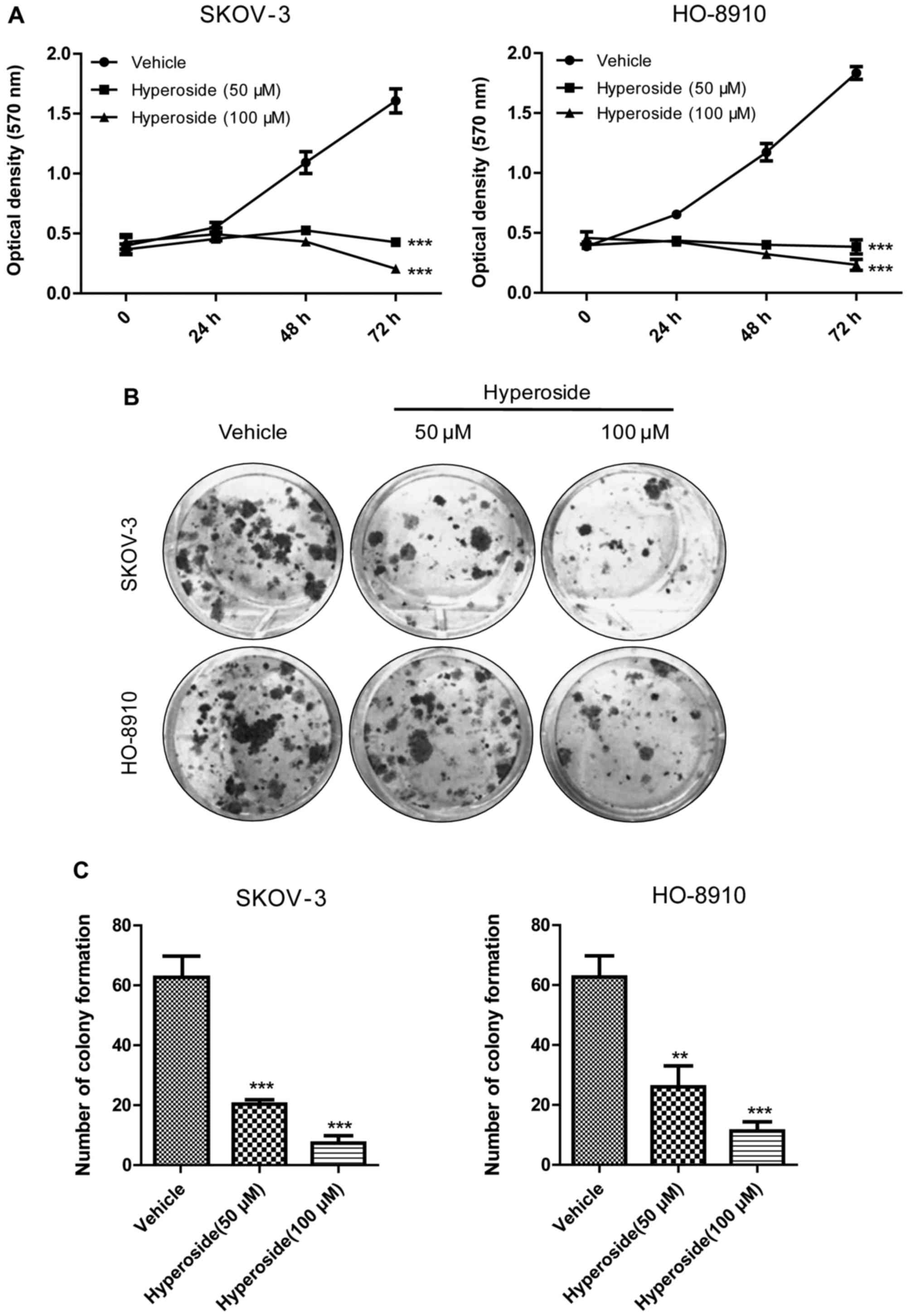
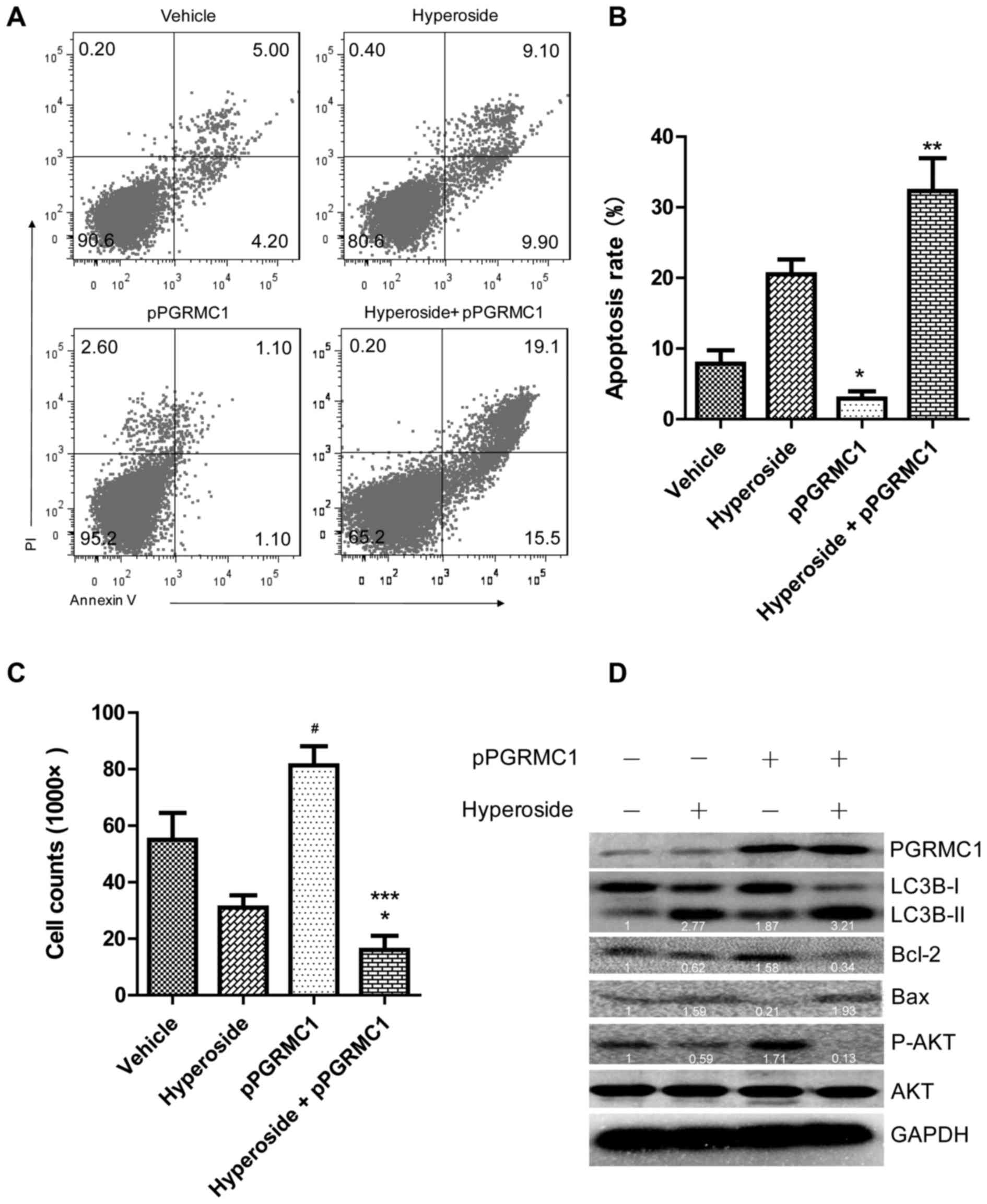
