Introduction
Epigenetic changes are widely observed in various
types of cancer. DNA methylation of gene promoter region can
suppress expression of cancer-related genes, e.g. tumor suppressor,
which may lead to carcinogenesis of many types of cancer including
endometrial cancer (1–3). On the other hand, aberrant DNA
hypermethylation in normally unmethylated sequences/promoters can
be regarded as epimutations. Epimutations are suspected as a cause
of some inherited cancer syndrome. Lynch syndrome is one such
inherited cancer syndrome related to endometrial cancer, and
germline mutations in DNA mismatch repair genes such as MLH1
are major cause of the disease. However, epimutations in
MLH1 gene may also cause Lynch syndrome (4–6).
It has been known that DNA methylation likely
contributes to both endometrial carcinogenesis and endometrial
cancer phenotype (7,8). We have identified aberrant DNA
methylations in promoters of various cancer-related genes in
endometrial cancer. Such concurrent DNA methylation of multiple
genes is observed in colorectal, breast, gastric and endometrial
cancers, and this is defined as the CpG island methylator phenotype
(CIMP) (9–11), but, methylation status, features
and causes of CIMP-positive endometrial cancer have not been well
understood. One can assume that cells of those CIMP-positive cancer
patients may have aberrant DNA methylation metabolism even in their
normal tissues before manifestation of the cancer, which may
trigger a series of epigenetic changes leading to carcinogenesis of
the CIMP-positive endometrial cancer. Based on this assumption, we
investigated DNA methylation status and tried to identify features
in normal tissue of CIMP-positive endometrial cancer compared to
CIMP-negative endometrial cancer.
CIMP was first identified in colorectal cancer by
the study of Toyota et al (12), and in the past decade CIMP-positive
colorectal cancer has been related to BRAF mutation,
MLH1 methylation and microsatellite instability (MSI)
(13,14). However, only a few reports have
described CIMP-positive endometrial cancer (10,15),
but good CIMP markers for endometrial cancer have not been
identified. A recent study also showed that there was no
BRAF mutation in endometrial cancer (16,17)
and suggested that CIMP markers for colorectal cancer were not
suitable for endometrial cancer. In previous studies (18–21),
we have shown that promoters of certain tumor suppressor genes were
methylated in endometrial cancer tissue, but not in normal
endometrium: MLH1, APC and CDH1 were most frequently
methylated in endometrial cancer and MLH1 and APC
were already methylated in atypical endometrial hyperplasia (AEH).
These results suggest that methylation of these gene promoters
plays an important role as early event in carcinogenesis of
endometrial cancer formation. Thus, we decided to examine
methylation status of these three genes as CIMP markers for
endometrial cancer in the present study.
The aim of the present study was to investigate
genomewide DNA methylation status of both CIMP-positive and
negative cancers. In addition, by comparing normal and cancer
tissues from these two CIMP classes, we attempted to identify
epimutation candidates in CIMP-positive endometrial cancer. The
epimutation candidate will be useful as a predictive marker for
CIMP-positive endometrial cancer. Further analysis of the
epimutation candidate may contribute to understanding the molecular
mechanisms underlying CIMP-positive endometrial cancer formation.
Furthermore, as DNA methylation is a reversible modification, the
results of the present study may contribute to development of
'epigenetic medicine' for cancer prevention.
Materials and methods
DNA and RNA extraction from patient
samples
The subjects were 25 Japanese patients diagnosed
with endometrial cancer at the Department of Obstetrics and
Gynecology, Keio University Hospital from December 2013 to March
2015. Patients aged under 20 years of age were excluded. The 25
patients had an age range of 33–76 years. Clinicopathological data
are shown in Table I. Fifty paired
peripheral blood and cancer tissue samples were collected from the
25 patients and stored at 4 and −80°C, respectively, until DNA or
RNA extraction using an AllPrep DNA/RNA/miRNA Universal kit
(Qiagen, Hilden, Germany). The study protocol (no. 2013258) was
approved by the Institutional Review Board of Keio University
School of Medicine, and the study was performed in compliance with
the Declaration of Helsinki. All participants gave written informed
consent.
 | Table IClinicopathological characteristics
in endometrial cancer patients. |
Table I
Clinicopathological characteristics
in endometrial cancer patients.
| Clinicopathological
characteristics | Data |
|---|
| Age (years) | |
| Average | 55.08 |
| Range | (33–76) |
| Histological
type | |
| Endometrioid
adenocarcinoma | 25 |
|
Differentiation | |
| G1 | 14 |
| G2 | 7 |
| G3 | 4 |
| Stage | |
| I | 17 |
| II | 6 |
| III | 2 |
Bisulfite treatment and methylation
specific polymerase chain reaction (MSP)
DNA (1 μg) in a volume of 50 μl was
denatured by adding 5.5 μl of 2N NaOH. After incubation of
the sample at 37°C for 15 min, 30 μl of 10 mM hydroquinone
(Sigma-Aldrich, St. Louis, MO, USA) and 520 μl of 2M sodium
bisulfate pH 5.5 (Sigma-Aldrich) were added. The sample was gently
mixed and centrifuged briefly, after which the solution was
overlaid with 200 μl of mineral oil and incubated at 50°C
for 20 h. After incubation, 1 ml of Wizard DNA Clean-up resin
(Promega, Madison, WI, USA) was added to the lower layer and mixed
for DNA purification. This procedure gave 50 μl of DNA
solution, to which 5.5 μl of 3N NaOH was added, and the
solution was incubated at 37°C for 20 min. Next, 66 μl of 5N
ammonium acetate (Sigma-Aldrich) and 243 μl of 95% ethanol
were added and the solution was incubated at −80°C for 1 h. After
centrifugation at 20,000 × g for 45 min at 4°C, the DNA pellet was
rinsed with 1 ml of 70% ethanol and centrifuged at 20,000 × g for
30 min at 4°C. Precipitated DNA was air dried and resuspended in 20
μl of Milli-Q water. Aliquots of this solution (2 μl)
were used as the MSP template. AmpliTaq Gold with 10X PCR Gold
Buffer and MgCl2 (Applied Biosystems, Foster City, CA,
USA) were used for MSP and the methylation status of each gene was
analyzed using a ProFlex PCR System (Applied Biosystems). CpGenome
Universal Methylated DNA and Unmethylated DNA (Millipore, Temecula,
CA, USA) were used as positive controls for methylated and
unmethylated PCR, respectively.
Each 25 μl PCR reaction mixture contained 1X
PCR buffer, 0.8 μM primers, 200 μM dNTP, 3 mM
MgCl2 and 1U Taq polymerase. The primers and PCR
conditions for MSP analysis were as follows: for MLH1,
M-forward, 5′-ACG TAG ACG TTT TAT TAG GGT CGC-3′ and M-reverse,
5′-CCT CAT CGT AAC TAC CCG CG-3′, U-forward, 5′-TTT TGA TGT AGA TGT
TTT ATT AGG GTT GT-3′ and U-reverse, 5′-ACC ACC TCA TCA TAA CTA CCC
ACA-3′, 95°C for 10 min, 5 cycles at 94°C for 30 sec, 60°C for 30
sec, 72°C for 30 sec, 30 cycles at 94°C for 30 sec, 55°C for 30
sec, 72°C for 30 sec, and 72°C for 10 min; for APC,
M-forward, 5′-TAT TGC GGA GTG CGG GTC-3′ and M-reverse, 5′-TCG ACG
AAC TCC CGA CGA-3′, U-forward, 5′-GTG TTT TAT TGT GGA GTG TGG
GTT-3′ and U-reverse, 5′-CCA ATC AAC AAA CTC CCA ACA A-3′, 95°C for
10 min, 35 cycles at 95°C for 30 sec, 68°C (M) or 66°C (U) for 30
sec, 72°C for 30 sec and 72°C for 10 min. A CpG WIZ E-cadherin
amplification kit (Millipore) was used for CDH1 MSP
analysis, using methylated and unmethylated primer sets supplied
with the kit and PCR conditions of 95°C for 10 min, 35 cycles at
95°C for 45 sec, 60°C for 45 sec, 72°C for 45 sec and 72°C for 10
min. PCR products were separated by electrophoresis on a 3% agarose
gel and stained with ethidium bromide.
Based on the MSP analysis, patients with methylation
of two of the three genes were defined as CIMP-High (CIMP-H), those
with methylation of one of the genes as CIMP-Low (CIMP-L) and those
with no methylation as CIMP-negative (CIMP(-)).
DNA methylation analysis using
next-generation sequencing (NGS)
A SureSelect Human Methyl-Seq Capture Library and a
SureSelect Target Enrichment kit (Agilent Technologies, Santa
Clara, CA, USA) were used to investigate the genomewide DNA
methylation status. The SureSelect Human Methyl-Seq kit captures 84
Mb of the human genome with 3.7 million individual CpG
dinucleotides covering ~91% CpG islands and ~141,000 promoters. We
used the kit for DNA library preparation with the SureSelect
Methyl-Seq and Postbisulfite Adaptor Tagging Protocol (http://www.chem-agilent.com/pdf/PBAT_SureSelect_Methyl_DraftB_19AUG15.pdf#search='SureSelectPBAT').
In brief, 100 ng of dsDNA measured with a Qubit dsDNA BR Assay kit
(Life Technologies, Carlsbad, CA, USA) was diluted in 130 μl
of TE buffer and fragmented into 500–600 bp with Covaris S2
(Covaris, Woburn, MA, USA). After sheared DNA purification with
AMPure XP beads (Beckman Coulter, Brea, CA, USA), the DNA was
hybridized with a biotinylated RNA probe and enriched with
Dynabeads MyOne Streptavidin T1 (Life Technologies). Bisulfite
conversion was performed using an EZ DNA Methylation-Gold kit (Zymo
Research, Irvine, CA, USA) according to the SureSelect-PBAT
protocol. After first and second strand synthesis with Klenow
fragment (3′→5′ exo-) (NEB, Ipswich, MA, USA), the concentration of
library DNA was measured with a Library Quantification kit for
Illumina (Kapa Biosystems, Boston, MA, USA). PCR amplification of
7–15 cycles was performed using a GeneAmp PCR System 9700 (Applied
Biosystems). After measuring the final concentration of template
DNA using qPCR, 10 pM DNA was used for sequencing. PhiX Control v3
(Illumina, San Diego, CA, USA) was spiked at a final concentration
of 1.6 pM and 2×75 paired-end sequencing was performed using a
MiSeq reagent kit v3 (Illumina).
