Introduction
Cancer cells are confronted with diverse
environmental stresses including hypoxia, nutrient deprivation, and
pH changes caused by metabolic byproducts and the tumour
microenvironment (1–3). Stress factors induce diverse
apoptotic signaling in cells, in which various pro-apoptotic
proteins can be activated. To overcome the apoptotic response,
cancer cells develop diverse ways to inhibit apoptotic signaling
(2,4,5).
Furthermore, these signaling alterations can also allow cancer
cells to resist chemo- or radiotherapeutic challenge (6).
The heat shock protein (HSP) 70 chaperone system is
upregulated in many cancers and facilitates the refolding or
degradation of proteins that are denatured as a result of stress
(7–9). In addition, Hsp70 can also directly
interfere with apoptosis pathways to protect cells (10–12).
The diverse protective mechanisms of Hsp70 are known to confer
resistance to some forms of stress-induced cell death.
Several pro-apoptotic proteins that are directly
inhibited by Hsp70 have been reported. Apoptotic
protease-activating factor 1 (Apaf-1), a key regulatory component
of the caspase-dependent apoptotic pathway, directly associates
with Hsp70 (11,12). This interaction prevents
oligomerization of Apaf-1 with procaspase-9, consequently
inhibiting apoptosome formation. Another pro-apoptotic protein,
apoptosis-inducing factor (AIF) is a mitochondrial intermembrane
protein that initiates one of the key mechanisms of
caspase-independent apoptosis (13). Under stress conditions, AIF, which
is normally well secluded in mitochondria, translocates to the
cytosol and ultimately to the nucleus where it induces
caspase-independent peripheral chromatin condensation and DNA
fragmentation (14).
Autophagic cell death is another type of programmed
cell death, which involves autophagy (15,16).
The crosstalk between autophagic and apoptotic cell death is a
current topic of heightened interest. In relation to the HSP
system, autophagy is a regulatory mechanism that maintains cellular
protein homeostasis by sequestering and delivering large protein
aggregates and whole damaged organelles to lysosomes for
degradation (17). Previous
studies have reported that autophagy is regulated by the heat shock
response. Depletion of HSF-1 potentiates starvation-induced LC3
lipidation, which is associated with the formation of
autophagosomal organelles, while Hsp70 overexpression inhibits this
process (18).
Recently, Hsp70 was reported to be acetylated by
acetyltransferase arrest defective (ARD) 1 (Naa10,
Nα-acetyltransferase 10) and this acetylation contributes to its
protective function under cellular stress (19). Here, we sought to investigate the
molecular mechanisms of Hsp70 acetylation-mediated cellular
protection.
Materials and methods
Cell culture and stimulation
SH-SY5Y, HeLa and HEK293T cells were obtained from
the American Type Culture Collection (ATCC). Cells were grown in
DMEM supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin in a 5% CO2 humidified
atmosphere at 37°C. To induce cellular stress, cells were treated
with 1 µg/ml doxorubicin or 0.3 mM
H2O2 for 24 h.
Plasmid construction
Full-length cDNAs for human Hsp70 (Genbank:
NM_005345.5) and human ARD1 (Genbank: NM_003491.3) were generated
by PCR and subcloned into pCDNA3.1 (FLAG-ARD1) or pEGFP-C3
(GFP-Hsp70) vectors for cellular expression. For the construction
of stable cell lines, cDNA constructs for Hsp70 and ARD1 were
co-inserted into the pIRES vector, purchased from Clontech.
Transfection
Transfection was carried out as described previously
(20). HEK293T cell transfection
used polyethyleneimine (PEI) at a ratio of 4:1 (ml PEI/mg plasmid
DAN) in basal media overnight, followed by a change of media. For
the establishment of stable cells, pEGFP-C3-Hsp70 and
pIRES-GFP-Hsp70-FLAG-ARD1 plasmids were transfected into SH-SY5Y
cells using PolyFect reagent (Qiagen), according to the
manufacturer's instructions. Transfected cells were maintained in
complete DMEM with G418 (500 µg/ml). After several days, the
surviving colonies were selected and amplified.
Antibodies
Anti-cleaved caspase-3 antibody (#9661, 1:3,000),
anti-PARP antibody (#9542, 1:3,000), anti-Apaf-1 (#8969, 1:2,000)
antibody, anti-AIF antibody (#4642, 1:2,000), anti-Atg12 antibody
(#4180, 1:3,000), anti-Beclin-1 antibody (#3495, 1:3,000), and
anti-LC3A/B antibody (#12741, 1:200) were purchased from Cell
Signaling Technology. Anti-Myc antibody (9E10, sc-40, 1:3,000) and
anti-lamin A antibody (4A58, sc-71481, 1:2,000) were purchased from
Santa Cruz. The anti-FLAG antibody (M2, F1804, 1:3,000),
anti-tubulin antibody (DM1A, T9016, 1:3,000) and anti-β-actin
(A2066, 1:3,000) antibody were purchased from Sigma. The anti-GFP
antibody (ab6556, 1:3,000) was from Abcam.