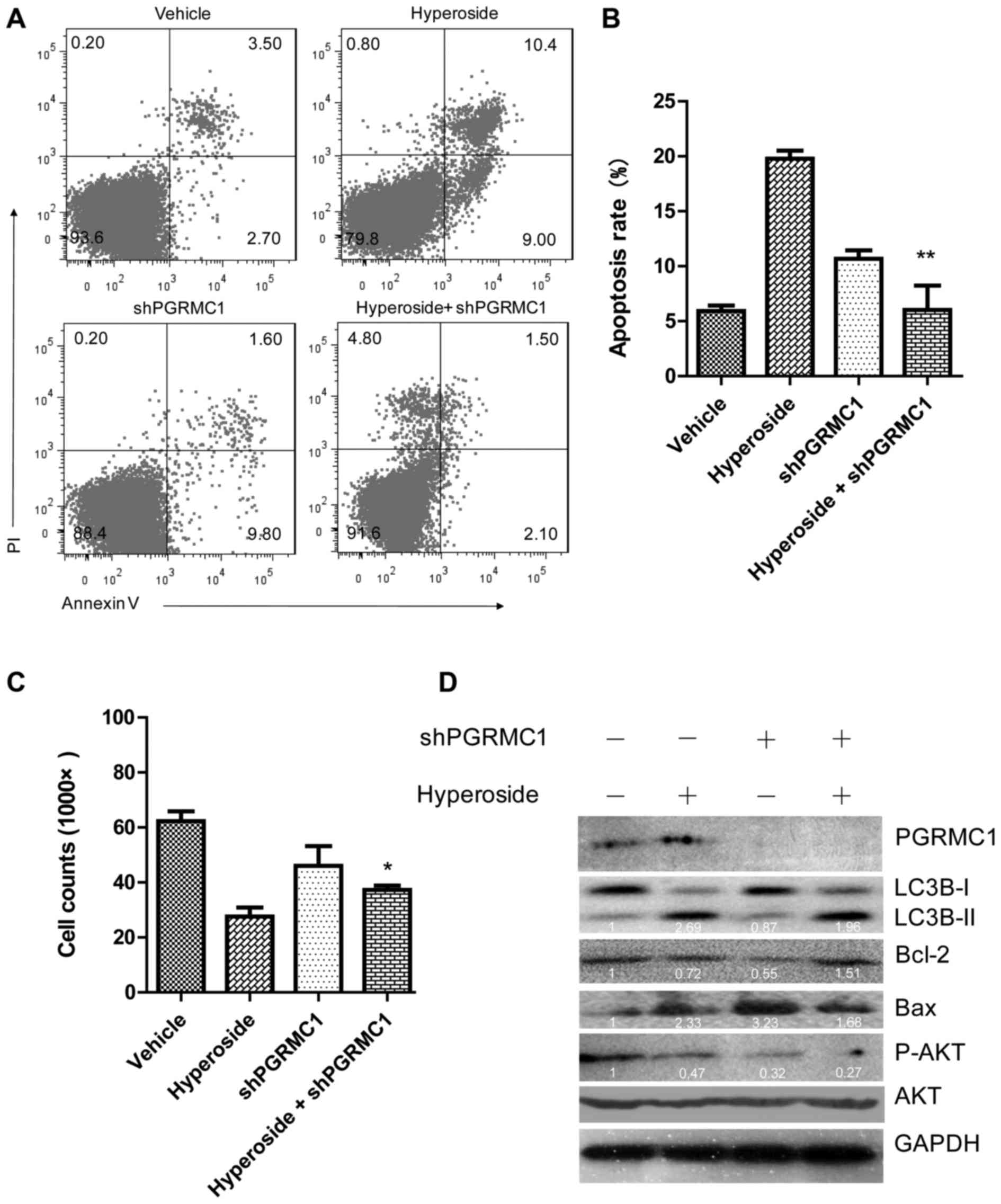
|
1
|
Salzberg M, Thurlimann B, Bonnefois H,
Fink D, Rochlitz C, von Moos R and Senn H: Current concepts of
treatment strategies in advanced or recurrent ovarian cancer.
