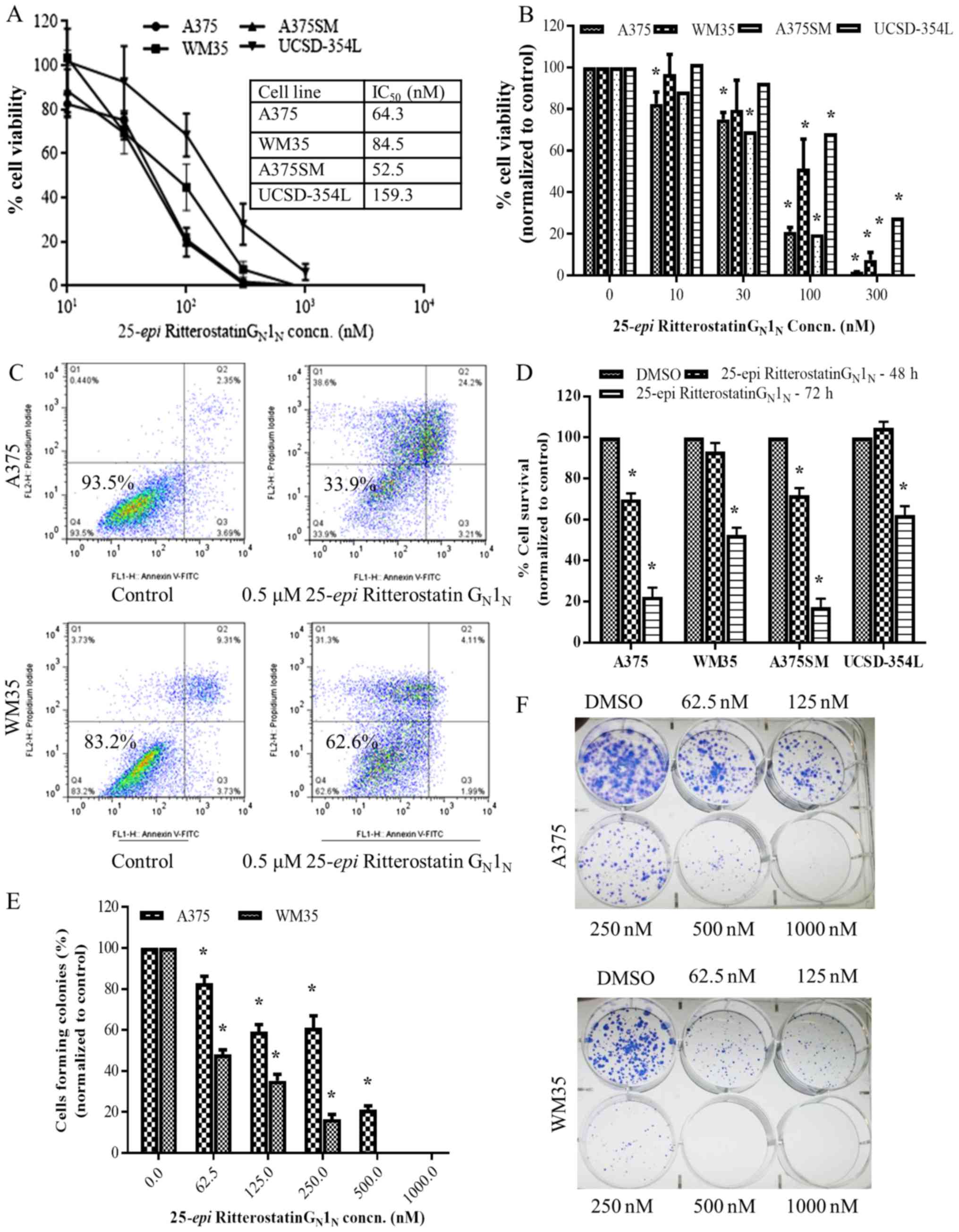
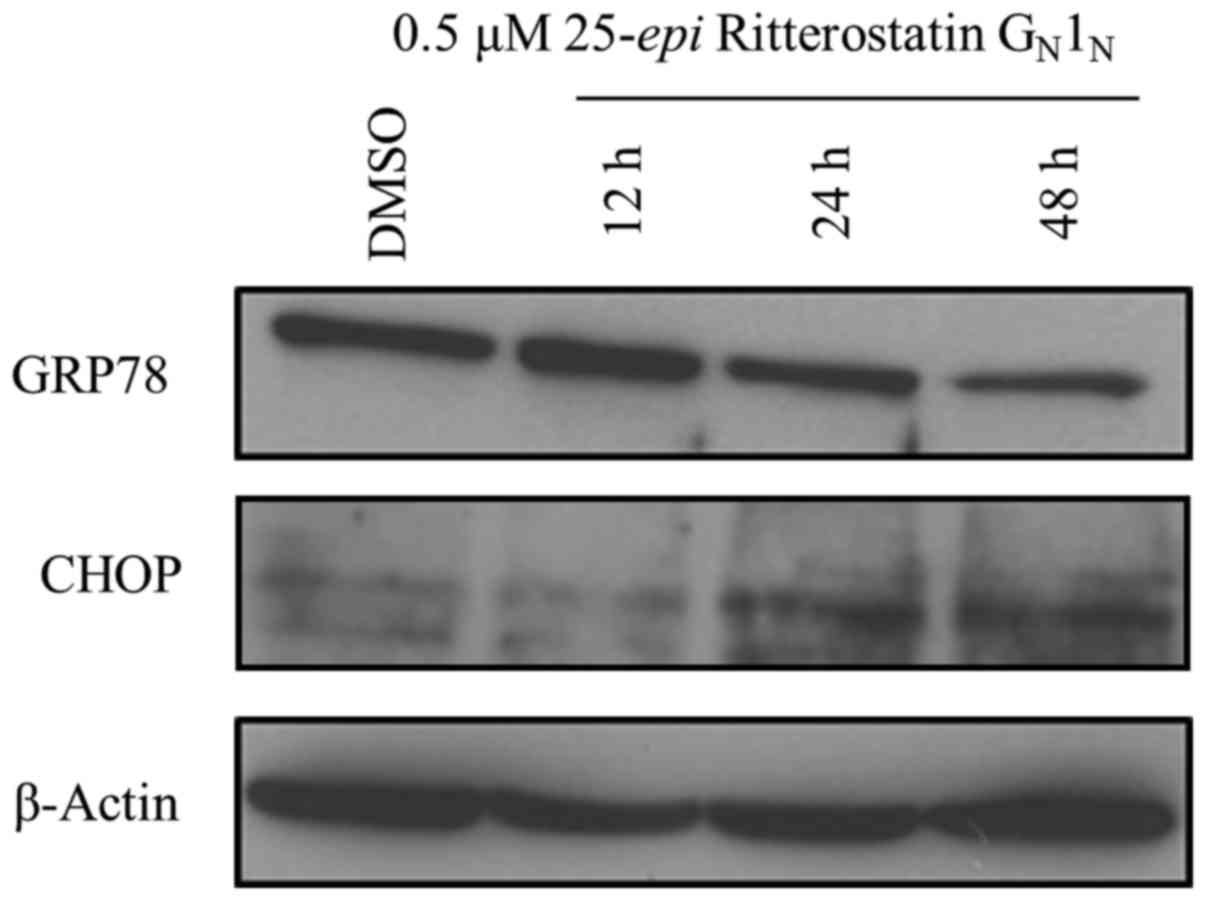
