Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignant tumors, especially in China, and has become the
second leading cause of cancer-related death (1,2). The
lack of effective early detection and high frequency of
postoperative metastasis and recurrence resulted in a very low
5-year survival rate (3). Thus, it
is essential to clarify the underlying mechanism of HCC
progression, which may contribute to identification of novel
targets for effective intervention.
Abelson interactor 1 (ABI1), also known as E3B1, was
originally identified as Abl kinase associating protein 1 (4) and later confirmed to be one of the
Bcr-Abl interactors (5). ABI1
regulated cell proliferation via the Ras small G-protein and affect
actin remodeling, cell adhesion, and cell migration via Rac
activation (6,7). It also plays a central role in
holding multiprotein complexes together (ABI-WAVE complex and
Sos1-Eps8-ABI1 complex) (8,9),
which mediate a number of pathways related to cell spreading and
migration (10–12). For the crucial role of ABI1 in
actin reorganization and cell migration, homozygous loss of this
protein leads to embryonic lethality in mice (9,13).
In cancers, previous studies showed that ABI1 participated in both
tumor progression and tumor suppression. Initially, ABI1 has been
proposed as a potential tumor suppressor based on the evidence that
ABI1 gene is deleted in prostate cancer and decreased in gastric
cancer and some types of leukemia (14–17).
ABI1 has been reported to be overexpressed and correlated with poor
prognosis in a wide variety of tumors, such as breast cancer,
BCR-Abl-induced leukemia, colon cancer, HCC and ovarian cancer
(18–25). These studies suggested that ABI1
may play a diverse role during cancer progression under various
circumstances. Although Liu et al (25) determined the expression of ABI1 in
40 HCC specimens, the role of ABI1 in regulating HCC progression as
well as its clinical significance remain largely unexplored.
In this study, we investigated the expression of
ABI1 both in human HCC tissues and cell lines. The prognostic value
of ABI1 in HCC patients after curative resection was also valuated.
Furthermore, we established ABI1 overexpression and knockdown
stable clones in HCC cell lines and for the first time explored the
functions of ABI1 in HCC cells both in vitro and in
vivo assays.
Materials and methods
Tissue samples
Paraffin-embedded HCC samples used in our study were
obtained from 124 HCC patients who underwent hepatic resection at
the Second Xiangya Hospital of Central South University between
March 2009 and October 2011. The main clinicopathologic variables
are shown in Table I. Tumor stage
was determined according to Barcelona Clinic Liver Cancer (BCLC)
staging classification. Tumor differentiation was graded by the
Edmondson grading system. Among these patients, 23 matched fresh
HCC tissues and adjacent non-tumor tissues were collected for
qRT-PCR and western blot analysis. Written informed consent was
obtained from all the patients. None of the patients received any
chemotherapy or radiotherapy before surgery. The study was approved
by the Ethics Committee of the Second Xiangya Hospital of Central
South University.
 | Table ICorrelations between ABI1 expression
and clinicopathologic characteristics of 124 HCC patients. |
Table I
Correlations between ABI1 expression
and clinicopathologic characteristics of 124 HCC patients.
| Clinicopathological
variables | Tumor ABI1
expression
| P-value |
|---|
| Low (49) | High (75) |
|---|
| Gender | | | 0.967 |
| Male | 43 | 66 | |
| Female | 6 | 9 | |
| Age | | | 0.752 |
| <50 | 28 | 45 | |
| ≥50 | 21 | 30 | |
| AFP | | | 0.146 |
| <20 ng/ml | 11 | 26 | |
| ≥20 ng/ml | 38 | 49 | |
| HBsAg | | | 0.640 |
| Negative | 10 | 18 | |
| Positive | 39 | 57 | |
| Liver
cirrhosis | | | 0.998 |
| Absent | 17 | 26 | |
| Present | 32 | 49 | |
| Tumor size | | | 0.041 |
| ≤5 cm | 21 | 19 | |
| >5 cm | 28 | 56 | |
| Tumor number | | |
<0.001 |
| Single | 32 | 23 | |
| Multiple | 17 | 52 | |
| Tumor
encapsulation | | |
<0.001 |
| Absent | 25 | 62 | |
| Present | 24 | 13 | |
| Edmondson
grade | | | 0.199 |
| I–II | 28 | 34 | |
| III–IV | 21 | 41 | |
| Microvascular
invasion | | | 0.101 |
| Absent | 34 | 41 | |
| Present | 15 | 34 | |
| BCLC stage | | | |
| 0-A | 16 | 10 | 0.010 |
| B-C | 33 | 65 | |
Follow-up
The follow-up was conducted by telephone or
outpatient visits regularly, every 3 months in the first three
years after operation and twice a year later. Recurrence or
metastasis were monitored by clinical examination,
alpha-fetoprotein (AFP) levels and ultrasonography, sometimes
high-resolution contrast-enhanced computed tomography (CT) or
magnetic resonance imaging (MRI) if necessary. Overall survival
(OS) was calculated from the date of surgery to the date of death
or the last follow-up. Time to recurrence(TTR) was calculated from
the date of surgery to the date of diagnosis of any type of relapse
(26). Patient follow-up was
terminated on November 10, 2015. The median follow-up time was 23.5
months, ranging from 1 to 68 months.
RNA isolation and quantitative real-time
PCR analysis
TRIzol reagents (Invitrogen, Carlsbad, CA, USA) was
used for isolating total RNA from cell lines or tissues. cDNA was
generated and quantitative real-time PCR was performed using a
standard protocol from the SYBR Green PCR kit (Toyobo, Osaka,
Japan). Each sample was analyzed in triplicate. The Primers of ABI1
were as follows: 5′-CGAATATGGAGCGCCCTGTA-3′ (forward);
5′-AGGACTTGGCGGTTTCTGAGT-3′ (reverse). GAPDH was used as an
internal control with the following primers:
5′-CTGGTAAAGTGGATATTGTTGCCAT-3′ (forward);
5′-TGGAATCATATTGGAACATGTAAACC-3′ (reverse). The 2−ΔΔCt
method was used to analyze the qRT-PCR results.
Western blot analysis
Total protein was extracted using RIPA lysis buffer
supplemented with 1% Phenylmethanesulfonyl (PMSF). After separated
by SDS-PAGE, the protein was transferred onto PVDF membranes. Then
the membranes were blocked with 5% skimmed milk followed by an
overnight incubation with primary antibodies against ABI1 (1:100,
Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (1:1000,
Sigma, St. Louis, MO, USA) at 4°C. After washing, membranes were
incubated with appropriate secondary antibodies conjugated to HRP
(1:10000, Zhongshan Goldenbridge Biotechnology, Beijing, China).
Signals were detected using the ECL detection systems (Thermo
Scientific, Rockford, IL, USA).
Immunohistochemistry (IHC)
Tissue sections (4 µm) from 124 HCC samples
were deparaffinized in xylene and rehydrated with graded ethanol,
then antigen retrieval with 0.01 M sodium citrate buffer (pH 6.0).
