Introduction
Angiogenesis occurs when new vessels are generated
from pre-existing vessels, this involves a complicated process,
including vascular destabilization, extracellular matrix
degradation, endothelial cell proliferation, migration, invasion,
tube formation and the recruitment of pericytes. Multiple
pro-angiogenic and anti-angiogenic factors, such as vascular
endothelial growth factor (VEGF), epidermal growth factor (EGF),
fibroblast growth factor (FGF), platelet-derived growth factor
(PDGF), angiopoietin 1 (Ang1) and angiopoietin 2 (Ang2), are
involved in the regulation of angiogenesis (1,2).
Physiological angiogenesis results from a fine-tuning balance
between these pro-angiogenic and anti-angiogenic factors and
establishes a well-organized vessel network to provide oxygen and
nutrients to tissues. When the balance is disturbed, vessel growth
is deregulated, resulting in physiological immature vessels, which
may lead to pathological angiogenesis, including cancer, psoriasis,
blindness, and arthritis (2,3). To
inhibit pathological angiogenesis, several inhibitors and
monoclonal antibodies targeting pro-angiogenic factors have been
developed and applied in clinic. However, there are limitations to
these anti-angiogenic agents, such as serious side effects and drug
resistance, which attenuate their efficacy and hamper drug
development (4,5).
Growth arrest-specific protein 6 (Gas6), which is a
member of the vitamin K-dependent protein family, is an important
pro-angiogenic factor. Gas6 has different affinities for
Tyro-Axl-Mer (TAM) receptor tyrosine kinases (RTKs) and shows
selectively high affinities for Axl receptor tyrosine kinase (Axl)
(6). The Gas/Axl axis is expressed
in endothelial cells (ECs), pericytes, and smooth muscle cells
(7–9), and has been demonstrated to
participate in multiple angiogenic processes including survival,
proliferation, migration, invasion and aggregation of ECs as well
as pericyte adhesion by regulating its downstream signaling
pathways, including the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR)
pathway, the mitogen-activated protein kinase (MEK)/extracellular
signal-regulated kinase (ERK) pathway, the nuclear factor-κB
(NF-κB) pathway and the janus kinase (JAK)/signal transducer and
activator of transcription (STAT) pathway (10–13).
Previously, many preclinical studies have shown that the inhibition
of the Gas6/Axl pathway may contribute to reduction in angiogenesis
(9). R428, which is a selective
inhibitor of the Axl kinase, has been demonstrated to block
Axl-dependent events, such as Akt phosphorylation, breast cancer
cell invasion, and pro-inflammatory cytokine production (14). Desacetylvinblastine monohydrazide,
which is a derivative of vinblastine, has also been reported to
suppress angiogenesis in vitro and in vivo by
suppressing the Gas6/Axl signaling pathway (15). In addition, the inhibition of
Gas6/Axl pathway enhances the effects of multiple anti-angiogenic
therapies in tumors (16).
Altogether, these findings suggest that the Gas/Axl pathway is a
therapeutic target for pathological angiogenesis.
Luteolin (3′, 4′, 5, 7-tetrahydroxy flavone) is
widely distributed in vegetables and fruits and serves as a common
dietary additive (17).
Preclinical studies have demonstrated that luteolin has multiple
pharmacological activities, such as anti-inflammation, anti-tumor,
and anti-microbial activities (18). Moreover, luteolin dietary additive
has been considered favorable in many pathological angiogenesis
therapies (19). Previously,
luteolin has been shown to inhibit tumor angiogenesis, including
human lung cancer, breast cancer, and prostate tumor, however, the
mechanism underlying this inhibition remains to be elucidated
(20). In the present study, we
reveal that luteolin suppresses Gas6-induced angiogenesis in
vitro, ex vivo and in vivo by downregulating the
Gas6/Axl signaling pathway. Our results show that luteolin inhibits
Gas6-mediated proliferation, motility and tube formation of human
microvascular endothelial cells (HMEC-1s), which are required for
neovascularization, and Gas6-induced recruitment of pericytes
during vessel maturation. In addition, using an aortic ring assay
and a chick chorioallantoic membrane (CAM) assay, we demonstrate
that luteolin suppresses Gas6-induced vascular sprouting and
neovascularization.
Materials and methods
Materials
Luteolin (≥98% pure) was purchased from
Sigma-Aldrich (St. Louis, MO, USA), dissolved in DMSO and stored at
−20°C. Fetal bovine serum (FBS) was obtained from Life Technology
(Carlsbad, CA, USA). Pentobarbital sodium was purchased from Merck
(Darmstadt, Germany). Matrigel was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). The antibodies against Axl, PI3K,
p-PI3KTyr458, Akt, p-AktThr308, mTOR,
p-mTORSer2448, p70S6k, p-p70S6kThr389, heat
shock protein 90 (HSP90), matrix metalloproteinase 2 (MMP-2),
matrix metalloproteinase 9 (MMP-9), and donkey anti-Rabbit IgG were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
p-AxlY779 antibody and recombinant human Gas6 were
obtained from R&D Systems (Minneapolis, MN, USA). The pericyte
medium (PM) was obtained from ScienCell Research Laboratories (San
Diego, CA, USA). The fertilized chicken eggs were purchased from
South China Agricultural University (Guangzhou, China). PKH 26, PKH
67 and other reagents were purchased from Sigma-Aldrich.
Cell lines and cell culture
Human microvascular endothelial cells (HMEC-1s) were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA) and cultured in RPMI-1640 medium (Gibco, Invitrogen Corp.,
Carlsbad, CA, USA) supplemented with 10% FBS. HBVPs were obtained
from ScienCell Research Laboratories and cultured in PM. All cells
were cultured at 37°C under humidified atmosphere containing 5%
CO2.
Animals
Adult male Sprague Dawley rats (weighing 180–220 g)
were obtained from the Guangdong Medical Experimental Animal Center
(Guangzhou, China). The animals were maintained in a specific
pathogen-free room with free access to water and standard
laboratory chow. All animal experiments were approved by the
laboratory animal ethics committee of Jinan University (Guangzhou,
China).
Cell viability assay
The effect of luteolin on the viability of HMEC-1s
was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay. HMEC-1s were seeded in
96-well plates at 1×104 cells per well and cultured for
24 h. Then, the cells were treated with various concentrations of
luteolin. After incubating for 24 and 48 h, the cell viability was
determined using the MTT assay. The Gas6-induced HMEC-1s
proliferation was measured by the MTT assay as previously described
(21). HMEC-1s were seeded in
96-well plates at 1×104 cells per well and cultured for
24 h. Adherent cells were starved with serum-free RPMI-1640 medium
for 6 h, then, the cells were treated with various concentrations
of luteolin, and Gas6 (100 ng/ml) was simultaneously added. After
incubating for 24 and 48 h, the cell viability was determined using
the MTT assay.
