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi E, Yang CK, Leem C, Park Y, Chang J-E,
Cho S and Jheon S: Clinical outcome of photodynamic therapy in
esophageal squamous cell carcinoma. J Photochem Photobiol B.
141:20–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shishkova N, Kuznetsova O and Berezov T:
Photodynamic therapy in gastroenterology. J Gastrointest Cancer.
44:251–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Höpfner M, Maaser K, Theiss A, Lenz M,
Sutter AP, Kashtan H, von Lampe B, Riecken EO, Zeitz M and Scherübl
H: Hypericin activated by an incoherent light source has
photodynamic effects on esophageal cancer cells. Int J Colorectal
Dis. 18:239–247. 2003.PubMed/NCBI
|
|
10
|
Castano AP, Demidova TN and Hamblin MR:
Mechanisms in photodynamic therapy: Part one-photosensitizers,
photochemistry and cellular localization. Photodiagn Photodyn Ther.
1:279–293. 2004. View Article : Google Scholar
|
|
11
|
Liu M, Tai C, Sain M, Hu AT and Chou F:
Photodynamic applications of phthalocyanines. J Photochem Photobiol
Chem. 165:131–136. 2004. View Article : Google Scholar
|
|
12
|
Josefsen LB and Boyle RW: Photodynamic
therapy: Novel third- generation photosensitizers one step closer?
Br J Pharmacol. 154:1–3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Z, Shao J, Yang T, Wang J and Jia L:
Pharmaceutical development, composition and quantitative analysis
of phthalocyanine as the photosensitizer for cancer photodynamic
therapy. J Pharm Biomed Anal. 87:98–104. 2014. View Article : Google Scholar
|
|
14
|
Kuzyniak W, Ermilov EA, Atilla D, Gürek
AG, Nitzsche B, Derkow K, Hoffmann B, Steinemann G, Ahsen V and
Höpfner M: Tetra-triethyleneoxysulfonyl substituted zinc
phthalocyanine for photodynamic cancer therapy. Photodiagn Photodyn
Ther. 13:148–157. 2016. View Article : Google Scholar
|
|
15
|
Atilla D, Saydan N, Durmuş M, Gürek AG,
Khan T, Rück A, Walt H, Nyokong T and Ahsen V: Synthesis and
photodynamic potential of tetra- and octa-triethyleneoxysulfonyl
substituted zinc phthalocyanines. J Photochem Photobiol Chem.
186:298–307. 2007. View Article : Google Scholar
|
|
16
|
Nitzsche B, Gloesenkamp C, Schrader M,
Ocker M, Preissner R, Lein M, Zakrzewicz A, Hoffmann B and Höpfner
M: Novel compounds with antiangiogenic and antiproliferative
potency for growth control of testicular germ cell tumours. Br J
Cancer. 103:18–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gloesenkamp C, Nitzsche B, Lim AR, Normant
E, Vosburgh E, Schrader M, Ocker M, Scherübl H and Höpfner M: Heat
shock protein 90 is a promising target for effective growth
inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol.
40:1659–1667. 2012.PubMed/NCBI
|
|
18
|
Höpfner M, Huether A, Sutter AP, Baradari
V, Schuppan D and Scherübl H: Blockade of IGF-1 receptor tyrosine
kinase has antineoplastic effects in hepatocellular carcinoma
cells. Biochem Pharmacol. 71:1435–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huether A, Höpfner M, Baradari V, Schuppan
D and Scherübl H: Sorafenib alone or as combination therapy for
growth control of cholangiocarcinoma. Biochem Pharmacol.
73:1308–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nitzsche B, Gloesenkamp C, Schrader M,
Hoffmann B, Zengerling F, Balabanov S, Honecker F and Höpfner M:
Anti-tumour activity of two novel compounds in cisplatin- resistant
testicular germ cell cancer. Br J Cancer. 107:1853–1863. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiss A, van Beijnum JR, Bonvin D,
Jichlinski P, Dyson PJ, Griffioen AW and Nowak-Sliwinska P:
Low-dose angiostatic tyrosine kinase inhibitors improve
photodynamic therapy for cancer: Lack of vascular normalization. J
Cell Mol Med. 18:480–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Jin H, Bao J, Fang F, Wei J and
Wang A: Intravenous repeated-dose toxicity study of
ZnPcS2P2-based-photodynamic therapy in Wistar rats. Photochem
Photobiol Sci. 5:1006–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tiwari DK, Jin T and Behari J:
Bio-distribution and toxicity assessment of intravenously injected
anti-HER2 antibody conjugated CdSe/ZnS quantum dots in Wistar rats.