The endogenous peroxidase was inactivated with 0.3% hydrogen
peroxide, next with 10% goat serum blocking for 30 min. ABI1
antibody (1:50, Santa Cruz Biotechnology) was incubated overnight
at 4°C in a humidified chamber and negative control slides were
incubated with PBS. Then followed by HRP conjugated secondary
antibody (Zhongshan Goldenbridge Biotechnology) incubation for 30
min at room temperature. Antibody binding was detected by DAB.
Tissue sections were dehydrated in graded ethanols and mounted.
All the immunostained sections were evaluated by two
investigators in a blinded manner. Both the staining intensity and
percentage were assessed. Staining intensity was graded on a 0 to 3
scale: 0 (absence of staining), 1 (weakly stained), 2 (moderately
stained), and 3 (strongly stained). The percent positivity was
scored as 1 (0–25%), 2 (26–50%), 3 (51–75%) and 4 (≥75%). A final
score was obtained for each case by multiplying the percentage and
the intensity score. Therefore, tumors with a multiplied score
exceeding 4 were deemed to be high ABI1 expression; all other
scores were considered to be low ABI1 expression.
Cell culture
The human HCC cell lines (Hep3B, HepG2, MHCC97H and
SMMC7721) and normal liver cell line L02 were purchased from the
Shanghai Institute of Cell Biology. The cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum(FBS) (Gibco, Australia) and 100 U/ml
penicillin-streptomycin mixture at 37°C in 5% CO2.
Construction of stable cell lines
Human Lenti-ABI1-GFP and three Lenti-shABI1-GFP as
well as their negative control lentiviruses were designed and
purchased from GenePharma Technologies (Shanghai, China). The
transfection was performed according to standard procedures.
Following lentiviral infection, single-cell clonal isolates were
selected in the presence of puromycin for 2–4 weeks. The 3
candidate hairpin sequences for ABI1 were as follows:
5′-GGAGTCTTCCATCAATCATAT-3′ (shRNA-1); 5′-GGTATATTCGGAAACCTATCG-3′
(shRNA-2); 5′-GCACACTGTCGAGAACAAATC-3′ (shRNA-3); The efficiency of
ABI1 overexpression or knockdown was confirmed by qRT-PCR and
western blotting after transfection, respectively.
Cell Counting Kit-8 assay and Colony
formation assay
Cell proliferation was determined by the Cell
Counting Kit-8 method (Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's instructions. The cell
proliferation curves were plotted using the absorbance at each time
point. Experiments were performed in triplicate.
For colony formation assay, 5×102 cells
were seeded in each well on 6-well plates and incubation for 14
days, then cells were washed twice with PBS, fixed with methanol
and stained with crystal violet. The number of colonies >40
µm in diameter was counted after the dishes were captured
with a camera.
EdU incorporation assay
EdU incorporation assay was carried out using the
Cell-Light EdU imaging kit (RiboBio, Guangzhou, China) according to
the manufacturer's protocol. Briefly, 5×103 cells were
seeded in each well on 96-well plates. After adherence, we added
EdU labeling medium and incubated for approximately 60 min. Then
cells were fixed with 4% formaldehyde for 15 min and successively
treated with 0.5% Triton X-100 for 10 min, Apollo reaction cocktail
for 30 min and Hoechst 33342 (5 µg/ml) for 30 min. The
results were visualized under a florescent microscope. For
quantification of HCC cell proliferative rate, five randomly
selected views from each sample image were used to calculate the
relative EdU-positive ratio.
Wound healing assay
Cells (1×105) were seeded in each well on
6-well plates and when the cell confluence reached approximately
90%, a line was scraped using the fine end of a 10 µl
pipette tip. Serum-free medium with Mitomycin-C (10 µg/ml)
was used to suppress cell proliferation (27). Wound healing within the scrape line
was observed and photographed every 12 h. Each experiment was
repeated three times.
Transwell invasion assay
For the cell invasion assay, Transwell chambers
(Corning, 8-µm pore size) were coated with 200 µl
Matrigel at 200 µg/ml and incubated overnight. Cells
(1×105) were suspended in serum-free DMEM and plated
into the upper chamber. The lower chamber was filled with DMEM
containing 10% FBS. After a 48 h incubation in 5% CO2 at
37°C, the cells in the upper chamber were removed and the bottom
surface of the polycarbonate membranes was stained using 0.1%
crystal violet dye. Cell invasion was determined by counting six
random fields under a microscope. All assays were carried out in
triplicates.
Immunofluorescence (IF)
For immunofluorescence of cytoskeleton, cells were
fixed in 4% paraformaldehyde, permeabilized using 0.5% Triton X-100
and incubated with Phalloidin (Sigma) according to the
manufacturer's protocol. The coverslips were counterstained with
DAPI and imaged with an inverted microscope.
Subcutaneous and lung metastasis tumor
models in nude mice
All the animal experiments were performed in
accordance with the guidelines of the Laboratory Animal Ethics
Committee of Central South University. For the in vivo
tumorigenesis model, cells (5×106) resuspended in 100
µl of PBS were injected subcutaneously to the left side of
nude mice (4 mice/per group). Four weeks later, the subcutaneous
tumors were resected, and tumor size was monitored by digital
caliper and tumor volume was calculated with the following formula:
1/2 length × width2.
For the in vivo lung metastasis model, cell
suspension at a concentration of 1×107 cells
ml−1 was injected into nude mice through tail veins (4
mice/per group). Six weeks later, the mice were sacrificed, and the
numbers of lung metastatic nodules were carefully examined and
counted under a microscope.
Statistical analysis
Statistical analysis was performed using SPSS18.0
(IBM, Chicago, IL, USA). All quantified data are presented as the
mean ± SD. Two-tailed Student's t-test was used for comparisons of
two independent groups. The association between ABI1 and
clinicopathological features were analyzed using the Chi-square
method. Kaplan-Meier curves were constructed, and the log-rank test
was used for analyzing the survival data. Univariate and
multivariate analyses were based on the Cox proportional hazards
regression model. P<0.05 was set as the statistical
significance.