Oncology. 68:293–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
3
|
Zou Y, Lu Y and Wei D: Antioxidant
activity of a flavonoid-rich extract of Hypericum perforatum L. in
vitro. J Agric Food Chem. 52:5032–5039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piao MJ, Kang KA, Zhang R, Ko DO, Wang ZH,
You HJ, Kim HS, Kim JS, Kang SS and Hyun JW: Hyperoside prevents
oxidative damage induced by hydrogen peroxide in lung fibroblast
cells via an antioxidant effect. Biochim Biophys Acta.
1780:1448–1457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ku SK, Zhou W, Lee W, Han MS, Na M and Bae
JS: Anti-inflammatory effects of hyperoside in human endothelial
cells and in mice. Inflammation. 38:784–799. 2015. View Article : Google Scholar
|
|
6
|
Ku SK, Kwak S, Kwon OJ and Bae JS:
Hyperoside inhibits high-glucose-induced vascular inflammation in
vitro and in vivo. Inflammation. 37:1389–1400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lü P: Inhibitory effects of hyperoside on
lung cancer by inducing apoptosis and suppressing inflammatory
response via caspase-3 and NF-κB signaling pathway. Biomed
Pharmacother. 82:216–225. 2016. View Article : Google Scholar
|
|
8
|
Boukes GJ and van de Venter M: The
apoptotic and autophagic properties of two natural occurring
prodrugs, hyperoside and hypoxoside, against pancreatic cancer cell
lines. Biomed Pharmacother. 83:617–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan J and Horvitz HR: A first insight
into the molecular mechanisms of apoptosis. Cell. 116:S53–S56.
51following S59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaacoub K, Pedeux R, Tarte K and
Guillaudeux T: Role of the tumor microenvironment in regulating
apoptosis and cancer progression. Cancer Lett. 378:150–159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed IS, Rohe HJ, Twist KE and Craven RJ:
PGRMC1 (progesterone receptor membrane component 1) associates with
epidermal growth factor receptor and regulates erlotinib
sensitivity. J Biol Chem. 285:24775–24782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kabe Y, Nakane T, Koike I, Yamamoto T,
Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K, et
al: Haem-dependent dimerization of PGRMC1/Sigma-2 receptor
facilitates cancer proliferation and chemoresistance. Nat Commun.
7:110302016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szczesna-Skorupa E and Kemper B:
Progesterone receptor membrane component 1 inhibits the activity of
drug-metabolizing cytochromes P450 and binds to cytochrome P450
reductase. Mol Pharmacol. 79:340–350. 2011. View Article : Google Scholar :
|
|
15
|
Zheng K, Li Y, Wang S, Wang X, Liao C, Hu
X, Fan L, Kang Q, Zeng Y, Wu X, et al: Inhibition of
autophagosome-lysosome fusion by ginsenoside Ro via the
ESR2-NCF1-ROS pathway sensitizes esophageal cancer cells to
5-fluorouracil-induced cell death via the CHEK1-mediated DNA damage
checkpoint. Autophagy. 12:1593–1613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kohli L, Kaza N, Lavalley NJ, Turner KL,
Byer S, Carroll SL and Roth KA: The pan erbB inhibitor PD168393
enhances lysosomal dysfunction-induced apoptotic death in malignant
peripheral nerve sheath tumor cells. Neurooncol. 14:266–277.
2012.
|
|
17
|
Ghadimi MP, Lopez G, Torres KE, Belousov
R, Young ED, Liu J, Brewer KJ, Hoffman A, Lusby K, Lazar AJ, et al:
Targeting the PI3K/mTOR axis, alone and in combination with
autophagy blockade, for the treatment of malignant peripheral nerve
sheath tumors. Mol Cancer Ther. 11:1758–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stanton MJ, Dutta S, Zhang H, Polavaram
NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH
and Datta K: Autophagy control by the VEGF-C/NRP-2 axis in cancer
and its implication for treatment resistance. Cancer Res.
73:160–171. 2013. View Article : Google Scholar :
|
|
20
|
de la Cruz-Morcillo MA, Valero ML,
Callejas-Valera JL, Arias-González L, Melgar-Rojas P, Galán-Moya
EM, García-Gil E, García-Cano J and Sánchez-Prieto R: P38MAPK is a
major determinant of the balance between apoptosis and autophagy
triggered by 5-fluorouracil: Implication in resistance. Oncogene.
