Introduction
Gastric cancer is one of the leading causes of
cancer-related deaths worldwide. Although the incidence rate
continues to decrease, approximately 723,000 patients died in 2012
due to gastric cancer (1–3). Currently, treatment of primary
gastric cancer consists of metastasectomy, gastrectomy,
radiofrequency ablation, and stereotactic body radiation therapy
(3,4). Despite the advanced surgical
resection techniques and anticancer drugs currently available to
treat early stage gastric cancer, the prognosis of patients with
gastric cancer remains poor. This is typically due to distant
metastasis such as liver or peritoneal and resistance to
traditional drugs (3,4). Thus, the 5-year survival rate of
gastric cancer patients after surgery has been poorly. Therefore,
there is a need to elucidate the mechanism of metastasis in order
to develop better treatments and to improve the survival rate in
patients with gastric cancer.
The epithelial to mesenchymal transition (EMT) is an
important process for the initiation of tumorigenesis. This is
characterized by a loss of polarity, cell-cell adhesion, and tight
junctions in polarized epithelial cells. Also occurring in
parallel, these cells become more mobile and invasive and exhibit
more of a mesenchymal phenotype. During the progression of tumors,
a variety of EMT regulators enhanced tumor formation and/or distant
metastasis (5). This involves
various molecular processes where transcription factors are
activated, cell surface proteins are overexpressed, and
cytoskeletal proteins are reorganized and overexpressed. These are
characteristic changes during EMT, resulting in the molecular
expression of EMT-related and mesenchymal cell markers that can be
used towards the prognosis of gastric cancer (5–8).
Additionally, abnormal control of reactive oxygen
species (ROS) has been implicated as a causal factor in >250
diseases and disorders, including cardiovascular disease, stroke,
neurodegenerative disease, diabetes, asthma, ageing, and cancer
(9,10). ROS include superoxide anion and
hydrogen peroxide, and are generated either by normal redox
reactions under aerobic metabolic processes in the mitochondria, or
through exposure to infectious and inflammatory stimuli such as
smoking, alcohol, pollutants, radiation, and carcinogens (11). Recent studies have suggested that
ROS can promote cell migration and invasion; thus, an imbalance of
redox homeostasis can result in cancer cells exhibiting EMT
properties (12). The long-term
presence of even a small amount of ROS can pose a risk to cells
because they can participate in pathophysiological processes such
as protein and DNA damage and lipid peroxidation. Furthermore, ROS
can participate in carcinogenesis at different stages such as
initiation, promotion, and progression; thus, ROS are considered a
key factor in tumorigenesis and can be a useful marker for the
diagnosis and prognosis of various kinds of cancer (2,12,13).
To counteract these damaging processes, cells can
use several protective mechanisms to either repairing the various
types of damage caused by ROS, such as by rescuing oxidized
targets, or by eliminating ROS such as through the catalytic
removal of free radicals, increasing free radical scavengers, and
the removal of Fe and Cu (9,11–15).
One of the most important protective mechanisms against ROS
involves antioxidant enzymes such as catalase, glutathione
peroxidase, and peroxiredoxins (PRXs) (15). A major common function of PRXs
involves enzymatic degradation of hydrogen peroxide, organic
hydroperoxides, and peroxynitrite (11). PRXs also play a key role in several
cellular functions such as protein and lipid protection against
oxidative injury, cell proliferation, differentiation, and
apoptosis (9–12). Furthermore, recent evidence
suggests that PRXs may be associated with tumor progression
(16–18). Overexpression of PRXs in tumors
have been suggested to be responsible for tumor progression,
prognosis, and resistance to chemotherapy and radiotherapy
(19,20). Overall, PRXs are peroxidases
containing high antioxidant efficacy and are associated with cancer
development and tumorigenesis in several kinds of cancer (21–23).
Lastly, PRXs are upregulated in various tumors in
the breast, bladder, lung, cervical, ovarian, prostate, esophageal,
and hepatocellular (19,21–23).
However, PRX expression and its impact on disease prognosis,
patient survival rate, and EMT have rarely been studied in the
context of human gastric cancer. Very few studies have addressed
how the expression and function of all six PRXs affect human
gastric cancer, disease progression or prognosis. Thus, to improve
our understanding of PRXs in human gastric cancer, we investigated
the expression of PRX1-5 in human gastric cancer tissues and
correlated their expression with clinicopathological
parameters.
Materials and methods
Tissue samples
Gastric cancer tissue samples were obtained from 210
consecutive patients who underwent elective surgery for gastric
cancer at the Chungnam National University Hospital between 2000
and 2003. The patients underwent R0 resection with at least a D1
lymph node dissection. Adenocarcinomas from the patients' stomachs
were isolated and histologically confirmed. The clinicopathological
parameters assessed were established by the Japanese Gastric Cancer
Association (24). All patients
signed informed consent for the therapy as well as for subsequent
tissue studies. The experiments received prior approval by the
institutional review board.
Tissue microarray construction
Paraffin blocks of the 210 human gastric cancer
samples were identified on corresponding H&E-stained sections.
Areas of interest that represented invasive adenocarcinomas were
identified and marked on the donor block. A 2-mm core from the area
of interest was transferred to the recipient master block using the
Tissue Microarrayer (Meditech Ind., Korea). Two stomach cancer
cores were arrayed per specimen. In addition, four cores of normal
gastric tissue were also sampled.
Immunohistochemistry
The stomach cancer tissue samples were fixed in 10%
buffered formalin, and routinely processed and embedded in
paraffin. Three-micrometer thick paraffin sections from the
paraffin TMA blocks were used for immunohistochemistry (IHC) with
the rabbit EnVision-HRP detection system (Dako, Carpinteria, CA,
USA). Polyclonal rabbit antibody for PRX5 (AbFrontier, Korea) was
used for IHC. All immunostaining steps were carried out at room
temperature. After deparaffinization, antigen retrieval was
performed with 10 mM sodium citrate buffer (pH 6.0) via a pressure
cooker at full power for 4 min. The tissue sections were then
treated with 3% hydrogen peroxide for 10 min. The primary antibody
was diluted PRX5, 1:500 with background reducing diluent (Dako) and
incubated for 30 min. Slides were then incubated with the EnVision
reagent for 30 min. The slides were then sequentially incubated
with DAB chromogen for 5 min, counterstained with Meyer's
hematoxylin, and mounted. Careful rinses with several changes of
TBS-0.3% Tween buffer were performed at each step. A rabbit IgG
isotype control without the primary antibody was used as a negative
control. Membranous, cytoplasmic, or nuclear staining was
considered positive staining for PRX5 in gastric carcinoma tissues.