Results
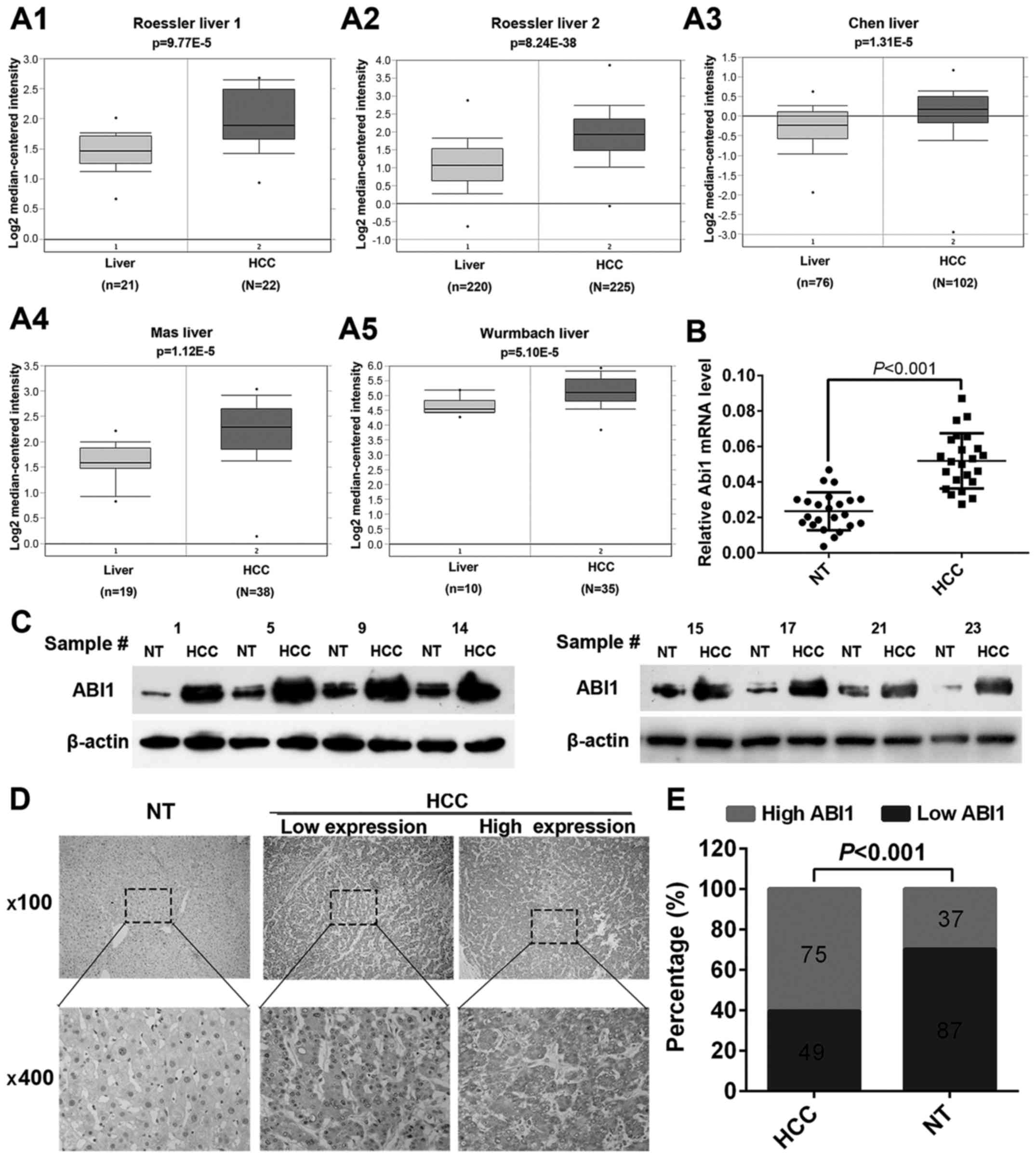
ABI1 is significantly upregulated in
human HCC tissues
To clarify the underlying role of ABI1 in HCC
progression, we first retrieved the expression of ABI1 from
Oncomine Database (www.oncomine.org). Data showed that the level of ABI1
mRNA was significantly upregulated in HCC tissues relative to
normal liver tissues in both Roessler liver 1 and 2 dataset
(Fig. 1A1 and A2). Convincingly,
similar results were also observed in Chen liver dataset, Mas liver
dataset and Wurmbach liver dataset, while P-values were 1.31E-5,
1.120E-5 and 5.10E-5, respectively (Fig. 1A3–A5). To further confirm the above
findings, quantitative real-time PCR (qRT-PCR) and western blot
were performed to detect the expression of ABI1 in 23 fresh-frozen
HCC samples. As shown in Fig. 1B and
C, both the mRNA and protein levels of ABI1 were significantly
higher in HCC tissues compared with the matched non-tumor tissues
(P<0.001). Furthermore, we performed immunohistochemistry (IHC)
to examine the expression of ABI1 protein in 124 paraffin-embedded
HCC samples. Representative images of ABI1 staining are shown in
Fig. 1D. The positive signal for
ABI1 was observed primarily in the cytoplasm of hepatic cells. Of
the 124 HCC specimens, 75 (60.5%) displayed high ABI1 expression
and 49 (39.5%) had low ABI1 expression. However, in adjacent
non-tumor tissues, only 37 (29.8%) exhibited high ABI1 expression.
The expression levels of ABI1 were significantly increased in HCC
tissues relative to matched non-tumor tissues (P<0.001; Fig. 1E). Taken together, the above
results suggest that ABI1 is significantly upregulated in HCC
tissues and may play an important role in HCC development.
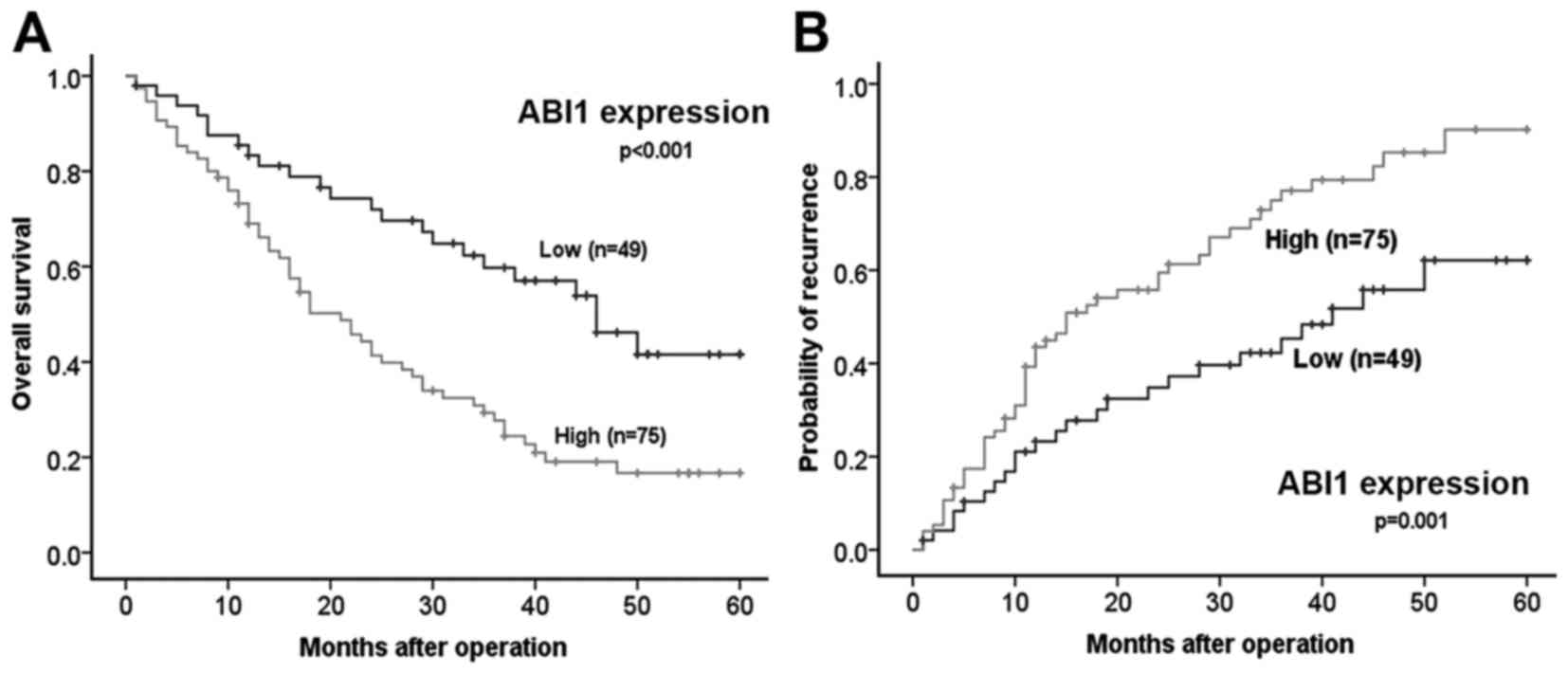
High ABI1 expression associates with
malignant clinicopathological features and predicts poor prognosis
in HCC patients
To illustrate the clinical significance of ABI1
expression in HCC, we first analyzed the correlation between ABI1
expression and clinicopathological features of HCC patients. As
shown in Table I, high ABI1
expression was significantly correlated with tumor size (P=0.041),
tumor number (P<0.001), tumor encapsulation (P<0.001), and
BCLC stage (P=0.010), but did not correlate with other
clinicopathologic characteristics including age, gender, serum AFP
level, HBsAg, liver cirrhosis, tumor differentiation and
microvascular invasion. Importantly, Kaplan-Meier analysis revealed
that patients with high ABI1 expression had shorter overall
survival time (median OS 25.7 vs. 40.2 months, P< 0.001) and a
higher tendency of tumor recurrence (median TTR 23.4 vs. 37.0
months, P=0.001) than those with low ABI1 expression (Fig. 2A and B). Strikingly, as shown in