Nuclear fractionation
Cultured cells were washed with PBS, homogenized in
buffer A [10 mM HEPES (pH 7.4), 1.5 mM MgCl2, 10 mM KCl,
0.5 mM DTT, 0.1% NP-40], and centri-fuged at 600 × g for 10 min at
4°C. The nuclear pellet was washed with buffer A, resuspended in
buffer C [10 mM HEPES (pH 7.4), 1.5 mM MgCl2, 0.5 mM
DTT, 20% glycerol, 0.5 mM PMSF, 0.2 mM EDTA, and 420 mM NaCl], and
centrifuged for 30 min at 15,000 rpm, before the supernatant was
isolated.
Immunoblotting and
immunoprecipitation
Proteins were extracted using lysis buffer
consisting of 20 mM Tris (pH 7.5), 150 mM NaCl, 0.1 mM EDTA, 0.2%
Triton X-100 and a protease inhibitor cocktail (Roche). Then, 20 mg
of cell extract was used for immunoblotting. For
immunoprecipitation, 1 mg of protein was incubated with a
corresponding antibody conjugated to A or G beads (Upstate)
overnight at 4°C. Beads were washed three times with washing buffer
containing 20 mM Tris (pH 7.5), 150 mM NaCl and 0.1 mM EDTA.
Following SDS-PAGE, membranes were immunoblotted using the
corresponding primary antibody overnight at 4°C. HRP-conjugated
secondary antibodies were incubated with the membranes for 1 h at
room temperature. Visualization was performed using ECL Plus
(Intron) and LAS-4000 (GE Healthcare).
Immunocytochemistry
HeLa cells were seeded onto glass coverslips in
24-well plates and transfected with GFP-Hsp70 WT and K77R. After
treatment of 0.3 mM H2O2 for 24 h, cells were
fixed in 4% PFA for 20 min and permeabilized in 0.3% Triton X-100
in PBS for 5 min at room temperature. Then, cells were incubated
with LC3A/B antibody (Cell Signaling Technology, #12741, 1:200) and
visualized with Alexa 546-conjugated IgG (Molecular Probes).
Nuclear staining was performed using Hoechst 33342 (Molecular
Probes). The immunofluorescence was visualized using an Axiovert
M200 microscope (Carl Zeiss).
Cell viability assay
Cell viability was calculated by measuring the
