1. Introduction
Gastric cancer remains third in ranking in cancer
death worldwide, although its overall incidence is declining in
recent years (1). In the past
decades, studies aimed at Helicobacter pylori infection
(2,3), hereditary susceptibility (4) and environmental factors (5) have made a great breakthrough in
investigating its precise pathogenesis. Recently, application of
various '-omics' technologies opened a new field to investigate the
mechanisms behind this disease.
With the emergency of metabolomics, major progress
has been made in the understanding of the relationship between
metabolic regulation and cancer. Warburg, in fact, showed a
characteristic metabolic pattern of tumors in the 1920s, that is,
tumor cells consume a large amount of glucose for glycolysis even
under the condition of sufficient oxygen (Warburg effect) (6). Extensive research also indicates that
metabolic reprogramming is one of the hallmarks of cancer (7), and intricately linked to oncogenesis
(8–10) and cancer immune escape (11–13).
On the other hand, study methods combined conventional oncology
research and metabolomics are more likely to provide deeper
insights in this field. The procedure of these methods is
illustrated in Fig. 1, and more
detailed information can be found in literature (14–16).
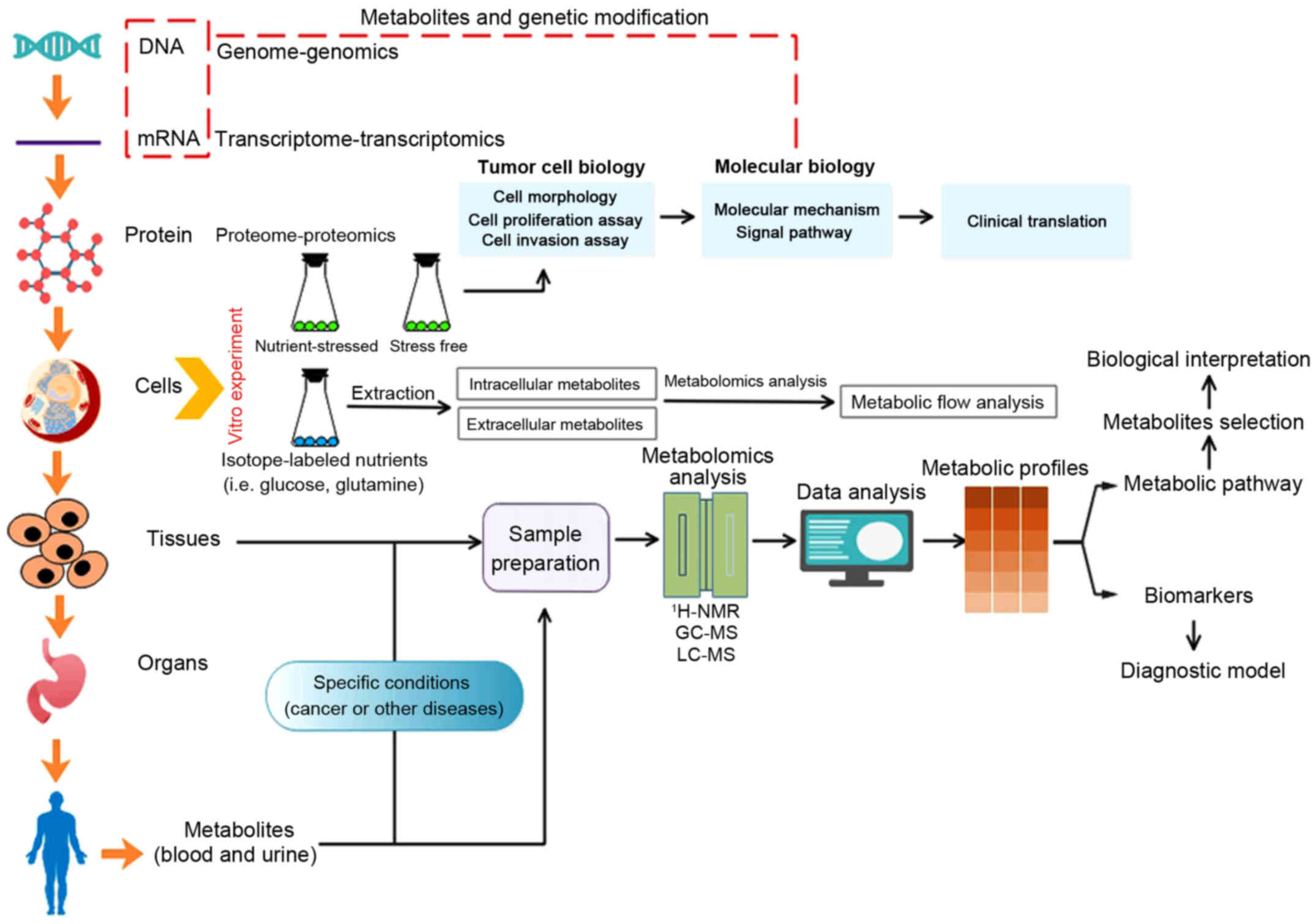 | Figure 1Metabolism and metabolomics in cancer
research. Concerning tumor cell lines cultured in vitro,
either conventional cell biology research or isotopic tracer
experiment is available. Tumor cytobiological methods (cell
morphology, cell proliferation assay and cell invasion assay) can
be utilized to assess cytobiological behaviors under specific
nutrient-stressed or stress-free condition, and further
investigations targeted at precise mechanism and significance can
be confirmed via molecular biology techniques. With regard to
isotopic tracer experiment, the flow of nutrients and metabolites
can be identified with isotopic tracer, then the significance of
specific nutrients or metabolites and its potential divergent fates
toward meeting the demands of either energetic utilization or
synthesizing macromolecules in cancer cells can be identified
(14). Metabolites that are
different between tumor groups and control groups are able to be
detected through metabolomics analysis (such as 1H-NMR,
1hydrogen-nuclear magnetic resonance; LC-MS, liquid
chromatography-mass spectrometry; GC-MS, gas chromatography-mass
spectrometry) and data analysis, metabolic biomarkers or metabolic
pathway that is specific to certain cancers were discovered to
benefit cancer research (15,16).
Of note, combination of genomics, transcriptomics and proteomics
plus metabolomics can further give us comprehensive understanding
of cancers toward systematic biology. |
Several excellent reviews have been published on
metabolomics application in different diseases (17–19)
especially cancer research (20–23).
Hence, this report presents fresh and profound insights into
metabolic changes in gastric cancer and possible mechanism behind
these alterations is further discussed. Then, we focus on some
studies including our data targeted on biomarkers involving
diagnosis, metastasis and prognosis, and treatment in this disease.
Finally, future directions are presented.
2. Metabolic alteration in gastric
cancer
Up to now, several studies aimed at identifiable
metabolic changes in macroenvironment-blood (24–29)
(Table I) and urine (30–34)
(Table II) or
microenvironment-carcinoma tissues (35–41)
(Table III) and gastric juice
(42–44) (Table
IV) have been done to map globally metabolic profiles and
interpret its possible mechanism in the process of gastric
carcinogenesis. Typical changes in metabolites of this disease are
illustrated in Fig. 2.
 | Figure 2Metabolic regulation in gastric
cancer. Altered metabolites in gastric can be categorized into four
main biomolecules: carbohydrates, amino acids, lipids and nucleic
acids. Activated glycolysis and impaired aerobic respiration shape
the altered glucose metabolism in this disease. For amino acid
metabolism, various amino acids (serine, valine, phenylalanine,
tryptophan, glycine, and proline) and some primary derivatives
(such as kynurenine, kynurenic acid, anthranilic acid and nicotinic
acid) are significantly higher in tissue specimens and gastric
content, but decreased concentration is observed in blood samples.