The immunohistochemical staining was then categorized according to
a scoring method. Tumors were classified into four grades based on
the staining intensity: −, 0–10%, no staining; +, 11–40%, weak
staining; ++, 41–70%, intermediate staining; +++, 71–100%, strong
staining. Cases with no staining (−) were assigned to the
PRX5-negative group, whereas those with a score of weak staining
(+) to strong staining (+++) were assigned to the PRX5-positive
group. Then, for deeper comparative analysis of PRX5 expression to
related 5-year survival rate of patients in gastric cancer, tumors
were categorized by high expression (>40% staining, ++ and +++)
and low expression (<40% staining, − and +) of PRX5.
Cell lines and cultures
Nine human gastric cancer cell lines were used in
the present study. MKN-28, MKN-45, MKN-74, SNU-1, SNU-16, SNU-216,
and SNU668 human gastric cancer cells were purchased from the
Korean Cell Line Bank (Seoul, Korea) and were maintained in
RPMI-1640 supplemented with 10% (v/v) fetal bovine serum (FBS)
according to the manufacturer's protocol.
Transfection and selection of stably
expressing cells
For all cell lines, 1×105 cells were
seeded onto 6-well plates. After 24 h, the cells in each well were
transfected with 2 µg of pLenti6.3-PRX5 by using Effectene
(Qiagen, CA, USA) according to the manufacturer's instructions.
After 24 h, the transfected cells were selected by supplementing 8
µg/ml blasticidin (Invitrogen, Carlsbad, CA, USA) into the
media.
RNA isolation and reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was isolated from SNU-216, SNU-668, and
PRX5-overexpressing SNU-216 cells using TRI-Solution (Bio Science
Technology, Seoul, Korea) according to the manufacturer's
instructions. Complementary DNA was synthesized from 2 µg of
total RNA by using a Reverse Transcription Premix (Bioneer,
Daejeon, Korea). PCR was performed using a PCR Premix (Bioneer).
The following PCR primers were used: E-cadherin forward,
5′-TTGACGCCGAGAGCTACAC-3′; reverse, 5′-GTCGACCGGTGCAATCTT-3′.
Vimentin forward, 5′-TACAGGAA GCTGCTGGAAGG-3′; reverse,
5′-ACCAGAGGGAGTGAATCCAG-3′. Snail forward,
5′-GCAACAAGGAATACCTCAGC-3′; reverse, 5′-TCTTGACATCTGAGTGGGTC-3′.
GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′; reverse,
5′-TCCACCACCCTGTTGCTGTA-3′.
Western blot analysis
Protein lysates were prepared using ice-cold
PRO-PREP protein extraction solution (iNtRON Biotechnology Inc.,
Seongnam, Korea). Protein quantification was performed using an
Infinite F50 microplate reader (Tecan Switzerland). For all
samples, 20 µg of the protein lysates were separated on a
10–15% SDS-polyacrylamide gel, transferred onto nitrocellulose
membranes (Pall Corp., NY, USA), and blocked with 5% skimmed milk
(BD Biosciences, CA, USA). Overnight incubations with primary
antibodies against β-actin (Santa Cruz Biotechnology Inc.), GAPDH
(Cell Signaling Biotechnology, MA, USA), PRX5, vimentin
(AbFrontier, Seoul, Korea), E-cadherin (BD Biosciences), Snail and
Slug (both from Santa Cruz Biotechnology Inc.) were performed at
4°C. The membranes were then washed 5 times with 10 mM Tris-HCl (pH
7.5) containing 150 mM NaCl and 0.1% Tween-20 (TBST). Finally, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit and anti-mouse antibodies (Thermo Fisher
Scientific, IL, USA) for 2 h at room temperature. After washing off
excess secondary antibodies, the membranes were washed 6 times with
TBST. Specific binding was detected using Clarity Western ECL
Substrate (Bio-Rad, CA, USA) according to the manufacturer's
instructions.
Xenograft assay
Four-week-old male athymic nude mice (BALB/c-nu)
were purchased from Central Lab. Animal Inc. (Seoul, Korea) and
used according to the Animal Care and Use Guidelines of Kyungpook
National University. When the mice were 5-weeks old,
5×106 SNU-216 and SNU-668 cells resuspended in 200
µl phosphate-buffered saline (PBS) were subcutaneously
injected using a 31-gauge needle. The sizes of the resulting tumors
were measured using a caliper and calculated using the following
formula: W (width)2 × L (length)/2. After we measured
tumor mass during 50 days, the mice were sacrificed.
Foci formation
SNU-216 and SNU-216_ PRX5 cell lines were seeded on
6-well plates. Two weeks after seeding, cells were washed twice
with PBS, fixed with 4% paraformaldehyde (Sigma, St. Louis, MO,
USA), and stained with 0.1% crystal violet (Sigma).
Proliferation assay and invasion
assay
To measure cell proliferation, cells were seeded
onto 96-well ImageLock plates (Essen Bioscience, Ann Arbor, MI,
USA) at a density of 5×104 cells/well and incubated in
DMEM with 10% normal FBS for 5 days. The plates were scanned on the
IncuCyte imager (Essen Bioscience) and the data was analyzed via
the IncuCyte Cell Proliferation assay software. Results are
representative of three independent experiments. For the invasion
assay, a scratch wound was generated using WoundMaker (Essen
Bioscience), and the resulting cells were washed twice with PBS.
The invasion assay was initiated by overlaying the cells with
Matrigel (BD Biosciences) and images were acquired with IncuCyte
Zoom (Essen Bioscience).
Statistical analysis
Quantitative data are presented as the mean ±
standard error of the mean (SEM) from at least three independent
experiments. Comparisons between groups were analyzed using the
unpaired two-tailed Student's t-test, or one-way or two-way ANOVA
with post hoc analyses when appropriate, as indicated. Survival
curves were visualized by applying Kaplan-Meier curves, and
P-values were determined by the log-rank test, P<0.05 was
considered significant. Multivariate analysis of survival was used
in a Cox proportional hazard regression model. All statistical
analysis was conducted using SPSS 13.0 (SPSS Inc., Chicago, IL,
USA).