Int J Nanomed. 6:463–475. 2011.
|
|
24
|
Gloesenkamp CR, Nitzsche B, Ocker M, Di
Fazio P, Quint K, Hoffmann B, Scherübl H and Höpfner M: AKT
inhibition by triciribine alone or as combination therapy for
growth control of gastroenteropancreatic neuroendocrine tumors. Int
J Oncol. 40:876–888. 2012.
|
|
25
|
Huang Z: A review of progress in clinical
photodynamic therapy. Technol Cancer Res Treat. 4:283–293. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fayter D, Corbett M, Heirs M, Fox D and
Eastwood A: A systematic review of photodynamic therapy in the
treatment of pre-cancerous skin conditions, Barrett's oesophagus
and cancers of the biliary tract, brain, head and neck, lung,
oesophagus and skin. Health Technol Assess. 14:1–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodrigues JR, Charris J, Ferrer R, Gamboa
N, Angel J, Nitzsche B, Hoepfner M, Lein M, Jung K and Abramjuk C:
Effect of quinolinyl acrylate derivatives on prostate cancer in
vitro and in vivo. Invest New Drugs. 30:1426–1433. 2012. View Article : Google Scholar
|
|
28
|
Qumseya BJ, David W and Wolfsen HC:
Photodynamic therapy for Barrett's esophagus and esophageal
carcinoma. Clin Endosc. 46:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mordon S, Cochrane C, Tylcz JB, Betrouni
N, Mortier L and Koncar V: Light emitting fabric technologies for
photodynamic therapy. Photodiagn Photodyn Ther. 12:1–8. 2015.
View Article : Google Scholar
|
|
30
|
Oleinick NL, Morris RL and Belichenko I:
The role of apoptosis in response to photodynamic therapy: What,
where, why, and how. Photochem Photobiol Sci. 1:1–21. 2002.
View Article : Google Scholar
|
|
31
|
Morgan J, Potter WR and Oseroff AR:
Comparison of photodynamic targets in a carcinoma cell line and its
mitochondrial DNA-deficient derivative. Photochem Photobiol.
71:747–757. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shao J, Dai Y, Zhao W, Xie J, Xue J, Ye J
and Jia L: Intracellular distribution and mechanisms of actions of
photosensitizer Zinc(II)-phthalocyanine solubilized in Cremophor EL
against human hepatocellular carcinoma HepG2 cells. Cancer Lett.
330:49–56. 2013. View Article : Google Scholar
|
|
33
|
Tynga IM, Houreld NN and Abrahamse H: The
primary subcellular localization of Zinc phthalocyanine and its
cellular impact on viability, proliferation and structure of breast
cancer cells (MCF-7). J Photochem Photobiol B. 120:171–176. 2013.
View Article : Google Scholar
|
|
34
|
Nanashima A, Isomoto H, Abo T, Nonaka T,
Morisaki T, Arai J, Takagi K, Ohnita K, Shoji H, Urabe S, et al:
How to access photodynamic therapy for bile duct carcinoma. Ann
Transl Med. 2:232014.PubMed/NCBI
|
|
35
|
Huang Z, Hsu Y, Li L, Wang L, Song X-D,
Yow CMN, Lei X, Musani AI, Luo R-C and Day BJ: Photodynamic therapy
of cancer - Challenges of multidrug resistance. J Innov Opt Health
Sci. 8:15300022015. View Article : Google Scholar
|
|
36
|
Juarranz A, Jaén P, Sanz-Rodríguez F,
Cuevas J and González S: Photodynamic therapy of cancer. Basic
principles and applications. Clin Transl Oncol. 10:148–154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: The roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar :
|
|
38
|
Nowis D, Makowski M, Stoktosa T, Legat M,
Issat T and Gołab J: Direct tumor damage mechanisms of photodynamic
therapy. Acta Biochim Pol. 52:339–352. 2005.PubMed/NCBI
|
|
39
|
Grimm S, Mvondo D, Grune T and Breusing N:
The outcome of 5-ALA-mediated photodynamic treatment in melanoma
cells is influenced by vitamin C and heme oxygenase-1. Biofactors.
37:17–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicholson DW: From bench to clinic with
apoptosis-based therapeutic agents. Nature. 407:810–816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abrahamse H and Hamblin MR: New
photosensitizers for photodynamic therapy. Biochem J. 473:347–364.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moeno S, Krause RWM, Ermilov EA, Kuzyniak
W and Höpfner M: Synthesis and characterization of novel zinc
phthalocyanines as potential photosensitizers for photodynamic
therapy of cancers. Photochem Photobiol Sci. 13:963–970. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang A, Li Y, Zhou L, Yuan L, Lu S, Lin Y,
Zhou J and Wei S: Charge dependent photodynamic activity of alanine
based zinc phthalocyanines. J Photochem Photobiol B. 141:10–19.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vargas A, Zeisser-Labouèbe M, Lange N,
Gurny R and Delie F: The chick embryo and its chorioallantoic
membrane (CAM) for the in vivo evaluation of drug delivery systems.
Adv Drug Deliv Rev. 59:1162–1176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nowak-Sliwinska P, van Beijnum JR, van
Berkel M, van den Bergh H and Griffioen AW: Vascular regrowth
following photodynamic therapy in the chicken embryo
chorioallantoic membrane. Angiogenesis. 13:281–292. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan WM, Lim TH, Pece A, Silva R and
Yoshimura N: Verteporfin PDT for non-standard indications - a
review of current literature. Graefes Arch Clin Exp Ophthalmol.
248:613–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fingar VH, Kik PK, Haydon PS, Cerrito PB,
Tseng M, Abang E and Wieman TJ: Analysis of acute vascular damage
after photodynamic therapy using benzoporphyrin derivative (BPD).
Br J Cancer. 79:1702–1708. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukumura D and Jain RK: Tumor
microvasculature and microenvironment: Targets for
anti-angiogenesis and normalization. Microvasc Res. 74:72–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|