Tables II and III, multivariate analysis further
confirmed that high ABI1 expression was an independent predictor
for both OS (hazard ratio 1.795, 95% confidence interval
1.077–2.990, P=0.025) and TTR (hazard ratio 1.893, 95% confidence
interval 1.152–3.109, P=0.012). These results indicate that ABI1
may be a very promising prognostic indicator for patients with
HCC.
 | Table IIUnivariate and multivariate analysis
of prognostic factors associated with OS in 124 HCC patients. |
Table II
Univariate and multivariate analysis
of prognostic factors associated with OS in 124 HCC patients.
| Clinicopathological
variables | OS
|
|---|
Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 0.912 | 0.456–1.826 | 0.796 | | | NA |
| Age (<50 vs.
≥50) | 0.761 | 0.482–1.200 | 0.240 | | | NA |
| AFP (<20 vs. ≥20
ng/ml) | 0.775 | 0.482–1.244 | 0.291 | | | NA |
| HBsAg (negative vs.
positive) | 1.390 | 0.793–2.438 | 0.250 | | | NA |
| Liver cirrhosis
(absent vs. present) | 0.807 | 0.511–1.274 | 0.357 | | | NA |
| Tumor number
(single vs. multiple) | 2.112 | 1.329–3.358 | 0.002 | 1.721 | 1.060–2.793 | 0.028 |
| Tumor size (≤5 vs.
>5 cm) | 1.609 | 0.982–2.635 | 0.059 | | | NA |
| Tumor encapsulation
(present vs. absent) | 1.570 | 0.927–2.660 | 0.093 | | | NA |
| Tumor
differentiation (I–II versus III–IV) | 1.650 | 1.060–2.567 | 0.026 | | | NS |
| Microvascular
invasion (absent vs. present) | 2.098 | 1.349–3.264 | 0.001 | 1.906 | 1.214–2.993 | 0.005 |
| BCLC stage (0-A vs.
B-C) | 1.897 | 1.026–3.508 | 0.041 | 2.058 | 1.103–3.841 | 0.023 |
| ABI1 expression
(low vs. high) | 2.327 | 1.428–3.792 | 0.001 | 1.795 | 1.077–2.990 | 0.025 |
 | Table IIIUnivariate and multivariate analysis
of prognostic factors associated with TTR in 124 HCC patients. |
Table III
Univariate and multivariate analysis
of prognostic factors associated with TTR in 124 HCC patients.
| Clinicopathological
variables | TTR
|
|---|
Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 0.793 | 0.369–1.590 | 0.514 | | | NA |
| Age (<50 vs.
≥50) | 0.850 | 0.540–1.335 | 0.480 | | | NA |
| AFP (<20 vs. ≥20
ng/ml) | 0.810 | 0.501–1.311 | 0.391 | | | NA |
| HBsAg (negative vs.
positive) | 1.725 | 0.950–3.130 | 0.073 | | | NA |
| Liver cirrhosis
(absent vs. present) | 0.774 | 0.491–1.219 | 0.269 | | | NA |
| Tumor number
(single vs. multiple) | 1.937 | 1.223–3.070 | 0.005 | | | NS |
| Tumor size (≤5 vs.
>5) | 1.351 | 0.839–2.174 | 0.216 | | | NA |
| Tumor encapsulation
(present vs. absent) | 1.310 | 0.793–2.165 | 0.292 | | | NA |
| Tumor
differentiation (I–II vs. III–IV) | 1.770 | 1.130–2.772 | 0.013 | 1.629 | 1.035–2.567 | 0.035 |
| Microvascular
invasion (absent vs. present) | 2.190 | 1.401–3.423 | 0.001 | 2.050 | 1.298–3.237 | 0.002 |
| BCLC stage (0-A vs.