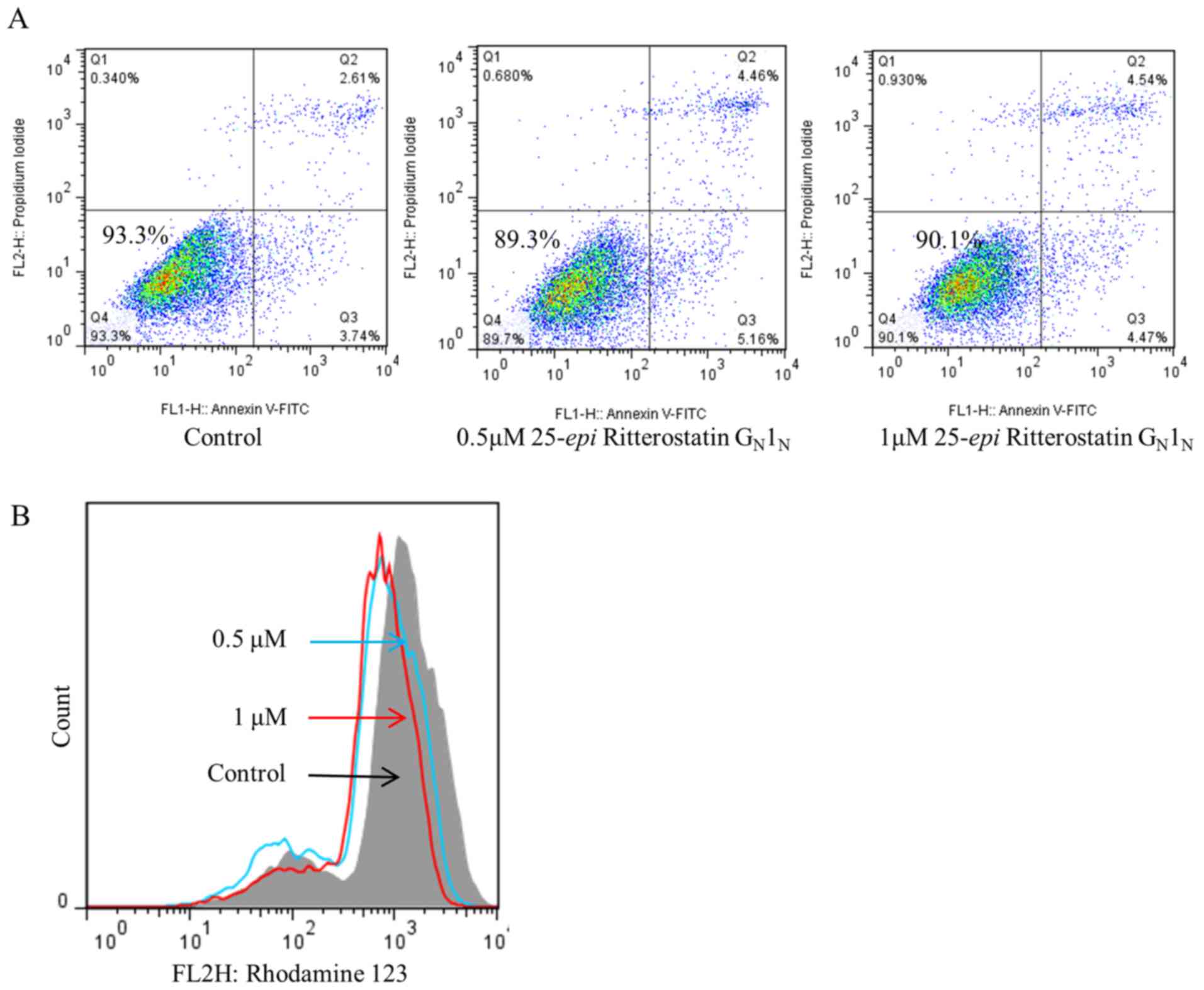
|
1
|
Brown TJ and Nelson BR: Malignant
melanoma: A clinical review. Cutis. 63:275–278. 281–284.