amount of lactate dehydrogenase (LDH) released from the cells into
the medium. Conditioned media from cultured cells were collected,
and LDH activity was determined with an LDH assay kit (DoGen).
Total cellular LDH activity was measured by solubilizing the cells
with 0.2% Triton X-100.
Statistical analysis
Results are expressed as the means ± SD P-values
were calculated by applying the two-tailed Student's t-test. A
difference was considered statistically significant at
P<0.05.
Results
Hsp70 acetylation protects cancer cells
from doxorubicin-induced cell death
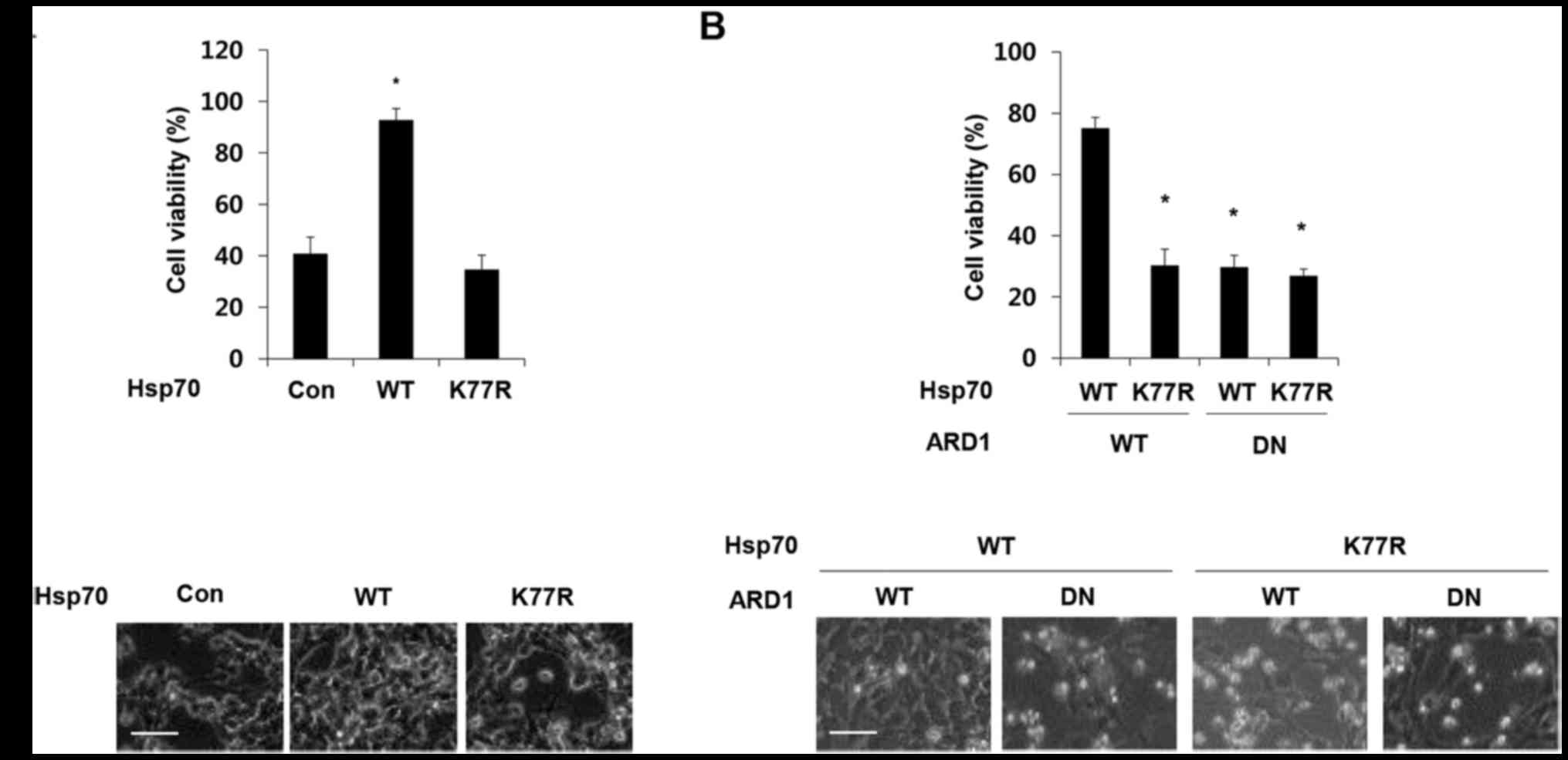
Hsp70 acetylation at residue K77 has been previously
reported to protect cells from stress. To confirm the protective
effect of Hsp70 acetylation, we treated Hsp70 wild-type (WT) and
K77R mutant-expressing SH-SY5Y cells with doxorubicin and analyzed
cell viability. Consistent with a previous report, overexpression
of wild-type Hsp70 protected cells against doxorubicin-induced cell
death, but this protective effect was eliminated by the presence of
the K77R mutation (Fig 1A). The
acetylation of Hsp70 at K77 is mediated by acetyltransferase ARD1
(19). To further validate the
relevance of ARD1 in the protective function of Hsp70, ARD1 WT and
a dominant-negative mutant (DN) that does not harbor
acetyltransferase activity was co-expressed with Hsp70 constructs
in SH-SY5Y cells, and cell viability was assessed after doxorubicin
treatment (21). As expected,
co-expression of the dominant-negative mutant ARD1 abolished the
protective effect of Hsp70 WT (Fig.
1B), indicating that ARD1-mediated Hsp70 acetylation protects
cancer cells from doxorubicin-induced cell death.
Hsp70 acetylation inhibits
caspase-dependent apoptosis
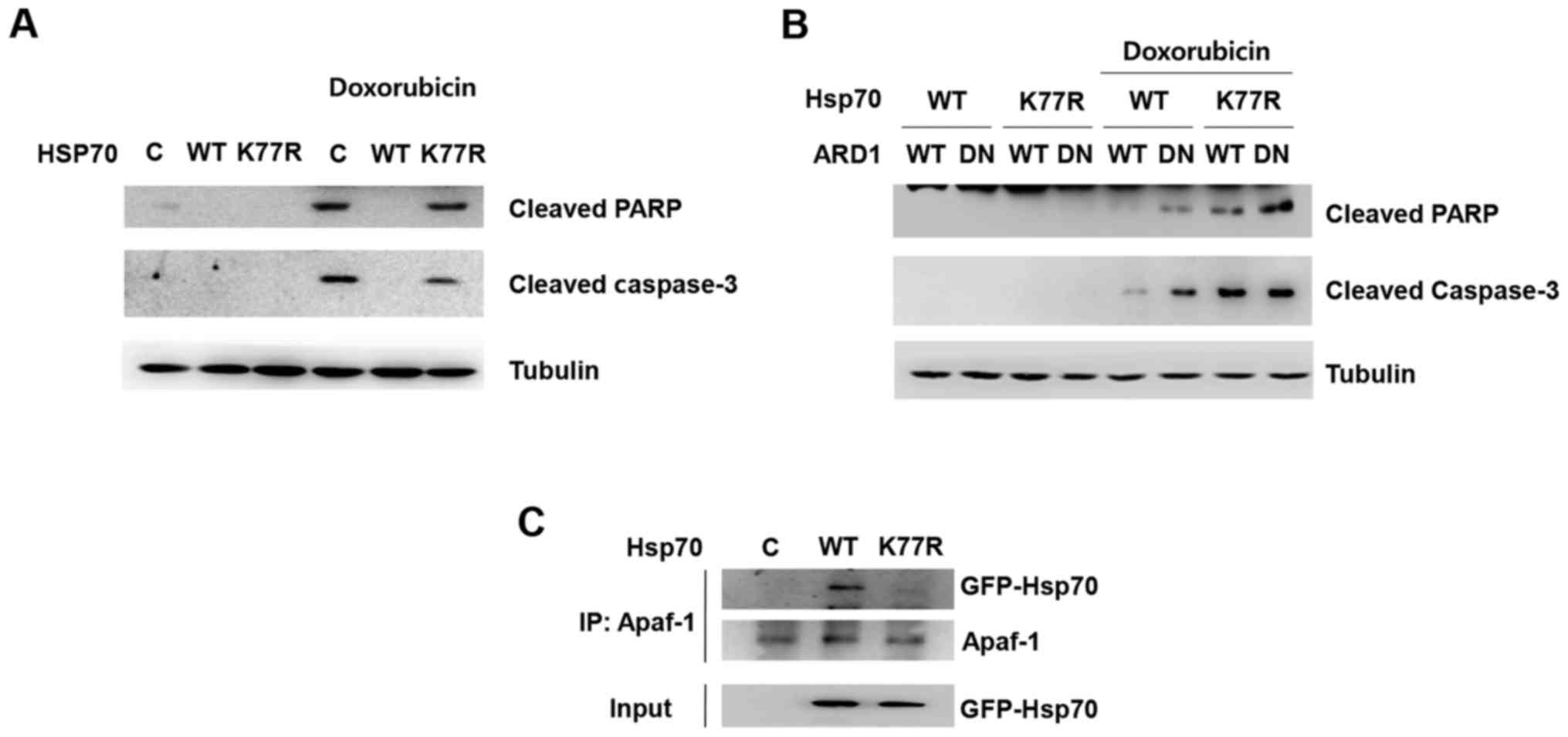
To further investigate the underlying mechanisms of
Hsp70 acetylation-mediated cellular protection, we first analyzed
caspase-dependent apoptosis. Doxorubicin treatment induced cleavage