Of note, glutamine is also the most greatly depleted. Increased
rate of lipogenesis, upregulation of fatty acid β-oxidation and
upregulated oxidative degradation are the typical characteristics
of lipid metabolism in this disease. Accumulation of the end
products of nucleotide catabolism is characterized by the higher
levels of uric acid. Moreover, there is correlation between these
four metabolisms. For instance, glycine, asparagine and glutamine
are used as building blocks of purines. |
 | Table IMetabolites in blood samples between
cancer groups and non-cancer groups. |
Table I
Metabolites in blood samples between
cancer groups and non-cancer groups.
| Year | Patients/animal
models | Samples | Sample size | Method | Major findings | Ref. |
|---|
| 2011 | Patients | Plasma | Healthy people
(n=985)
Gastric cancer (n=199) | HPLC-ESI-MS | i) Concentration of
amino acids was significantly decreased in plasma of gastric cancer
patients and this change occurs in early stage regardless of the
subsequent progression and poor nutrition
ii) Plasma-free amino acids have shared alteration among gastric
cancer, lung cancer, colorectal cancer, breast cancer and prostate
cancer, but specific profiles in gastric cancer were also
detected | (24) |
| 2011 | Patients | Plasma | Chronic superficial
gastritis (n=19)
Chronic atrophic gastritis (n=10)
Intestinal metaplasia (n=10)
Dysplasia (n=15)
Gastric cancer (n=22) | GC-TOFMS | i) 15 metabolites
(increase, glutamate, asparagine, ornithine, pyroglutamate,
2-hydroxybutyrate, azelaic acid, 11-eicosenoic acid,
1-monohexadecanoylglycerol-γ-tocopherol, urate; decrease:
creatinine, threonate) were different between chronic superficial
gastritis and gastric cancer group
ii) Metabolic phenotype of gastric cancer was greatly similar to
intestinal metaplasia | (25) |
| 2012 | Patients | Serum | Healthy people
(n=30)
Gastric cancer (n=30) | GC-MS | i) 18 metabolites
were different between gastric cancer and healthy control group,
including fumaric acid, glutamine, valine, sarcosine,
9,12-octadecadienoic acid, 9-octadecenoic acid,
trans-13-octadecenoic acid, nonahexacontanoic, cholesta-3,5-diene,
cholesterol pentafluoropionate, cholesterol, Cholest-5-en-3-ol,
2-O-mesyl arabinose, hexadecanenitrile, benzeneacetonitrile,
2-amino-4-hydroxy-pteridinone, 1,2,4-benzenetricarboxylic acid and
hexanedioic acid
ii) Valine showed the greatest fold change and hexanedioic acid was
the most depleted | (26) |
| 2012 | Patients | Serum | Healthy people
(n=12)
Gastric cancer (n=11) | GC-MS | i) Pyruvic acid,
3-hydroxypropionic acid, 3-hydroxyisobutyric acid, octanoic acid,
phosphoric acid significantly changed in gastric cancer
patients
ii) Levels of 3-hydroxypropionic acid and pyruvic acid could
discriminate gastric cancer from esophageal or colorectal
cancer | (27) |
| 2012 | Patients | Plasma | Chronic superficial
gastritis (n=20)
Gastric cancer (n=17)
Post-operation of gastric cancer (n=15) | GC-TOFMS | i) 15
discriminatory metabolites (increase, β-hydroxybutyrate,
β-D-methylglucopyranoside, heptanoic acid; decrease, succinate,
malate, fumarate, citrate, serine, glycine, cysteine, and
S-methyl-cysteine, docosahexaenoic acid, inositol-phosphate,
octadecenoic acid, and 9-(Z)-hexadecenoic acid) were identified
between gastric cancer and chronic superficial gastritis
group
ii) Surgical removal of the cancer tissues could change levels of
many metabolites that characterized the metabolic phenotype of the
cancer group | (28) |
| 2016 | Patients | Serum | Chronic superficial
gastritis (n=17)
Gastric cancer (n=32) | LC-MS | Gastric cancer
displays upregulated kynurenine pathway of tryptophan metabolism:
increase, indole-3-lactic acid, anthranilic acid, kynurenic acid;
decrease, kynurenine, 3-indoxyl-sulfate | (29) |
 | Table IIMetabolites in urine samples between
cancer groups and non-cancer groups. |
Table II
Metabolites in urine samples between
cancer groups and non-cancer groups.
| Year | Patients/animal
models | Sample size | Method | Major findings | Ref |
|---|
| 2011 | SCID mice (male)
SCG-7901 cell line | Gastric cancer
(n=16) (metastasis group (=8 and non-metastasis (=8)
Control group (n=8) | GC-MS | i) 10 metabolites
were different between cancer group (metastasis and non-metastasis)
and control group: lactic acid, malic acid, citric acid, glycerol,
hexadecanoic acid, pyrimidine, uric acid, butanoic acid, propanoic,
butanedioic acid
ii) 7 metabolites were characteristic between metastasis and
non-metastasis groups: alanine, L-proline, glycerol, butanoic acid,
butanedioic acid, L-threonic acid, myo-inositol. iii) Changes in
lactic acid and butanoic acid showed diagnostic value | (30) |
| 2014 | Patients | Training
set/validation set
Gastric cancer (n=50/23)
Healthy people (n=50/31) |
1H-NMR | i) Altered
metabolites in urine samples of gastric cancer are mainly related
to amino acids and lipid metabolism; levels of
4-hydroxyphenylacetate, alamine, phenylacetylglycine, manmitol,
glycolate and arginine are related to T stage of gastric
cancer
ii) Hypoxanthine is able to predict a recovery trend in
postoperative groups of gastric cancer | (31) |
| 2015 | Patients | Gastric cancer
(n=13)
Healthy people (n=9) | LC-MS | 16 metabolites were
differently expressed between cancer and healthy group: succinic
acid, malic acid, alanine, glycine, L-proline, hexadecanoic acid,
pyrimidine, uric acid, glycocholic acid, hippurate, urea,
4-deoxythreonic acid, phenylacetylglycine, taurine,
2-oxoglutarate | (32) |
| 2016 | Patients | Gastric cancer
(n=43)
Healthy people (n=40)
Barrett's esophagus (n=40) |
1H-NMR | i) Levels of
sucrose, dimethylamine, 1-methylnicotinamide, 2-furoylgylycine,
N-acetyl-serotonin, trans-aconitate, formate and serotonin greatly
altered in urine samples of gastric cancer
ii) Alanine, 2-hydroxyisobutyrate and 3-indoxylsulfate produced a
discriminatory model regarding discriminating cancer and control
group | (33) |
| 2016 | Patients | Gastric cancer
(n=199)
Healthy people (n=87) | GC-MS | i) 17 metabolites
are largely different between cancer and control groups in training
set: glycine, valine, isoleucine, serine, threonine, proline,
methionine, tyrosine, tryptophan, ethyl 2-methylacetoacetate,
levulinic acid, p-cresol, benzylmalonic acid, 4-hydroxybenzoic
acid, hippuric acid, benzil, alamine
ii) 14 of them show diagnostic value that is better than classic
blood biomarkers on validation set, most of which were related to
amino acid metabolism
iii) Proline, p-cresol and 4-hydroxybenzoic acid produce
outcome-prediction value by survival analysis | (34) |
 | Table IIIMetabolites in tissue samples between
cancer groups and non-cancer groups. |
Table III
Metabolites in tissue samples between
cancer groups and non-cancer groups.
| Year | Patients/animal
models | Sample size | Method | Major findings | Ref. |
|---|
| 2009 | Patients | 12 pairs of matched
tumor and normal gastric tissues | CE-TOFMS | i) Extremely low
glucose, high lactate and glycolytic intermediate concentrations
were found in both colon and stomach tumor tissues
ii) Significant accumulation of all amino acids except glutamine in
the tumor tissues | (35) |
| 2010 | Male SCID
mice
SCG-7901 cell line | Cancer
group
(Non-metastasis 8 Metastasis 8)
Control group 6 | GC-MS | i) 29 metabolites
were differently expressed between metastasis and non-metastasis
group: glucose, succinate, malicacid, lactate, alanine, glycine,
valine, leucine, dimethylglycine, isoleucine, propanamide,
butanedioic, proline, methionnine, serine, threonine, asparagine,
glutamine, phosphoserine, glutamate, lysine, arginine, docosanoic,
octadecanoic, pyrimidine, hypoxanthine, inositol, propanedioc,
pyrrolidine
ii) Serine and proline metabolisms were highlighted in metastatic
group | (36) |
| 2010 | Patients | 18 pairs of matched
tumor and normal gastric tissues | GC-MS | i) L-glutamine,
phosphoserine, L-valine, L-isoleucine, serine, heptanedioic acid,
propanoic acid, phenanthrenol, butanetriol, acetamid, butenoic
acid, oxazolethione, naphthalene, L-altrose, L-mannofuranose,
galactofuranoside, myo-inositol, D-ribofuranose were detected
differently between the malignant tissues and the adjacent
non-malignant tissues of gastric mucosa
ii) 5 of them were detected differently between the non-invasive
tumors and the invasive tumors: higher levels of L-cysteine,
L-tyrosine, hypoxanthine and lower levels of phenanthrenol,
butanoic acid in the invasive group | (37) |
| 2010 | Patients | 65 pairs of matched
gastric cardiac cancer and adjacent normal tissues | GC-TOFMS | i) Dysregulation of
pyruvic acid efflux was an important glucose metabolic signature in
the development of gastric cardiac cancer
ii) Transition from glycolysis to the Krebs cycle had an inhibitory
effect on GCC progression, which could be served as a potential
therapeutic target for gastric cardiac cancer | (38) |
| 2011 | Patients | 30 pairs of matched
tumor and normal gastric tissues | GC-MS | i) 15 differential
metabolites were identified: α-ketoglutaric acid, fumaric acid,
valric acid, 9-hexadecennoic acid, 3-hydroxybutanoic acid,
hexadecanoic acid, octadecanoic acid, cis-vaccenic acid,
arachidonic acid, 1-phenanthrene-carboxylic acid, 9-octadecenamide,
squalene, xylonic acid, benzenepropanoic acid
ii) Current models could not discriminate normal mucosa and
different pathological stages of GC tissues based on their
identified metabolic profiles | (39) |
| 2012 | Patients | Gastric cancer
(n=17)
Chronic superficial gastritis (n=20) | GC-TOFMS | Discriminating
metabolites associated with glucose, amino acids, lipid and
nucleotide metabolism were detected between two groups: increase,
citrate, malate, fumarate, succinate), cysteine, 2-aminoadipate,
9-(Z)-hexadecenoic acid, docosahexaenoic acid, β-hydroxybutyrate,
uracil, monomethylphosphate; decrease, glucose, maltose, ribose,
β-D-methylglucopyranoside, fructose-6-phosphate, inositol and
ribitol, glyceric acid-2,3-diphosphate, nonesterified cholesterol,
uridine | (28)
(31) |
| 2014 | Patients | 30 pairs of matched
tumor and normal gastric tissues | HR-MAS-NMR | Lipid metabolites
were significantly lower, while some amino acids (such as
isoleucine, glutamate, leucine, valine, alanine, lysine and
phenylalanine), taurine and lactate were significantly higher in
tumor tissues | |
 | Table IVMetabolites in gastric juice between
cancer groups and non-cancer groups. |
Table IV
Metabolites in gastric juice between
cancer groups and non-cancer groups.