1999.PubMed/NCBI
|
|
2
|
Alberts B, Lewis J, Raff M, Roberts K and
Walter P: Molecular Biology of the Cell. 5th edition. Garland
Science; New York, NY: pp. 13922008
|
|
3
|
Braakman I and Bulleid NJ: Protein folding
and modification in the mammalian endoplasmic reticulum. Annu Rev
Biochem. 80:71–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
Dev Biol. 18:716–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang CC, Chen LH, Gillespie S, Kiejda KA,
Mhaidat N, Wang YF, Thorne R, Zhang XD and Hersey P: Tunicamycin
sensitizes human melanoma cells to tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis by up-regulation of
TRAIL-R2 via the unfolded protein response. Cancer Res.
67:5880–5888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noda I, Fujieda S, Seki M, Tanaka N,
Sunaga H, Ohtsubo T, Tsuzuki H, Fan GK and Saito H: Inhibition of
N-linked glycosylation by tunicamycin enhances sensitivity to
cisplatin in human head-and-neck carcinoma cells. Int J Cancer.
80:279–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Denmeade SR, Jakobsen CM, Janssen S, Khan
SR, Garrett ES, Lilja H, Christensen SB and Isaacs JT:
Prostate-specific antigen-activated thapsigargin prodrug as
targeted therapy for prostate cancer. J Natl Cancer Inst.
95:990–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Treiman M, Caspersen C and Christensen SB:
A tool coming of age: Thapsigargin as an inhibitor of
sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci.
19:131–135. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson AJ, Hsu AL, Lin HP, Song X and
Chen CS: The cyclo-oxygenase-2 inhibitor celecoxib perturbs
intracellular calcium by inhibiting endoplasmic reticulum
Ca2+-ATPases: A plausible link with its anti-tumour
effect and cardiovascular risks. Biochem J. 366:831–837. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
11
|
Lee AS: The glucose-regulated proteins:
Stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing X, Lai M, Wang Y, Xu E and Huang Q:
Overexpression of glucose-regulated protein 78 in colon cancer.
Clin Chim Acta. 364:308–315. 2006. View Article : Google Scholar
|
|
14
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA and Patierno SR:
Overexpression of the glucose-regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shuda M, Kondoh N, Imazeki N, Tanaka K,
Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al:
Activation of the ATF6, XBP1 and grp78 genes in human
hepatocellular carcinoma: A possible involvement of the ER stress
pathway in hepatocarcinogenesis. J Hepatol. 38:605–614. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu
MY, Che CM and Fan ST: Proteomic profiling of hepatocellular
carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27,
Hsp70, GRP78) up-regulation and their associated prognostic values.
Proteomics. 6:1049–1057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arap MA, Lahdenranta J, Mintz PJ, Hajitou
A, Sarkis AS, Arap W and Pasqualini R: Cell surface expression of
the stress response chaperone GRP78 enables tumor targeting by
circulating ligands. Cancer Cell. 6:275–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhuang L, Scolyer RA, Lee CS, McCarthy SW,
Cooper WA, Zhang XD, Thompson JF and Hersey P: Expression of
glucose-regulated stress protein GRP78 is related to progression of
melanoma. Histopathology. 54:462–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Ridder GG, Ray R and Pizzo SV: A murine
monoclonal antibody directed against the carboxyl-terminal domain
of GRP78 suppresses melanoma growth in mice. Melanoma Res.
22:225–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ranganathan AC, Zhang L, Adam AP and
Aguirre-Ghiso JA: Functional coupling of 38-induced up-regulation
of BiP and activation of RNA-dependent protein kinase-like
endoplasmic reticulum kinase to drug resistance of dormant
carcinoma cells. Cancer Res. 66:1702–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rutkowski DT, Arnold SM, Miller CN, Wu J,
Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D and Kaufman RJ:
Adaptation to ER stress is mediated by differential stabilities of
pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol.