Results
The expression of PRX5 is associated with
poor prognosis of patients with gastric cancer
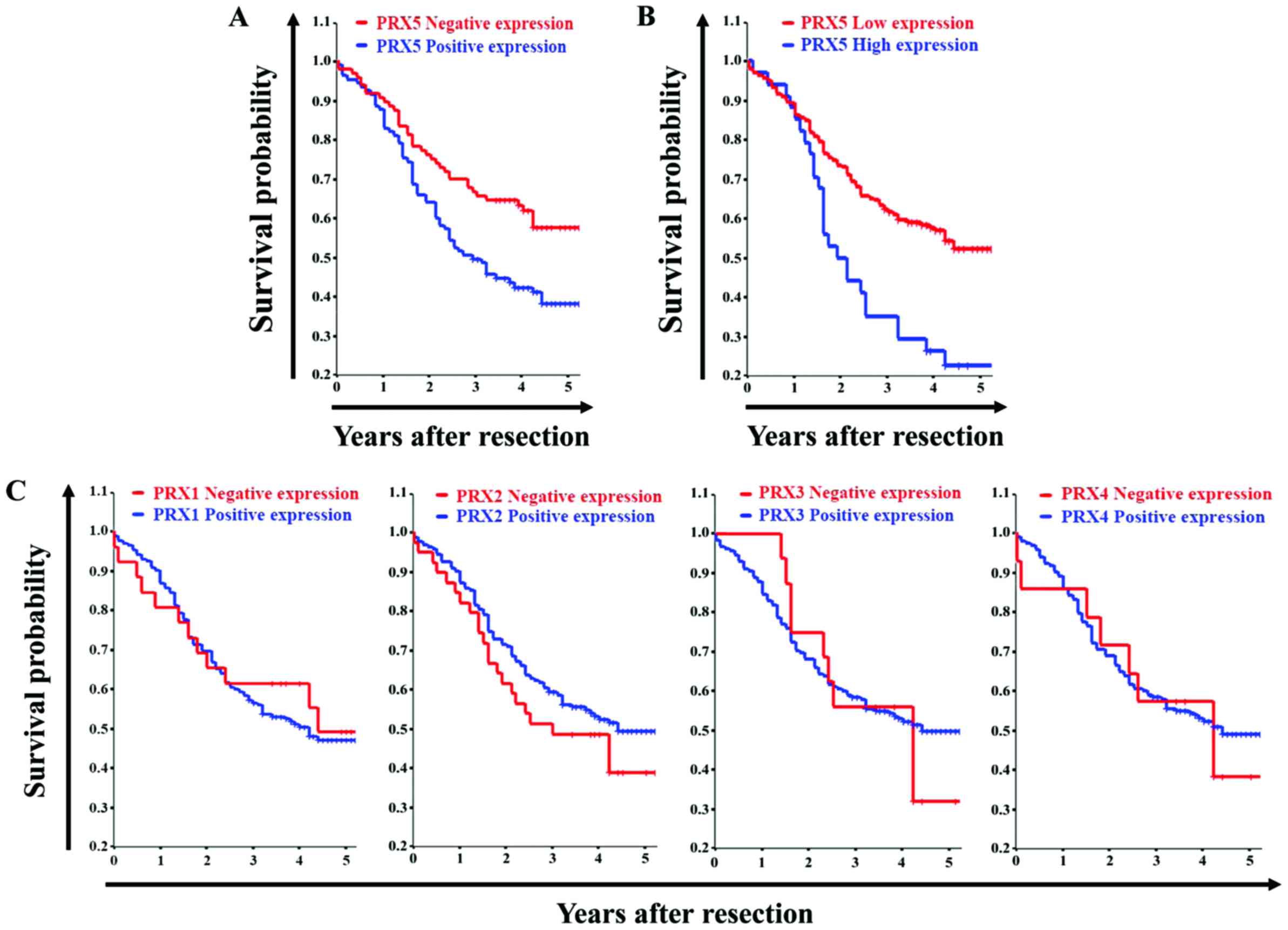
Between 2000 and 2003, we followed up 210 patients
that underwent elective surgery for gastric cancer. The 5-year
follow-up was conducted in two different groups. Patients with
gastric cancer that showed PRX5 positive expression typically had a
reduced 5-year survival of 36.8% compared to 58.7% of patients with
gastric cancer that showed PRX5-negative expression, as depicted in
the survival curve (log-rank test, P=0.007; Fig. 1A). In addition, patients with high
PRX5 expressing tumors showed a reduced 5-year survival of 22.7%
compared to 52.4% for patients with low PRX5-expressing tumors
(log-rank test, P=0.0004; Fig.
1B). However, no significant correlation was found between
PRX1-4 expression and changes in survival rates (Fig. 1C). Also, as expected, we estimated
that no significant correlation between PRX1-4 expression levels
and changes in 5-year survival rates. Overall, the 5-year survival
data analyzed via the log-rank test indicated that overexpression
of PRX5 is correlated with poor prognosis, and that this difference
was statistically significant.