Data analysis
To avoid low quality reads and contamination by
adapter sequences, quality control and trimming were performed
using FASTQ Toolkit ver. 2.0.0 (BaseSpace on the Illumina web
site). After trimming of adapter sequences, the read pairs for
which the 3′ end quality score was <30 were excluded. After
adapter sequence trimming, 15 bps were trimmed from both the 5′ and
3′ ends. A quality control check of trimmed read pairs was
performed using FASTQC ver. 1.0.0 (BaseSpace on Illumina web
site).
Reads were aligned to the reference human genome
(hg19) using Bismark ver. 0.14.5, Bowtie2 ver. 2.2.6 and Samtools
ver. 1.2 with settings of --pbat, --score_min L, −0.6, −0.6 -D 150
-X 1000. PCR duplicates were removed using default Bismark
settings. Methylation calling was also processed using Methylation
Extractor in the Bismark module for visualization on IGV. The
SureSelect Human Methyl-Seq kit captures 84 Mb of the human genome
with 3.7 million individual CpG dinucleotides. Thus, the on-target
rate against a designed target sequence was measured using Bedtool
ver. 2.19.1. After deduplication by Bismark, the output SAM file
was used as input to methylKit ver. 0.9.2. (22) with default settings. Correlation
plots and detection and annotation of differentially methylated
CpGs (DMCs) and differentially methylated regions (DMRs) were
performed in MethylKit. The minimum read coverage was set to 10 to
measure DMCs and DMRs. A 25% methylation difference has been shown
to induce a 2-fold repression in gene expression (23). Thus, we used a methylation
difference >25% and a cut-off of q<0.01. DMRs were identified
using a 50-bp window and a 10-bp step size.
Validation of genome-wide bisulfite
sequencing
Bisulfite treated DNA was amplified by PCR with the
following primers and condition; miR-663aBS forward,
5′-GTTTGTAGAGGA ATTTTTTTTAGTT-3′ and reverse, 5′-ACCACAACCACA
AACTCAAC-3′, 95°C for 10 min, 35 cycles at 95°C for 30 sec, 60°C
for 30 sec, 72°C for 30 sec and 72°C for 10 min. Other PCR settings
were same as MSP. PCR products were separated by electrophoresis on
a 3% agarose gel and stained with ethidium bromide, and purified
with a NucleoSpin Gel and PCR Clean-up kit (Takara Bio, Tokyo,
Japan) according to the manufacturer's instructions. The PCR
products were TA cloned by using the pGEM-T Easy Vector System
(Promega) according to the manufacturer's instructions. PCR with
universal T7 and SP6 primers was performed on transformed colonies
and correctly inserted clonal amplicons were sent to The Core
Instrumentation Facility in Keio University for sequencing.
Semi-quantitative RT-PCR and RT-qPCR
cDNA for miR-663a expression analysis was
synthesized with 0.5 μg of total RNA using a Mir-X miRNA
First-Strand Synthesis kit (Clontech Laboratories, Mountain View,
CA, USA). AmpliTaq Gold with 10X PCR Gold Buffer and
MgCl2 (Applied Biosystems) were used in the
semi-quantitative RT-PCR analysis. PCR amplification was performed
using a ProFlex PCR System (Applied Biosystems). Each 25 μl
PCR reaction mixture contained 1X PCR buffer, 1.0 μM
primers, 200 μM dNTP, 3 mM MgCl2 and 1U Taq
polymerase, with 5% dimethyl sulfoxide (DMSO) spiked in the
reaction mix for miR-663a analysis. The primers and the PCR
conditions for semi-quantitative RT-PCR were forward primer for
miR-663a 5′-AGG CGG GGC GCC GCG GGA CCG C-3′, reverse primer for
miR-663a and U6 control primers supplied with the kit, 95°C for 10
min, 28 cycles of 95°C for 20 sec, 68°C for 20 sec, 72°C for 20 sec
and 72°C for 10 min. PCR products were separated by electrophoresis
on a 3% agarose gel and stained with ethidium bromide. Signals were
quantified using an E-BOX VX2 system and E-Capt software (Bilber
Lourmat, Marne-la-Vallée, France). miR-663a relative expression was
calculated using U6 as an internal control.
cDNA for GAPDH, TGF-β, DNMT1, DNMT3a and
DNMT3b expression analysis was synthesized with 1 μg
of total RNA using a Superscript First-Strand Synthesis system for
RT-PCR kit (Invitrogen, Carlsbad, CA, USA), with 1 μl of
synthesized First-Strand cDNA as template. Thunderbird SYBR qPCR
Mix (Toyobo, Co., Ltd., Tokyo, Japan) was used for RT-qPCR, with 10
μl of PCR reaction mixture containing 1X qPCR mix and 0.3
μM primers. The primers and PCR conditions used for RT-qPCR
were as follows: for GAPDH forward, 5′-GAA GGT GAA GGT CGG
AGT C-3′ and reverse, 5′-GAA GAT GGT GAT GGG ATT TC-3′; for
TGF-β forward, 5′-AGT GGA CAT CAA CGG GTT CAG-3′ and
reverse, 5′-CAT GAG AAG CAG GAA AGG CC-3′; for DNMT1
forward, 5′-AAG GGA AGG GCA AGG GAA AAG G-3′ and reverse, 5′-AGA
AAA CAC ATC CAG GGT CCG CAG-3′; for DNMT3a forward, 5′-GAT
TGA TGC CAA AGA AGT GTC AG-3′ and reverse, 5′-CAT TCA CAG TGG ATG
CCA AC-3′; for DNMT3b forward, 5′-AAT GTG AAT CCA GCC AGG
AAA GGC-3′ and reverse, 5′-ACT GGA TTA CAC TCC AGG AAC CGT-3′; 95°C
for 30 sec, 40 or 50 cycles of 95°C for 5 sec, 60°C for 30 sec.
Quantification was performed using a LightCycler® 480
system (Roche Diagnostics, Basel, Switzerland). Expression of
TGF-β and DNMTs was calculated by the ΔΔCq method
using GAPDH as an internal control.
Results
Defining CIMP classes based on the MSP
analysis of MLH1, APC and CDH1
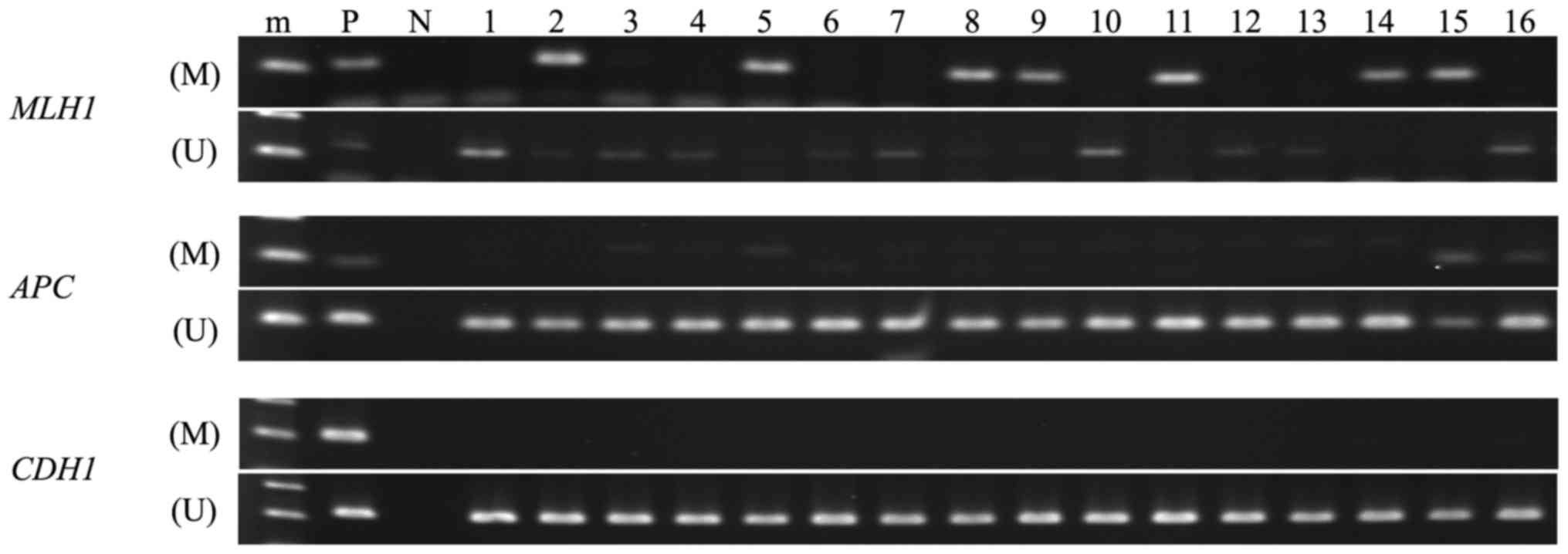
All endometrial cancer tissues were analyzable by
MSP (Fig. 1 and Table II). The methylation frequencies in
the MLH1, APC and CDH1 promoter regions in these
tissues were 32.0% (8/25), 12.0% (3/25) and 0.0% (0/25),
respectively. Based on the MSP analysis, we defined CIMP classes as
follows: cases with hypermethylation in more than two of the three
promoter regions are defined as CIMP-high, whereas cases with no
hypermethylation in the corresponding regions are CIMP(-). If only
one of the regions is hypermethylated, we defined these cases as
CIMP-low. According to these criteria, we identified 2 CIMP-H
(8.0%; 2/25), 7 CIMP-L (28.0%; 7/25) and 16 CIMP(-) (64.0%;
16/25).
 | Table IIAberrant DNA méthylation of three
genes in endometrial cancer. |
Table II
Aberrant DNA méthylation of three
genes in endometrial cancer.