4:e3742006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto M, Minami M, Takeda K, Sakao Y
and Akira S: Ectopic expression of CHOP (GADD153) induces apoptosis
in M1 myeloblastic leukemia cells. FEBS Lett. 395:143–147. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho HY, Thomas S, Golden EB, Gaffney KJ,
Hofman FM, Chen TC, Louie SG, Petasis NA and Schönthal AH: Enhanced
killing of chemo-resistant breast cancer cells via controlled
aggravation of ER stress. Cancer Lett. 282:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rabik CA, Fishel ML, Holleran JL, Kasza K,
Kelley MR, Egorin MJ and Dolan ME: Enhancement of cisplatin
[cisdiammine dichloroplatinum (II)] cytotoxicity by
O6-benzylguanine involves endoplasmic reticulum stress. J Pharmacol
Exp Ther. 327:442–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sánchez AM, Martínez-Botas J,
Malagarie-Cazenave S, Olea N, Vara D, Lasunción MA and Díaz-Laviada
I: Induction of the endoplasmic reticulum stress protein
GADD153/CHOP by capsaicin in prostate PC-3 cells: A microarray
study. Biochem Biophys Res Commun. 372:785–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ravikumar B, Futter M, Jahreiss L,
Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM,
Narayanan U, Renna M, et al: Mammalian macroautophagy at a glance.
J Cell Sci. 122:1707–1711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo JY, Chen HY, Mathew R, Fan J,
Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM,
Karantza V, et al: Activated Ras requires autophagy to maintain
oxidative metabolism and tumorigenesis. Genes Dev. 25:460–470.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maiuri MC, Malik SA, Morselli E, Kepp O,
Criollo A, Mouchel PL, Carnuccio R and Kroemer G: Stimulation of
autophagy by the p53 target gene Sestrin2. Cell Cycle. 8:1571–1576.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tasdemir E, Maiuri MC, Galluzzi L, Vitale
I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C,
Harper F, et al: Regulation of autophagy by cytoplasmic p53. Nat
Cell Biol. 10:676–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gozuacik D, Bialik S, Raveh T, Mitou G,
Shohat G, Sabanay H, Mizushima N, Yoshimori T and Kimchi A:
DAP-kinase is a mediator of endoplasmic reticulum stress-induced
caspase activation and autophagic cell death. Cell Death Differ.
15:1875–1886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zalckvar E, Berissi H, Mizrachy L,
Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R
and Kimchi A: DAP-kinase-mediated phosphorylation on the BH3 domain
of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and
induction of autophagy. EMBO Rep. 10:285–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zalckvar E, Berissi H, Eisenstein M and
Kimchi A: Phosphorylation of Beclin 1 by DAP-kinase promotes
autophagy by weakening its interactions with Bcl-2 and Bcl-XL.
Autophagy. 5:720–722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen J, Chen X, Hendershot L and Prywes R:
ER stress regulation of ATF6 localization by dissociation of
BiP/GRP78 binding and unmasking of Golgi localization signals. Dev
Cell. 3:99–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okada T, Yoshida H, Akazawa R, Negishi M
and Mori K: Distinct roles of activating transcription factor 6
(ATF6) and double-stranded RNA-activated protein kinase-like
endoplasmic reticulum kinase (PERK) in transcription during the
mammalian unfolded protein response. Biochem J. 366:585–594. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding WX, Ni HM, Gao W, Hou YF, Melan MA,
Chen X, Stolz DB, Shao ZM and Yin XM: Differential effects of
endoplasmic reticulum stress-induced autophagy on cell survival. J
Biol Chem. 282:4702–4710. 2007. View Article : Google Scholar
|
|
42
|
Yorimitsu T, Nair U, Yang Z and Klionsky
DJ: Endoplasmic reticulum stress triggers autophagy. J Biol Chem.