of caspase-3 and poly(ADP-ribose) polymerase (PARP), which are
hallmarks of apoptosis (Fig. 2A).
Consistent with the doxorubicin-induced cell death shown in
Fig. 1, Hsp70 WT overexpression
prevented doxorubicin-induced cleavage of caspase-3 and PARP,
whereas the presence of the K77R mutation abolished the protective
effect. Moreover, co-expression of the dominant-negative mutant
ARD1 diminished Hsp70 acetylation-mediated inhibition of PARP and
caspase cleavage (Fig. 2B).
Since Hsp70 is reported to inhibit apoptosome
formation via binding with Apaf-1, a key molecule of
caspase-dependent apoptosis, we next investigated whether
acetylation at K77 can modulate Apaf-1 binding. Hsp70 WT or the
K77R mutant was overexpressed in HEK293T cells and their affinity
to Apaf-1 was assessed by co-immunoprecipitation. Consistent with
previous reports, Hsp70 wild-type was co-immunoprecipitated with
Apaf-1 in cell extracts. Interestingly, however, mutation at K77
abrogated its binding to Apaf-1, implying that Hsp70 acetylation
contributes to Hsp70/Apaf-1 association, subsequently leading to
inhibition of functional apoptosome assembly and caspase activation
(Fig. 2C). These results suggest
that Hsp70 acetylation enables the association of Apaf-1 with Hsp70
and prevents apoptosis.
Hsp70 acetylation prevents
caspase-independent apoptosis
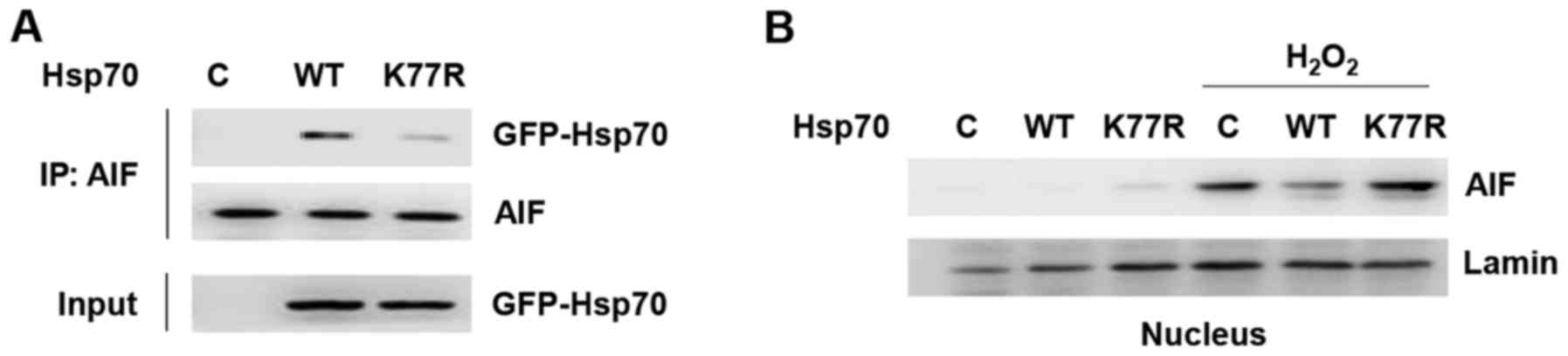
Another cell death pathway in which Hsp70 is
involved is AIF-dependent apoptosis. Hsp70 inhibits
caspase-independent cell death by sequestering AIF and blocking its
induction of apoptosis. To examine its relevance to
caspase-independent apoptosis, we analyzed AIF binding to Hsp70 WT
and the K77R mutant by co-immunoprecipitation (Fig. 3A). Interestingly, compared to Hsp70
WT, AIF binding to the K77R mutant was significantly reduced,
implying that Hsp70 acetylation can interfere with AIF-mediated
caspase-independent apoptosis.