| No. | Result of MSP
| Classification of
CIMP | Age (years) | Histological
type |
Differentiation | Stage |
|---|
| hMLH1 | APC | CDH1 |
|---|
| 1 | U | U | U | CIMP (-) | 69 | Endometrioid
adenocarcinoma | G3 | IIIc2 |
| 2 | M | U | U | CIMP-L | 47 | Endometrioid
adenocarcinoma | G2 | Ia |
| 3a | U | U | U | CIMP (-) | 50 | Endometrioid
adenocarcinoma | G2 | Ia |
| 4 | U | U | U | CIMP (-) | 66 | Endometrioid
adenocarcinoma | G2 | Ib |
| 5 | M | U | U | CIMP-L | 50 | Endometrioid
adenocarcinoma | G1 | Ia |
| 6 | U | U | U | CIMP (-) | 65 | Endometrioid
adenocarcinoma | G1 | Ia |
| 7 | U | U | U | CIMP (-) | 70 | Endometrioid
adenocarcinoma | G2 | Ia |
| 8 | M | U | U | CIMP-L | 50 | Endometrioid
adenocarcinoma | G2 | II |
| 9 | M | U | U | CIMP-L | 53 | Endometrioid
adenocarcinoma | G3 | II |
| 10 | U | U | U | CIMP (-) | 76 | Endometrioid
adenocarcinoma | G2 | Ia |
| 11 | M | U | U | CIMP-L | 54 | Endometrioid
adenocarcinoma | G3 | Ia |
| 12 | U | U | U | CIMP (-) | 63 | Endometrioid
adenocarcinoma | G1 | II |
| 13 | U | U | U | CIMP (-) | 42 | Endometrioid
adenocarcinoma | G1 | Ia |
| 14 | M | U | U | CIMP-L | 58 | Endometrioid
adenocarcinoma | G1 | Ia |
| 15a | M | M | U | CIMP-H | 56 | Endometrioid
adenocarcinoma | G2 | Ia |
| 16 | U | M | U | CIMP-L | 56 | Endometrioid
adenocarcinoma | G1 | Ia |
| 17 | U | U | U | CIMP (-) | 64 | Endometrioid
adenocarcinoma | G3 | II |
| 18 | U | U | U | CIMP (-) | 54 | Endometrioid
adenocarcinoma | G1 | II |
| 19 | U | U | U | CIMP (-) | 55 | Endometrioid
adenocarcinoma | G1 | IIIc2 |
| 20 | U | U | U | CIMP (-) | 45 | Endometrioid
adenocarcinoma | G1 | Ia |
| 21 | U | U | U | CIMP (-) | 65 | Endometrioid
adenocarcinoma | G1 | Ib |
| 22 | U | U | U | CIMP (-) | 44 | Endometrioid
adenocarcinoma | G1 | Ia |
| 23a | U | U | U | CIMP (-) | 46 | Endometrioid
adenocarcinoma | G1 | Ia |
| 24 | U | U | U | CIMP (-) | 46 | Endometrioid
adenocarcinoma | G1 | II |
| 25a | M | M | U | CIMP-H | 33 | Endometrioid
adenocarcinoma | G1 | Ia |
Genome-wide bisulfite sequencing in
CIMP-H and CIMP(-) cases
The SureSelect Human Methyl-Seq method adopting PBAT
protocol was used for genome-wide bisulfite targeted sequencing
analysis. The SureSelect method captures 84 Mb of the human genome
containing 3.7 million individual CpG dinucleotides in theory. The
captured regions cover >90% of the CpG islands and ~141,000
regions corresponding to the gene promoters defined by GENCODE.
Cancer-specific or tissues-specific differentially methylated
regions (DMRs) can also be analyzed by this method. PBAT protocol
was used to construct the libraries, as this protocol requires less
amount of materials compared to the Methyl-Seq procedures (24). Using this genome-wide analytical
method, we investigated DNA methylation status of the paired
samples of PBC and cancer from two CIMP-H and two CIMP(-) patients.
The two CIMP(-) patients were selected based on patient age, cancer
stage and differentiation status. We sequenced total of eight
bisulfite-converted DNA libraries and an average of 13.6 million
paired end sequence reads were obtained from each library. The
mapping rate against the human reference genome (hg19) ranged from
20.4 to 37.9%, which is consistent with the fact that sequences
obtained from PBAT library give lower mapping rate compared to
those from other bisulfite sequencing libraries (https://sequencing.qcfail.com/articles/). The
duplication rate, which varies depending on the number of
amplification PCR cycles used, was 15.91–85.93%. Despite the high
duplication rate, the average on-target rate for all 8 samples was
84.24% (Table III).
 | Table IIISummary of mapping of Methyl-Seq
Libraries. |
Table III
Summary of mapping of Methyl-Seq
Libraries.
| No. of reads
(pair) | Mapping
| Duplication
| On-target
|
|---|
| No. of reads
(pair) | Mapping rate