31:1073–1085. 2012. View Article : Google Scholar
|
|
21
|
Weidhaas JB, Babar I, Nallur SM, Trang P,
Roush S, Boehm M, Gillespie E and Slack FJ: MicroRNAs as potential
agents to alter resistance to cytotoxic anticancer therapy. Cancer
Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Cao L, Yang L, Kang R, Lotze M and
Tang D: microRNA 30A promotes autophagy in response to cancer
therapy. Autophagy. 8:853–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, He Z, von Rütte T, Yousefi S,
Hunger RE and Simon HU: Down-regulation of autophagy-related
protein 5 (ATG5) contributes to the pathogenesis of early-stage
cutaneous melanoma. Sci Transl Med. 5:202ra1232013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leng S, Hao Y, Du D, Xie S, Hong L, Gu H,
Zhu X, Zhang J, Fan D and Kung HF: Ursolic acid promotes cancer
cell death by inducing Atg5-dependent autophagy. Int J Cancer.
133:2781–2790. 2013.PubMed/NCBI
|
|
25
|
Mir SU, Schwarze SR, Jin L, Zhang J,
Friend W, Miriyala S, St Clair D and Craven RJ: Progesterone
receptor membrane component 1/Sigma-2 receptor associates with
MAP1LC3B and promotes autophagy. Autophagy. 9:1566–1578. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu X, Han Y, Fang Z, Wu W, Ji M, Teng F,
Zhu W, Yang X, Jia X and Zhang C: Progesterone protects ovarian
cancer cells from cisplatin-induced inhibitory effects through
progesterone receptor membrane component 1/2 as well as AKT
signaling. Oncol Rep. 30:2488–2494. 2013.PubMed/NCBI
|
|
27
|
Zhu X, Guo Y, Yao S, Yan Q, Xue M, Hao T,
Zhou F, Zhu J, Qin D and Lu C: Synergy between Kaposi's
sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein
in promotion of angiogenesis and oncogenesis: Role of the AKT
signaling pathway. Oncogene. 33:1986–1996. 2014. View Article : Google Scholar
|
|
28
|
Xue M, Yao S, Hu M, Li W, Hao T, Zhou F,
Zhu X, Lu H, Qin D, Yan Q, et al: HIV-1 Nef and KSHV oncogene K1
synergistically promote angiogenesis by inducing cellular miR-718
to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic Acids Res.
42:9862–9879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H and Zhang Y: Life and death partners
in post-PCI restenosis: Apoptosis, autophagy, and the cross-talk
between them. Curr Drug Targets. Jun 24–2016.Epub ahead of print.
PubMed/NCBI
|
|
30
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang RC, Wei Y, An Z, Zou Z, Xiao G,
Bhagat G, White M, Reichelt J and Levine B: Akt-mediated regulation
of autophagy and tumorigenesis through Beclin 1 phosphorylation.
Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cahill MA, Jazayeri JA, Catalano SM,
Toyokuni S, Kovacevic Z and Richardson DR: The emerging role of
progesterone receptor membrane component 1 (PGRMC1) in cancer
biology. Biochim Biophys Acta. 1866:339–349. 2016.PubMed/NCBI
|
|
33
|
Grabowski JP and Sehouli J: Current
management of ovarian cancer. Minerva Med. 106:151–156.
2015.PubMed/NCBI
|
|
34
|
Herzog TJ: The current treatment of
recurrent ovarian cancer. Curr Oncol Rep. 8:448–454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Absolom K, Eiser C, Turner L, Ledger W,
Ross R, Davies H, Coleman R, Hancock B, Snowden J and Greenfield D;
Late Effects Group Sheffield: Ovarian failure following cancer
treatment: Current management and quality of life. Hum Reprod.