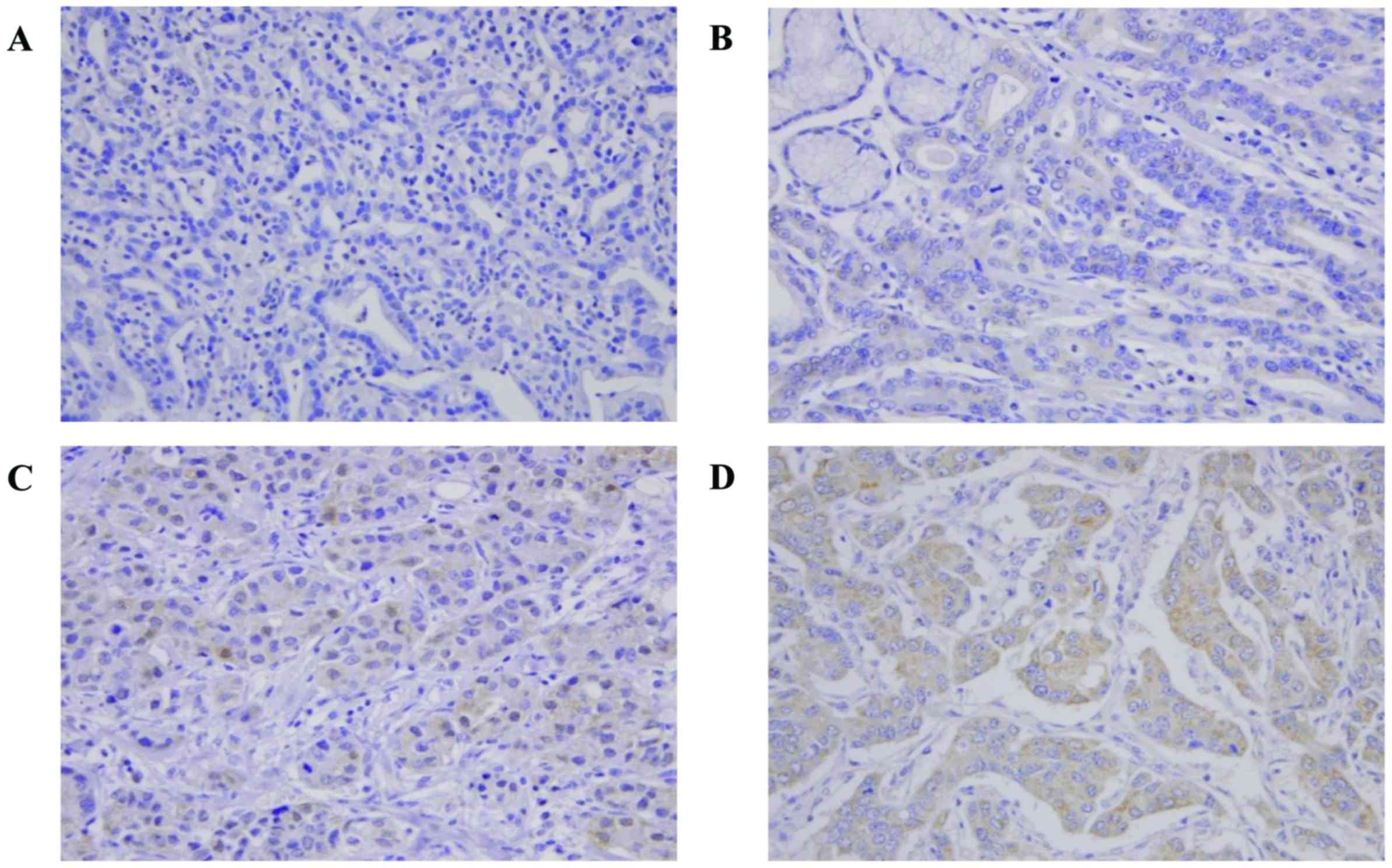
We also examined whether upregulation of PRX5 is
linked to poor prognosis of gastric cancer, immunohistochemical
analysis was performed in 210 paired gastric cancer tissues using
PRX5 specific antibody. As shown in Fig. 2, PRX5 expression was mainly located
in the membrane, cytoplasm, and nucleus of gastric cancer tissues.
In the case of heterogeneous staining within the samples, tumor
tissues were classified into four grades based on the staining
intensity: PRX5 was weakly located in the cytoplasm, weak staining;
PRX5 was located in the membrane and cytoplasm, intermediate
staining; PRX5 was strongly located in the membrane, cytoplasm, and
nucleus, strong staining. These data suggested that PRX5 is a
potential factor that may contribute to poor prognosis of gastric
cancer. The relationships between PRX5 expression and prognostic
factors, such as expression levels of PRX5 and tumor size and grade
are summarized in Table I. The
prognostic factors in patient' survival were tumor size (P=0.001),
high expression of PRX5 (P=0.001) and later tumor stage III/IV
(P<0.001; Table I). The
difference was statistically significant.
 | Table IMultivariate analysis of the
prognostic factors in patient survival. |
Table I
Multivariate analysis of the
prognostic factors in patient survival.
| Factors | HR (95% CI) | P-value |
|---|
| Tumor size (>5
cm vs. ≤5 cm) | 2.171
(1.186–3.977) | <0.01 |
| Stage (III, IV vs
I, II) | 4.502
(2.468–8.214) | <0.01 |
| PRX% expression
(positive vs. negative) | 1.528
(0.875–2.749) | <0.01 |
Correlation of PRX5 expression and
clinicopathological parameters
The relationship between PRX5 expression and
clinicopathological parameters, such as tumor size, differentiation
status, invasion and tumor grade are summarized in Table II. No significant associations
with gender and age were observed with the clinicopathological
parameters analyzed including clinical stages and lymph node
metastasis. Furthermore, there were no significant differences in
tumor location, lymphatic invasion, venous invasion, and proportion
of tumor size >5 cm, depth of tumor and TNM stages in PRX1-4
expressing tumors. However, positive expression of PRX3 and PRX4
tend to be associated with more aggressive tumor differentiation
(PRX3; P=0.02, PRX4; P=0.01). In addition, expression of PRX5 was
significantly correlated with tumor size (P=0.001), depth of tumor
(T status; P=0.001), lymph node involvement (N status; P=0.03), and
TNM* stage (P=0.003). Finally, high expression of PRX5
was associated with lymphatic invasion (PRX5: P=0.03; Table II). These data are consistent with
the hypothesis that PRX5 is a potential factor that may contribute
to the poor prognosis of gastric cancer.
 | Table IIClinicopathological features of
patients with gastric carcinoma organized by PRX1-5 expression. |
Table II
Clinicopathological features of
patients with gastric carcinoma organized by PRX1-5 expression.