(%) | No. of duplicated
reads (pair) | Duplication rate
(%) | Leftover sequences
(single) | No. of reads
(single) | On-target rate
(%) |
|---|
| CIMP-H | | | | | | | | |
| no. 15 PBC | 9,247,852 | 1,923,159 | 20.8 | 305,967 | 15.91 | 3,234,384 | 2,795,612 | 86.43 |
| no. 15 cancer | 18,617,848 | 5,638,156 | 30.3 | 4,705,597 | 83.46 | 1,865,098 | 1,466,259 | 78.62 |
| no. 25 PBC | 8,738,237 | 1,785,247 | 20.4 | 300,533 | 16.83 | 2,969,426 | 2,583,578 | 87.01 |
| no. 25 cancer | 18,123,262 | 5,882,527 | 32.5 | 4,536,000 | 77.11 | 2,693,030 | 2,270,036 | 84.29 |
| CIMP (-) | | | | | | | | |
| no. 3 PBC | 12,788,054 | 3,681,561 | 28.8 | 1,185,309 | 32.20 | 4,992,504 | 4,249,381 | 85.12 |
| no. 3 cancer | 13,742,323 | 3,015,433 | 21.9 | 2,591,097 | 85.93 | 848,654 | 705,353 | 83.11 |
| no. 23 PBC | 10,010,082 | 2,477,429 | 24.7 | 541,780 | 21.87 | 3,871,296 | 3,335,224 | 86.15 |
| no. 23 cancer | 17,277,985 | 6,543,732 | 37.9 | 4,182,685 | 63.92 | 4,722,094 | 3,926,204 | 83.15 |
Identification of DMCs and DMRs in CIMP-H
and CIMP(-) cases
After alignment of the sequence reads, we calculated
methylation % of each read and used the methylKit program to
analyze global DNA methylation profiles among the samples. For this
analysis, only CpG sites with sequence depth of >10 were used.
The average number of the analyzed CpGs here was 35,000 only
(range, 4,104–103,761), ~1% of CpG sites covered by the SureSelect
method (although the method used would be able to analyze 3.7
million CpGs, due to the small number of sequence reads, we could
analyze ~35,000 CpGs on average). The global methylation profiles
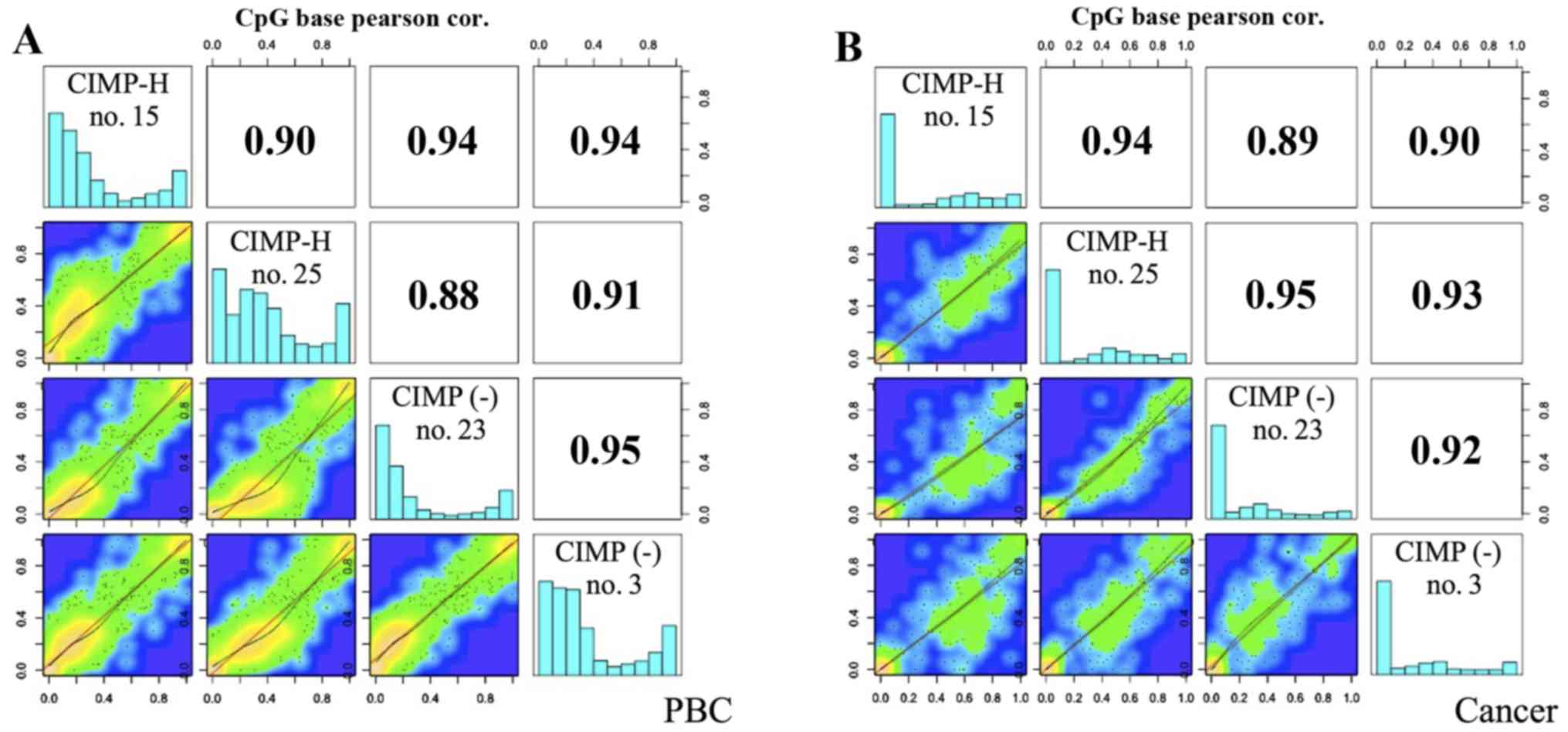
were similar between the PBC samples and the cancer samples,
showing a high positive correlation between CIMP-H and CIMP(-)
cancer (Pearson correlation coefficient: 0.89–0.95). As shown in
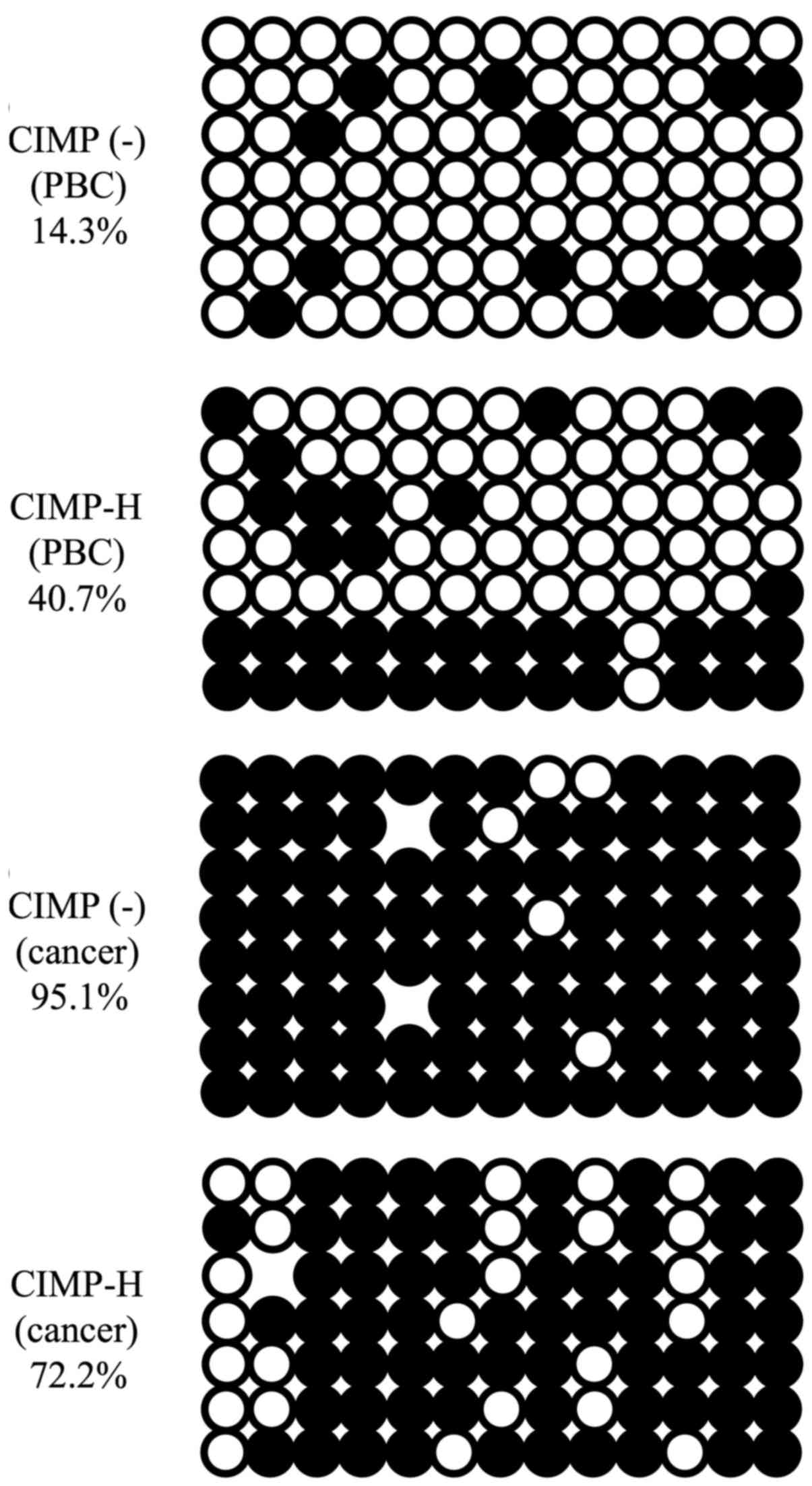
the histograms of Fig. 2, normal
tissues, i.e. PBCs, of both CIMP-H and CIMP(-) cases show bimordal
distribution with peaks at low (<30%) and high (>90%) DNA
methylation. On the contrary, cancer tissues show relatively
hypomethylated status, demonstrating a peak at very low (<10%)
DNA methylation level. This trend is true for both CIMP-H and
CIMP(-), and is consistent with the notion that cancer genome is
generally hypomethylated (25,26).
DMCs and DMRs between the CIMP-H and
CIMP(-) samples were also identified based on a q-value <0.01
and a méthylation difference >25%
Table IV shows
differentially methylated CpG sites among the samples. For example,
in comparison of CIMP-H cancer with CIMP(-) cancer, 9 out of 573
informative CpG sites were found to be significantly
hypermethylated in CIMP-H. There is no hypomethylated CpG site in
CIMP-H relative to CIMP(-) in this comparison. Six out of these 9
DMCs are located in the gene promoter regions. DMR analysis also
showed similar results, demonstrating that the DMRs are always
hypermethylated in the CIMP-H cases (Table V). Notably, when PBCs of the CIMP-H
cases were compared with those of the CIMP(-), 7 hypermethylated
sites were detected in the CIMP-H, of which 4 were in the promoter
regions. In contrast to cancer tissues, 8 out of the 14 DMRs
detected between CIMP-H and CIMP(-) PBCs are hypomethylated in the
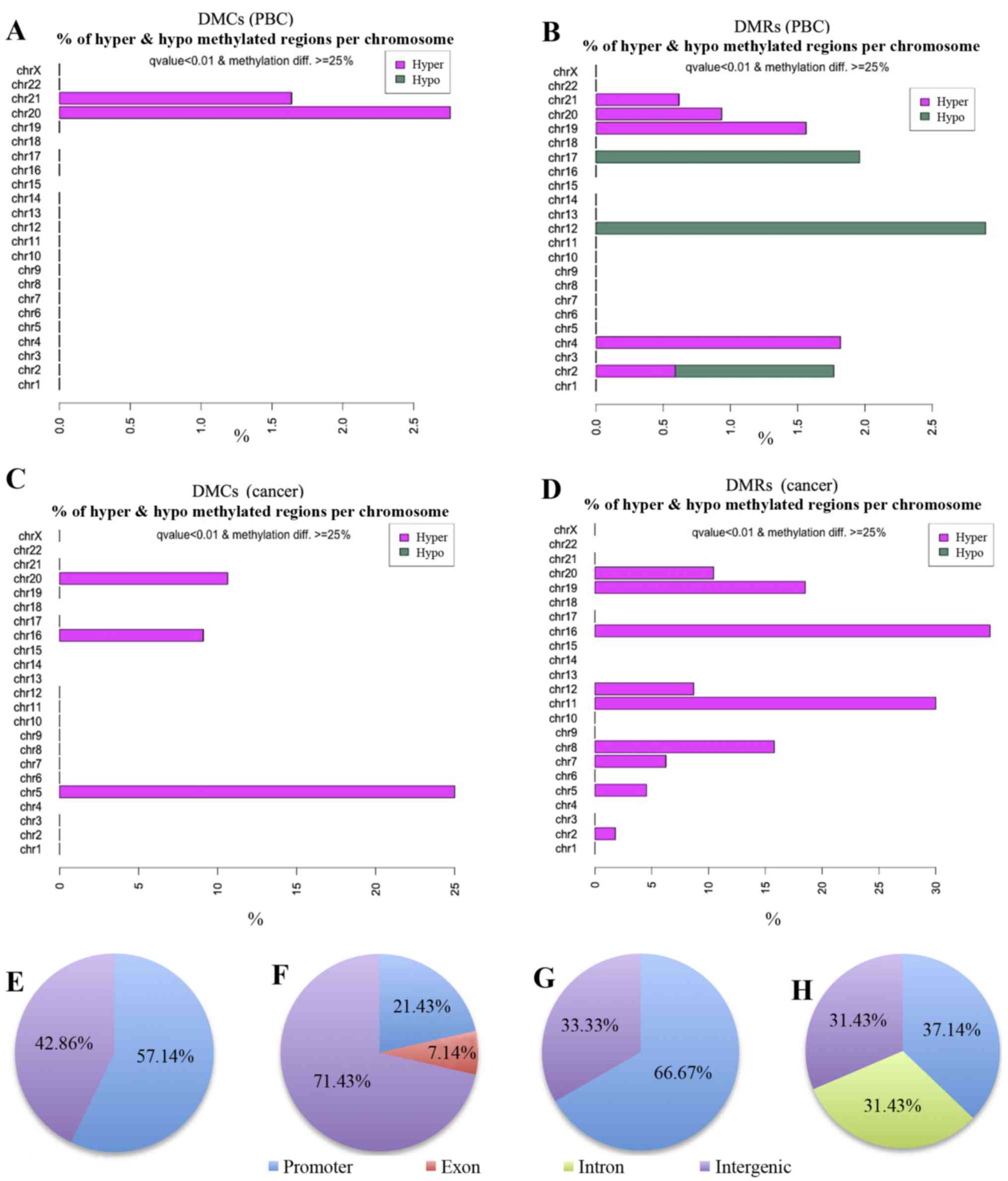
CIMP-H cases (Table V). Fig. 3 shows chromosomal distribution of
the DMCs and DMRs detected by the methylKit. Seven DMCs detected in
the comparison of CIMP-H PBCs with CIMP(-) PBCs are located on
chromosomes 20 and 21.
 | Table IVDetails of DMCs in each
comparison. |
Table IV
Details of DMCs in each
comparison.
| No. of DMC
(meth.diff >1%) | (meth.diff >25%,
q-value <0.01)
|
|---|
| Hypermethylated
CpGs | Hypomethylated
CpGs | Hypermethylated
CpGs in promoter region | Hypomethylated CpGs
in promoter region |
|---|
| CIMP-H PBC vs.