We next verified whether this change in binding
affinity was indeed relevant for AIF function. Nuclear
translocation of AIF is a key final step in the AIF pathway
(14). To validate the difference
in binding affinity and whether it influences AIF nuclear
translocation, Hsp70-expressing cells were treated with hydrogen
peroxide and nuclear translocation of AIF was greatly increased
(Fig. 3B). Moreover, Hsp70 WT
reduced H2O2-induced nuclear translocation of
AIF but the K77R mutant could not. These results demonstrate that
Hsp70 K77 acetylation suppresses apoptosis by preventing AIF from
nuclear translocation, consequently inhibiting caspase-independent
cell death. Collectively, these findings indicate that Hsp70
acetylation protects cancer cells against apoptosis through
inhibition of both caspase-dependent and -independent apoptotic
pathways.
Hsp70 acetylation attenuates autophagic
cell death
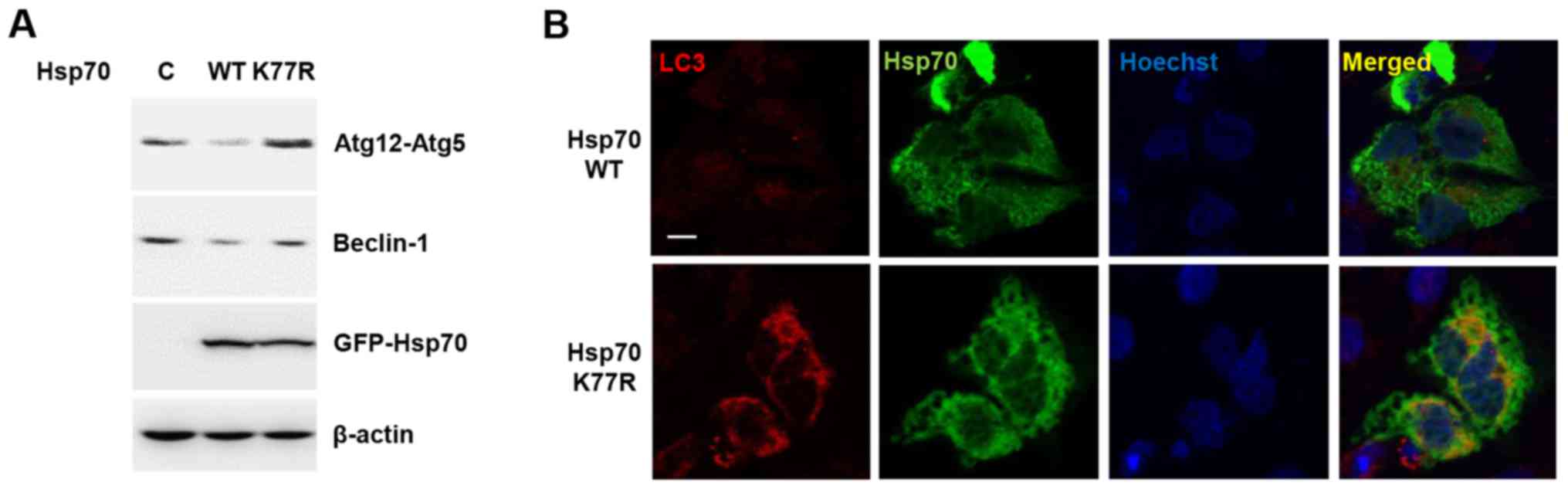
Autophagic cell death is another type of programmed
cell death that is controlled by Hsp70. Recent studies of
stress-induced Hsp70 acetylation and its modulatory role in
autophagosome formation led us to hypothesize that Hsp70
acetylation at K77 can also regulate autophagy (22). To test this, we treated
H2O2 to Hsp70 WT and K77R mutant-expressing
cells and assessed autophagy-related proteins. Autophagy-related
genes (Atg) are universal markers for autophagic induction
(23). During stepwise autophagy
induction, Beclin-1 (the mammalian orthologue of yeast
Atg6)-mediated core complex formation and Atg12-Atg5 conjugation
are key processes, in nucleation and elongation of autophagosome
formation, respectively (24,25).