| Year | Patients/animal
models | Sample size | Method | Major findings | Ref. |
|---|
| 2011 | Patients | Benign gastric
diseases (n=68)
Gastric malignancies (n=33) | HPLC
LC-MS | i) Aromatic amino
acids in gastric juice can be used as diagnostic biomarkers to
screen gastric malignancies, areas under receiver operating
characteristic curves for tyrosine, phenylalanine and tryptophan
were 0.838, 0.856 and 0.816, respectively
ii) The sensitivity and specificity of gastric malignancy detection
with phenylalanine reached 87.9% and 79.4% | (40) |
| 2012 | Patients | Non-neoplastic
gastric disease (n=70)
Early gastric cancer (n=49)
Advanced gastric cancer (n=66) | HPLC | Levels of tyrosine,
phenylalanine and tryptophan in gastric juice increased in the
early phase of gastric carcinogenesis, which could function as a
biomarker to screen this disease at early stage in the general
population | (41) |
| 2016 | Patients | Chronic superficial
gastritis (n=17)
Gastric cancer (n=32) | LC-MS | Upregulated
kynurenine pathway of tryptophan metabolism: levels of tryptophan,
anthranilic acid, nicotinic acid, kynurenic acid, kynurenine and
indole-3-lactic acid (P>0.05) were increased | (29) |
Glucose metabolism
Cumulative evidence demonstrates that concentration
of lactic acid shows a consistent increase in urine (30) or tissue (31,35,36,38)
samples of gastric cancer groups, but glucose is considerably
depleted compared with those healthy counterparts or non-malignant
patients (like chronic superficial gastritis and chronic atrophic
gastritis without intestinal metaplasia) (35,36).
The high lactate level might be attributed to the special
metabolism of most cancer cells, known as 'Warburg effect' we
mentioned above (6). Scarce
glucose might result from the overexpression of glucose
transporters (42) and type II
hexokinase (43), which are both
confirmed in gastric cancer tissues. Higher
fructose-6-phosphokinase (6-FPK) activity can also result in low
glucose in gastric cancer tissues (44), as it regulates the output of
glucose to glycolysis pathway. The glycolytic switch has been
identified to be associated with oncogenic transformation and
molecular signal transduction, such as hypoxia-inducible factor
pathway, insulin signaling pathway and PI3K-Akt-mTOR pathway
(45). Furthermore, overexpression
of pyruvate kinase and lactate dehydrogenase is positively
associated with tumor proliferation and poor prognosis,
downregulation of them in vitro experiment can impair tumor
invasion (38,46–49).
On the other hand, such special microenvironment might be the
requirement of rapid propagation of tumor cells. To our
understanding, it has been reported that accumulated lactic acid
moderates the activity of proteases that decompose extracellular
matrix, which can produce some peptides and amino acids that are
consumable for energy generation (44). Acidosis microenvironment is also
ascribed to the formation of cancer blood vessels, meeting the
plentiful supply of nutrients and leading to tumor invasion and
metastasis (50). Moreover,
tumor-derived lactate shows strongly negative effects on cytotoxic
T-cell/NK cell function (11,51)
and blocks differentiation of monocytes to dendritic cells
(52), finally leading to tumor
immune escape. However, such outcome demands further verification
in gastric cancer.
Considering tricarboxylic acid cycle (TCA)
intermediates, an increase of five metabolites (α-ketoglutaric
acid, malic acid, fumarate, succinate, citric acid) is noticed
regardless of blood (26,28), urine (30,32)
or tissue (28,35,36,39)
samples in gastric cancer. There are some possible reasons that can
explain this phenomenon. One account is that cancer cells still use
a small portion of glucose for oxidative phosphorylation. Secondly,
cancer cells might also utilize fumarate respiration to generate
energy under special conditions of glucose deprivation and severe
hypoxia in microenvironment (53),
and succinate is one of the byproducts in this process except for
originating from TCA. Hence, it provides a likely explanation for
the accumulation of fumarate and succinate. Another reason is that
some amino acids, such as glutamine, threonine, phenylalanine,
tyrosine or proline, can be converted into these intermediates
involving in TCA (Fig. 2).
Additionally, elevated levels of citric acid can be used in the
de novo fatty acid synthesis, but it is noted that citrate
can also induce apoptosis in two gastric cancer cell lines in
vitro experiment (54,55).
Amino acid metabolism
Availability of amino acids is pivotal for cellular
protein biosynthesis and cytoskeleton formation, while it has been
pointed out that amino acids especially those linking to TCA
(Fig. 2) are an alternative energy
source of cancer cell proliferation (56). By employing metabolomics
technologies, levels of various amino acids (including serine,
valine, phenylalanine, tryptophan, glycine, and proline) and their
primary derivatives (such as kynurenine, kynurenic acid,
anthranilic acid and nicotinic acid) are significantly higher in
tissue specimens (31,36,37)
and gastric content (29,40,41),
but decreased concentration in some of them is observed in blood
(24). The overexpression of
L-type amino acid transporter 1 (LAT1) might be proposed to explain
this dissimilarity (57). Free
amino acids are greatly assimilated to cancer tissues via LAT1 from
bloodstream, resulting in the low accumulation of amino acid in
contrast to normal counterparts. Malnourishment may also be a
contributing factor to these reduced levels of plasma amino acids.
Apart from these, degradation of extracellular matrix mediated by
the overexpressed matrix metalloproteinases (MMPs) and activated
autophagic degradation of intracellular proteins are considered as
the potential source of accumulative amino acids in tumor tissues
(58–60).
Elevated amino acids in microenvironment are
contributing factors in carcinogenesis. Most strikingly, it is
indicated that many cancer cell lines cannot survive in the absence
of glutamine (61), because it is
required for anabolic growth of mammalian cells through its ability
to control the master regulator of protein translation mTORC1
(62). Reprogramming of glutamine
metabolism further contributes to the proliferative and metabolic
responses regulated by oncogenic transcription factor c-MYC
(63). In addition, it is also the
nitrogen donor for several key metabolic enzymes and for the de
novo synthesis of both purines and pyrimidines (Fig. 2). Serine also participates in the
de novo synthesis of nucleotides by serving one carbon unit.
Functional genomics further indicates that serine biosynthesis
pathway is significant for breast cancer event, which can be
attributable to the overexpression of phosphoglycerate
dehydrogenase (PHGDH) that controls the flow of intermediates
originated from glycolysis (64).
Inhibition of PHGDH in cells can result in lower serine and
decrease cellular proliferation in vitro. However, this
remains unclear in gastric cancer. Tryptophan and its downstream
metabolites (mainly including kynurenine, kynurenic acid,
anthranilic acid, nicotinic acid) via kynurenine pathway are
related to the pathogenesis and prognosis of various malignancies
including gastric cancer (65,66).