Wound-healing migration assay
HMEC-1s were grown to 100% confluence in 6-well
plates. The cells were starved with serum-free RPMI-1640 medium for
6 h, and then scratched with pipette tips. The cells were cultured
with 1% FBS RPMI-1640 medium containing various dilutions of
luteolin in the presence or absence of Gas6 (100 ng/ml) for 8 h.
Images of the cells were taken 0 and 8 h after wounding.
Transwell migration and Matrigel invasion
assay
The in vitro migration and invasion were
assessed using a Transwell assay with or without Matrigel as
previously described (22).
Briefly, 2×104 HMEC-1s were suspended in 100 µl
of the serum-free RPMI-1640 medium with or without various
concentrations of luteolin (10 and 20 µM) added to the upper
chamber insert, the bottom chamber was filled with 600 µl of
fresh RPMI-1640 medium supplemented with or without Gas6 (100
ng/ml). After 24-h incubation, the upper chamber was fixed with 4%
paraformaldehyde for 30 min and the cells were stained with 0.1%
crystal violet. The non-migrated HMEC-1s that remained on the inner
side of the upper chamber were removed with a cotton swab. The
cells on the lower surface were photographed under an Olympus IX70
inverted microscope. For the cell invasion assay, the upper chamber
was pre-coated with 35 µl of diluted Matrigel (Matrigel was
diluted to a ratio of 3:1 with PBS) for 1 h at 37°C, and the assay
was performed according to the procedures described above. The
migrated and invasive cells were quantified using Image-Pro Plus
6.0.
Tube formation assay
The HMEC-1s were seeded in Matrigel-coated 96-well
plates at a density of 2.5×104 cells per well with or
without the presence of the indicated concentrations of luteolin
(10 and 20 µM), and Gas6 (100 ng/ml) was simultaneously
added. After 8 h, capillary-like tubes were observed and
photographed under an Olympus IX70 inverted microscope, and the
number of tubes was calculated by Image-Pro Plus 6.0.
Aortic ring assay
The dorsal aortas were isolated from the
Sprague-Dawley rats as previously described (15). Then the dorsal aortas were cut into
1- to 1.5-mm-long rings. Next, the rinsed aortic rings were placed
in Matrigel-coated 96-well plates and sealed with an overlay of 60
µl of Matrigel. After 2-h incubation, fresh RPMI-1640 medium
containing Gas6 (300 ng/ml) was added, and the rings were incubated
for 48 h. Then, the aortic rings were treated with luteolin (10
µM or 20 µM) for 7 days. The microvessels were
photographed under an Olympus IX70 inverted microscope, and the
microvessel structures that extend outward from the aortic ring
were quantified using Image-Pro Plus 6.0.
Three-dimensional co-cultures of HMEC-1s
and HBVPs
The three-dimensional (3D) co-culture of the HMEC-1s
and HBVPs was performed as previously described with slight
modifications (23). Briefly, the
HMEC-1s were labeled with PKH 26 (λex=551 nm, λem=567 nm) following
the manufacturer's instructions. Next, 2×104 HMEC-1s
were seeded into Matrigel-coated 96-well plates and incubated for 2
h to allow the capillary network to form. Then, 2×104
HBVPs, which were pre-treated with luteolin for 4 h and labeled
with PKH 67 (λex=490 nm, λem=502 nm), were added to the capillary
network and incubated for another 10 h to allow the HBVPs to adhere
to the capillary network and form complicated and solid tubes. The
capillary networks were observed and captured under an Olympus IX70
inverted microscope at 0, 2 and 10 h after the addition of
HBVPs.
CAM assay
The CAM assay was performed as previously described
with slight modifications (24).
Fertilized chicken eggs were incubated at 37.8°C in a humidified
incubator containing 60–65% humidity for 5 days (blunt end up). A
small window approximately 1×1 cm, was carefully drilled in the
eggshell on the gas chamber side to create an artificial gas
chamber. Then, the shell and shell membrane were removed to expose
the CAM. Next, a piece of filter membrane containing Gas6 (300
ng/ml) and the indicated concentrations of luteolin (10 and 20
µM) dissolved in 20 µl PBS, was placed in the center
of the CAM. The window was sealed with transparent tape and the CAM
was incubated for another 48 h. The microvessels in the CAM were
observed under an Olympus SZX18 dissecting microscope, and the
microvessels were quantified with Image-Pro Plus 6.0.
Western blot analysis
HMEC-1s were starved for 6 h in serum-free RPMI-1640
medium and HBVPs were treated with serum-free PM in the same way,
then the cells were treated with luteolin (10 and 20 µM) for
4 h. After luteolin was removed, cells were stimulated with Gas6
(100 ng/ml) for another 1 h. Then, the cells were harvested and
lysed with RIPA buffer at 4°C. Proteins were analyzed by
electrophoresis on 10% SDS-polyacrylamide gels and then detected by
western blot as previously described (25). Protein gray values were measured
with ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All experiments were performed in triplicate. The
data are expressed as the mean ± SEM, and the statistical analyses
were performed with GraphPad Prism 5.0 (GraphPad Software, La
Jolla, CA, USA). Significant differences were evaluated with
one-way ANOVA followed by Tukey's test. P<0.05 was considered
statistically significant.