Consistent with previous reports, Hsp70 WT overexpression decreased
H2O2-induced Beclin-1 and Atg12-Atg5
conjugation, suggesting that Hsp70 plays a regulatory role in the
modulation of autophagy (Fig. 4A).
However, the Hsp70 K77R mutant could not prevent autophagy as
demonstrated by an increase in Beclin-1 and Atg12-Atg5 conjugation.
This observation implies that Hsp70 acetylation contributes to the
attenuation of autophagic cell death.
We also analyzed microtubule-associated protein
light chain 3 (LC3) to monitor autophagic induction. Upon
autophagy, the unconjugated cytosolic form of LC3-I is converted to
the phosphatidylethanolamine-conjugated form of LC3-II that forms
the autophagosomal membrane (26,27).
Therefore, the transition of LC3 from a diffusive cytoplasm pattern
to the punctated membrane pattern is a hallmark of autophagy
induction, indicating the formation of autophagic vacuoles
(27). When compared to WT, the
Hsp70 mutant caused an increase in LC3 expression and perinuclear
autophagic vacuole formation (Fig.
4B). These results suggest that Hsp70 acetylation may play a
role in the prevention of autophagic cell death.
Discussion
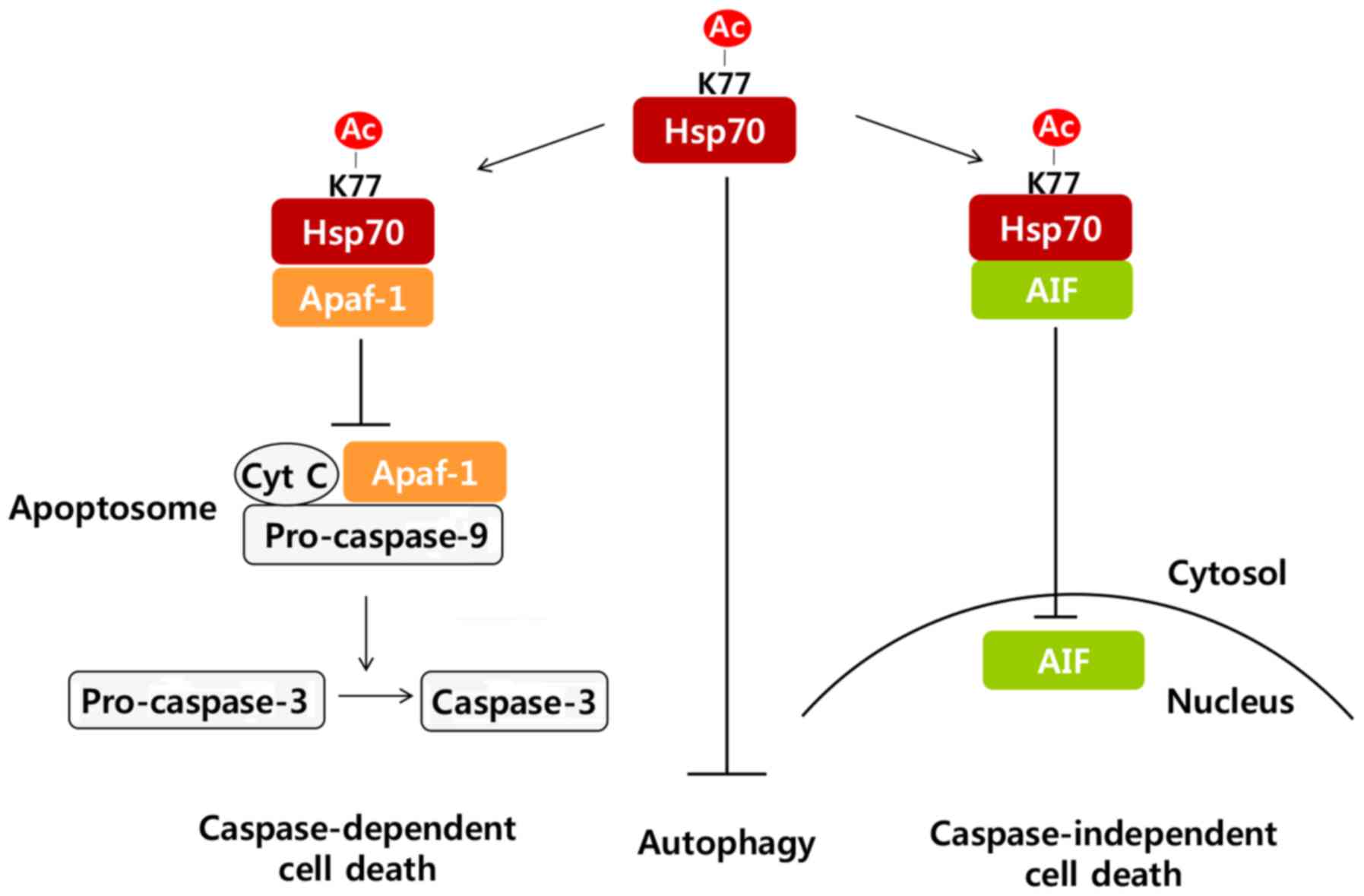
In response to stress, Hsp70 acetylation on K77
residue facili-tated Hsp70 interaction with Apaf-1 and AIF, and
inhibited Apaf-1 and AIF-dependent apoptosis. Moreover, acetylation
of Hsp70 attenuated autophagy, observed via Atg12-Atg5 complex
formation, Beclin-1 expression and perinuclear LC3 puncta
formation, resulting in the inhibition of autophagic cell death.