B-C) | 1.947 | 1.051–3.605 | 0.034 | 1.982 | 1.054–3.730 | 0.034 |
| ABI1 expression
(low vs. high) | 2.142 | 1.318–3.479 | 0.002 | 1.893 | 1.152–3.109 | 0.012 |
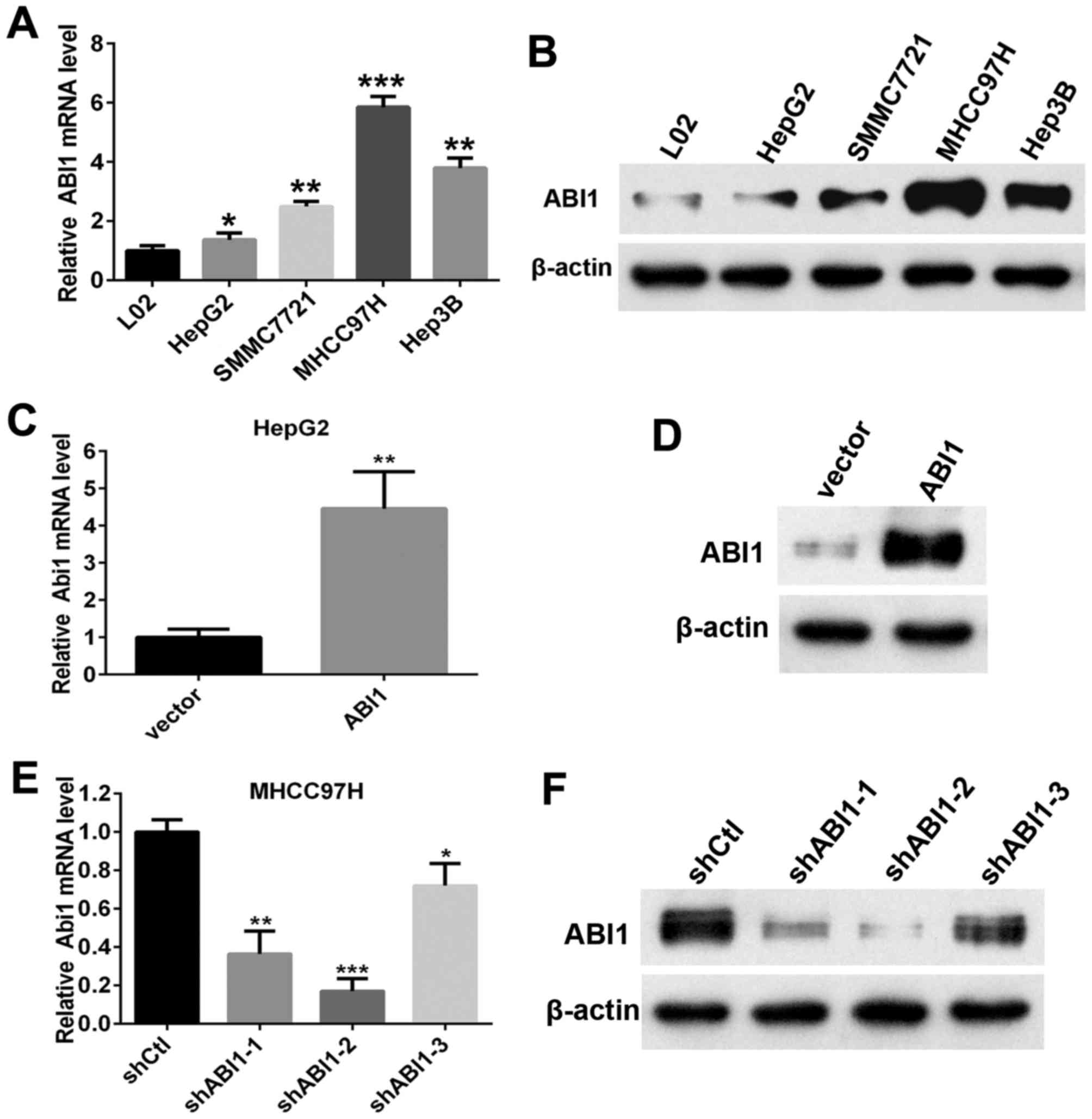
Construction of stable cell lines
displaying ABI1 overexpression or knockdown
We next analyzed the expression of ABI1 in four
different human HCC cell lines (HepG2, Hep3B, SMMC7721 and MHCC97H)
and immortalized hepatocytes (L02). Results showed that both levels
of ABI1 mRNA and protein in HCC cell lines were evidently higher
than those in L02 (Fig. 3A and B).
Noteworthy, we found that the highly metastatic cell line (MHCC97H
and Hep3B) exhibited stronger signals of ABI1 than the lowly
metastatic cell line (HepG2 and SMMC7721). To further elucidate the
biological function of ABI1 in HCC cells, we chose HepG2 and
MHCC97H cells for further research, which expressed the lowest and
highest levels of ABI1 among the four examined cell lines,
respectively. Then we stably overexpressed ABI1 in HepG2 and
silenced ABI1 in MHCC97H cells by lentivirus transfection. After
transfection, qRT-PCR and western blot were used to evaluate the
efficiency of ABI1 overexpression or knockdown in HCC cells.
Results showed that transfection of ABI1 expressing lentiviral
plasmid increased the expression of ABI1 in HepG2 cells (Fig. 3C and D). Among the three shRNAs,
ABI1 was significantly knocked down by shRNA-2 (Fig. 3E and F), which was chosen for
further study.
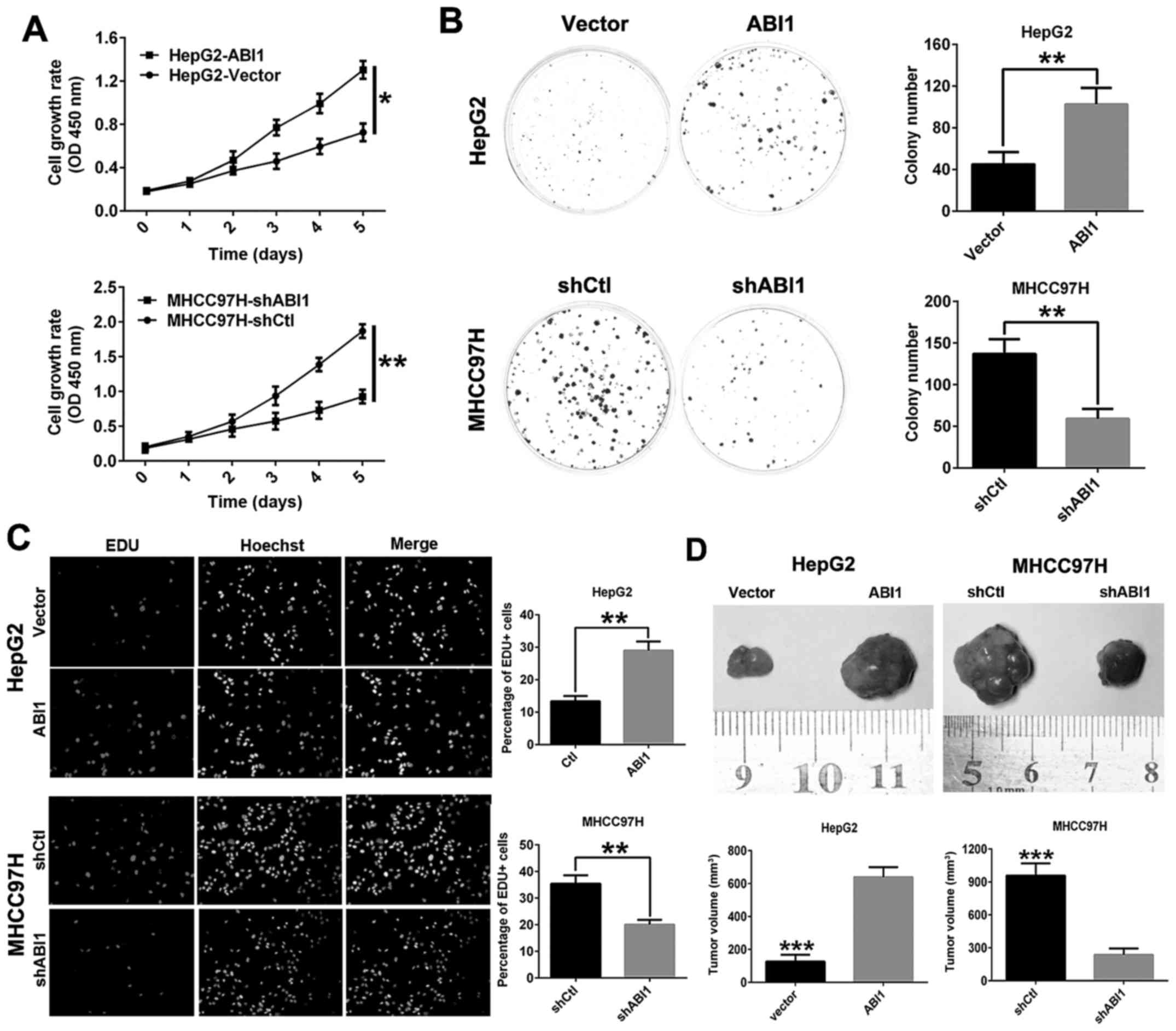
ABI1 promotes HCC cell growth in vitro
and in vivo
To evaluate the function of ABI1 on cell
proliferation in HCC, Cell Counting Kit-8 assay, clone formation
and EdU assays in vitro were performed. The results of CCK8
assay and clone formation assay showed that overexpression of ABI1
in HepG2 cells significantly promoted cell viability and colony
formation compared with HepG2vector cells, whereas
knockdown of ABI1 in MHCC97H cells had the opposite effect
(Fig. 4A and B). In addition,
similar results were observed in EdU incorporation assays (Fig. 4C).