Results
Luteolin inhibits the Gas6-induced
proliferation of HMEC-1s
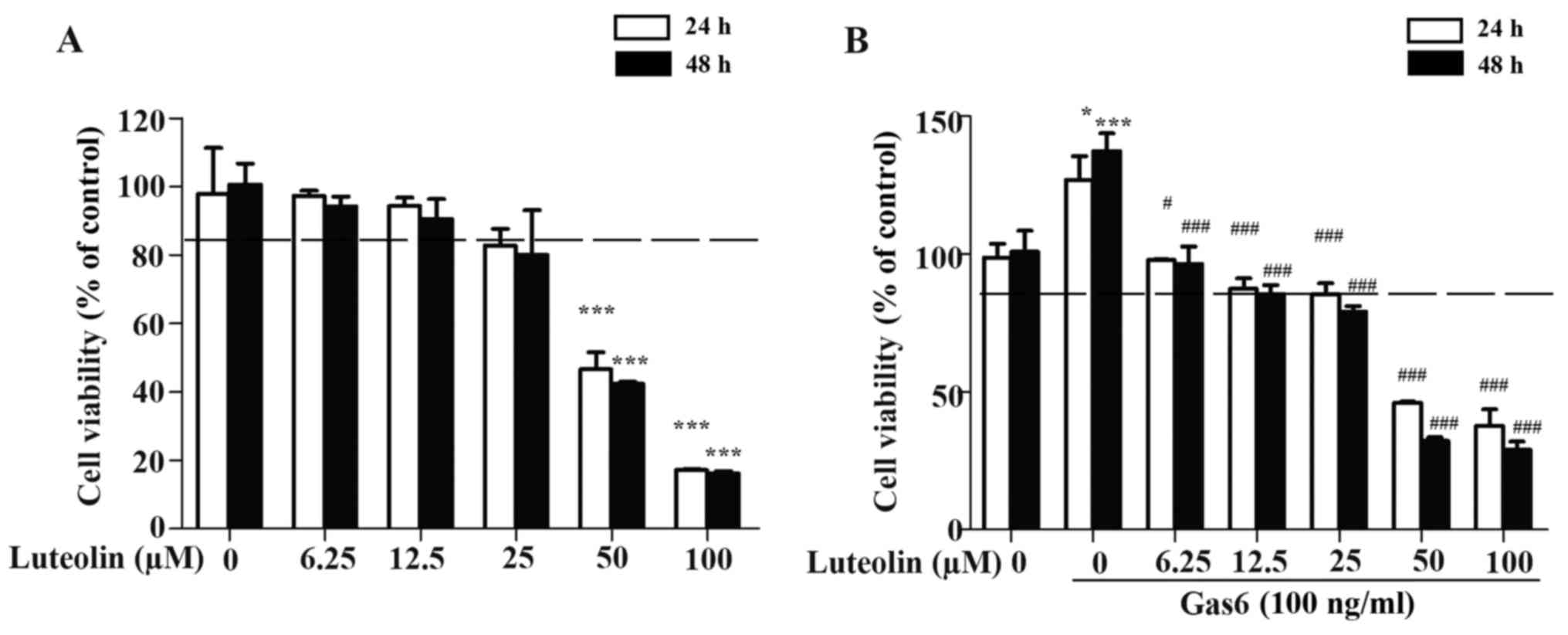
First, the non-toxic concentration of luteolin in
HMEC-1s was determined by the MTT assay. Our results showed that
luteolin had a negligible effect on the proliferation of HMEC-1s at
a dose range of 10 to 20 µM (Fig. 1A). Therefore, the concentrations of
10 and 20 µM were identified as non-toxic doses and used in
the subsequent in vitro, ex vivo and in vivo
experiments. Then, we examined the effect of luteolin on the
Gas6-induced proliferation of HMEC-1s. We found that Gas6
significantly promoted the proliferation of HMEC-1s. In addition,
luteolin caused a concentration-dependent inhibitory effect on
Gas6-induced growth of the HMEC-1s (Fig. 1B).
Luteolin inhibits the Gas6-induced
migration and invasion of HMEC-1s
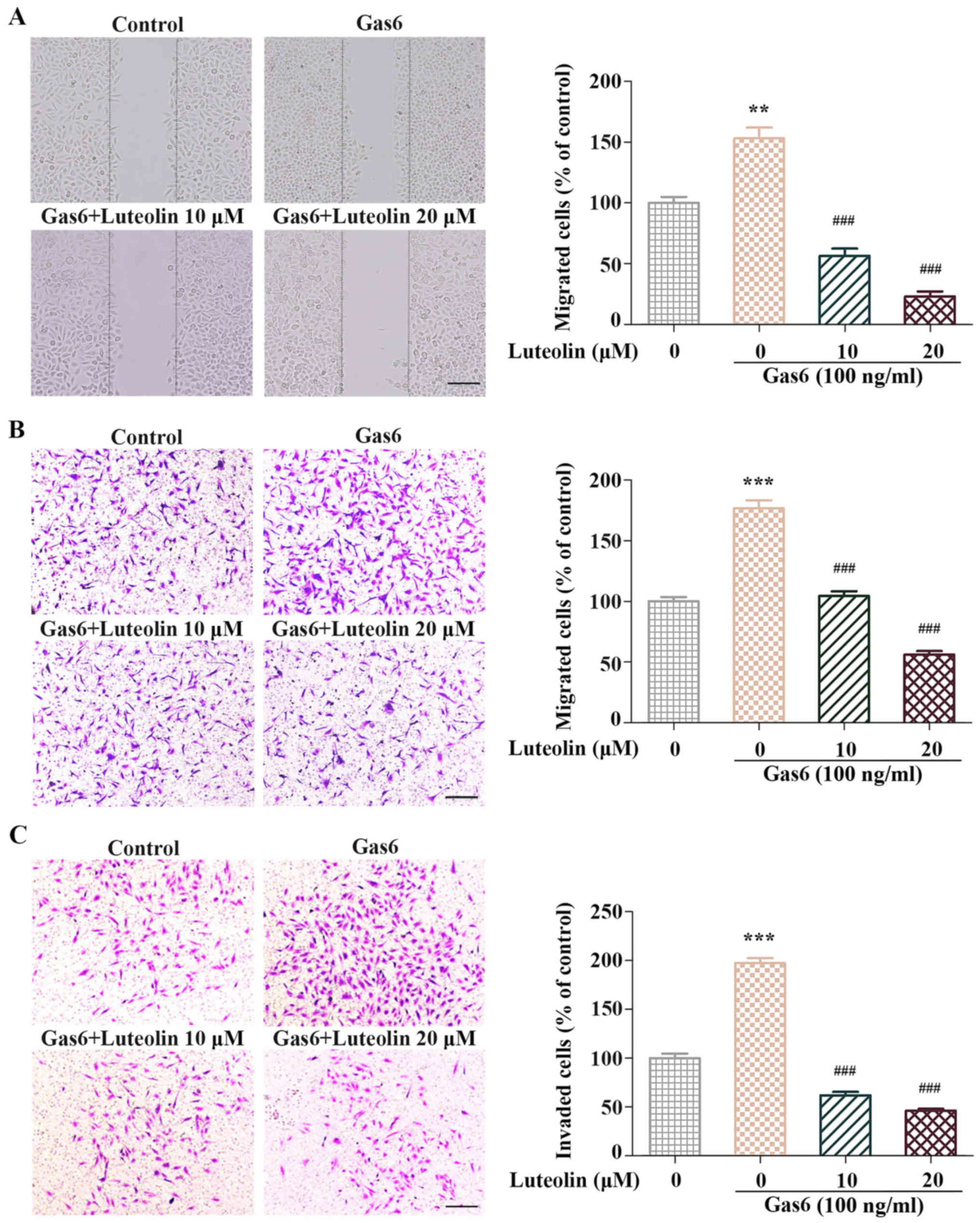
The effect of luteolin on the Gas6-induced migration
was evaluated by the wound-healing migration assay and Transwell
assay. Confluent HMEC-1s were starved with serum-free RPMI-1640
medium for 6 h and scratched with pipette tips. Then, the cells
were treated with or without luteolin and Gas6 (100 ng/ml). In the
Gas6-treated group, the number of migrated HMEC-1s increased
compared with the control group; however, the luteolin treatment
suppressed the Gas6-induced migration of HMEC-1s (Fig. 2A). Similar results were observed in
the migration chamber and invasion chamber assay. Gas6 facilitated
the migration and invasion of HMEC-1s, and the stimulatory effect
of Gas6 was attenuated by luteolin (Fig. 2B and C). Taken together, luteolin
significantly suppressed the Gas6-induced motilities of
HMEC-1s.