Taken together, our results suggest that Hsp70 acetylation inhibits
cell death by at least three different mechanisms: i) attenuation
of caspase-dependent pathways by interacting with Apaf-1 and
blocking apoptosome formation, ii) inhibition of
caspase-independent pathways by interacting with AIF and preventing
its nuclear translocation, and iii) attenuation of autophagic cell
death (Fig. 5).
Previously, Hsp70 acetylation induced by cellular
stress was reported to increase its protein refolding chaperone
activity. This is mediated by increased association of Hsp70 with
co-chaperones assisting protein refolding such as Hsp90 and Hop
(19). In addition to co-chaperone
binding, in this study, Hsp70 acetylation facilitated binding of
pro-apoptotic proteins as well, implying a broad impact for Hsp70
acetylation on its overall functionality.
Autophagy, together with HSP systems, represents a
major protein quality control system. To cope with stress-induced
cell damage, Hsp70 maintains protein homeostasis primarily by
facilitating protein refolding and prevent aggregation, while
autophagy results in protein and whole-organelle degradation.
However, the role of autophagy in cell death and survival has long
been controversial (3,28). It has been accepted as a cell
survival mechanism in response to cellular stresses like
starvation. However, recent molecular approaches have provided
evidence that autophagy contributes to programmed cell death
(29,30). Hsp70 has been suggested to play a
crucial role in autophagy regulation, although the underlying
mechanisms need further investigation (18,22).
This study elucidates Hsp70 acetylation as a new regulatory
mechanism in autophagic induction and also adds evidence for the
contribution of autophagy to programmed cell death. Furthermore, it
also suggests the possible linkage between HSP and the autophagy
system mediated by Hsp70 acetylation, although the precise
causality in physiological signals and underlying mechanisms
requires further investigation.
How Hsp70 acetylation at K77 can affect its target
protein affinity is another issue that needs to be addressed. The
nucleotide binding domain (NBD) of Hsp70 that contains K77 is
required for Hsp70/Apaf-1 interaction, whereas the Hsp70/AIF
interaction appears to be independent of NBD (13). Previously, we suggested that K77
acetylation in NBD may induce allosteric conformational changes in
other domains of Hsp70, resulting in overall changes to target
protein binding. The significant location of acetylation site K77
at interdomain contacts increases the interesting possibility that
acetylation may modulate the Hsp70 conformational changes important
for its protein domain interactions and overall activity. Although
detailed studies are needed to elucidate the exact mechanisms
involved, our results provide insight into the acetylation-mediated
allosteric regulation of Hsp70.
In conclusion, we have described cancer cell
survival mechanisms mediated by Hsp70 acetylation under stress. The
findings offer rationale for the development of an Hsp70 inhibitor
that minimizes disturbance of the normal cellular function of
Hsp70. Regulation of Hsp70 K77 acetylation might be helpful in
treating various diseases that involve Hsp70, including cancer,
inflammatory diseases and neurodegenerative diseases.
Acknowledgments
This study was supported by the Global Research
Laboratory Program (2011-0021874), Brain Korea 21 Program, the
Global Core Research Center (GCRC) Program (20110030001), Bio &
Medical Technology Development Program (2015M3A9E6028949), NRF
grant (2015R1C1A2A01054446) through the National Research
Foundation of Korea (NRF) funded by the Ministry of Science, ICT
and Future Planning (MSIP), and Basic Science Research Program
(2013R1A1A2058956, 2016R1D1A1B03935560) through the NRF funded by
the Ministry of Education.
References
|
1
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jezierska-Drutel A, Rosenzweig SA and
Neumann CA: Role of oxidative stress and the microenvironment in
breast cancer development and progression. Adv Cancer Res.
119:107–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Xia X and Pan H: Active autophagy in
the tumor microenvironment: A novel mechanism for cancer
metastasis. Oncol Lett. 5:411–416. 2013.PubMed/NCBI
|
|
4
|
Vaughn AE and Deshmukh M: Glucose
metabolism inhibits apoptosis in neurons and cancer cells by redox
inactivation of cytochrome c. Nat Cell Biol. 10:1477–1483. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Borchert GL, Donald SP, Surazynski
A, Hu CA, Weydert CJ, Oberley LW and Phang JM: MnSOD inhibits
proline oxidase-induced apoptosis in colorectal cancer cells.