To further verify the growth-enhancing effect of
ABI1 in vivo, HepG2ABI1, MHCC97HshABI1
and their control cells were injected subcutaneously into nude mice
for xenotrans-plantation. Data showed that ABI1 overexpression in
HepG2 cells resulted in a marked increase in tumor size compared
with the control transfectants. Conversely, ABI1 knockdown in
MHCC97H cells decreased the tumor size (Fig. 4D). These results provided evidence
that ABI1 accelerates HCC cell proliferation both in vitro
and in vivo.
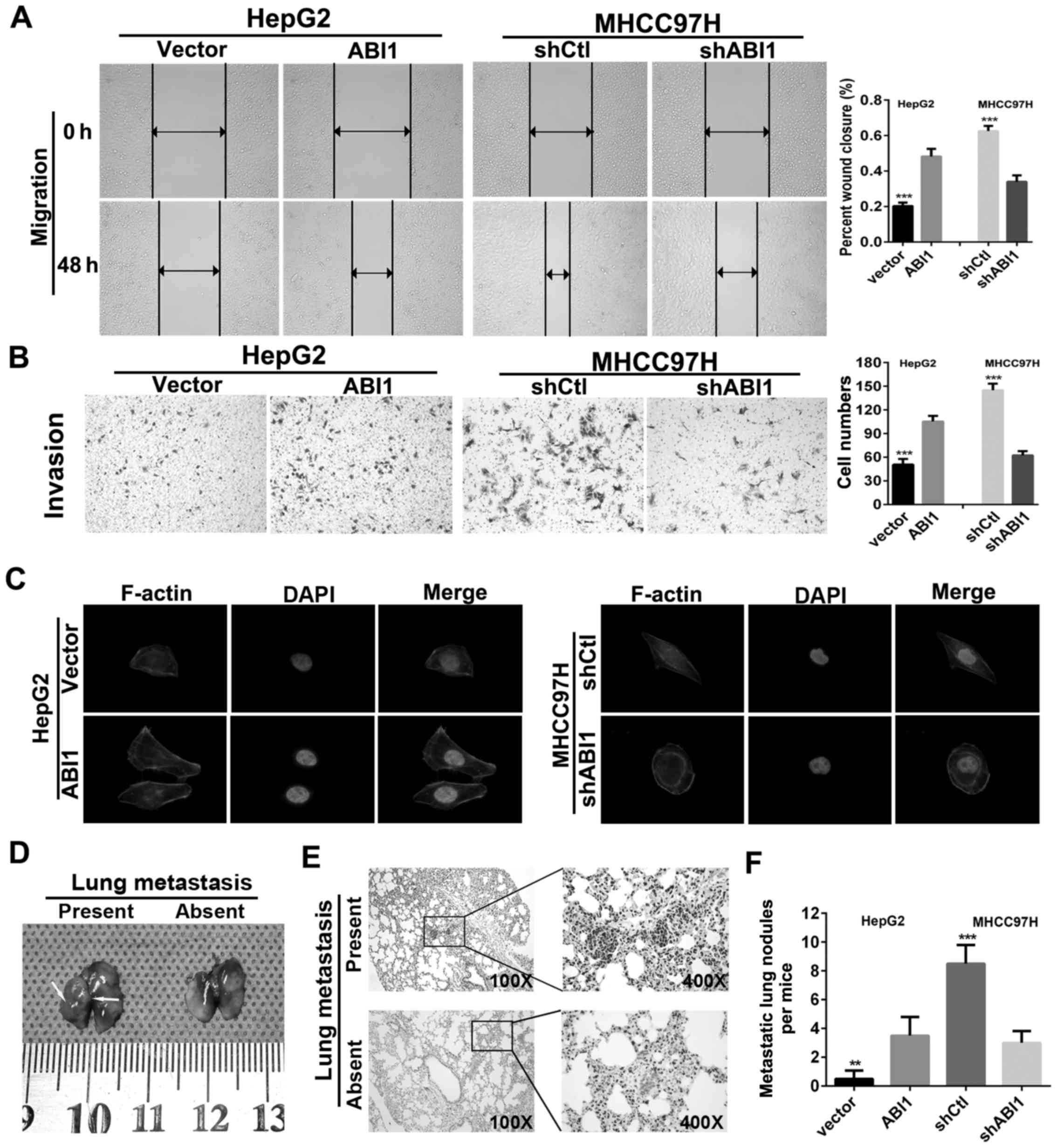
ABI1 enhances HCC cell migration,
invasion and lung metastasis
In order to investigate the effects of ABI1 on HCC
cell motility, wound healing and Transwell invasion assays were
performed. The results showed that HepG2ABI1 cells had
significantly higher percentage of wound closure compared with the
control cells (Fig. 5A). Moreover,
HepG2ABI1 cells also showed a high degree of invasion
through Matrigel (Fig. 5B). By
contrast, silencing ABI1 markedly decreased the percentage of wound
closure and number of invasion cells in MHCC97H (Fig. 5A and B). Since previous studies
showed that ABI1 plays an important role in the cell cytoskeleton
rearrangements (28,29), which is crucial for the invasive
and metastatic spread of tumor cells, we performed
rhodamine-phalloidin fluorescent staining to examine morphologic
changes of HCC cells. As shown in Fig.