| Clinicopathological
features | PRX1 | PRX2 | PRX3 | PRX4 | PRX5 |
|---|
| Sex
(female/male) | 0.82 | 0.62 | 0.85 | 0.06 | 0.31 |
| Location | 0.61 | 0.73 | 0.41 | 0.31 | 0.98 |
| Tumor size (>5
cm/≤5 cm) | 0.09 | 0.67 | 0.85 | 0.53 | <0.01 |
| Differentiation
(differentiated/undifferentiated) | 0.59 | 0.053 | 0.02 | 0.01 | 0.46 |
| Lymphatic invasion
(yes/no) | 0.65 | 0.96 | 0.1 | 0.14 | 0.07 |
| Venous invasion
(yes/no) | 0.97 | 0.71 | 0.67 | 0.14 | 0.24 |
| T stage (T3, 4/T1,
2) | 0.12 | 0.48 | 0.97 | 0.34 | <0.01 |
| Lymph node
involvement (yes/no) | 0.32 | 0.85 | 0.18 | 0.69 | <0.01 |
| TNM stage (III, IV
vs I, II) | 0.87 | 0.09 | 0.66 | 0.43 | <0.01 |
High expression of PRX5 is induced to
mesenchymal phenotype in gastric cancer
Recently, increasing evidence suggests that EMT may
also contribute to poor prognosis in patients with gastric cancer
(25,26). EMT-related markers are
significantly expressed and can also act as prognostic factors
(5–8). The 5-year survival rate of patients
suggests that tumors positive for PRX5 are associated with a lower
survival probability. Thus, we decided to focus on studying the
relationship between PRX5 and EMT. First, we estimated the
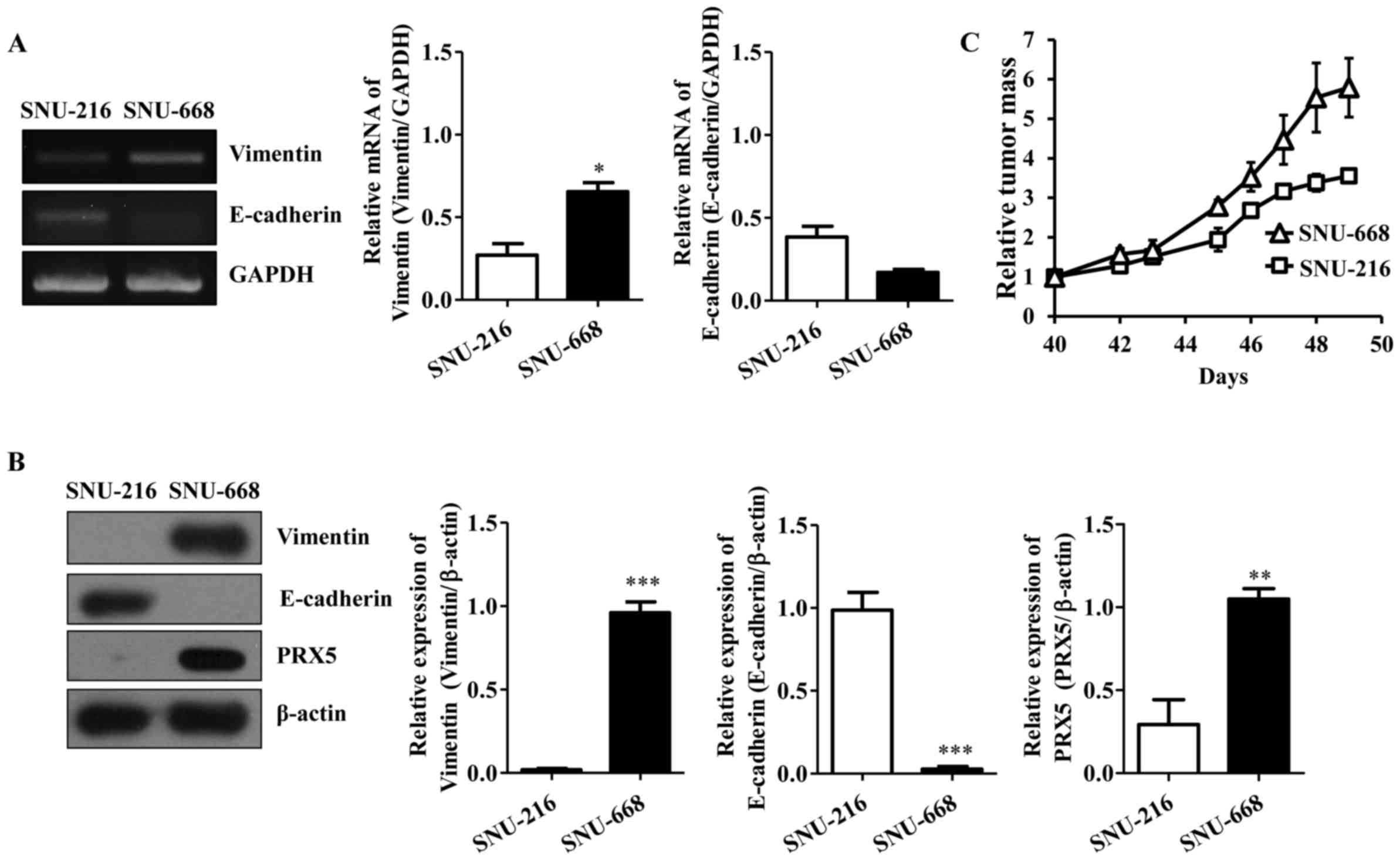
expression of PRX5 in various gastric cancer cell lines. SNU-216
cells showed the lowest expression of PRX5 whereas SNU-668 cells
showed the highest expression of PRX5 (data not shown). We then
analyzed EMT in these cell lines, which is a requirement for
several molecular expression changes. Vimentin is regulated to
change the cell adhesion and migration capacity in cancer cells,
and loss of E-cadherin is required for tumor invasion. Thus,
expression changes in vimentin and E-cadherin are typical features
of EMT (6–8,16,17).
We determined the molecular level of vimentin and E-cadherin.
Interestingly, both RT-PCR and western blot analysis showed high
levels of vimentin and low levels of E-cadherin in SNU-668 cells
expressing high levels of endogenous PRX5, which was not observed
in SNU-216 cells expressing low levels of endogenous PRX5 (Fig. 3A and B). To investigate whether
PRX5 influenced tumor growth in vivo, we performed the tumor
xenograft assay. Tumor size was measured ~50 days after the gastric
cancer cell injection. SNU-668 cells gave rise to larger tumors
than the SNU-216 cells (Fig. 3C).
This suggests that PRX5 may contribute to the mesenchymal phenotype
in gastric cancer.