Kynurenine pathway catalyzed by indoleamine-2, 3-dioxygenase (IDO)
plays a key role in adapting the tumor microenvironment to favor
cancer progression because higher IDO expression is associated with
an increase in immunosuppressive T-regulatory cell activity
(67), and its immunosuppressive
role inhibits T-cell mediated cytotoxicity and cell proliferation
of gastric cell lines in vitro (68). Additionally, 3-hydroxyanthanilie
acid (downstream metabolites in kynurenine pathway) also has
suppressive effects on inflammation and immune response (69). Glycine used in living organism as
building blocks of purines is strongly correlated with the rapid
proliferation rates, and then antagonizing glycine uptake and its
mitochondrial biosynthesis preferentially impair rapidly
proliferating cells (70). The
indirect anti-angiogenic impact of glycine is also identified in
vitro (71,72) and in vivo (73,74),
possibly because it might inhibit the proliferation of vascular
endothelial cells, finally leading to angiogenesis (74). Elevated proline in tumor tissues
might begin with the activation of MMPs and degradation of
micro-environmentally extracellular matrix (ECM), subsequently the
degradation of collagen catalyzed by proline dehydrogenase (PRODH)
that can be regulated under conditions of nutrient stress linked to
mTOR signaling system (75). Other
elevated amino acids, such as tyrosine, valine and cysteine, can be
converted into the TCA intermediates (except for citric acid,
isocitrate, succinyl-CoA, oxaloacetate) to generate energy
(Fig. 2).
Lipid metabolism
The notable feature of lipid metabolism in cancer
cells is an increased rate of lipogenesis and the upregulation of
mitochondrial fatty acid β-oxidation, gastric cancer shows a
similar tendency and presents typical changes regarding various
metabolites involving in lipid metabolism.
Fatty acids, such as hexadecenoic acid,
docosahexaenoic acid, eptanoic acid and β-hydroxybutyrate, are
significantly larger in gastric cancer tissues than in benign
tissues (like chronic superficial gastritis) (28). Octadecanoic acid is also found to
be elevated in blood specimens obtained from gastric cancer
patients (39). Of them,
β-hydroxybutyrate is the common product of fatty acid degradation
via β-oxidation, suggesting more intensive decomposition of fatty
acids in microenvironment. The accelerated metabolism from lipids
to fatty acids and finally ketone bodies consumes fat, which might
explain the fact that patients become very thin in later stages of
gastric cancer. This signature also has been identified by the
xenograft animal models with gastric cancer showing elevated levels
of glycerol and hexadecanoic acid (30), resulting from the high activation
of adipocyte lipolysis in cancer cells as well as enhanced
expression and function of adipocyte hormone-sensitive lipase in
cancer cachexia (76). In
contrast, some data show that unsaturated fatty acids such as
9-hexadecenoic acid, cis-vaccenic acid, arachidinic acid,
hexadecanoic acid and 3-hydroxybutanoic acid are found to be
significantly decreased in cancer tissue samples (26). Of note, the level of
O-acetylcarnitine, which increases the β-oxidation of fatty
acid, shows a declining trend as the early gastric cancer
progresses into advanced stage (31). Accordingly, it seems that decreased
O-acetylcarnitine might explain the impaired fatty acids
β-oxidation in stage III/IV gastric cancer, which is characterized
by the decline in unsaturated fatty acids we discussed above
(9-hexadecenoic acid, cis-vaccenic acid, arachidinic acid,
hexadecanoic acid and 3-hydroxybutanoic acid). However, this
discrepancy between different research needs further elucidation
with larger samples and different analytical methods. On the other
hand, free fatty acids in plasma, including palmitic acid, stearic
acid, 9-(Z)-hexadecenoic acid, oleic acid, linoleic acid,
docosahexaenoic acid and arachidonic acid, are equivalent in both
gastric cancer and gastric benign disorders (25). Therefore, it infers that free fatty
acids in blood might be not utilized by tumor cells.
Upregulated lipid peroxides are also confirmed in
this disease. Accumulation of 4-hydroxyphenylacetate resulting from
the oxidative degradation of lipids was observed in the study of
Jung et al (31). Elevation
of azelaic acid in blood samples, which is the end product of
linoleic acid when subjected to peroxide decomposition (77) and can serve as a marker of lipid
peroxidation (78), as observed by
Yu et al (25).
Based on that indicated above, these signatures show
that cancer cells utilize massive fatty acids to meet the demand of
cell membrane synthesis, mainly for lipid raft and lipid-modified
signaling molecules (79); and a
large fraction of their membrane lipids are biosynthesized de
novo rather than scavenging from extracellular sources. In
de novo lipogenesis, fatty acid synthase (FAS) catalyzes the
synthesis of palmitate from acetyl-CoA or malonyl-CoA in the
presence of NADPH as a redox equivalent. FAS expression is commonly
low in non-proliferating cells that typically import lipids from
the extracellular milieu. In contrast, actively proliferating
cells, especially tumor cells, have increased demands for lipids,
which is highly dependent on de novo synthesis. So FAS is
frequently upregulated in many types of tumors (80–82)
including gastric cancer (83,84);
and increased FAS expression is linked to tumor proliferation,
chemoresistance and poorer prognosis in cancers (85–88).
Thus, this key enzyme implicated in lipogenesis has been studied as
potential target in anti-neoplastic therapy (84). On the other hand, enhancement of
fatty acid-β oxidation is also considered to be an important
metabolic reprogramming in the early stage of some cancer types
(89), as it produces more ATP and
acetyl coenzyme A which in turn can accelerate the rate of citric
acid oxidation and serve as the energy source (90). Furthermore, production of
polyunsaturated fatty acids, to some extent, is also associated
with tumor cell proliferation, apoptosis and angiogenesis (91,92).
Nucleotide metabolism
Tumor cells are in a state of such rapid
proliferation and differentiation that frequent nucleotide
synthesis and metabolism are upregulated significantly.
Accumulation of the end products of nucleotide catabolism is
characterized by the higher levels of uric acid or urate (25,30)
in gastric cancer patients or animal models. Other purines
compounds like hypoxanthine and guanosine were also increased
(35,37), but Aa et al showed decreases
in uridine (an RNA building block) (28). Nucleotides are also associated with
energy metabolism, mainly in the form of ATP and GTP. Of tumor
cells, adequate energy should be supplied to meet their
proliferation. In this way, it is assumed that nucleotide
phosphates should increase in cancer tissues compared with normal
tissues. However, Hirayama and colleagues (35), identified that there was no
noticeable difference between gastric cancer tissues and adjacent
normal tissues with regard to most nucleotide phosphates (ATP, ADP,
GTP, and GDP), total adenylate and energy charge. Accordingly, it
infers that cancer cells gain growth superiority over their normal
counterparts by switching metabolic patterns of energy to anaerobic
glycolysis and possibly fumarate respiration that we have discussed
above, instead of securing more ATP.
Other altered metabolisms
Except for the changed metabolisms mentioned above,
other metabolite concentrations also show increased or decreased
trend in the development of gastric cancer. Increased level of
creatinine, a waste product of muscle metabolism, was detected in
urine samples of tumor groups (33), which might be induced by lower
total body skeletal mass among cachectic patients (93,94).
Changes in inositol level of gastric malignancy patients are
investigated in either tumor tissues (28,36,37)
or urine samples (30), but its
mechanism and significance are poorly understood.
3. Metabolomics in diagnosis, treatment and
prognostic prediction of gastric cancer
Diagnosis
Early diagnosis is the key element determining the
outcome of treatment in cancer research, but current application of
cancer biomarkers, endoscopy and imaging is still not satisfactory.
Serum biomarkers, like CEA and CA19-9, are not effective given
their poor sensitivity or specificity. Inconsistent diagnostic
efficacy at endoscopy that results from the variations in skill and
experience of endoscopists and pathologist might lead to missed
diagnosis at early phase, while positive results displayed on
imaging examination (such as barium meal and computer tomography)
are prone to advanced stage. Interestingly, utility of various
-omics technologies open a new field to discover potential
biomarkers for gastric cancer diagnosis, especially based on
metabolomics.
Exploration of gastric cancer biomarkers in blood or
urine is more appreciated because of its non-invasive priority. Yu
et al demonstrated that metabolic profiles were quite
different in gastric cancer patients with different pathological
types in the Correa model, but intestinal metaplasia shared similar
metabolic phenotype (threonate, glutamate and azelaic acid) in
plasma with neoplastic groups (25,95).
Ikeda et al also identified that there were obvious
variations in serum metabolic profiles of gastrointestinal cancers
(including esophageal, gastric and colorectal) in contrast to
healthy volunteers (27). In
particular, changes in the levels of 3-hydroxypropionic acid and
pyruvic acid were sufficient to differentiate gastric cancer from
esophageal and colorectal cancer, and showed high values for both
sensitivity (84.6 and 70.0%) and specificity (71.4 and 90.0%)
compared with conventional biomarkers (CA19-9 and CEA) (27). The diagnostic potential of serum
metabolic profiles between gastric cancer and non-cancer groups was
also confirmed by Song et al, and these alterations occurred
at early stage of gastric carcinogenesis (26).