CIMP(-) PBC | 1,218 | 7 | 0 | 4 | 0 |
| CIMP-H cancer vs.
CIMP-H PBC | 745 | 132 | 12 | 67 | 7 |
| CIMP(-) cancer vs.
CIMP(-) PBC | 396 | 31 | 8 | 14 | 2 |
| CIMP-H cancer vs.
CIMP(-) cancer | 573 | 9 | 0 | 6 | 0 |
 | Table VDetails of DMRs in each
comparison |
Table V
Details of DMRs in each
comparison
| No. of DMR
(meth.diff >1%) | (meth.diff >25%,
q-value <0.01)
|
|---|
| Hypermethylated
regions | Hypomethylated
regions | Hypermethylated
regions in promoter region | Hypomethylated
regions in promoter region |
|---|
| CIMP-H PBC vs.
CIMP(-) PBC | 2,427 | 6 | 8 | 3 | 0 |
| CIMP-H cancer vs.
CIMP-H PBC | 269 | 232 | 37 | 114 | 14 |
| CIMP(-) cancer vs.
CIMP(-) PBC | 919 | 51 | 17 | 22 | 5 |
| CIMP-H cancer vs.
CIMP(-) cancer | 1,311 | 35 | 0 | 13 | 0 |
Next, the DMCs and DMRs were classified into
annotated genomic domains such as promoters, introns, exons and
intergenic regions (Fig. 3E–H).
Here the promoter regions are defined as +1000 to −1000 bp from the
transcription start sites (TSS). Fifty-seven percent of the DMCs
(4/7) and 21% of DMRs (3/14) detected in the PBC group comparisons
were found to be located in promoter regions (Fig. 3E and F and Tables IV and V). Since methylation of promoter region
DNA suppresses gene expression, expression of genes with methylated
promoters may be already suppressed in the CIMP-H PBCs. Moreover,
all 4 hypermethylated DMCs and 1 of the 3 hypermethylated DMRs in
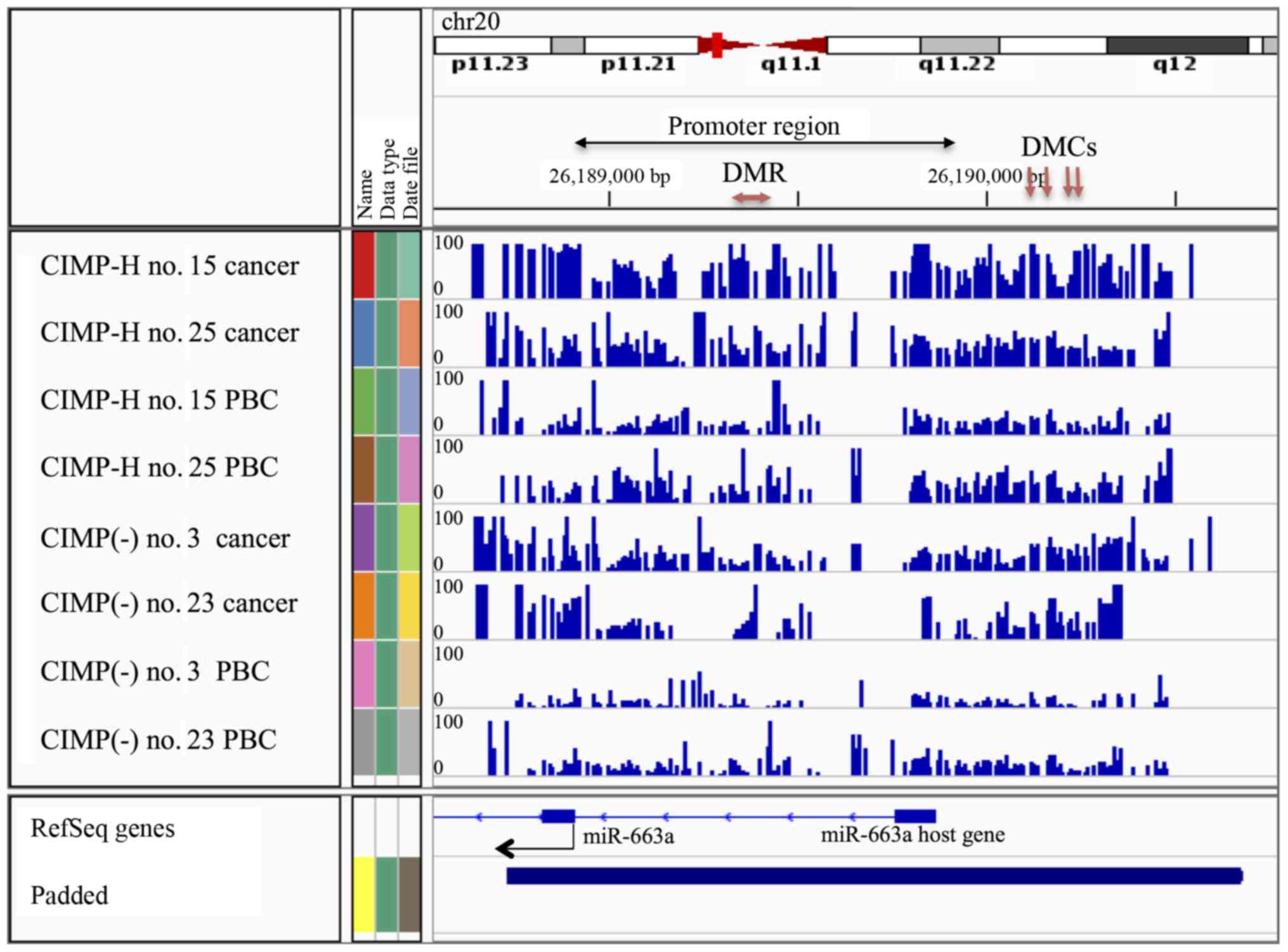
the CIMP-H PBCs were located on the MIR663A host gene of human
chromosome 20 (Fig. 4 and Tables VI and VII). MIR663A host gene harbors the
miR-663a microRNA sequence. There are two other hypermethylated
DMRs with gene annotation of microRNA 3648-1 and MTR-1-P. A
previous genome-wide human epigenome study showed that the miR-663a
region is normally hypomethylated in all the tissues tested
(27). These results suggested
that the identified differential DNA methylation is an aberrant
modification occurring in the CIMP-H patients and may represent a
candidate of epimutation.
 | Table VIAnnotation of hypermethylated DMCs
(CIMP-H PBCs vs. CIMP(-) PBCs). |
Table VI
Annotation of hypermethylated DMCs
(CIMP-H PBCs vs. CIMP(-) PBCs).
| Feature.name | Chromosome | Feature.
strand | Start | End | q-value | Meth.diff | Gene name |
|---|
| NR_040095 | 20p11.1 | – | 26190118 | 26190118 | 0.007784562 | 33.33333 | MIR663A host
gene |
| NR_040095 | 20p11.1 | – | 26190161 | 26190161 | 0.000695757 | 26.59274 | MIR663A host
gene |
| NR_040095 | 20p11.1 | – | 26190239 | 26190239 | 0.000907851 | 26.28239 | MIR663A host
gene |
| NR_040095 | 20p11.1 | – | 26190246 | 26190246 | 0.007784562 | 26.42045 | MIR663A host
gene |
 | Table VIIAnnotation of hypermethylated DMRs
(CIMP-H PBCs vs. CIMP(-) PBCs). |
Table VII
Annotation of hypermethylated DMRs
(CIMP-H PBCs vs. CIMP(-) PBCs).
| Feature.name | Chromosome | Feature.
strand | Start | End | q-value | Meth.diff | Gene name |
|---|
| NR 037421 | 21 | + | 98225451 | 9825500 | 0.000009127 | 26.02800 | microRNA
3648-1 |
| NM_032285 | 19p13.2 | + | 13875071 | 13875120 | 0.005663958 | 25.67164 | MTR-1-P |
| NR_040095 | 20p11.1 | – | 26189381 | 26189430 | 0.000312766 | 25.48611 | MIR663A host
gene |
There were 67 hypermethylated DMCs and 114
hypermethylated DMRs in the CIMP-H cancer group compared to the
CIMP-H PBC group, whereas only 14 hypermethylated DMCs and 22
hypermethylated DMRs in the CIMP(-) cancer group compared to the
CIMP(-) PBC group. The number of hypermethylated DMCs/DMRs in the
CIMP-H group was ~5-fold (4.8-fold for DMCs, 5.3-fold for DMRs)
higher than that in the CIMP(-) group (Tables IV and V). These results suggest that the CIMP-H
cases appear to gain hypermethylation in gene promoters during
endometrial cancer development, and that in general the CIMP-H
cases have elevated DNA methylation in promoter regions compared to
the CIMP(-) cases.
Validation of methylation status in
miR-663a promoter region
As described above, MIR663A host gene that includes
miR-663a promoter region is hypermethylated in the PBCs of the
CIMP-H cases, though this region is known to be hypomethylated in
PBCs of normal individuals. Human miR-663a is a microRNA possibly
involved in tumorigenesis (28,29).
Therefore, aberrant DNA methylation in the miR-663a promoter may be
involved in CIMP-H endometrial cancer formation. We next validated
methylation status of miR-663a promoter region in the CIMP-H and
the CIMP(-) samples by conventional bisulfite sequencing.
Consistent with the genomewide bisulfite sequencing, the CIMP-H
PBCs showed higher level of methylation than the CIMP(-) PBCs.
Furthermore, the correspondig region in the cancer tissues of the
CIMP-H and the CIMP(-) cases demonstrated that this region is
almost completely methylated (Fig.
5).