Carcinogenesis. 26:1335–1342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fodale V, Pierobon M, Liotta L and
Petricoin E: Mechanism of cell adaptation: When and how do cancer
cells develop chemoresistance? Cancer J. 17:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldberg AL: Protein degradation and
protection against misfolded or damaged proteins. Nature.
426:895–899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartl FU, Bracher A and Hayer-Hartl M:
Molecular chaperones in protein folding and proteostasis. Nature.
475:324–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beere HM, Wolf BB, Cain K, Mosser DD,
Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM and Green DR:
Heat-shock protein 70 inhibits apoptosis by preventing recruitment
of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saleh A, Srinivasula SM, Balkir L, Robbins
PD and Alnemri ES: Negative regulation of the Apaf-1 apoptosome by
Hsp70. Nat Cell Biol. 2:476–483. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ravagnan L, Gurbuxani S, Susin SA, Maisse
C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C,
et al: Heat-shock protein 70 antagonizes apoptosis-inducing factor.
Nat Cell Biol. 3:839–843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Candé C, Cohen I, Daugas E, Ravagnan L,
Larochette N, Zamzami N and Kroemer G: Apoptosis-inducing factor
(AIF): A novel caspase-independent death effector released from
mitochondria. Biochimie. 84:215–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daugas E, Susin SA, Zamzami N, Ferri KF,
Irinopoulou T, Larochette N, Prévost MC, Leber B, Andrews D,
Penninger J, et al: Mitochondrio-nuclear translocation of AIF in
apoptosis and necrosis. FASEB J. 14:729–739. 2000.PubMed/NCBI
|
|
15
|
Liu Y and Levine B: Autosis and autophagic
cell death: The dark side of autophagy. Cell Death Differ.
22:367–376. 2015. View Article : Google Scholar :
|
|
16
|
Tsujimoto Y and Shimizu S: Another way to
die: Autophagic programmed cell death. Cell Death Differ. 12(Suppl
2): 1528–1534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dokladny K, Zuhl MN, Mandell M,
Bhattacharya D, Schneider S, Deretic V and Moseley PL: Regulatory
coordination between two major intracellular homeostatic systems:
Heat shock response and autophagy. J Biol Chem. 288:14959–14972.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo JH, Park JH, Lee EJ, Vo TT, Choi H,
Kim JY, Jang JK, Wee HJ, Lee HS, Jang SH, et al: ARD1-mediated
Hsp70 acetylation balances stress-induced protein refolding and
degradation. Nat Commun. 7:128822016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cha JH, Wee HJ, Seo JH, Ahn BJ, Park JH,
Yang JM, Lee SW, Lee OH, Lee HJ, Gelman IH, et al: Prompt meningeal
reconstruction mediated by oxygen-sensitive AKAP12 scaffolding
protein after central nervous system injury. Nat Commun.
5:49522014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo JH, Park JH, Lee EJ, Vo TT, Choi H,
Jang JK, Wee HJ, Ahn BJ, Cha JH, Shin MW, et al: Autoacetylation
regulates differentially the roles of ARD1 variants in
tumorigenesis. Int J Oncol. 46:99–106. 2015.
|
|
22
|
Yang Y, Fiskus W, Yong B, Atadja P,
Takahashi Y, Pandita TK, Wang HG and Bhalla KN: Acetylated hsp70
and KAP1-mediated Vps34 SUMOylation is required for autophagosome
creation in autophagy. Proc Natl Acad Sci USA. 110:6841–6846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamb CA, Yoshimori T and Tooze SA: The
autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol
Cell Biol. 14:759–774. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanada T, Noda NN, Satomi Y, Ichimura Y,
Fujioka Y, Takao T, Inagaki F and Ohsumi Y: The Atg12-Atg5
conjugate has a novel E3-like activity for protein lipidation in
autophagy. J Biol Chem. 282:37298–37302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lamoureux F, Thomas C, Crafter C, Kumano
M, Zhang F, Davies BR, Gleave ME and Zoubeidi A: Blocked autophagy
using lysosomotropic agents sensitizes resistant prostate tumor
cells to the novel Akt inhibitor AZD5363. Clin Cancer Res.
19:833–844. 2013. View Article : Google Scholar
|
|
28
|
Yonekawa T and Thorburn A: Autophagy and
cell death. Essays Biochem. 55:105–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu L, Alva A, Su H, Dutt P, Freundt E,
Welsh S, Baehrecke EH and Lenardo MJ: Regulation of an ATG7-beclin
1 program of autophagic cell death by caspase-8. Science.
304:1500–1502. 2004. View Article : Google Scholar : PubMed/NCBI
|