281:30299–30304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bernales S, McDonald KL and Walter P:
Autophagy counterbalances endoplasmic reticulum expansion during
the unfolded protein response. PLoS Biol. 4:e4232006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ogata M, Hino S, Saito A, Morikawa K,
Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K,
et al: Autophagy is activated for cell survival after endoplasmic
reticulum stress. Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kanduluru AK, Banerjee P, Beutler JA and
Fuchs PL: A convergent total synthesis of the potent
cephalostatin/ritterazine hybrid -25-epi ritterostatin
GN1N. J Org Chem. 78:9085–9092. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shoemaker RH: The NCI60 human tumour cell
line anticancer drug screen. Nat Rev Cancer. 6:813–823. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
von Schwarzenberg K and Vollmar AM:
Targeting apoptosis pathways by natural compounds in cancer: Marine
compounds as lead structures and chemical tools for cancer therapy.
Cancer Lett. 332:295–303. 2013. View Article : Google Scholar
|
|
48
|
Fan C, Wang W, Zhao B, Zhang S and Miao J:
Chloroquine inhibits cell growth and induces cell death in A549
lung cancer cells. Bioorg Med Chem. 14:3218–3222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA
and Koh JY: Induction of lysosomal dilatation, arrested autophagy,
and cell death by chloroquine in cultured ARPE-19 cells. Invest
Ophthalmol Vis Sci. 51:6030–6037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Papalas JA, Vollmer RT, Gonzalez-Gronow M,
Pizzo SV, Burchette J, Youens KE, Johnson KB and Selim MA: Patterns
of GRP78 and MTJ1 expression in primary cutaneous malignant
melanoma. Mod Pathol. 23:134–143. 2010. View Article : Google Scholar
|
|
53
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Su R, Li Z, Li H, Song H, Bao C, Wei J and
Cheng L: Grp78 promotes the invasion of hepatocellular carcinoma.
BMC Cancer. 10:202010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Govindarajan B, Sligh JE, Vincent BJ, Li
M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L,
Zhang Y, et al: Overexpression of Akt converts radial growth
melanoma to vertical growth melanoma. J Clin Invest. 117:719–729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum: Coordination of gene
transcriptional and translational controls. Genes Dev.
13:1211–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schindler AJ and Schekman R: In vitro
reconstitution of ER-stress induced ATF6 transport in COPII
vesicles. Proc Natl Acad Sci USA. 106:17775–17780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen J, Snapp EL, Lippincott-Schwartz J
and Prywes R: Stable binding of ATF6 to BiP in the endoplasmic
reticulum stress response. Mol Cell Biol. 25:921–932. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Haze K, Yoshida H, Yanagi H, Yura T and
Mori K: Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Reddy RK, Mao C, Baumeister P, Austin RC,
Kaufman RJ and Lee AS: Endoplasmic reticulum chaperone protein
GRP78 protects cells from apoptosis induced by topoisomerase
inhibitors: Role of ATP binding site in suppression of caspase-7
activation. J Biol Chem. 278:20915–20924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun FC, Wei S, Li CW, Chang YS, Chao CC
and Lai YK: Localization of GRP78 to mitochondria under the
unfolded protein response. Biochem J. 396:31–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sivridis E, Koukourakis MI, Mendrinos SE,
Karpouzis A, Fiska A, Kouskoukis C and Giatromanolaki A: Beclin-1
and LC3A expression in cutaneous malignant melanomas: A biphasic
survival pattern for beclin-1. Melanoma Res. 21:188–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang J, Pan XL, Ding LJ, Liu DY, Da-Peng
Lei and Jin T: Aberrant expression of Beclin-1 and LC3 correlates
with poor prognosis of human hypopharyngeal squamous cell
carcinoma. PLoS One. 8:e690382013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang X, Bai HM, Chen L, Li B and Lu YC:
Reduced expression of LC3B-II and Beclin 1 in glioblastoma
multiforme indicates a downregulated autophagic capacity that
relates to the progression of astrocytic tumors. J Clin Neurosci.
17:1515–1519. 2010. View Article : Google Scholar : PubMed/NCBI
|