Recently, one urine metabolomics in gastric cancer
found that 14 out of 17 metabolites detected from training set (94
urine samples) via GC-MS showed diagnostic value better than
classic blood biomarkers on validation set (199 urine samples)
(34). Six of them (L-alanine,
L-isoleucine, L-serine, L-threonine, L-proline and L-methionine)
revealed satisfactory diagnostic values with the area under the ROC
of >0.75. Chan et al also revealed that gastric cancer
has a unique urine metabolic profiles in contrast to benign gastric
diseases and healthy patients, especially 2-hydroxyisobutyrate,
3-indoxylsulfate and alanine, producing a discriminatory model with
the area under the curve (AUC) of 0.95 (33). Another study reported that
metabolites altered in urinary data of gastric cancer patients was
predicted with higher sensitivity than CA19-9 and CEA (31).
In tissue testing, Wu and colleagues indicated that
18 metabolites were detected differently between the malignant
tissues and the adjacent non-malignant tissues of gastric mucosa
with AUC value of 0.9629 (37),
but tissue testing was not a non-invasive approach in contrast to
blood or urine testing. Our data, on the other hand, showed that
higher levels of tyrosine, phenylalanine and tryptophan in the
gastric juice were detected in the early phase of gastric
carcinogenesis (40), and the
sensitivity and specificity for gastric cancer detection with
phenylalanine was 87.9 and 79.4% respectively (41).
Metastasis and prognosis
Most gastric cancer-related deaths occur as a result
of metastasis, even among patients undergoing gastrectomy.
Unfortunately, no molecular markers for predicting metastasis and
prognosis are accessible.
Based on metabolomics, Wu and colleagues showed that
five metabolites (increased L-cysteine, hypoxanthine and
L-tyrosine; decreased phenanthrenol and butanoic acid) were
detected differently between non-invasive (T1 and T2) and invasive
(T3 and T4) groups, furthermore, 4-hydroxyphenyl-acetate, alanine,
phenylacetylglycine, mannitol, glycolate and arginine levels were
significantly correlated with cancer T stage (37). By establishing animal models with
gastric cancer cell line SGC-7901, Chen et al confirmed that
metabolites correlated to proline and serine metabolism could
distinguish metastatic from non-metastatic specimens with an AUC
value of 1.0 (36). Study
conducted by Hu et al suggested that decreased levels of
alanine, glycerol, L-proline, butanoic acid and L-threonic acid as
well as increased levels of butanediotic acid and myo-inositol
could detect non-metastatic and metastatic groups (AUC=1.00)
(30).
Significantly, Chen and coworkers recently evaluated
the prognostic value of 17 urinary metabolites, which have been
identified differently between gastric cancer group and normal
group, by following up 82 out of 112 gastric cancer cases for 3–5
years after surgery (34). They
discovered that patients with higher levels of proline, p-cresol
and 4-hydroxybenzoic acid display poor prognosis with median
survival time 16, 15 and 15 months, respectively. Furthermore, the
concentration of p-cresol closely correlated with gastric cancer
stage, which was gradually increased with the stage of the
patients.
It is possible that changes in proline might be
essential in tumor metastasis. As we have mentioned above, proline
in tumor tissues might result from the degradation of collagen
(73). This process mainly begins
with the activation of MMPs and degradation of microenvironmental
ECM, which partially accounts for the tumor invasion and metastasis
(96). In this respective,
elevated proline serving as metastatic biomarker for gastric cancer
is possible, but further research is necessary.
Treatment
Chemosensitivity prediction that aims to maximize
the therapeutic response and minimize adverse effects is a
difficult task in the treatment of advanced tumors. One of
classical approaches for predicting the activity of anticancer
agents is cell culture testing, which is mainly based on clone
formation, cell metabolic activity assays, proliferation and tumor
growth in vitro experiments. However, it must be noted that
these methods still fail to fully reproduce the tumor
microenvironment, although current patient-derived primary cell
culture or patient-derived tumor xenograft models are able to
retain cellular heterogeneity of original tumors (97).
Lu et al, in particular, suggested that some
conventional cytotoxic anticancer agents (vincristine, taxol,
5-fluorouracil, doxorubicin, cisplatin, camptothecin) lost their
efficacy apparently when cultured PNAC-1 cells (pancreatic cancer)
in vitro were deprived of glucose (98). Similarly, a recent study also
identified that high glucose conditions promoted SGC-7901
proliferation in vitro and reduced chemosensitivity in
vivo or in vitro (99).
We could speculate that responses of gastric cancer against
anticancer drugs in actual microenvironment in vivo might be
considerably different from what we expect in culture condition.
Therefore, utilizing metabolomics is considered to be a promising
tool to assess the sensitivity of chemotherapy in virtual
conditions and discovering therapeutic targets regarding specific
tumor metabolism (20,21).
Wang et al applied high performance liquid
chromatography coupled with a quadrupole time-of-flight mass
spectrometer to predict chemotherapy response in a human xenograft
model of gastric cancer administered with cisplatin plus
5-fluorouracil (5-FU) (100).
Consequently, 1-acyl-lysophosphatidylcholine and polyunsaturated
fatty acid were proposed to surveil gastric cancer
chemosensitivity, since 1-acyl-lysophosphatidylcholine can regulate
the activity of enzymes like phospholipase A2 (PLA2) and
lysophosphatidylcholine acetyltransferases. PLA2 catalyzes the
production of arachidonic acid that is likely to promote cell cycle
arrest and apoptosis dependent on ceramide pathway (101,102), while lysophosphatidylcholine
acetyltransferases catalyzes phospholipid synthesis linked to tumor
cell proliferation. Another study suggested that proline was
reduced while glutamate increased dramatically, and PRODH
(catalyzes the metabolic production of glutamate from proline
proceeds) mRNA expression was upregulated 2-fold after 5-FU
administration; but they were less affected in 5-FU-resistant cells
(103). Thus PRODH might make it
possible to be a marker for assessing intracellular dynamic
responses to 5-FU. Additionally, Kim and colleagues utilized
1H-NMR to investigate the metabolic changes in urine
sample following Adriamycin (ADR) treatment for gastric
adenocarcinoma in an animal model (104). This study revealed that levels of
trimethylamine oxide, hippurate and taurine, which all decreased in
tumor group without treatment, were increased dramatically after
ADR disposal; while 2-oxoglutarate, 3-indoxylsulfate, trigonelline,
trimethylamine and citrate recovered to those of normal group
(104). Alterations in these
metabolites might be ascribed to the pharmacological activity of
ADR that activates apoptotic process of gastric cancer cells via
ADR-induced genotoxic stress.
In another study, dysregulation of pyruvic acid
efflux in gastric cardia cancer was observed with the combination
of proteomics and metabolomics (38). Furthermore, Cai et al also
found that downregulation of lactate dehydrogenase A (LDH-A) and
overexpression of pyruvate dehydrogenase B (PDH-B) could force
pyruvic acid into the Krebs cycle rather than the glycolysis
process in gastric cancer cell line AGS, consequently inhibiting
cell growth and migration (38).
In view of the above, LDH or PDH might serve as a therapeutic
target in gastric cancer treatment.
4. Current perspectives and future
directions
As we indicated above, cumulative studies employing
metabolomics have yielded initial and promising results in gastric
cancer research. However, inconsistent results across studies can
be observed, probably because of the different sensitivity of
metabolomics methods (105),
variety of experimental subjects (patients, animal models or in
vitro cell culture), and the number of samples. Additionally,
values of those biomarkers should be further validated with larger
cohorts and normalized metabolomics analysis. Furthermore, it
should be noted that investigations targeted at the mechanism of
the altered metabolism and specific metabolic pathways in gastric
cancer are relatively deficient at present, so it is difficult to
draw a clear dividing line on metabolism for common cancers and
this disease based on a handful of studies that looked also at the
role of metabolomics. Overall, exploring the metabolic disorders
and gastric carcinogenesis still has far to go.
On the other hand, metabolomics locate at the
downstream of genomics, transcriptomics and proteomics, mapping the
complete metabolic changes under specific conditions associated
with pathogenic factors, host or environmental co-effectors.