Expression analysis of miR-663a and its
target genes
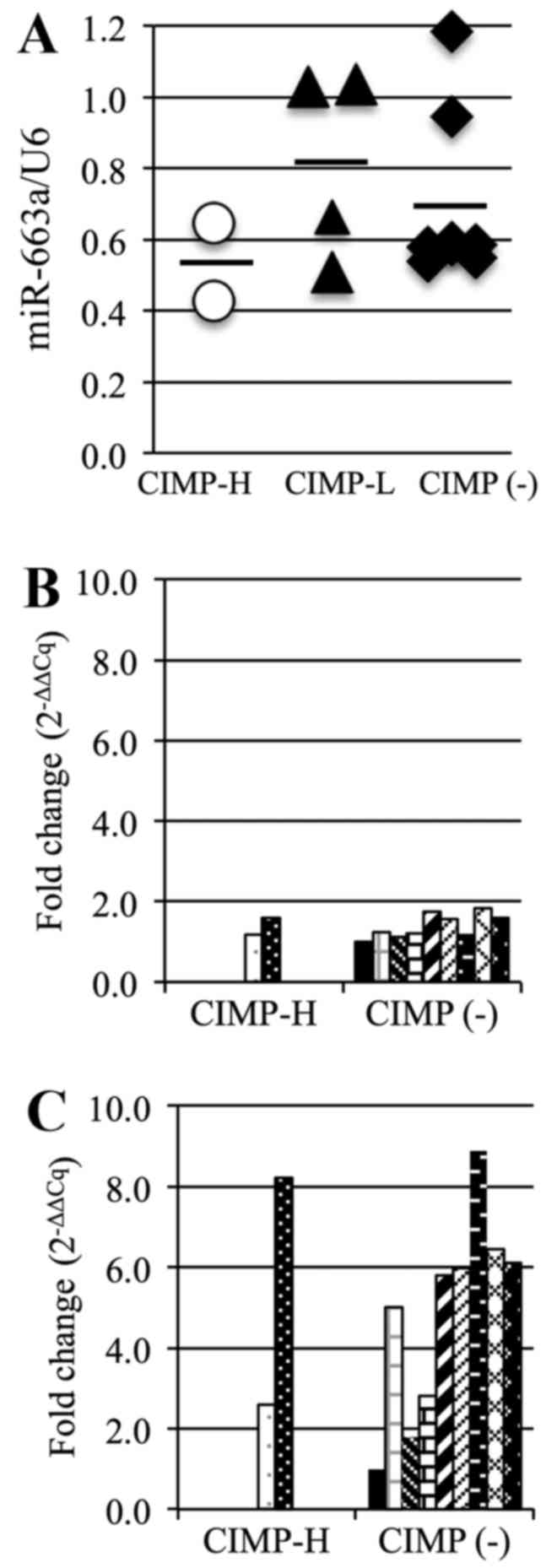
The average miR-663a expression level in the CIMP-H
PBCs (0.54) was lower than that in the other PBCs (CIMP-L, 0.81,
CIMP(-), 0.70; Fig. 6A). This
suggests that miR-663a expression in the CIMP-H PBCs is reduced by
the methylation of the promoter region. We also analyzed expression
of TGF-β, a possible target gene of miR-663a, using RT-qPCR.
However, there was no significant difference in TGF-β mRNA
expression between the CIMP-H and the CIMP(-) PBCs (Fig. 6B), with an average expression level
of 1.4 in each group. However, the average TGF-β expression in
CIMP-H cancer samples (5.4) was higher than that in the CIMP(-)
cancer samples (4.9) (Fig.
6C).
Analysis of DNMT expression in CIMP-H and
CIMP(-) patient samples
TGF-β induces global DNA methylation through
upregulation of DNMTs (30). Thus, we suspected that DNMT
expression may contribute to the development of CIMP-H methylation
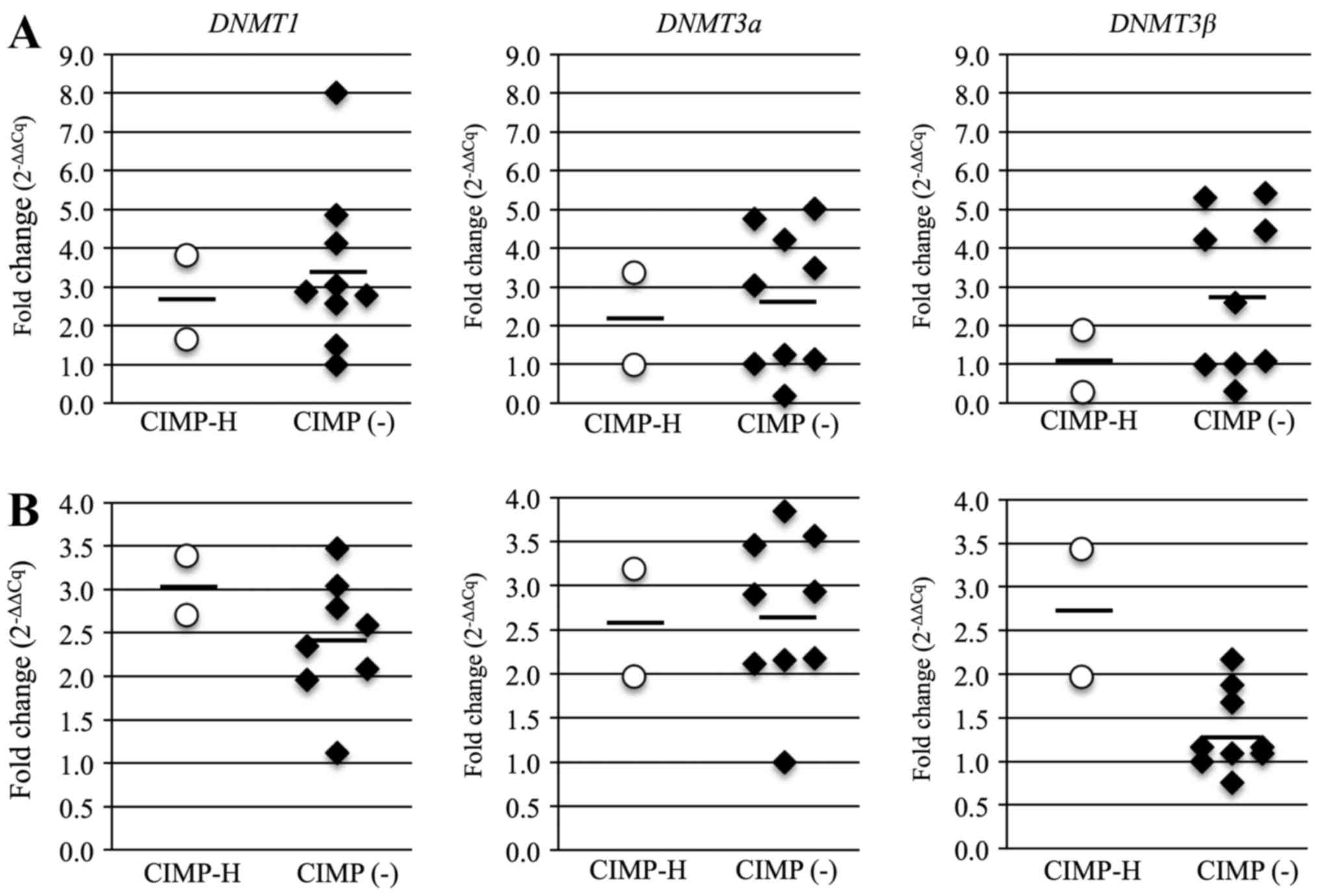
phenotype. The average mRNA expression levels for all three
DNMTs was lower in the CIMP-H PBCs than in the CIMP(-) PBCs,
but the average levels of DNMT1 and DNMT3b in the
CIMP-H cancer samples was higher than that in the CIMP(-) cancer
samples (Fig. 7). The average mRNA
levels in the CIMP-H and the CIMP(-) cases were 2.7 and 3.4 for
DNMT1, 2.1 and 2.7 for DNMT3a, and 1.1 and 2.8 for
DNMT3b in PBCs; and 3.1 and 2.4 for DNMT1, 2.6 and
2.7 for DNMT3a, and 2.7 and 1.3 for DNMT3b in cancer
samples. These results suggest that expression of DNMTs,
especially DNMT3b, are linked to the CIMP-H endometrial
cancer phenotype.
Discussion
Endometrial cancer is known to have a CIMP phenotype
(10,15,31),
similarly to other cancers. There have been several reports on the
relationship of CIMP-positive cancer with genetic mutations and
clinicopathological features (9,11,32),
but the mechanism of carcinogenesis in CIMP-positive cancer is
still unknown. The present study is the first to focus on the cause
of CIMP-positive endometrial cancer and demonstrated for the first
time that aberrant DNA methylation occurs in the miR-663a promoter
region in normal tissue, i.e. PBCs, of patients with CIMP-H
endometrial cancer. The miR-663a promoter was fully methylated in
the cancer tissues of both CIMP-H and CIMP(-) cases examined in
this study. The miR-663a promoter region is known to be
unmethylated in all the tissues of normal individuals (33), and miR-663a is thought to be
involved in the formation of certain cancers. Therefore, it is
possible that aberrant DNA methylation in the miR-663a promoter is
involved in endometrial cancinogenesis.
Methylation levels of miR-663a promoter DNA is
higher in the CIMP-H than in the CIMP(-) cases, suggesting that
aberrant DNA methylation may be associated with the CIMP-H
phenotype, and that it could serve as an epigenetic marker for
endometrial cancer diagnosis or prediction. We do not know at
present that this epimutation in the miR-663a promoter occurs de
novo during development (primary epimutation), or is involved
in a genetic mutation (secondary epimutation). Primary epimutations
are defined as constitutional epimutations that are independent of
genetic mutation. On the other hand, secondary epimutations are
caused by genetic mutation in other loci controlling its epigenetic
modification (34). To address
these issues, it will be important to analyze genetic alterations
of regions adjacent to the miR-663a locus and to perform systematic
survey of the miR-663a epimutation in larger number of patients in
future.
The proportion of CIMP-H, CIMP-L and CIMP(-)
endometrial cancer in our samples were 8.0, 28.0 and 64.0%,
respectively. The frequencies of MLH1, APC and CDH1
methylation were consistent with previous studies (15,35),
but the frequency of CIMP-positive endometrial cancer was found to
be lower in this study than in the previous studies, in which 49 or
75% of the cases were found to be CIMP-H (6,7).