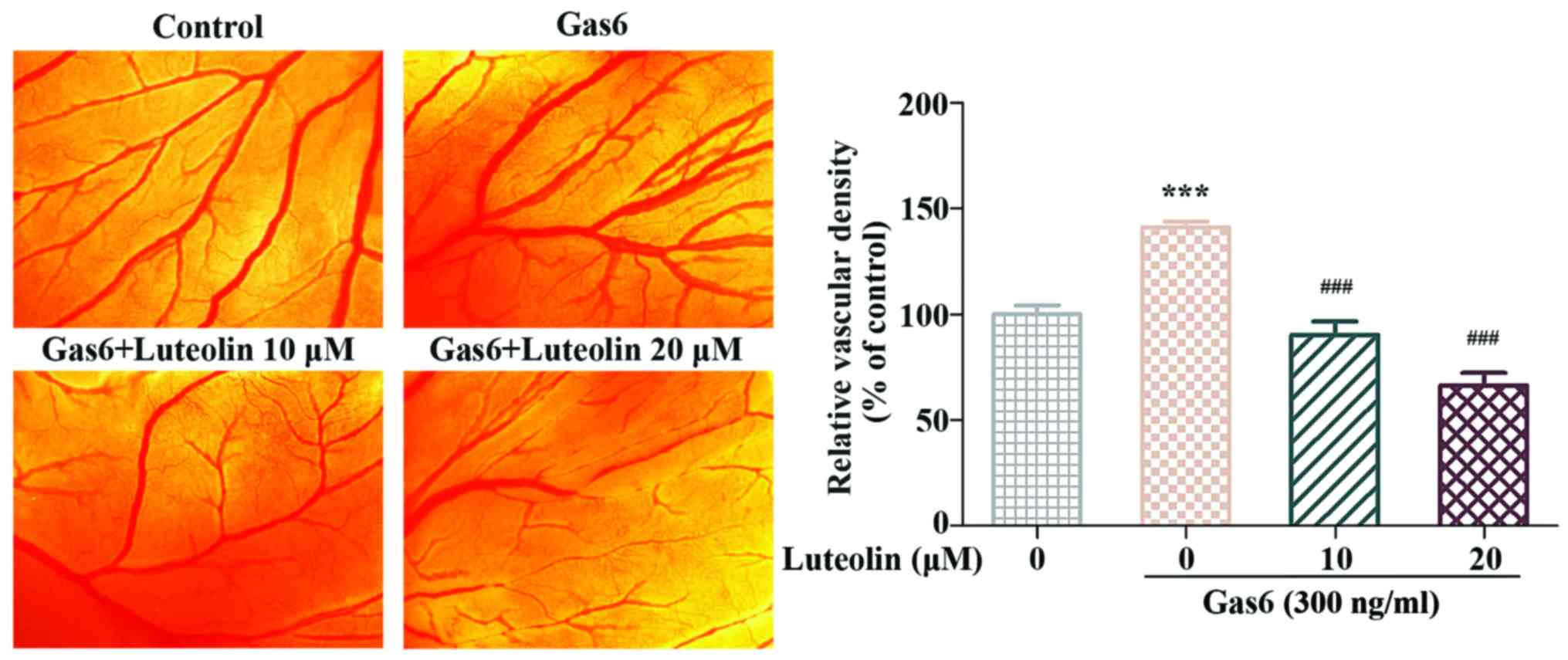
Luteolin inhibits the Gas6-induced
angiogenesis ex vivo
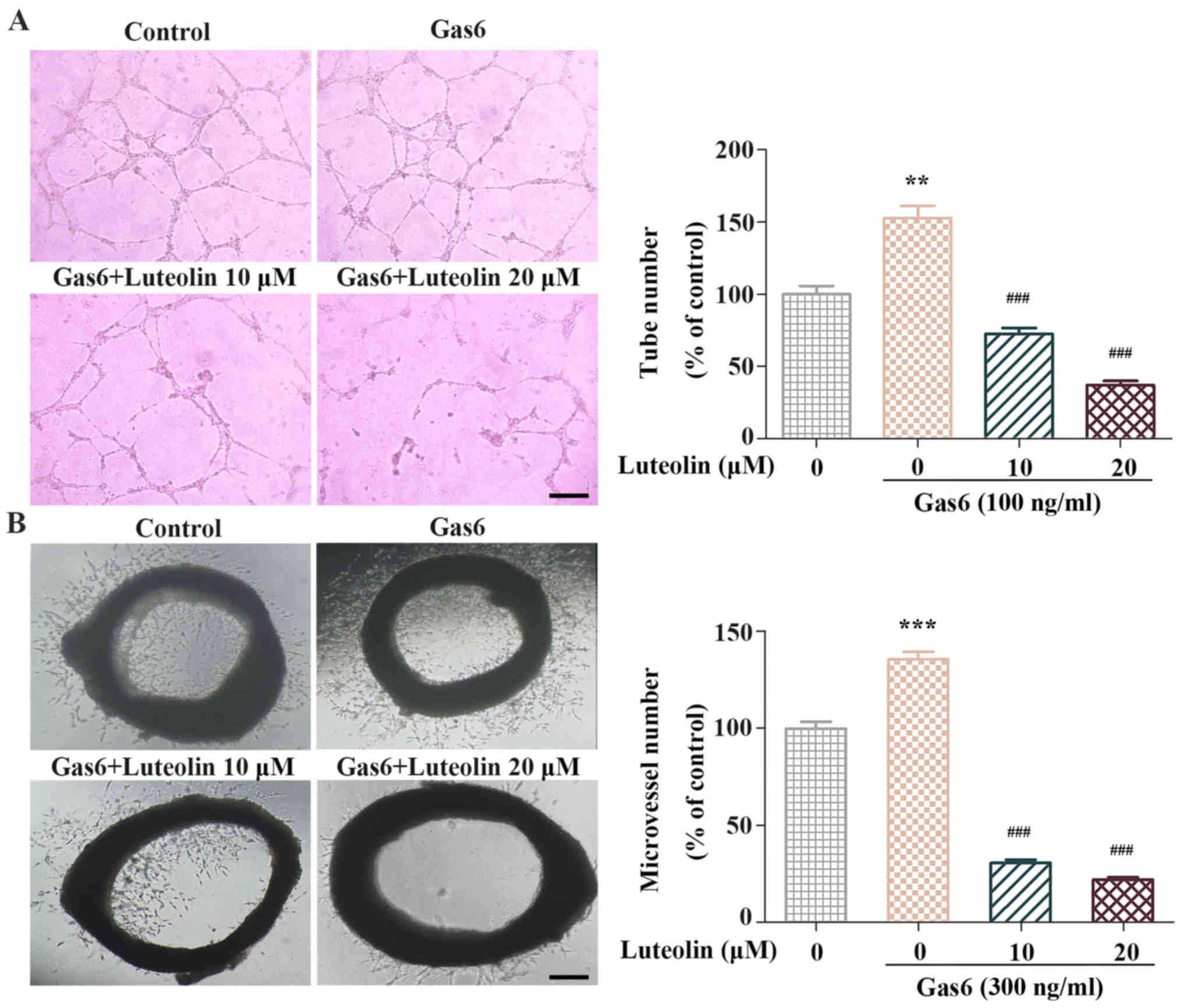
We performed a tube formation assay and an aortic
ring assay to examine the anti-angiogenic effects of luteolin ex
vivo. In the tube formation assay, we found that the
capillary-like tube networks in the Gas6-treated group were more
solid and complicated than those in the control group. In contrast,
luteolin significantly inhibited Gas6-induced tube formation. The
number of tubes was significantly decreased after luteolin exposure
(Fig. 3A). Next, we used a mouse
aortic ring angiogenesis assay to investigate whether luteolin
suppressed the Gas6-induced outgrowth of microvessels. The
outgrowth of microvessels in the aortic ring assay was remarkably
enhanced following Gas6 treatment. In contrast, luteolin markedly
suppressed Gas6-induced microvessel sprouting (Fig. 3B). These results suggested that
luteolin inhibited Gas6-induced angiogenesis ex vivo.