Exogenous overexpression of PRX5
aggravates carcinogenicity in gastric cancer cells
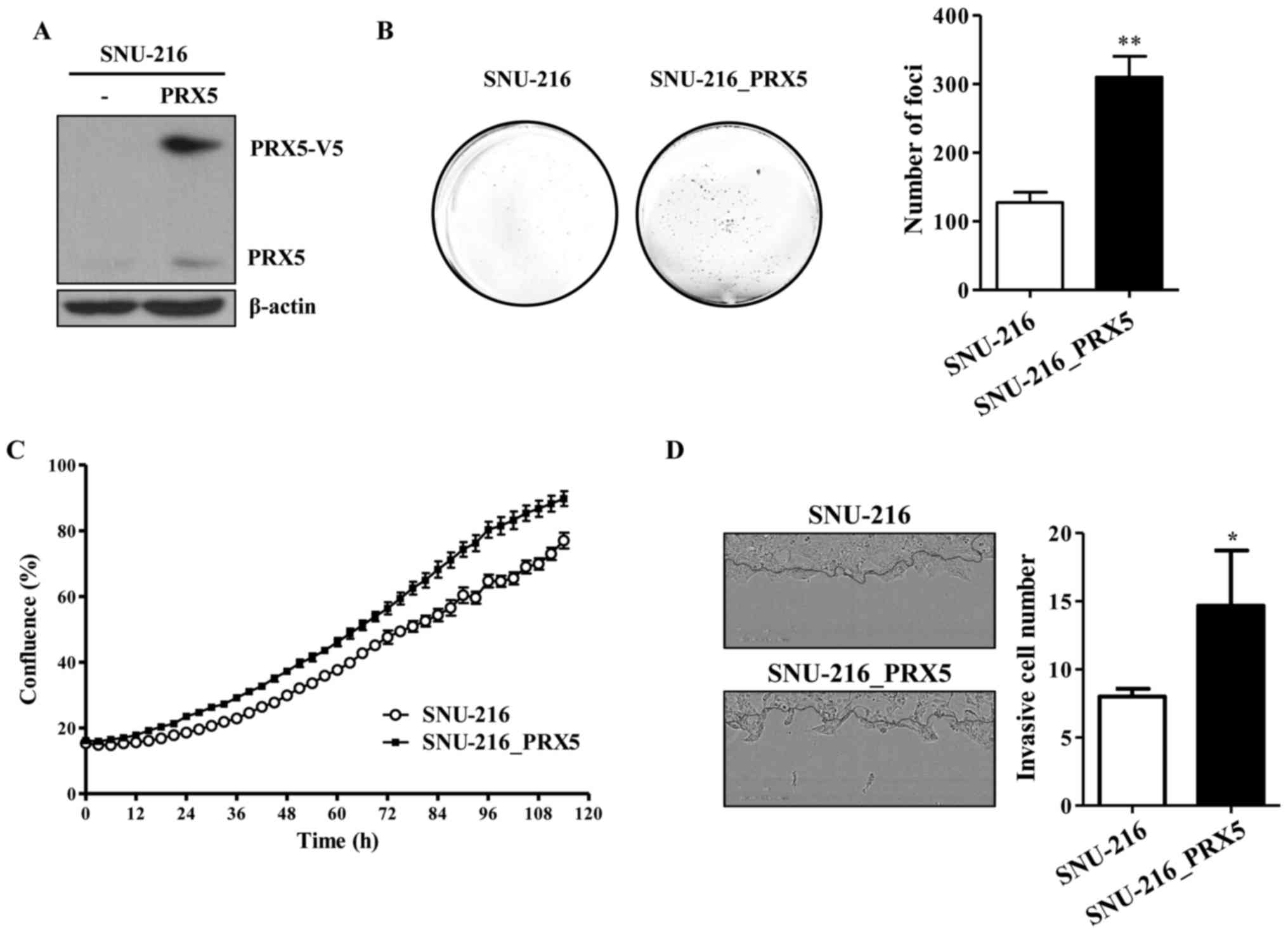
To investigate the functional effect of PRX5 in
carcinogenesis, we overexpressed V5-tagged PRX5 (PRX5-V5) in
SNU-216 cells (SNU-216_PRX5) which was low expressing levels of
endogenous PRX5. SNU-216_ PRX5 was then confirmed via western
blotting (Fig. 4A). To analyze the
carcinogenic capacity between SNU-216 cells and SNU-216_PRX5 cells,
we performed the foci formation assay and invasion assay to assess
their proliferation. The number of foci was increased in
SNU-216_PRX5 cells compared with SNU-216 cells (Fig. 4B). Moreover, we observed higher
proliferation rate in SNU-216_PRX5 cells than in SNU-216 cells. The
proliferation rate of SNU-216_PRX5 cells was also faster than
SNU-216 cells (Fig. 4C). Moreover,
cancer cell invasion was also higher in SNU-216_PRX5 cells than in
SNU-216 cells (Fig. 4D).
Therefore, these results suggest that overexpression of PRX5
enhanced carcinogenicity by increasing the proliferation and
invasiveness of gastric cancer cells.
EMT may be enhanced by PRX5 in gastric
cancer cells
Downregulation of E-cadherin is considered an
important step during EMT. E-cadherin also acts as a suppressor
during tumor progression (16,17).
Thus, we examined the mRNA and protein levels of E-cadherin in
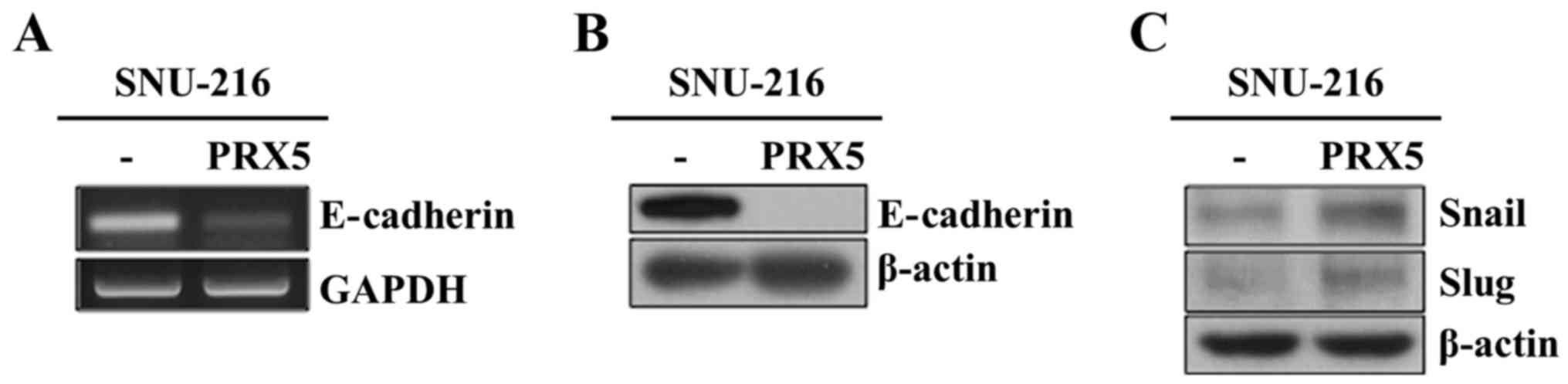
SNU-216_PRX5 cells. As expected, the mRNA levels of E-cadherin was
decreased (Fig. 5A). Moreover, the
protein level of E-cadherin was also declined (Fig. 5B). We also investigated how PRX5
may be regulating E-cadherin expression by analyzing the expression
level of Snail, a known E-cadherin repressor (7). As expected, the protein levels of
Snail and Slug increased in SNU-216_PRX5 cells (Fig. 5C). As a result, we speculated that
PRX5 may upregulate Snail and Slug, consequently resulting in
downregulation of E-cadherin.