However, it is essential to combine metabolomics with other-omics
methods to get a more integrated understanding of gastric
carcinogenesis (Fig. 1). For
instance, metabolomic genome-wide association studies (mGWAS) have
their priority in quantifying metabolic data and uncovering genetic
variants affecting metabolite levels (106). Impacts of the microbiome on the
metabolome are also an area of increasing interest, because
perturbation of gastrointestinal microbiota composition or function
including Helicobacter pylori has been proved to play a role
in gastric carcinogenesis (107,108). Furthermore, microbe-derived
metabolites also produce effects on cancer cells, such as butanoic
acid. Some research revealed that it can modulate immune response
via the differentiation of colonic regulatory T cells (109) and inhibit colonic tumor cells
(110,111), although the signaling mechanism
was not clearly understood. Thus, it can explain the fact that some
changed metabolites in gastric cancer such as butanoic acid
(37), mannitol (37) and p-cresol (34) that are commonly thought of
artificial substances, can originate from fermentation by
microorganism in gastric flora. Given this, it is reasonable to
presume that gastric flora might be incorporated into an in-depth
study of the prominent disorders of metabolism in gastric cancer,
but there is still a gap in further research.
5. Conclusions
Gastric cancer is one of the most malignant tumors
worldwide, and remains a major global health threat. Though its
pathogenesis is unknown, promising discoveries have been made with
the emergence of -omics studies. Most strikingly, metabolomics
provides us in-depth information on metabolic perturbation between
healthy and neoplastic states in the stomach, and further help us
discovery disease-specific biomarkers. As technology advances and
our understanding of metabolic perturbation in gastric cancer
grows, new diagnostic and therapeutic targets will undoubtedly
emerge. Ultimately, these advances can be translated into clinical
practice to realize the goal of truly personalized cancer
treatment.
Acknowledgments
This study was supported by National Natural Science
Foundation of China (no. 81672410).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Conteduca V, Sansonno D, Lauletta G, Russi
S, Ingravallo G and Dammacco F: H. pylori infection and gastric
cancer: State of the art (Review). Int J Oncol. 42:5–18. 2013.
|
|
3
|
Amieva M and Peek RM Jr: Pathobiology of
Helicobacter pylori-induced gastric cancer. Gastroenterology.
150:64–78. 2016. View Article : Google Scholar
|
|
4
|
Kim J, Yum S, Kang C and Kang SJ:
Gene-gene interactions in gastrointestinal cancer susceptibility.
Oncotarget. 7:67612–67625. 2016.PubMed/NCBI
|
|
5
|
Raei N, Behrouz B, Zahri S and
Latifi-Navid S: Helicobacter pylori infection and dietary factors
act synergistically to promote gastric cancer. Asian Pac J Cancer
Prev. 17:917–921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yun J, Rago C, Cheong I, Pagliarini R,
Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S,
Zhou S, et al: Glucose deprivation contributes to the development
of KRAS pathway mutations in tumor cells. Science. 325:1555–1559.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim
SH, Ito S, Yang C, Wang P, Xiao MT, et al: Oncometabolite
2-hydroxyglutarate is a competitive inhibitor of
α-ketoglutarate-dependent dioxygenases. Cancer Cell. 19:17–30.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 465:9662010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dietl K, Renner K, Dettmer K, Timischl B,
Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart
LA, Oefner PJ, et al: Lactic acid and acidification inhibit TNF
secretion and glycolysis of human monocytes. J Immunol.
184:1200–1209. 2010. View Article : Google Scholar
|
|
13
|
Herber DL, Cao W, Nefedova Y, Novitskiy
SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et
al: Lipid accumulation and dendritic cell dysfunction in cancer.
Nat Med. 16:880–886. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Ahn WS, Gameiro PA, Keibler MA,
Zhang Z and Stephanopoulos G: 13C isotope-assisted methods for
quantifying glutamine metabolism in cancer cells. Methods Enzymol.
542:369–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beger RD: A review of applications of
metabolomics in cancer. Metabolites. 3:552–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Putri SP, Yamamoto S, Tsugawa H and
Fukusaki E: Current metabolomics: Technological advances. J Biosci
Bioeng. 116:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duarte IF, Diaz SO and Gil AM: NMR
metabolomics of human blood and urine in disease research. J Pharm
Biomed Anal. 93:17–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ussher JR, Elmariah S, Gerszten RE and
Dyck JR: The emerging role of metabolomics in the diagnosis and
prognosis of cardiovascular disease. J Am Coll Cardiol.
68:2850–2870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guma M, Tiziani S and Firestein GS:
Metabolomics in rheumatic diseases: Desperately seeking biomarkers.
Nat Rev Rheumatol. 12:269–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herrmann K, Walch A, Balluff B, Tänzer M,
Höfler H, Krause BJ, Schwaiger M, Friess H, Schmid RM and Ebert MP:
Proteomic and metabolic prediction of response to therapy in
gastrointestinal cancers. Nat Clin Pract Gastroenterol Hepatol.
6:170–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armitage EG and Southam AD: Monitoring
cancer prognosis, diagnosis and treatment efficacy using
metabolomics and lipidomics. Metabolomics. 12:1462016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jayavelu ND and Bar NS: Metabolomic
studies of human gastric cancer (Review). World J Gastroenterol.
20:8092–8101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan AW, Gill RS, Schiller D and Sawyer
MB: Potential role of metabolomics in diagnosis and surveillance of
gastric cancer. World J Gastroenterol. 20:12874–12882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyagi Y, Higashiyama M, Gochi A, Akaike
M, Ishikawa T, Miura T, Saruki N, Bando E, Kimura H, Imamura F, et
al: Plasma free amino acid profiling of five types of cancer
patients and its application for early detection. PLoS One.
6:e241432011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu L, Aa J, Xu J, Sun M, Qian S, Cheng L,
Yang S and Shi R: Metabolomic phenotype of gastric cancer and
precancerous stages based on gas chromatography time-of-flight mass
spectrometry. J Gastroenterol Hepatol. 26:1290–1297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song H, Peng JS, Dong-Sheng Y, Yang ZL,
Liu HL, Zeng YK, Shi XP and Lu BY: Serum metabolic profiling of
human gastric cancer based on gas chromatography/mass spectrometry.
Braz J Med Biol Res. 45:78–85. 2012. View Article : Google Scholar
|
|
27
|
Ikeda A, Nishiumi S, Shinohara M, Yoshie
T, Hatano N, Okuno T, Bamba T, Fukusaki E, Takenawa T, Azuma T, et
al: Serum metabolomics as a novel diagnostic approach for
gastrointestinal cancer. Biomed Chromatogr. 26:548–558. 2012.
View Article : Google Scholar
|
|
28
|
Aa J, Yu L, Sun M, Liu L, Li M, Cao B, Shi
J, Xu J, Cheng L, Zhou J, et al: Metabolic features of the tumor
microenvironment of gastric cancer and the link to the systemic
macroenvironment. Metabolomics. 8:164–173. 2012. View Article : Google Scholar
|
|
29
|
Choi JM, Park WS, Song KY, Lee HJ and Jung
BH: Development of simultaneous analysis of tryptophan metabolites
in serum and gastric juice - an investigation towards establishing
a biomarker test for gastric cancer diagnosis. Biomed Chromatogr.
30:1963–1974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu JD, Tang HQ, Zhang Q, Fan J, Hong J, Gu
JZ and Chen JL: Prediction of gastric cancer metastasis through
urinary metabolomic investigation using GC/MS. World J
Gastroenterol. 17:727–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung J, Jung Y, Bang EJ, Cho SI, Jang YJ,
Kwak JM, Ryu DH, Park S and Hwang GS: Noninvasive diagnosis and
evaluation of curative surgery for gastric cancer by using
NMR-based metabolomic profiling. Ann Surg Oncol. 21(Suppl 4):
S736–S742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang Q, Wang C and Li B: Metabolomic
analysis using liquid chromatography/mass spectrometry for gastric
cancer. Appl Biochem Biotechnol. 176:2170–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan AW, Mercier P, Schiller D, Bailey R,
Robbins S, Eurich DT, Sawyer MB and Broadhurst D: (1)H-NMR urinary
metabolomic profiling for diagnosis of gastric cancer. Br J Cancer.
114:59–62. 2016. View Article : Google Scholar
|
|
34
|
Chen Y, Zhang J, Guo L, Liu L, Wen J, Xu
L, Yan M, Li Z, Zhang X, Nan P, et al: A characteristic
biosignature for discrimination of gastric cancer from healthy
population by high throughput GC-MS analysis. Oncotarget.
7:87496–87510. 2016.PubMed/NCBI
|
|
35
|
Hirayama A, Kami K, Sugimoto M, Sugawara
M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et
al: Quantitative metabolome profiling of colon and stomach cancer
microenvironment by capillary electrophoresis time-of-flight mass
spectrometry. Cancer Res. 69:4918–4925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen JL, Tang HQ, Hu JD, Fan J, Hong J and
Gu JZ: Metabolomics of gastric cancer metastasis detected by gas
chromatography and mass spectrometry. World J Gastroenterol.