Luteolin inhibits the Gas6-induced
pericyte recruitment to endothelial tubes
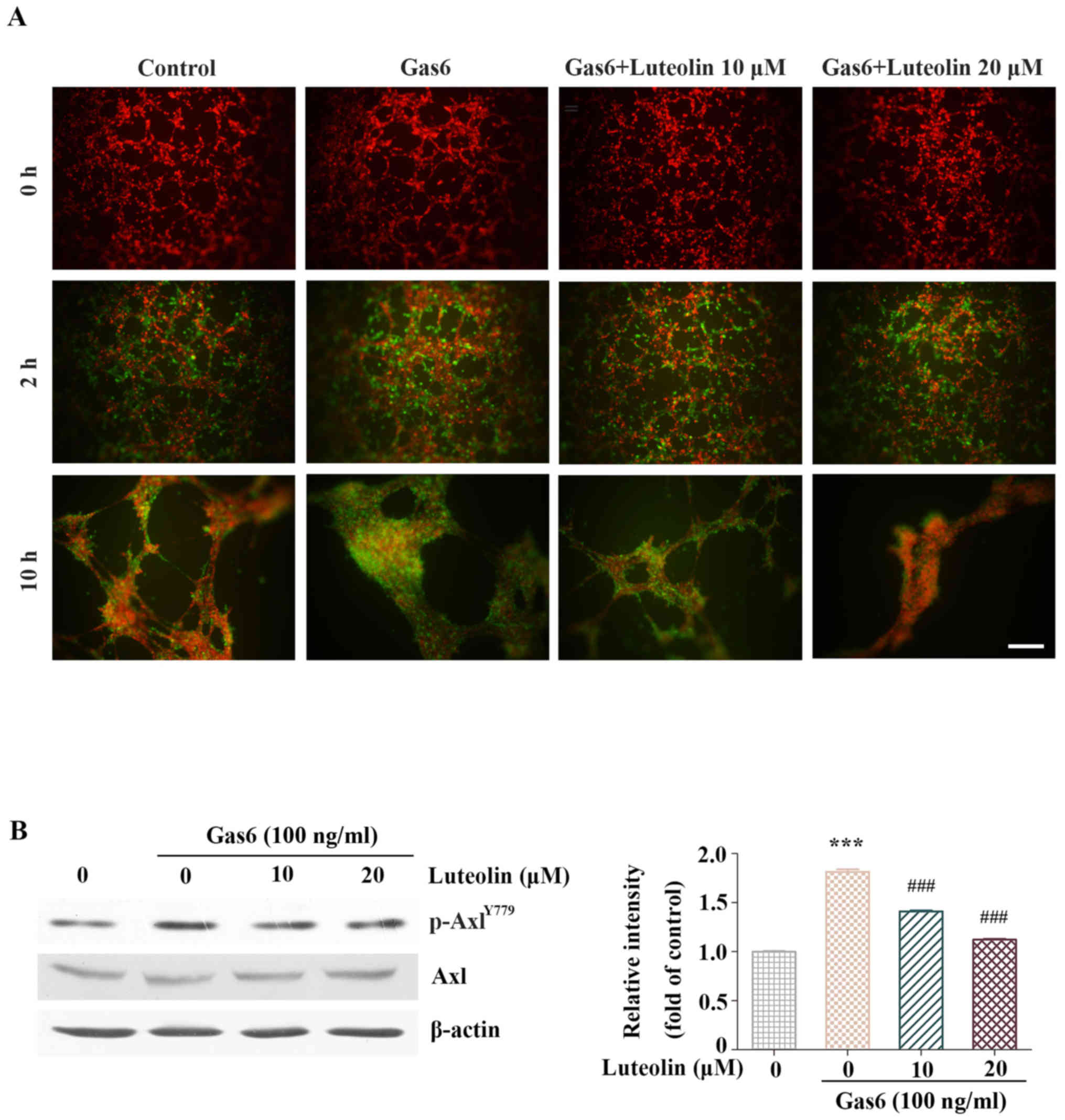
Since pericytes are crucial for the maturation of
neovessels during late-stage angiogenesis and Axl is overexpressed
in pericytes (26,27), we conducted a 3D co-culture of
HMEC-1s and HBVPs to evaluate the effect of luteolin on pericyte
recruitment to the endothelial tubes. A large number of HBVPs
migrated to the endothelial tubes within 2 h in the control group.
Noteworthy, almost all Gas6-treated HBVPs migrated and adhered to
the formed endothelial tubes. However, few HBVPs were recruited to
the endothelial tubes in the luteolin-treated group. In addition,
the tubes in the Gas6-treated group were more complex and solid
after an additional 10-h incubation compared with those in the
control group. However, only a few HBVP-supported tubes were
observed after the luteolin treatment. These results suggested that
luteolin inhibited Gas6-induced HBVP recruitment to the endothelial
tubes (Fig. 4A). Then, the
inhibitory effect of luteolin on the activation of Axl in HBVPs was
examined by western blotting. Our results showed that luteolin
significantly suppressed the Gas6-induced phosphorylation of Axl in
HBVPs (Fig. 4B).