Discussion
In this study, we investigated the correlation
between PRX5 and carcinogenesis in gastric cancer cells. We noted
that the 5-year survival rates of patients with PRX5-positive
expressing gastric cancer tumors are poorer than the survival rates
of those with PRX5-negative expressing tumors (log-rank test,
P=0.007; Fig. 1A). We also showed
that the overexpressionof PRX5 is related to poor prognosis in
patients with gastric cancer. To show the correlation between PRX5
and carcinogenesis, we analyzed two gastric cancer cell lines. The
highest expression of PRX5 (SNU-668) and the lowest expression of
PRX5 (SNU-216) gastric cancer cell line were selected, because we
wanted to confirm precise difference between low and high
expression of PRX5 gastric cancer cell lines (data not shown). We
observed that tumor growth of SNU-668 was faster than that of
SNU-216 (Fig. 3C). Moreover,
exogenous expression of PRX5 in SNU-216 cells induced cell
proliferation, invasion, and foci formation (Fig. 4). These results suggested that high
level of PRX5 expression might enhance the tumorigenic phenotype in
gastric cancer cells.
Previous studies have shown that EMT is related to
cancer progression and metastasis (25,27–29).
Changes in expression are also observed in several molecular
markers for EMT such as E-cadherin and vimentin, as well as
transcription factors such as Snail (6–8).
These changes result in a poorer prognosis for patients with
gastric cancer. Vimentin is an important factor of mesenchymal
cells and is critically involved in cell-cell adhesion, migration
and EMT-related signaling pathways. Vimentin also cooperates with
membrane integrins to promote the mesenchymal architecture.
Therefore, vimentin expression correlates with tumor progression
and the survival rate of patients with gastric cancer (16,17).
E-cadherin plays a critical role in cell-cell adhesion and the
maintenance of cell polarity in epithelial cells. The loss of
E-cadherin is associated with cancer cell invasion and worse
survival (19,21–23).
In addition, several reports even suggest that regulation of EMT
may be the key to curing cancer (28–31).
In the present study, we investigated how PRX5 may be regulating
tumorigenicity of gastric cancer cells by analyzing correlations
between PRX5 expression and EMT markers. First, we confirmed mRNA
and protein level of E-cadherin and vimentin in both SNU-216 and
SNU-668 cells (Fig. 3A and B).
SNU-216 cells showed higher levels of E-cadherin and lower levels
of vimentin, whereas SNU-668 cells showed lower levels of
E-cadherin and higher levels of vimentin. In addition,
overexpression of PRX5 in SNU-216 cells resulted in a significant
reduction of E-cadherin (Fig. 5A and
B). Furthermore, to investigate how PRX5 regulates E-cadherin
expression, we analyzed the protein expression level of Snail and
Slug, a well-known repressor of E-cadherin (7). Snail and Slug expression were
significantly increased in SNU-216_PRX5 (Fig. 5C). Our data indicated that PRX5
enhanced the expression of Snail and Slug by which the expression
of E-cadherin was subsequently repressed. Thus our these data
suggest that overexpression of PRX5 significantly correlated with
poorer prognosis in gastric cancer.
Furthermore, previous studies have also suggested
that reactive oxygen species (ROS) and reactive nitrogen species
(RNS) are generated in high amounts during cancer development.
Thus, factors involved in these processes may provide useful
candidate diagnostic and prognostic markers for many types of
cancers (2). Excessive ROS and RNS
may also enhance damaging reactions within lipids, proteins, and
DNA, which can then lead to tumor progression (12–15).
Normally, certain signaling pathways are activated to control ROS
and RNS concentrations within the cell. These signaling pathways
include enzymes such as catalase, superoxide dismutase, and
glutathione peroxidase, as well as other factors such as
antioxidants and reducing agents (15). A large number of researchers have
reported that PRXs is an antioxidant peroxidase protecting against
ROS and ROS-induced damage (14,15,18).
However, other studies have also suggested that PRXs may
significantly enhance tumorigenic efficacy and the metastatic
capability of various cancer cells independent on ROS (19–23).
Finally, PRX5 has been reported to be the type of
PRX family that reacts with both hydrogen peroxide and
peroxynitrite (19,20). To date, six isoforms of PRXs
(PRX1-6) have been identified in mammalian tissues. Also, they are
divided into three major subclasses: typical 2-cysteine (PRX1-4),
atypical 2-cysteine PRX (PRX5), and 1-cysteine PRX (PRX6) (10). These isoenzymes are widely
distributed throughout several subcellular structures, including
the mitochondria, peroxisomes, ER, protoplasm, and the cell
membrane (9). PRX family proteins
are also upregulated in many types of tumors. For instance, PRX1-6
are upregulated in breast, lung and malignant mesothelioma cancer
(21–23). PRX1, 2, and 6 are upregulated in
ovarian cancer tissues, and PRX2-4 are upregulated in prostate
cancer tissues (14,15). In breast cancer, PRX5 is associated
with a larger tumor size, positive lymph nodes, and TNM
classification (16). In
colorectal cancer, PRX1, -2 and -5 are associated with an advanced
stage of cancer and lymph node metastasis (30). Overall, the PRX family of proteins
have been linked to tumor development and progression (19,21–23,30).
In the present study, high expression of PRX5 was significantly
correlated with tumor cell proliferation, tumor size, foci
formation, invasion, lymph node metastasis, and advanced TNM
classification. Patients with higher levels of PRX5 expression in
gastric cancer cells also showed a shorter 5-year survival rate. In
the Cox hazard regression covariate analysis, expression of PRX5
was shown to have an impact on survival.
Taken together, PRX5 is implicated in
multifunctional mechanisms that promote EMT and tumorigenic
phenotype in gastric cancer cells. In addition, PRX5 is an
important factor of diagnosis that may contribute to poor
prognosis. Finally, PRX5 is a putative therapeutic target and
clinical strategy for various cancers overexpressing PRX5.