23:2506–2512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rodriguez-Freixinos V, Mackay HJ,
Karakasis K and Oza AM: Current and emerging treatment options in
the management of advanced ovarian cancer. Expert Opin
Pharmacother. 17:1063–1076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peluso JJ: Non-genomic actions of
progesterone in the normal and neoplastic mammalian ovary. Semin
Reprod Med. 25:198–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peluso JJ: Progesterone signaling mediated
through progesterone receptor membrane component-1 in ovarian cells
with special emphasis on ovarian cancer. Steroids. 76:903–909.
2011.PubMed/NCBI
|
|
39
|
Oda S, Nakajima M, Toyoda Y, Fukami T and
Yokoi T: Progesterone receptor membrane component 1 modulates human
cytochrome p450 activities in an isoform-dependent manner. Drug
Metab Dispos. 39:2057–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Albrecht C, Huck V, Wehling M and Wendler
A: In vitro inhibition of SKOV-3 cell migration as a distinctive
feature of progesterone receptor membrane component type 2 versus
type 1. Steroids. 77:1543–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahmed IS, Rohe HJ, Twist KE, Mattingly MN
and Craven RJ: Progesterone receptor membrane component 1 (Pgrmc1):
A heme-1 domain protein that promotes tumorigenesis and is
inhibited by a small molecule. J Pharmacol Exp Ther. 333:564–573.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peluso JJ, Liu X, Gawkowska A, Lodde V and
Wu CA: Progesterone inhibits apoptosis in part by PGRMC1-regulated
gene expression. Mol Cell Endocrinol. 320:153–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Waarde A, Rybczynska AA, Ramakrishnan
NK, Ishiwata K, Elsinga PH and Dierckx RA: Potential applications
for sigma receptor ligands in cancer diagnosis and therapy. Biochim
Biophys Acta. 1848B:2703–2714. 2015. View Article : Google Scholar
|
|
44
|
Lin L and Baehrecke EH: Autophagy, cell
death, and cancer. Mol Cell Oncol. 2:e9859132015. View Article : Google Scholar
|
|
45
|
Gewirtz DA: Cytoprotective and
nonprotective autophagy in cancer therapy. Autophagy. 9:1263–1265.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Petiot A, Ogier-Denis E, Blommaart EF,
Meijer AJ and Codogno P: Distinct classes of phosphatidylinositol
3′-kinases are involved in signaling pathways that control
macroautophagy in HT-29 cells. J Biol Chem. 275:992–998. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rubinstein AD, Eisenstein M, Ber Y, Bialik
S and Kimchi A: The autophagy protein Atg12 associates with
antiapoptotic Bcl-2 family members to promote mitochondrial
apoptosis. Mol Cell. 44:698–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mann SS and Hammarback JA: Molecular
characterization of light chain 3. A microtubule binding subunit of
MAP1A and MAP1B. J Biol Chem. 269:11492–11497. 1994.PubMed/NCBI
|
|
50
|
Tsukada M and Ohsumi Y: Isolation and
characterization of autophagy-defective mutants of Saccharomyces
cerevisiae. FEBS Lett. 333:169–174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ostenfeld MS, Høyer-Hansen M, Bastholm L,
Fehrenbacher N, Olsen OD, Groth-Pedersen L, Puustinen P,
Kirkegaard-Sørensen T, Nylandsted J, Farkas T, et al: Anti-cancer
agent siramesine is a lysosomotropic detergent that induces
cytoprotective autophagosome accumulation. Autophagy. 4:487–499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ishisaka R, Kanno T, Akiyama J, Yoshioka
T, Utsumi K and Utsumi T: Activation of caspase-3 by lysosomal
cysteine proteases and its role in
2,2′-azobis-(2-amidinopropane)dihydrochloride (AAPH)-induced
apoptosis in HL-60 cells. J Biochem. 129:35–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stoka V, Turk B, Schendel SL, Kim TH,
Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson
M, et al: Lysosomal protease pathways to apoptosis. Cleavage of
bid, not pro-caspases, is the most likely route. J Biol Chem.
276:3149–3157. 2001. View Article : Google Scholar
|