Luteolin inhibits the Gas6-induced
angiogenesis in CAM
We then conducted a CAM assay to examine the
anti-angiogenic effect of luteolin in vivo. The CAM assay is
a widely used and accessible system in angiogenesis studies
(28). We found that the blood
vessels in the Gas6-treated group formed a dense and
spatially-oriented branching network, and the number of blood
vessels in the embryonic neovascularization increased significantly
compared with control group. However, in the luteolin-treated
group, the number of blood vessels was significantly decreased
compared with Gas6 group (Fig. 5).
These results suggested that luteolin suppressed the Gas6-induced
angiogenesis in vivo.
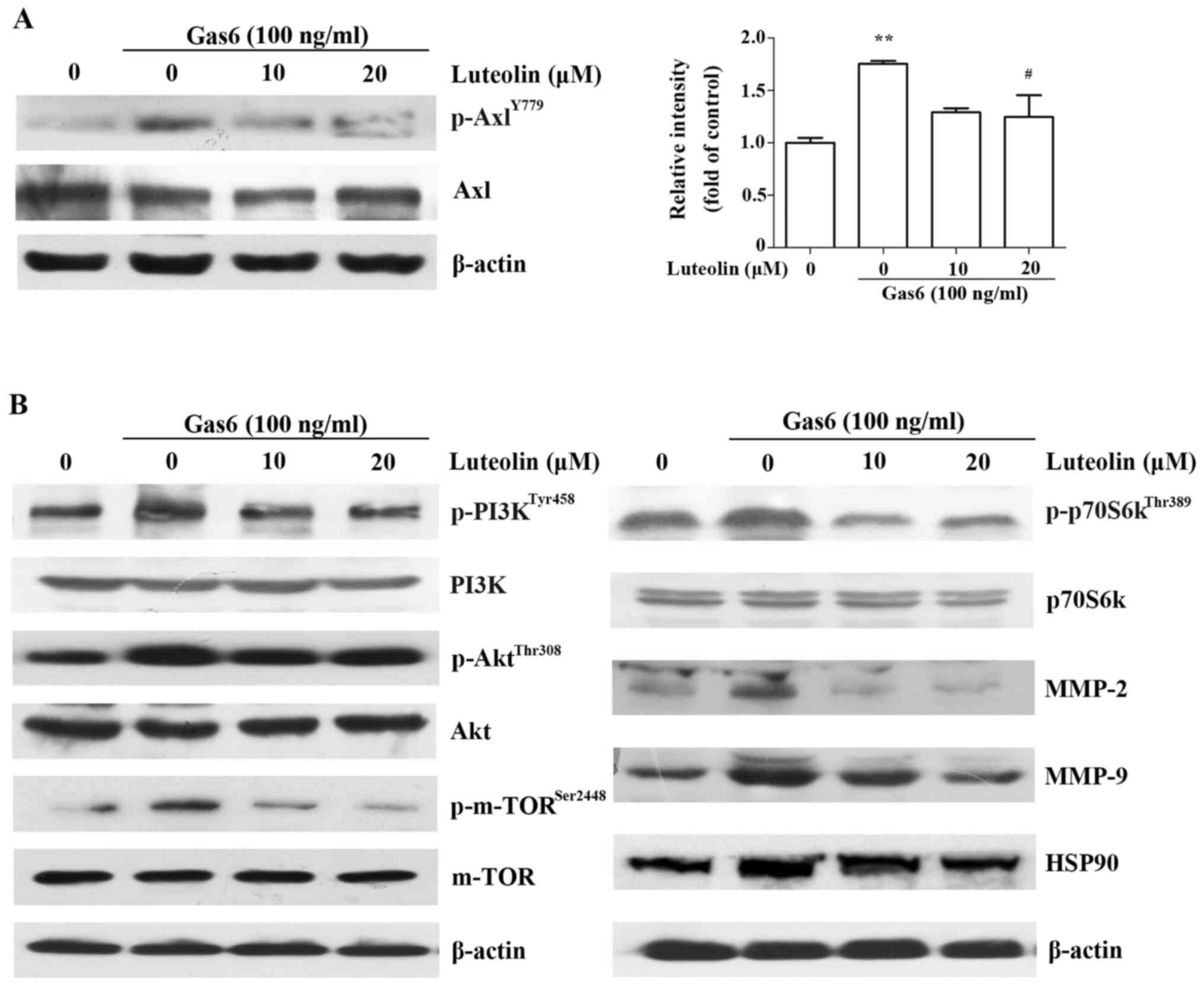
Luteolin inhibits the Gas6/Axl signaling
pathway
The above mentioned results demonstrated that
luteolin suppressed Gas6-induced angiogenesis in vitro,
ex vivo and in vivo. Thus, we investigated whether
this effect was associated with the suppression of the Gas6/Axl
signaling pathway and explored its downstream molecular mechanisms.
We found that luteolin significantly suppressed the Gas6-mediated
phosphorylation of Axl, followed by the downregulation of Gas6/Axl
mediated phosphorylation of the PI3K, Akt, mTOR and p70S6k
(Fig. 6A and B). Luteolin also
markedly inhibited the expression of MMP-2 and MMP-9 (Fig. 6B), which play crucial roles in
various physiological processes, such as wound healing and vessel
sprouting (29). In addition,
luteolin also inhibited the expression of HSP90 (Fig. 6B), which has been shown to promote
angiogenesis (30). These data
indicated that luteolin inhibited Gas6-induced angiogenesis by
inactivating the Gas6/Axl signaling pathway in HMEC-1s.
Discussion
Epidemiological and preclinical evidence indicates
that the dietary intake of flavonoids, including luteolin and
catchin, contributes to the treatment of multiple pathological
angiogenesis involving cancer, arthritis and cataractogenesis.
Previously, luteolin has been widely investigated to validate its
anti-angiogenic effects, but the underlying mechanism is not fully
understood (9,19,31,32).
In the present study, we found that luteolin inhibited growth
arrest-specific protein 6 (Gas6)-induced angiogenesis by inhibiting
the Gas6/Axl signaling pathway. Noteworthy, we found that luteolin
not only inhibited the Gas6-induced motilities of HMEC-1s
(proliferation, migration, invasion and tubulogenesis) that are
required for neovascularization but also suppressed the
Gas6-induced recruitment of pericytes to well-established
endothelial tubes, which is vital for the maturation of neovessels
during the late-stage of angiogenesis. Our study provides new
evidence regarding novel anti-angiogenic mechanism of luteolin and
also contributes to the notion that the dietary intake of luteolin
is beneficial for the treatment of pathological angiogenesis.
Gas6/Axl is expressed in blood vascular system
containing endothelial cells (ECs), pericytes and smooth muscle
cells (7–9). The Gas6/Axl pathway can promote
proliferation, migration, invasion and tube formation of endothelia
cells by regulating the PI3K/Akt/mTOR signaling pathway (26,33).