Acknowledgments
This study was supported by a grant from the Korean
Health Technology R&D Project, Ministry of Health & Welfare
(HI15C0789), by a Chungnam National University Hospital Research
Fund, 2012, grants from the KRIBB Research Initiative Program
(KGM4611714), and the National Research Foundation of Korea funded
by the Republic of Korea Government (NRF-2014R1A2A1A11054095 and
NRF-2017R1A2B4008176).
References
|
1
|
Yoon JH, Choi WS, Kim O, Choi BJ, Nam SW,
Lee JY and Park WS: Gastrokine 1 inhibits gastric cancer cell
migration and invasion by downregulating RhoA expression. Gastric
Cancer. 20:274–285. 2017. View Article : Google Scholar
|
|
2
|
Burlaka AP, Ganusevich II, Gafurov MR,
Lukin SM and Sidorik EP: Stomach Cancer: interconnection between
the redox state, activity of MMP-2, MMP-9 and stage of tumor
growth. Cancer Microenviron. 9:27–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ,
Park DJ, Kim JH, Kim HH and Lee JS: Survival benefit of gastrectomy
± metastasectomy in patients with metastatic gastric cancer
receiving chemotherapy. Gastric Cancer. 14:130–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH,
Kim HR, Ahn JB, Roh JK, Noh SH and Chung HC: Survival benefit of
combined curative resection of the stomach (D2 resection) and liver
in gastric cancer patients with liver metastases. Ann Oncol.
19:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otsuki S, Inokuchi M, Enjoji M, Ishikawa
T, Takagi Y, Kato K, Yamada H, Kojima K and Sugihara K: Vimentin
expression is associated with decreased survival in gastric cancer.
Oncol Rep. 25:1235–1242. 2011.PubMed/NCBI
|
|
7
|
Rosivatz E, Becker KF, Kremmer E, Schott
C, Blechschmidt K, Höfler H and Sarbia M: Expression and nuclear
localization of Snail, an E-cadherin repressor, in adenocarcinomas
of the upper gastrointestinal tract. Virchows Arch. 448:277–287.
2006. View Article : Google Scholar
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halliwell B, Gutteridge JM and Cross CE:
Free radicals, antioxidants, and human disease: Where are we now? J
Lab Clin Med. 119:598–620. 1992.PubMed/NCBI
|
|
10
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grek CL and Tew KD: Redox metabolism and
malignancy. Curr Opin Pharmacol. 10:362–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poli G, Leonarduzzi G, Biasi F and
Chiarpotto E: Oxidative stress and cell signalling. Curr Med Chem.
11:1163–1182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tehan L, Taparra K and Phelan S:
Peroxiredoxin overexpression in MCF-7 breast cancer cells and
regulation by cell proliferation and oxidative stress. Cancer
Invest. 31:374–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kropotov A, Gogvadze V, Shupliakov O,
Tomilin N, Serikov VB, Tomilin NV and Zhivotovsky B: Peroxiredoxin
V is essential for protection against apoptosis in human lung
carcinoma cells. Exp Cell Res. 312:2806–2815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caldeira J, Figueiredo J, Brás-Pereira C,
Carneiro P, Moreira AM, Pinto MT, Relvas JB, Carneiro F, Barbosa M,
Casares F, et al: E-cadherin-defective gastric cancer cells depend
on Laminin to survive and invade. Hum Mol Genet. 24:5891–5900.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY,
Lai KH, Tseng HH, Chou NH, Huang MS, Tseng CJ, et al: Loss of
E-cadherin expression correlates with poor differentiation and
invasion into adjacent organs in gastric adenocarcinomas. Cancer
Lett. 201:97–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tölle A, Schlame M, Charlier N, Guthmann F
and Rüstow B: Vitamin E differentially regulates the expression of
peroxire-doxin-1 and -6 in alveolar type II cells. Free Radic Biol
Med. 38:1401–1408. 2005. View Article : Google Scholar
|
|
19
|
Kim B, Park J, Chang KT and Lee DS:
Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal
cell death by inhibiting ERK-Drp1-mediated mitochondrial
fragmentation. Free Radic Biol Med. 90:184–194. 2016. View Article : Google Scholar
|
|
20
|
Knoops B, Goemaere J, Van der Eecken V and
Declercq JP: Peroxiredoxin 5: Structure, mechanism, and function of
the mammalian atypical 2-Cys peroxiredoxin. Antioxid Redox Signal.
15:817–829. 2011. View Article : Google Scholar
|
|
21
|
Li L, Zhang YG and Chen CL: Anti-apoptotic
role of peroxiredoxin III in cervical cancer cells. FEBS Open Bio.
3:51–54. 2012. View Article : Google Scholar
|
|
22
|
Whitaker HC, Patel D, Howat WJ, Warren AY,
Kay JD, Sangan T, Marioni JC, Mitchell J, Aldridge S, Luxton HJ, et
al: Peroxiredoxin-3 is overexpressed in prostate cancer and
promotes cancer cell survival by protecting cells from oxidative
stress. Br J Cancer. 109:983–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi H, Chang JW and Jung YK:
Peroxiredoxin 6 interferes with TRAIL-induced death-inducing
signaling complex formation by binding to death effector domain
caspase. Cell Death Differ. 18:405–414. 2011. View Article : Google Scholar :
|
|
24
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma. 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar
|
|
25
|
Han G, Lu SL, Li AG, He W, Corless CL,
Kulesz-Martin M and Wang XJ: Distinct mechanisms of
TGF-beta1-mediated epithelial-to-mesenchymal transition and
metastasis during skin carcinogenesis. J Clin Invest.
115:1714–1723. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
29
|
Sung WJ, Park KS, Kwak SG, Hyun DS, Jang
JS and Park KK: Epithelial-mesenchymal transition in patients of
pulmonary adenocarcinoma: Correlation with cancer stem cell markers
and prognosis. Int J Clin Exp Pathol. 8:8997–9009. 2015.PubMed/NCBI
|
|
30
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han MW, Lee JC, Kim YM, Cha HJ, Roh JL,
Choi SH, Nam SY, Cho KJ, Kim SW and Kim SY: Epithelial-mesenchymal
transition: Clinical implications for nodal metastasis and
prognosis of tongue cancer. Otolaryngol Head Neck Surg. 152:80–86.
2015. View Article : Google Scholar
|