16:5874–5880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Xue R, Tang Z, Deng C, Liu T, Zeng
H, Sun Y and Shen X: Metabolomic investigation of gastric cancer
tissue using gas chromatography/mass spectrometry. Anal Bioanal
Chem. 396:1385–1395. 2010. View Article : Google Scholar
|
|
38
|
Cai Z, Zhao JS, Li JJ, Peng DN, Wang XY,
Chen TL, Qiu YP, Chen PP, Li WJ, Xu LY, et al: A combined
proteomics and metabolomics profiling of gastric cardia cancer
reveals characteristic dysregulations in glucose metabolism. Mol
Cell Proteomics. 9:2617–2628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song H, Wang L, Liu HL, Wu XB, Wang HS,
Liu ZH, Li Y, Diao DC, Chen HL and Peng JS: Tissue metabolomic
fingerprinting reveals metabolic disorders associated with human
gastric cancer morbidity. Oncol Rep. 26:431–438. 2011.PubMed/NCBI
|
|
40
|
Deng K, Lin S, Zhou L, Geng Q, Li Y, Xu M
and Na R: Three aromatic amino acids in gastric juice as potential
biomarkers for gastric malignancies. Anal Chim Acta. 694:100–107.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng K, Lin S, Zhou L and Li Y, Chen M,
Wang Y and Li Y: High levels of aromatic amino acids in gastric
juice during the early stages of gastric cancer progression. PLoS
One. 7:e494342012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koukourakis MI, Pitiakoudis M,
Giatromanolaki A, Tsarouha A, Polychronidis A, Sivridis E and
Simopoulos C: Oxygen and glucose consumption in gastrointestinal
adenocarcinomas: Correlation with markers of hypoxia, acidity and
anaerobic glycolysis. Cancer Sci. 97:1056–1060. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pedersen PL, Mathupala S, Rempel A,
Geschwind JF and Ko YH: Mitochondrial bound type II hexokinase: A
key player in the growth and survival of many cancers and an ideal
prospect for therapeutic intervention. Biochim Biophys Acta.
1555:14–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yuan LW, Yamashita H and Seto Y: Glucose
metabolism in gastric cancer: The cutting-edge. World J
Gastroenterol. 22:2046–2059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Israelsen WJ and Vander Heiden MG:
Pyruvate kinase: Function, regulation and role in cancer. Semin
Cell Dev Biol. 43:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu J, Hu L, Chen M, Cao W, Chen H and He
T: Pyruvate kinase M2 overexpression and poor prognosis in solid
tumors of digestive system: Evidence from 16 cohort studies. Onco
Targets Ther. 9:4277–4288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Augoff K, Hryniewicz-Jankowska A and
Tabola R: Lactate dehydrogenase 5: An old friend and a new hope in
the war on cancer. Cancer Lett. 358:1–7. 2015. View Article : Google Scholar
|
|
49
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhup S, Dadhich RK, Porporato PE and
Sonveaux P: Multiple biological activities of lactic acid in
cancer: Influences on tumor growth, angiogenesis and metastasis.
Curr Pharm Des. 18:1319–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lardner A: The effects of extracellular pH
on immune function. J Leukoc Biol. 69:522–530. 2001.PubMed/NCBI
|
|
52
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar
|
|
53
|
Chen Z, Lu W, Garcia-Prieto C and Huang P:
The Warburg effect and its cancer therapeutic implications. J
Bioenerg Biomembr. 39:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Costello LC and Franklin RB: 'Why do
tumour cells glycolyse?': From glycolysis through citrate to
lipogenesis. Mol Cell Biochem. 280:1–8. 2005. View Article : Google Scholar
|
|
55
|
Lu Y, Zhang X, Zhang H, Lan J, Huang G,
Varin E, Lincet H, Poulain L and Icard P: Citrate induces apoptotic
cell death: A promising way to treat gastric carcinoma? Anticancer
Res. 31:797–805. 2011.PubMed/NCBI
|
|
56
|
Weljie AM and Jirik FR: Hypoxia-induced
metabolic shifts in cancer cells: Moving beyond the Warburg effect.
Int J Biochem Cell Biol. 43:981–989. 2011. View Article : Google Scholar
|
|
57
|
Ichinoe M, Yanagisawa N, Mikami T, Hana K,
Nakada N, Endou H, Okayasu I and Murakumo Y: L-Type amino acid
transporter 1 (LAT1) expression in lymph node metastasis of gastric
carcinoma: Its correlation with size of metastatic lesion and Ki-67
labeling. Pathol Res Pract. 211:533–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang M, Zhu GY, Gao HY, Zhao SP and Xue
Y: Expression of tissue levels of matrix metalloproteinases and
tissue inhibitors of metalloproteinases in gastric adenocarcinoma.
J Surg Oncol. 103:243–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sampieri CL, León-Córdoba K and
Remes-Troche JM: Matrix metalloproteinases and their tissue
inhibitors in gastric cancer as molecular markers. J Cancer Res
Ther. 9:356–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian HR and Yang Y: Functional role of
autophagy in gastric cancer. Oncotarget. 7:17641–17651.
2016.PubMed/NCBI
|
|
61
|
Hensley CT, Wasti AT and DeBerardinis RJ:
Glutamine and cancer: Cell biology, physiology, and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nicklin P, Bergman P, Zhang B,
Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson
C, et al: Bidirectional transport of amino acids regulates mTOR and
autophagy. Cell. 136:521–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu W, Le A, Hancock C, Lane AN, Dang CV,
Fan TW and Phang JM: Reprogramming of proline and glutamine
metabolism contributes to the proliferative and metabolic responses
regulated by oncogenic transcription factor c-MYC. Proc Natl Acad
Sci USA. 109:8983–8988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Possemato R, Marks KM, Shaul YD, Pacold
ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et
al: Functional genomics reveal that the serine synthesis pathway is
essential in breast cancer. Nature. 476:346–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Godin-Ethier J, Hanafi LA, Piccirillo CA
and Lapointe R: Indoleamine 2,3-dioxygenase expression in human
cancers: Clinical and immunologic perspectives. Clin Cancer Res.
17:6985–6991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wiggins T, Kumar S, Markar SR, Antonowicz
S and Hanna GB: Tyrosine, phenylalanine, and tryptophan in
gastroesophageal malignancy: A systematic review. Cancer Epidemiol
Biomarkers Prev. 24:32–38. 2015. View Article : Google Scholar
|
|
67
|
Bauer TM, Jiga LP, Chuang JJ, Randazzo M,
Opelz G and Terness P: Studying the immunosuppressive role of
indoleamine 2,3-dioxygenase: Tryptophan metabolites suppress rat
allogeneic T-cell responses in vitro and in vivo. Transpl Int.
18:95–100. 2005. View Article : Google Scholar
|
|
68
|
Zhang R, Li H, Yu J, Zhao J, Wang X, Wang
G, Yao Z, Wei F, Xue Q and Ren X: Immunoactivative role of
indoleamine 2,3-dioxygenase in gastric cancer cells in vitro. Mol
Med Rep. 4:169–173. 2011.PubMed/NCBI
|
|
69
|
McGaha TL, Huang L, Lemos H, Metz R,
Mautino M, Prendergast GC and Mellor AL: Amino acid catabolism: A
pivotal regulator of innate and adaptive immunity. Immunol Rev.
249:135–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jain M, Nilsson R, Sharma S, Madhusudhan
N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB and Mootha
VK: Metabolite profiling identifies a key role for glycine in rapid
cancer cell proliferation. Science. 336:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rose ML, Madren J, Bunzendahl H and
Thurman RG: Dietary glycine inhibits the growth of B16 melanoma
tumors in mice. Carcinogenesis. 20:793–798. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Amin K, Li J, Chao WR, Dewhirst MW and
Haroon ZA: Dietary glycine inhibits angiogenesis during wound
healing and tumor growth. Cancer Biol Ther. 2:173–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bruns H, Petrulionis M, Schultze D, Al
Saeedi M, Lin S, Yamanaka K, Ambrazevičius M, Strupas K and
Schemmer P: Glycine inhibits angiogenic signaling in human
hepatocellular carcinoma cells. Amino Acids. 46:969–976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bruns H, Kazanavicius D, Schultze D,
Saeedi MA, Yamanaka K, Strupas K and Schemmer P: Glycine inhibits
angiogenesis in colorectal cancer: Role of endothelial cells. Amino
Acids. 48:2549–2558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Phang JM, Donald SP, Pandhare J and Liu Y:
The metabolism of proline, a stress substrate, modulates
carcinogenic pathways. Amino Acids. 35:681–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Agustsson T, Rydén M, Hoffstedt J, van
Harmelen V, Dicker A, Laurencikiene J, Isaksson B, Permert J and
Arner P: Mechanism of increased lipolysis in cancer cachexia.