Gas6-induced angiogenesis is associated with various pathological
angiogenesis, including cancer, psoriasis, blindness and arthritis
(2,34). Gas6 and its receptor play a crucial
role in tumor angiogenesis, which functions in a variety of tumors
including breast cancer, prostatic cancer and non-small cell lung
cancer (3). Therefore, it is
meaningful to develop an inhibitor that targets the Gas6-induced
angiogenesis. Herein, we sought to determine whether the
anti-angiogenic effect of luteolin is correlated with the Gas6/Axl
signaling pathway. We demonstrated that luteolin significantly
inhibited multiple processes of Gas6-induced HMEC-1s during
angiogenesis. Luteolin also suppressed the Gas6-stimulated
recruitment of HBVPs to the endothelial tubes, which promoted the
maturation and stabilization of neovessels. In addition, luteolin
suppressed the Gas6-induced newly branched blood vessels in the
CAM. These results were due to the inactivation of the Gas6/Axl
pathway, resulting in the downregulation of the PI3K/Akt/mTOR
signaling pathway. Luteolin also reduced the expression of MMP-2,
MMP-9 and HSP90, which are vital for the degradation of
extracellular matrix (ECM) components (29). Taken together, our study provides
new insight for further exploring the anti-angiogenic effect of
luteolin and contributes to the understanding of its mechanism. Our
study also indicates the potential of luteolin in the treatment of
cancer, psoriasis, blindness and arthritis involvement of Gas6/Axl
activation.
Previous studies have demonstrated that the Gas6/Axl
is involved in chemoresistance in breast cancer and indicated that
the inactivation of the Gas6/Axl pathway may contribute to the
activity of chemotherapeutic drugs (35,36).
Recent studies have demonstrated that the inhibition of the
Gas6/Axl pathway improved the efficacy of chemotherapies in
preclinical models of advanced pancreatic and ovarian cancer
(37). Thus, the dietary intake of
luteolin during therapy likely contributes to the decreased risk of
resistance. In addition, luteolin has been shown to inhibit
VEGF/VEGFR2-mediated angiogenesis (20,38),
combined with our results, luteolin may achieve a synergistic
anti-angiogenic efficacy which is similar to that achieved by
combinational therapy with inhibitors targeting the Gas6/Axl and
VEGF/VEGFR2 signaling pathways in anti-angiogenesis therapies.
Currently, angiogenic inhibitors are widely used in
cancer therapy in clinic, including bevacizumab, a monclone
antibody of VEGF, and the multi-targeted tyrosine kinase
inhibitors, such as sunitinib, and sorafenib (39). However, it was reported that many
side effects emerged after the treatments, the severely adverse
reactions including nausea, vomiting, and drug resistance in the
advanced stage, which lead to treatment failure (40). In addition, the drugs used for the
treatment of angiogenesis, such as bevacizumab and ranibizumab, are
quite expensive, thus many patients can not afford the high
treatment expense. However, luteolin is widely distributed in the
vegetables and fruits (17), it
can be obtained in our daily life as a nutritional additive thus
preventing from pathological angiogenesis in clinic.
In conclusion, we demonstrate that luteolin
significantly suppresses Gas6-induced angiogenesis in vitro,
ex vivo and in vivo. This study investigated the
potential of luteolin in clinic as a therapy for angiogenesis and
it also provides evidence that dietary intake of luteolin may
contribute to reducing the risk of pathological angiogenesis.
Abbreviations:
|
HMEC-1s
|
human microvascular endothelial
cells
|
|
HBVPs
|
human brain vascular pericytes
|
|
ECs
|
endothelial cells
|
|
Gas6
|
growth arrest-specific protein 6
|
|
Axl
|
Axl receptor tyrosine kinase
|
|
VEGF
|
vascular endothelial growth factor
|
|
EGF
|
epidermal growth factor
|
|
FGF
|
fibroblast growth factor
|
|
PDGF
|
platelet-derived growth factor
|
|
Ang1
|
angiopoietin 1
|
|
Ang2
|
angiopoietin 2
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
protein kinase B
|
|
mTOR
|
mammalian target of rapamycin
|
|
RTK
|
receptor tyrosine kinases
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
HSP90
|
heat shock protein 90
|
|
PM
|
pericyte medium
|
|
MEK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
NF-κB
|
nuclear factor κB
|
|
JAK
|
janus kinase
|
|
STAT
|
signal transducer and activator of
transcription
|
|
CAM
|
chick chorioallantoic membrane
|
|
MTT
|
3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide
|
Acknowledgments
This study was supported by the Science and
Technology Program of China (2012ZX09103101-053), the national
Science Foundation of China (81573455) and Guangdong Province
(S2013050014183 and 2013CXZDA006), the Program for new Century
Excellent Talents in University and Pear River Scholar Funded
Scheme (D.Z.) and the Project for Constuction of Traditional
Chinese Medicine Strong Province (20132114).
References
|
1
|
Dong R, Yang GD, Luo NA and Qu YQ: HuR: A
promising therapeutic target for angiogenesis. Gland Surg.
3:203–206. 2014.PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gavalas NG, Liontos M, Trachana SP,
Bagratuni T, Arapinis C, Liacos C, Dimopoulos MA and Bamias A:
Angiogenesis-related pathways in the pathogenesis of ovarian
cancer. Int J Mol Sci. 14:15885–15909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thanapprapasr D, Hu W, Sood AK and Coleman
RL: Moving beyond VEGF for anti-angiogenesis strategies in
gynecologic cancer. Curr Pharm Des. 18:2713–2719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alameddine RS, Yakan AS, Skouri H,
Mukherji D, Temraz S and Shamseddine A: Cardiac and vascular
toxicities of angiogenesis inhibitors: The other side of the coin.
Crit Rev Oncol Hematol. 96:195–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kariolis MS, Miao YR, Jones DS II, Kapur
S, Mathews II, Giaccia AJ and Cochran JR: An engineered Axl 'decoy
receptor' effectively silences the Gas6-Axl signaling axis. Nat
Chem Biol. 10:977–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Avanzi GC, Gallicchio M, Bottarel F,
Gammaitoni L, Cavalloni G, Buonfiglio D, Bragardo M, Bellomo G,
Albano E, Fantozzi R, et al: GAS6 inhibits granulocyte adhesion to
endothelial cells. Blood. 91:2334–2340. 1998.PubMed/NCBI
|
|
8
|
Manfioletti G, Brancolini C, Avanzi G and
Schneider C: The protein encoded by a growth arrest-specific gene
(gas6) is a new member of the vitamin K-dependent proteins related
to protein S, a negative coregulator in the blood coagulation
cascade. Mol Cell Biol. 13:4976–4985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fotsis T, Pepper M, Adlercreutz H, Hase T,
Montesano R and Schweigerer L: Genistein, a dietary ingested
isoflavonoid, inhibits cell proliferation and in vitro
angiogenesis. J Nutr. 125(Suppl 3): 790S–797S. 1995.PubMed/NCBI
|
|
10
|
Zuo PY, Chen XL, Lei YH, Liu CY and Liu
YW: Growth arrest-specific gene 6 protein promotes the
proliferation and migration of endothelial progenitor cells through
the PI3K/AKT signaling pathway. Int J Mol Med. 34:299–306.