This is probably due to the use of different CIMP markers in each
study. Thus, further investigation is required to identify the best
CIMP markers with higher detection power for CIMP-H phenotype. In
this study, MLH1, APC and CDH1 were used for
definition of the CIMP phenotype because we have shown that these
genes are frequently methylated in endometrial cancer (19), of which MLH1 promoter is
most frequently methylated in endometrial carcinogenesis.
MLH1 and APC methylation is also observed in AEH.
Here we demonstrated that the CIMP-H cases defined by MLH1
and APC had hypermethylated DMCs and DMRs compared to
CIMP(-) endometrial cancer, suggesting usefulness of MLH1
and APC for detection of CIMP-H endometrial cancers.
Genome-wide bisulfite sequencing was performed to
identify DMCs and DMRs in CIMP-H endometrial cancer. Since total
number of sequence reads obtained in this study were low, we could
analyze only ~35,000 CpGs on average. Despite such low number of
analyzed CpGs, we identified aberrant DNA methylation in the
miR-663a promoter of CIMP-H PBCs, which is normally unmethylated.
Therefore, deeper sequencing analysis of the CIMP-H normal tissues
is likely to reveal more aberrant DNA methylation or
epimutation.
DNA methylation status of miR-663a promoter region
in both CIMP-H and CIMP(-) cases was validated by conventional
bisulfite sequencing. Expression analysis of miR-663a and its
target genes revealed that miR-663a expression in the CIMP-H normal
tissue was lower than that in the CIMP(-) normal tissue. Since
peripheral blood cells and endometrium are of the same mesoderm
origin, the DNA methylation status of PBCs is likely to share those
of normal endometrium and other mesoderm-derived tissues.
miRNAs are small non-coding RNAs that regulate
expression of gene products through translational inhibition or
cleavage of target mRNAs. miRNAs have been associated with
oncogenesis and tumor progression in many cancer types (36–40),
and miR-663a has roles in cell proliferation, immunity and cancer,
and acts as a tumor suppressor miRNA in gastric cancer (41). miR-663a is downregulated by
promoter methylation in breast, hepatocellular and other cancers
(42–44). The CpG island located 1 kb upstream
of the pre-miR-663 sequence shows promoter activity (45), and a DMR identified in the
comparison of CIMP-H and CIMP(-) PBC was included in this
region.
TGF-β is a direct target of miR-663a
(46) and has oncogenic activities
and is upregulated in endometrial cancer (47). We found no inverse correlation
between miR-663a and TGF-β expression levels detected by
qRT-PCR in the PBCs. However, miR-663a has been shown to inhibit
TGF-β expression at the post-transcriptional level (29). We could not analyze the TGF-β
protein level because of the limitation in the amounts of cancer
samples in this study. We also analyzed expression of DNMTs
using RT-qPCR. TGF-β induces DNMT expression in cancers
(48,49) and DNMTs are upregulated in
CIMP-positive cancers (50–52).
In particular, the DNMT3b expression level was higher in
CIMP-H than in CIMP(-) endometrial cancer. This suggests that
DNMT upregulation might contribute to the development of
CIMP-positive endometrial cancer.
In summary, this is the first report of aberrant DNA
methylation in the miR-663a promoter region in normal tissue of
patients with CIMP-H endometrial cancer. Both peripheral blood and
endometrium originated from the same germ layer, i.e. mesoderm.
Therefore, miR-663a methylation may be an epimutation candidate in
the development of endometrial cancer. To evaluate whether miR-663a
methylation is a primary or a secondary epimutation, a larger-scale
analysis is required, and there is also a need to show a role of
miR-663a in endometrial cancer using a functional assay. However,
the present study is significant in showing a potential basis for
the development of CIMP-H endometrial cancer, and this finding may
contribute to the prevention or the therapy for CIMP-H endometrial
cancer using miR-663a demethylation.
Abbreviations:
|
PBC
|
peripheral blood cell
|
|
CIMP
|
CpG island methylator phenotype
|
|
CIMP-H, -L, (-)
|
CIMP-high, -low, negative
|
|
MSI
|
microsatellite instability
|
|
PCR
|
polymerase chain reaction
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MSP
|
methylation specific polymerase chain
reaction
|
|
DMC
|
differentially methylated CpG
|
|
DMR
|
differentially methylated region
|
Acknowledgments
The present study was supported by a grant to M.Y.
and K.B. from JSPS KAKENHI Grants-in-Aid for Scientific Research
(C) (grant nos. 15K10727 and 16K11154), and in part by a grant to
K.A. from JSPS KAKENHI Grant-in-Aid for Scientific Research on
Innovative Areas (26112515 and 16H01225).
References
|
1
|
Fiolka R, Zubor P, Janusicova V, Visnovsky
J, Mendelova A, Kajo K, Lasabova Z, Plank L and Danko J: Promoter
hypermethylation of the tumor-suppressor genes RASSF1A, GSTP1 and
CDH1 in endometrial cancer. Oncol Rep. 30:2878–2886.
2013.PubMed/NCBI
|
|
2
|
Guida M, Sanguedolce F, Bufo P, Di Spiezio
Sardo A, Bifulco G, Nappi C and Pannone G: Aberrant DNA
hypermethylation of hMLH-1 and CDKN2A/p16 genes in benign,
premalignant and malignant endometrial lesions. Eur J Gynaecol
Oncol. 30:267–270. 2009.PubMed/NCBI
|
|
3
|
Kanaya T, Kyo S, Maida Y, Yatabe N, Tanaka
M, Nakamura M and Inoue M: Frequent hypermethylation of MLH1
promoter in normal endometrium of patients with endometrial
cancers. Oncogene. 22:2352–2360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crépin M, Dieu MC, Lejeune S, Escande F,
Boidin D, Porchet N, Morin G, Manouvrier S, Mathieu M and Buisine
MP: Evidence of constitutional MLH1 epimutation associated to
transgenerational inheritance of cancer susceptibility. Hum Mutat.
33:180–188. 2012. View Article : Google Scholar
|
|
5
|
Ward RL, Dobbins T, Lindor NM, Rapkins RW
and Hitchins MP: Identification of constitutional MLH1 epimutations
and promoter variants in colorectal cancer patients from the Colon
Cancer Family Registry. Genet Med. 15:25–35. 2013. View Article : Google Scholar
|
|
6
|
Cini G, Carnevali I, Quaia M, Chiaravalli
AM, Sala P, Giacomini E, Maestro R, Tibiletti MG and Viel A:
Concomitant mutation and epimutation of the MLH1 gene in a Lynch
syndrome family. Carcinogenesis. 36:452–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bischoff J, Ignatov A, Semczuk A,
Schwarzenau C, Ignatov T, Krebs T, Küster D, Przadka-Rabaniuk D,
Roessner A, Costa SD, et al: hMLH1 promoter hypermethylation and
MSI status in human endometrial carcinomas with and without
metastases. Clin Exp Metastasis. 29:889–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romero-Pérez L, López-García MÁ,
Díaz-Martín J, Biscuola M, Castilla MÁ, Tafe LJ, Garg K, Oliva E,
Matias-Guiu X, Soslow RA, et al: ZEB1 overexpression associated
with E-cadherin and microRNA-200 downregulation is characteristic
of undifferentiated endometrial carcinoma. Mod Pathol.
26:1514–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SY, Kook MC, Kim YW, Cho NY, Jung N,
Kwon HJ, Kim TY and Kang GH: CpG island hypermethylator phenotype
in gastric carcinoma and its clinicopathological features. Virchows
Arch. 457:415–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang QY, Yi DQ, Zhou L, Zhang DH and Zhou
TM: Status and significance of CpG island methylator phenotype in
endometrial cancer. Gynecol Obstet Invest. 72:183–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weisenberger DJ, Levine AJ, Long TI,
Buchanan DD, Walters R, Clendenning M, Rosty C, Joshi AD, Stern MC,
Le Marchand L, et al for the Colon Cancer Family Registry:
Association of the colorectal CpG island methylator phenotype with
molecular features, risk factors, and family history. Cancer
Epidemiol Biomarkers Prev. 24:512–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toyota M, Ahuja N, Ohe-Toyota M, Herman
JG, Baylin SB and Issa JP: CpG island methylator phenotype in
colorectal cancer. Proc Natl Acad Sci USA. 96:8681–8686. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samowitz WS, Albertsen H, Herrick J, Levin
TR, Sweeney C, Murtaugh MA, Wolff RK and Slattery ML: Evaluation of
a large, population-based sample supports a CpG island methylator
phenotype in colon cancer. Gastroenterology. 129:837–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weisenberger DJ, Siegmund KD, Campan M,
Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D,
Buchanan D, et al: CpG island methylator phenotype underlies
sporadic microsatellite instability and is tightly associated with
BRAF mutation in colorectal cancer. Nat Genet. 38:787–793. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitcomb BP, Mutch DG, Herzog TJ, Rader
JS, Gibb RK and Goodfellow PJ: Frequent HOXA11 and THBS2 promoter
methylation, and a methylator phenotype in endometrial
adenocarcinoma. Clin Cancer Res. 9:2277–2287. 2003.PubMed/NCBI
|
|
16
|
Kawaguchi M, Yanokura M, Banno K,
Kobayashi Y, Kuwabara Y, Kobayashi M, Nomura H, Hirasawa A, Susumu
N and Aoki D: Analysis of a correlation between the BRAF V600E
mutation and abnormal DNA mismatch repair in patients with sporadic
endometrial cancer. Int J Oncol. 34:1541–1547. 2009.PubMed/NCBI
|
|
17
|
Peterson LM, Kipp BR, Halling KC, Kerr SE,
Smith DI, Distad TJ, Clayton AC and Medeiros F: Molecular
characterization of endometrial cancer: a correlative study
assessing microsatellite instability, MLH1 hypermethylation, DNA
mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and
braf mutation analysis. Int J Gynecol Pathol. 31:195–205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banno K, Kisu I, Yanokura M, Masuda K,
Kobayashi Y, Ueki A, Tsuji K, Yamagami W, Nomura H, Susumu N, et
al: Endometrial cancer and hypermethylation: Regulation of DNA and
MicroRNA by epigenetics. Biochem Res Int. 2012:7382742012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banno K, Yanokura M, Susumu N, Kawaguchi
M, Hirao N, Hirasawa A, Tsukazaki K and Aoki D: Relationship of the
aberrant DNA hypermethylation of cancer-related genes with
carcinogenesis of endometrial cancer. Oncol Rep. 16:1189–1196.