5C, ectopic expression of ABI1 in HepG2 cells displayed
fibroblast-like spindle shape with long stretched F-actin fibers.
However, silenced ABI1 in MHCC97H cells exhibited cobblestone-like
appearance with shrunken F-actin fibers.
To verify these findings in vivo, we
generated lung metastasis model in nude mice by injecting
HepG2ABI1, MHCC97HshABI1 and their parental
control cells intravenously in the tail vein. Six weeks later, the
mice were sacrificed and the metastatic nodules in the lungs were
counted. The representative images of lungs with/without metastasis
are shown in Fig. 5D. Hematoxylin
and eosin (H&E) staining confirmed that the nodules in the
lungs were metastatic tumors (Fig.
5E). Further analysis showed that nude mice inoculated with
HepG2ABI1 cells displayed more micrometastatic lesions
in lungs as compared with HepG2vector cells. However,
nude mice in MHCC97HshABI1 groups exhibited less
micrometastatic lesions than MHCC97HshCtl groups
(Fig. 5F). Hence, these
observations suggest that ABI1 is crucial for promoting HCC
invasion and metastasis.
Discussion
The major obstacle for improving the outcome of
cancer patients is recurrence and metastasis. Given that actin
polymerization and lamellipodia formation are critical steps in
cell migration during metastasis (30), while ABI1 was initially reported to
exert its regulatory role in actin reorganization and lamellipodia
formation, the function of ABI1 in cancer development have
attracted increased attention. Accumulating evidence has indicated
that ABI1 was frequently dysregulated in the development of cancer,
however, it remains controversial whether ABI1 acts as a tumor
promoter or suppressor. On the one hand, some studies reported that
ABI1 over expression was closely associated with tumor progression
in breast cancer (20), colon
cancer (22,23), HCC (25) and ovarian cancer (24). which revealed a potential
carcinogenic role of ABI1 in cancers. However, some other studies
showed that ABI1 expression was decreased in human prostate cancer,
gastric cancer and some types of leukemia (14–17)
compared to corresponding normal tissues. These contradictory
results indicated that the ABI1 function as oncogenes or tumor
suppressor genes was context dependent. However, there are limited
studies on the expression, significance, and function of ABI1 in
HCC.
In this study, we for the first time evaluated the
ABI1 expression and clinical significance in a large cohort of HCC
patients. Before the experiments started, we first collected ABI1
information through bioinfomatic analysis from Oncomine database
and found that the levels of ABI1 mRNA were significantly increased
in five independent liver cancer datasets. Next, our results in 23
paired HCC samples further confirmed the upregulation of ABI1 in
HCC tissues. When evaluated the level of ABI1 protein by IHC in an
expanded population with 124 pairs of HCC samples, we also observed
that the ABI1 protein was increased in 60.5% of paraffin-embedded
HCC tissues, which was predominantly detected in the cytoplasm of
HCC cells. Our findings are consistent with the result from Liu
et al in 40 HCC samples (25) but more convincing. Collectively,
our results unambiguously confirmed that ABI1 is overexpressed in
HCC tissues, which may be an oncogene in HCC.
Moreover, several pieces of evidence in this study
support a close association between ABI1 expression and HCC
progression. Firstly, we provided evidence that increased
expression of ABI1 was significantly associated with invasive
characteristics of HCC, including multiple tumor nodes, larger
tumor size, lack of tumor encapsulation and advanced BCLC stage.
Secondly, survival analysis demonstrated that HCC patients with
high ABI1 expression had significantly shorter OS time and TTR than
did patients with low ABI1 expression. Moreover, multivariate
analyses disclosed that ABI1 expression level was an independent
risk factor for poor survival of HCC patients after curative
resection. These clinical data strongly suggest that ABI1
contributes to the malignant progression of HCC, thus making it a
useful prognostic biomarker. Actually, our results are consistent
with previous studies in other cancers. Wang et al (20) performed IHC in a tissue microarrays
including 988 invasive breast carcinoma patients and found that
tumors expressing high levels of ABI1 were significantly associated
with early recurrence and worse survival. Steinestel et al
(22,23) reported ABI1 overexpression in
primary human colorectal carcinoma associated with an infiltrative
phenotype and high-grade tumor cell budding. Zhang et al
(24) observed that upregulation
of ABI1 predicted poor outcome in epithelial ovarian cancer.
Although previous studies found that overexpression
of ABI1 promotes cell proliferation, migration, and invasion in
some cancer types (18,19,21),
Kumar et al recently reported that ABI1 negatively regulated
Tyr251 phosphorylation of Crk and inhibited invasive behavior of
glioblastoma cells (31). Thus,
these characteristics of ABI1 are further needed to confirm in HCC
cells for detailed phenotypes. Firstly, we determined the
expression pattern of ABI1 in HCC cell lines and manipulated the
expression of ABI1 in HCC cells by lentivirus transfection.
Moreover, we explored the potential roles of ABI1 in tumor cell
proliferation, migration and invasion. In our study, we found that
ABI1 overexpression significantly promoted HCC cell proliferation,
migration and invasion in vitro. Otherwise, ABI1 knockdown
prominently reduced them. Furthermore, the mouse model experiments
revealed that ABI1 not only promoted HCC growth, but also enhanced
metastatic potential. Altogether, dysregulation of ABI1 may play a
fundamental role in tumor growth and metastasis. To our knowledge,
this is the first study exploring the biological function of ABI1
in HCC. However, the potential mechanism by which ABI1 affects HCC
progression remains elusive and will require future
investigation.
In conclusion, our study revealed that ABI1 was
frequently upregulated in HCC tissues and significantly associated
with disease progression and poor post-operative outcome of HCC
patients. Functionally, ABI1 promoted HCC growth and metastasis
both in vitro and in vivo. Thus, ABI1 acts as an
oncogene in HCC progression and is a novel prognostic molecular
marker for patients with HCC.
Abbreviations:
|
ABI1
|
Abelson interactor 1
|
|
HCC
|
hepatocellular carcinoma
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
IHC
|
immunohistochemistry
|
|
mRNA
|
microRNA
|
|
shRNA
|
short hairpin RNA
|
|
PBS
|
phosphate buffered saline
|
|
FBS
|
fetal bovine serum
|
Acknowledgments
This study was supported by grants from the
Fundamental Research Funds for the Central Universities of Central
South University (2014zzts088).
References
|
1
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
2
|
He J, Gu D, Wu X, Reynolds K, Duan X, Yao
C, Wang J, Chen CS, Chen J, Wildman RP, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Y, Alin K and Goff SP:
Abl-interactor-1, a novel SH3 protein binding to the
carboxy-terminal portion of the Abl protein, suppresses v-abl
transforming activity. Genes Dev. 9:2583–2597. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brehme M, Hantschel O, Colinge J, Kaupe I,
Planyavsky M, Köcher T, Mechtler K, Bennett KL and Superti-Furga G:
Charting the molecular network of the drug target Bcr-Abl. Proc
Natl Acad Sci USA. 106:7414–7419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scita G, Nordstrom J, Carbone R, Tenca P,
Giardina G, Gutkind S, Bjarnegård M, Betsholtz C and Di Fiore PP:
EPS8 and E3B1 transduce signals from Ras to Rac. Nature.