Cancer Res. 67:5531–5537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Raghavamenon A, Garelnabi M, Babu S,
Aldrich A, Litvinov D and Parthasarathy S: Alpha-tocopherol is
ineffective in preventing the decomposition of preformed lipid
peroxides and may promote the accumulation of toxic aldehydes: A
potential explanation for the failure of antioxidants to affect
human atherosclerosis. Antioxid Redox Signal. 11:1237–1248. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schallreuter KU and Wood JM: Azelaic acid
as a competitive inhibitor of thioredoxin reductase in human
melanoma cells. Cancer Lett. 36:297–305. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Muñoz-Pinedo C, El Mjiyad N and Ricci JE:
Cancer metabolism: Current perspectives and future directions. Cell
Death Dis. 3:e2482012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Swinnen JV, Roskams T, Joniau S, Van
Poppel H, Oyen R, Baert L, Heyns W and Verhoeven G: Overexpression
of fatty acid synthase is an early and common event in the
development of prostate cancer. Int J Cancer. 98:19–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Flavin R, Peluso S, Nguyen PL and Loda M:
Fatty acid synthase as a potential therapeutic target in cancer.
Future Oncol. 6:551–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hao Q, Li T, Zhang X, Gao P, Qiao P, Li S
and Geng Z: Expression and roles of fatty acid synthase in
hepatocellular carcinoma. Oncol Rep. 32:2471–2476. 2014.PubMed/NCBI
|
|
83
|
Kusakabe T, Nashimoto A, Honma K and
Suzuki T: Fatty acid synthase is highly expressed in carcinoma,
adenoma and in regenerative epithelium and intestinal metaplasia of
the stomach. Histopathology. 40:71–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ito T, Sato K, Maekawa H, Sakurada M,
Orita H, Shimada K, Daida H, Wada R, Abe M, Hino O, et al: Elevated
levels of serum fatty acid synthase in patients with gastric
carcinoma. Oncol Lett. 7:616–620. 2014.PubMed/NCBI
|
|
85
|
Lin HP, Cheng ZL, He RY, Song L, Tian MX,
Zhou LS, Groh BS, Liu WR, Ji MB, Ding C, et al: Destabilization of
fatty acid synthase by acetylation inhibits de novo lipogenesis and
tumor cell growth. Cancer Res. 76:6924–6936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Takahiro T, Shinichi K and Toshimitsu S:
Expression of fatty acid synthase as a prognostic indicator in soft
tissue sarcomas. Clin Cancer Res. 9:2204–2212. 2003.PubMed/NCBI
|
|
87
|
Menendez JA, Lupu R and Colomer R:
Inhibition of tumor-associated fatty acid synthase hyperactivity
induces synergistic chemosensitization of HER-2/neu-overexpressing
human breast cancer cells to docetaxel (taxotere). Breast Cancer
Res Treat. 84:183–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Duan J, Sun L, Huang H, Wu Z, Wang L and
Liao W: Overexpression of fatty acid synthase predicts a poor
prognosis for human gastric cancer. Mol Med Rep. 13:3027–3035.
2016.PubMed/NCBI
|
|
89
|
Khasawneh J, Schulz MD, Walch A, Rozman J,
Hrabe de Angelis M, Klingenspor M, Buck A, Schwaiger M, Saur D,
Schmid RM, et al: Inflammation and mitochondrial fatty acid
beta-oxidation link obesity to early tumor promotion. Proc Natl
Acad Sci USA. 106:3354–3359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu Y: Fatty acid oxidation is a dominant
bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic
Dis. 9:230–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hyde CA and Missailidis S: Inhibition of
arachidonic acid metabolism and its implication on cell
proliferation and tumour-angiogenesis. Int Immunopharmacol.
9:701–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lu X, Yu H, Ma Q, Shen S and Das UN:
Linoleic acid suppresses colorectal cancer cell growth by inducing
oxidant stress and mitochondrial dysfunction. Lipids Health Dis.
9:1062010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Swaminathan R, Major P, Snieder H and
Spector T: Serum creatinine and fat-free mass (lean body mass).
Clin Chem. 46:1695–1696. 2000.PubMed/NCBI
|
|
94
|
Eisner R, Stretch C, Eastman T, Xia J, Hau
D, Damaraju S, Greiner R, Wishart D and Baracos V: Learning to
predict cancer-associated skeletal muscle wasting from
1H-NMR profiles of urinary metabolites. Metabolomics.
7:25–34. 2011. View Article : Google Scholar
|
|
95
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
96
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lau V, Wong AL, Ng C, Mok Y, Lakshmanan M
and Yan B: Drug sensitivity testing platforms for gastric cancer
diagnostics. J Clin Pathol. 69:93–96. 2016. View Article : Google Scholar
|
|
98
|
Lu J, Kunimoto S, Yamazaki Y, Kaminishi M
and Esumi H: Kigamicin D, a novel anticancer agent based on a new
anti-austerity strategy targeting cancer cells' tolerance to
nutrient starvation. Cancer Sci. 95:547–552. 2004. View Article : Google Scholar
|
|
99
|
Zhao W, Chen R, Zhao M, Li L, Fan L and
Che XM: High glucose promotes gastric cancer chemoresistance in
vivo and in vitro. Mol Med Rep. 12:843–850. 2015.PubMed/NCBI
|
|
100
|
Wang X, Yan SK, Dai WX, Liu XR, Zhang WD
and Wang JJ: A metabonomic approach to chemosensitivity prediction
of cisplatin plus 5-fluorouracil in a human xenograft model of
gastric cancer. Int J Cancer. 127:2841–2850. 2010. View Article : Google Scholar
|
|
101
|
Ilsley JN, Nakanishi M, Flynn C, Belinsky
GS, De Guise S, Adib JN, Dobrowsky RT, Bonventre JV and Rosenberg
DW: Cytoplasmic phospholipase A2 deletion enhances colon
tumorigenesis. Cancer Res. 65:2636–2643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ganesan K, Ivanova T, Wu Y, Rajasegaran V,
Wu J, Lee MH, Yu K, Rha SY, Chung HC, Ylstra B, et al: Inhibition
of gastric cancer invasion and metastasis by PLA2G2A, a novel
beta-catenin/TCF target gene. Cancer Res. 68:4277–4286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sasada S, Miyata Y, Tsutani Y, Tsuyama N,
Masujima T, Hihara J and Okada M: Metabolomic analysis of dynamic
response and drug resistance of gastric cancer cells to
5-fluorouracil. Oncol Rep. 29:925–931. 2013.
|
|
104
|
Kim KB, Yang JY, Kwack SJ, Kim HS, Ryu DH,
Kim YJ, Bae JY, Lim DS, Choi SM, Kwon MJ, et al: Potential
metabolomic biomarkers for evaluation of adriamycin efficacy using
a urinary 1H-NMR spectroscopy. J Appl Toxicol.
33:1251–1259. 2013.
|
|
105
|
Büscher JM, Czernik D, Ewald JC, Sauer U
and Zamboni N: Cross-platform comparison of methods for
quantitative metabolomics of primary metabolism. Anal Chem.
81:2135–2143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Adamski J and Suhre K: Metabolomics
platforms for genome wide association studies - linking the genome
to the metabolome. Curr Opin Biotechnol. 24:39–47. 2013. View Article : Google Scholar
|
|
107
|
Lofgren JL, Whary MT, Ge Z, Muthupalani S,
Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, et
al: Lack of commensal flora in Helicobacter pylori-infected INS-GAS
mice reduces gastritis and delays intraepithelial neoplasia.
Gastroenterology. 140:210–220. 2011. View Article : Google Scholar
|
|
108
|
Lertpiriyapong K, Whary MT, Muthupalani S,
Lofgren JL, Gamazon ER, Feng Y, Ge Z, Wang TC and Fox JG: Gastric
colonisation with a restricted commensal microbiota replicates the
promotion of neoplastic lesions by diverse intestinal microbiota in
the Helicobacter pylori INS-GAS mouse model of gastric
carcinogenesis. Gut. 63:54–63. 2014. View Article : Google Scholar :
|
|
109
|
Furusawa Y, Obata Y, Fukuda S, Endo TA,
Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et
al: Commensal microbe-derived butyrate induces the differentiation
of colonic regulatory T cells. Nature. 504:446–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Vanhoutvin SA, Troost FJ, Hamer HM,
Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K and Brummer RJ:
Butyrate-induced transcriptional changes in human colonic mucosa.
PLoS One. 4:e67592009. View Article : Google Scholar :
|
|
111
|
Singh N, Gurav A, Sivaprakasam S, Brady E,
Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et
al: Activation of Gpr109a, receptor for niacin and the commensal
metabolite butyrate, suppresses colonic inflammation and
carcinogenesis. Immunity. 40:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
