2006.PubMed/NCBI
|
|
20
|
Yanokura M, Banno K, Kawaguchi M, Hirao N,
Hirasawa A, Susumu N, Tsukazaki K and Aoki D: Relationship of
aberrant DNA hypermethylation of CHFR with sensitivity to taxanes
in endometrial cancer. Oncol Rep. 17:41–48. 2007.
|
|
21
|
Yanokura M, Banno K, Susumu N, Kawaguchi
M, Kuwabara Y, Tsukazaki K and Aoki D: Hypermethylation in the p16
promoter region in the carcinogenesis of endometrial cancer in
Japanese patients. Anticancer Res. 26(2A): 851–856. 2006.PubMed/NCBI
|
|
22
|
Akalin A, Kormaksson M, Li S,
Garrett-Bakelman FE, Figueroa ME, Melnick A and Mason CE:
methylKit: A comprehensive R package for the analysis of
genome-wide DNA methylation profiles. Genome Biol. 13:R872012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avraham A, Cho SS, Uhlmann R, Polak ML,
Sandbank J, Karni T, Pappo I, Halperin R, Vaknin Z, Sella A, et al:
Tissue specific DNA methylation in normal human breast epithelium
and in breast cancer. PLoS One. 9:e918052014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miura F, Enomoto Y, Dairiki R and Ito T:
Amplification-free whole-genome bisulfite sequencing by
post-bisulfite adaptor tagging. Nucleic Acids Res. 40:e1362012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feinberg AP and Vogelstein B:
Hypomethylation distinguishes genes of some human cancers from
their normal counterparts. Nature. 301:89–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alvarez H, Opalinska J, Zhou L, Sohal D,
Fazzari MJ, Yu Y, Montagna C, Montgomery EA, Canto M, Dunbar KB, et
al: Widespread hypomethylation occurs early and synergizes with
gene amplification during esophageal carcinogenesis. PLoS Genet.
7:e10013562011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kundaje A, Meuleman W, Ernst J, Bilenky M,
Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ,
et al: Roadmap Epigenomics Consortium: Integrative analysis of 111
reference human epigenomes. Nature. 518:317–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yi C, Wang Q, Wang L, Huang Y, Li L, Liu
L, Zhou X, Xie G, Kang T, Wang H, et al: MiR-663, a microRNA
targeting p21WAF1/CIP1, promotes the proliferation and
tumorigenesis of nasopharyngeal carcinoma. Oncogene. 31:4421–4433.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Cheng Q, Chen Z, Peng R, Chen R, Ma
Z, Wan X, Liu J, Meng M, Peng Z, et al: MicroRNA-663 inhibits the
proliferation, migration and invasion of glioblastoma cells via
targeting TGF-β1. Oncol Rep. 35:1125–1134. 2016.PubMed/NCBI
|
|
30
|
Cardenas H, Vieth E, Lee J, Segar M, Liu
Y, Nephew KP and Matei D: TGF-β induces global changes in DNA
methylation during the epithelial-to-mesenchymal transition in
ovarian cancer cells. Epigenetics. 9:1461–1472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suga Y, Sugai T, Uesugi N, Kawasaki T,
Fukagawa T, Yamamoto E, Ishida K, Suzuki H and Sugiyama T:
Molecular analysis of isolated tumor glands from endometrial
endometrioid adenocarcinomas. Pathol Int. 65:240–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Phipps AI, Limburg PJ, Baron JA,
Burnett-Hartman AN, Weisenberger DJ, Laird PW, Sinicrope FA, Rosty
C, Buchanan DD, Potter JD, et al: Association between molecular
subtypes of colorectal cancer and patient survival.
Gastroenterology. 148:77–87.e2. 2015. View Article : Google Scholar
|
|
33
|
Lister R, Pelizzola M, Dowen RH, Hawkins
RD, Hon G, TontiFilippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al:
Human DNA methylomes at base resolution show widespread epigenomic
differences. Nature. 462:315–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hitchins MP: Constitutional epimutation as
a mechanism for cancer causality and heritability? Nat Rev Cancer.
15:625–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Muraki Y, Banno K, Yanokura M, Kobayashi
Y, Kawaguchi M, Nomura H, Hirasawa A, Susumu N and Aoki D:
Epigenetic DNA hypermethylation: Clinical applications in
endometrial cancer (Review). Oncol Rep. 22:967–972. 2009.PubMed/NCBI
|
|
36
|
Chen QY, Jiao DM, Wang J, Hu H, Tang X,
Chen J, Mou H and Lu W: miR-206 regulates cisplatin resistance and
EMT in human lung adenocarcinoma cells partly by targeting MET.
Oncotarget. 7:24510–24526. 2016.PubMed/NCBI
|
|
37
|
Feng S, Zhu X, Fan B, Xie D, Li T and
Zhang X: miR-p19a-3p targets PMEPA1 and induces prostate cancer
cell proliferation, migration and invasion. Mol Med Rep.
13:4030–4038. 2016.PubMed/NCBI
|
|
38
|
Ge X, Liu X, Lin F, Li P, Liu K, Geng R,
Dai C, Lin Y, Tang W, Wu Z, et al: MicroRNA-421 regulated by HIF-1α
promotes metastasis, inhibits apoptosis, and induces cisplatin
resistance by targeting E-cadherin and caspase-3 in gastric cancer.
Oncotarget. 7:24466–24482. 2016.PubMed/NCBI
|
|
39
|
Ma M, He M, Jiang Q, Yan Y, Guan S, Zhang
J, Yu Z, Chen Q, Sun M, Yao W, et al: MiR-487a promotes
TGF-β1-induced EMT, the migration and invasion of breast bancer
bells by directly targeting MAGI2. Int J Biol Sci. 12:397–408.
2016. View Article : Google Scholar :
|
|
40
|
Zhang L, Wang W, Li X, He S, Yao J, Wang
X, Zhang D and Sun X: MicroRNA-155 promotes tumor growth of human
hepatocellular carcinoma by targeting ARID2. Int J Oncol.
48:2425–2434. 2016.PubMed/NCBI
|
|
41
|
Pan J, Hu H, Zhou Z, Sun L, Peng L, Yu L,
Sun L, Liu J, Yang Z and Ran Y: Tumor-suppressive miR-663 gene
induces mitotic catastrophe growth arrest in human gastric cancer
cells. Oncol Rep. 24:105–112. 2010.PubMed/NCBI
|
|
42
|
Lehmann U, Hasemeier B, Christgen M,
Müller M, Römermann D, Länger F and Kreipe H: Epigenetic
inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J
Pathol. 214:17–24. 2008. View Article : Google Scholar
|
|
43
|
Potapova A, Albat C, Hasemeier B,
Haeussler K, Lamprecht S, Suerbaum S, Kreipe H and Lehmann U:
Systematic crossvalidation of 454 sequencing and pyrosequencing for
the exact quantification of DNA methylation patterns with single
CpG resolution. BMC Biotechnol. 11:62011. View Article : Google Scholar
|
|
44
|
Yan-Fang T, Jian N, Jun L, Na W, Pei-Fang
X, Wen-Li Z, Dong W, Li P, Jian W, Xing F, et al: The promoter of
miR-663 is hypermethylated in Chinese pediatric acute myeloid
leukemia (AML). BMC Med Genet. 14:742013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Wang LL, Li YH, Gao XN, Liu Y and
Yu L: Effect of CpG island methylation on microRNA expression in
the k-562 cell line. Biochem Genet. 50:122–134. 2012. View Article : Google Scholar
|
|
46
|
Hong Q, Yu S, Geng X, Duan L, Zheng W, Fan
M, Chen X and Wu D: High concentrations of uric acid inhibit
endothelial cell migration via miR-663 which regulates phosphatase
and tensin homolog by targeting transforming growth Factor-beta1.
Microcirculation. 22:306–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Narkiewicz J, Lapinska-Szumczyk S,
Zurawa-Janicka D, Skorko-Glonek J, Emerich J and Lipinska B:
Expression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in
primary endometrial cancer. Oncol Rep. 21:1529–1537.
2009.PubMed/NCBI
|
|
48
|
Kogure T, Kondo Y, Kakazu E, Ninomiya M,
Kimura O and Shimosegawa T: Involvement of miRNA-29a in epigenetic
regulation of transforming growth factor-beta-induced
epithelialmesenchymal transition in hepatocellular carcinoma.
Hepatol Res. 44:907–919. 2014. View Article : Google Scholar
|
|
49
|
Zhang Q, Chen L, Helfand BT, Jang TL,
Sharma V, Kozlowski J, Kuzel TM, Zhu LJ, Yang XJ, Javonovic B, et
al: TGF-β regulates DNA methyltransferase expression in prostate
cancer, correlates with aggressive capabilities, and predicts
disease recurrence. PLoS One. 6:e251682011. View Article : Google Scholar
|
|
50
|
Kanai Y, Ushijima S, Kondo Y, Nakanishi Y
and Hirohashi S: DNA methyltransferase expression and DNA
méthylation of CPG islands and peri-centromeric satellite regions
in human colorectal and stomach cancers. Int J Cancer. 91:205–212.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roll JD, Rivenbark AG, Jones WD and
Coleman WB: DNMT3b overexpression contributes to a hypermethylator
phenotype in human breast cancer cell lines. Mol Cancer. 7:152008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nosho K, Shima K, Irahara N, Kure S, Baba
Y, Kirkner GJ, Chen L, Gokhale S, Hazra A, Spiegelman D, et al:
DNMT3B expression might contribute to CpG island methylator
phenotype in colorectal cancer. Clin Cancer Res. 15:3663–3671.
2009. View Article : Google Scholar : PubMed/NCBI
|