401:290–293. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scita G, Tenca P, Areces LB, Tocchetti A,
Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M and Di
Fiore PP: An effector region in Eps8 is responsible for the
activation of the Rac-specific GEF activity of Sos-1 and for the
proper localization of the Rac-based actin-polymerizing machine. J
Cell Biol. 154:1031–1044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotula L: Abi1, a critical molecule
coordinating actin cytoskeleton reorganization with PI-3 kinase and
growth signaling. FEBS Lett. 586:2790–2794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubielecka PM, Ladwein KI, Xiong X,
Migeotte I, Chorzalska A, Anderson KV, Sawicki JA, Rottner K,
Stradal TE and Kotula L: Essential role for Abi1 in embryonic
survival and WAVE2 complex integrity. Proc Natl Acad Sci USA.
108:7022–7027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steffen A, Rottner K, Ehinger J, Innocenti
M, Scita G, Wehland J and Stradal TE: Sra-1 and Nap1 link Rac to
actin assembly driving lamellipodia formation. EMBO J. 23:749–759.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kheir WA, Gevrey JC, Yamaguchi H, Isaac B
and Cox D: A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich
membrane protrusions and migration in macrophages. J Cell Sci.
118:5369–5379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Wu X, Pan ZK and Huang S:
Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer
metastasis. Cancer Res. 70:9979–9990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ring C, Ginsberg MH, Haling J and
Pendergast AM: Abl-interactor-1 (Abi1) has a role in cardiovascular
and placental development and is a binding partner of the alpha4
integrin. Proc Natl Acad Sci USA. 108:149–154. 2011. View Article : Google Scholar
|
|
14
|
Macoska JA, Xu J, Ziemnicka D, Schwab TS,
Rubin MA and Kotula L: Loss of expression of human spectrin src
homology domain binding protein 1 is associated with 10p loss in
human prostatic adenocarcinoma. Neoplasia. 3:99–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai Z, Quackenbush RC, Courtney KD, Grove
M, Cortez D, Reuther GW and Pendergast AM: Oncogenic Abl and Src
tyrosine kinases elicit the ubiquitin-dependent degradation of
target proteins through a Ras-independent pathway. Genes Dev.
12:1415–1424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui M, Yu W, Dong J, Chen J, Zhang X and
Liu Y: Downregulation of ABI1 expression affects the progression
and prognosis of human gastric carcinoma. Med Oncol. 27:632–639.
2010. View Article : Google Scholar
|
|
17
|
Jenei V and Jakus J: The role of EGF
receptor-dependent e3B1/Abi1 protein as a tumor suppressor protein
in malignant tumors. Orv Hetil. 146:1293–1299. 2005.In Hungarian.
PubMed/NCBI
|
|
18
|
Wang C, Navab R, Iakovlev V, Leng Y, Zhang
J, Tsao MS, Siminovitch K, McCready DR and Done SJ: Abelson
interactor protein-1 positively regulates breast cancer cell
proliferation, migration, and invasion. Mol Cancer Res.
5:1031–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Li C, Zhuang C, Gilmore WC, Cobos
E, Tao Y and Dai Z: Abl interactor 1 regulates Src-Id1-matrix
metalloproteinase 9 axis and is required for invadopodia formation,
extracellular matrix degradation and tumor growth of human breast
cancer cells. Carcinogenesis. 30:2109–2116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Tran-Thanh D, Moreno JC, Cawthorn
TR, Jacks LM, Wang DY, McCready DR and Done SJ: Expression of Abl
interactor 1 and its prognostic significance in breast cancer: A
tissue-array-based investigation. Breast Cancer Res Treat.
129:373–386. 2011. View Article : Google Scholar
|
|
21
|
Yu W, Sun X, Clough N, Cobos E, Tao Y and
Dai Z: Abi1 gene silencing by short hairpin RNA impairs
Bcr-Abl-induced cell adhesion and migration in vitro and
leukemogenesis in vivo. Carcinogenesis. 29:1717–1724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinestel K, Brüderlein S, Steinestel J,
Märkl B, Schwerer MJ, Arndt A, Kraft K, Pröpper C and Möller P:
Expression of Abelson interactor 1 (Abi1) correlates with
inflammation, KRAS mutation and adenomatous change during colonic
carcinogenesis. PLoS One. 7:e406712012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinestel K, Brüderlein S, Lennerz JK,
Steinestel J, Kraft K, Pröpper C, Meineke V and Möller P:
Expression and Y435-phosphorylation of Abelson interactor 1 (Abi1)
promotes tumour cell adhesion, extracellular matrix degradation and
invasion by colorectal carcinoma cells. Mol Cancer. 13:1452014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Tang L, Chen Y, Duan Z, Xiao L,
Li W, Liu X and Shen L: Upregulation of Abelson interactor protein
1 predicts tumor progression and poor outcome in epithelial ovarian
cancer. Hum Pathol. 46:1331–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu SY, Wu F, Tao YM and Yang LY:
Increased expression of Abi1 in hepatocellular carcinoma and its
correlation with poor prognosis of hepatocellular carcinoma.
Zhonghua Wai Ke Za Zhi. 47:1732–1735. 2009.In Chinese.
|
|
26
|
Chen W, Chen L, Cai Z, Liang D, Zhao B,
Zeng Y, Liu X and Liu J: Overexpression of annexin A4 indicates
poor prognosis and promotes tumor metastasis of hepatocellular
carcinoma. Tumour Biol. 37:9343–9355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao S, Chang RM, Yang MY, Lei X, Liu X,
Gao WB, Xiao JL and Yang LY: Actin-like 6A predicts poor prognosis
of hepatocellular carcinoma and promotes metastasis and
epithelial-mesenchymal transition. Hepatology. 63:1256–1271. 2016.
View Article : Google Scholar :
|
|
28
|
Stradal T, Courtney KD, Rottner K, Hahne
P, Small JV and Pendergast AM: The Abl interactor proteins localize
to sites of actin polymerization at the tips of lamellipodia and
filopodia. Curr Biol. 11:891–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zipfel PA, Bunnell SC, Witherow DS, Gu JJ,
Chislock EM, Ring C and Pendergast AM: Role for the Abi/wave
protein complex in T cell receptor-mediated proliferation and
cytoskeletal remodeling. Curr Biol. 16:35–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
31
|
Kumar S, Lu B, Dixit U, Hossain S, Liu Y,
Li J, Hornbeck P, Zheng W, Sowalsky AG, Kotula L, et al: Reciprocal
regulation of Abl kinase by Crk Y251 and Abi1 controls invasive
phenotypes in glioblastoma. Oncotarget. 6:37792–37807.
2015.PubMed/NCBI
|