2014.PubMed/NCBI
|
|
11
|
Stenhoff J, Dahlbäck B and Hafizi S:
Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth
of cardiac fibroblasts. Biochem Biophys Res Commun. 319:871–878.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son BK, Kozaki K, Iijima K, Eto M, Nakano
T, Akishita M and Ouchi Y: Gas6/Axl-PI3K/Akt pathway plays a
central role in the effect of statins on inorganic
phosphate-induced calcification of vascular smooth muscle cells.
Eur J Pharmacol. 556:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melaragno MG, Fridell YW and Berk BC: The
Gas6/Axl system: A novel regulator of vascular cell function.
Trends Cardiovasc Med. 9:250–253. 1999. View Article : Google Scholar
|
|
14
|
Holland SJ, Pan A, Franci C, Hu Y, Chang
B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, et al: R428, a
selective small molecule inhibitor of Axl kinase, blocks tumor
spread and prolongs survival in models of metastatic breast cancer.
Cancer Res. 70:1544–1554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei X, Chen M, Nie Q, Hu J, Zhuo Z, Yiu A,
Chen H, Xu N, Huang M, Ye K, et al: In vitro and in vivo
antiangiogenic activity of desacetylvinblastine monohydrazide
through inhibition of VEGFR2 and Axl pathways. Am J Cancer Res.
6:843–858. 2016.PubMed/NCBI
|
|
16
|
Ye X, Li Y, Stawicki S, Couto S,
Eastham-Anderson J, Kallop D, Weimer R, Wu Y and Pei L: An anti-Axl
monoclonal antibody attenuates xenograft tumor growth and enhances
the effect of multiple anticancer therapies. Oncogene.
29:5254–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clere N, Faure S, Martinez MC and
Andriantsitohaina R: Anticancer properties of flavonoids: Roles in
various stages of carcinogenesis. Cardiovasc Hematol Agents Med
Chem. 9:62–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pratheeshkumar P, Son YO, Budhraja A, Wang
X, Ding S, Wang L, Hitron A, Lee JC, Kim D, Divya SP, et al:
Luteolin inhibits human prostate tumor growth by suppressing
vascular endothelial growth factor receptor 2-mediated
angiogenesis. PLoS One. 7:e522792012. View Article : Google Scholar
|
|
21
|
Zhang DM, Liu JS, Tang MK, Yiu A, Cao HH,
Jiang L, Chan JY, Tian HY, Fung KP and Ye WC: Bufotalin from
Venenum Bufonis inhibits growth of multidrug resistant HepG2 cells
through G2/M cell cycle arrest and apoptosis. Eur J Pharmacol.
692:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ming J, Zhou Y, Du J, Fan S, Pan B, Wang
Y, Fan L and Jiang J: Identification of miR-200a as a novel
suppressor of connexin 43 in breast cancer cells. Biosci Rep.
35:352015. View Article : Google Scholar
|
|
23
|
Darland DC and D'Amore PA: TGF beta is
required for the formation of capillary-like structures in
three-dimensional cocultures of 10T1/2 and endothelial cells.
Angiogenesis. 4:11–20. 2001. View Article : Google Scholar
|
|
24
|
Staton CA, Stribbling SM, Tazzyman S,
Hughes R, Brown NJ and Lewis CE: Current methods for assaying
angiogenesis in vitro and in vivo. Int J Exp Pathol. 85:233–248.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi JM, Bai LL, Zhang DM, Yiu A, Yin ZQ,
Han WL, Liu JS, Li Y, Fu DY and Ye WC: Saxifragifolin D induces the
interplay between apoptosis and autophagy in breast cancer cells
through ROS-dependent endoplasmic reticulum stress. Biochem
Pharmacol. 85:913–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collett G, Wood A, Alexander MY, Varnum
BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW and Canfield
AE: Receptor tyrosine kinase Axl modulates the osteogenic
differentiation of pericytes. Circ Res. 92:1123–1129. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stratman An, Malotte KM, Mahan RD, Davis
MJ and Davis GE: Pericyte recruitment during vasculogenic tube
assembly stimulates endothelial basement membrane matrix formation.
Blood. 114:5091–5101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
García-Caballero M, Cañedo L,
Fernández-Medarde A, Medina MA and Quesada AR: The marine fungal
metabolite, AD0157, inhibits angiogenesis by targeting the Akt
signaling pathway. Mar Drugs. 12:279–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davidson B, Goldberg I, Gotlieb WH,
Kopolovic J, Risberg B, Ben-Baruch G and Reich R: Coordinated
expression of integrin subunits, matrix metalloproteinases (MMP),
angiogenic genes and Ets transcription factors in advanced-stage
ovarian carcinoma: A possible activation pathway? Cancer Metastasis
Rev. 22:103–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang DJ, An H, Kim KS, Kim HH, Jung J,
Lee JM, Kim NJ, Han YT, Yun H, Lee S, et al: Design, synthesis, and
biological evaluation of novel deguelin-based heat shock protein 90
(HSP90) inhibitors targeting proliferation and angiogenesis. J Med
Chem. 55:10863–10884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Negrão R, Costa R, Duarte D, Gomes TT,
Coelho P, Guimarães JT, Guardão L, Azevedo I and Soares R:
Xanthohumol-supplemented beer modulates angiogenesis and
inflammation in a skin wound healing model. Involvement of local
adipocytes. J Cell Biochem. 113:100–109. 2012. View Article : Google Scholar
|
|
32
|
Sreelakshmi V, Sasikala V and Abraham A:
Luteolin supplementation prevents selenite-induced cataractogenesis
in Sprague Dawley rat pups. Chem Biodivers. 12:1881–1890. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J,
Kallop D, Ludlam MJ and Pei L: Axl as a potential therapeutic
target in cancer: Role of Axl in tumor growth, metastasis and
angiogenesis. Oncogene. 28:3442–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim YS, Jung SH, Jung DH, Choi SJ, Lee YR
and Kim JS: Gas6 stimulates angiogenesis of human retinal
endothelial cells and of zebrafish embryos via ERK1/2 signaling.
PLoS One. 9:e839012014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Jin H, Wang N, Fan S, Wang Y,
Zhang Y, Wei L, Tao X, Gu D, Zhao F, et al: Gas6/Axl axis
contributes to chemoresistance and metastasis in breast cancer
through Akt/GSK-3β/β-catenin signaling. Theranostics. 6:1205–1219.
2016. View Article : Google Scholar :
|
|
36
|
Roberts CM, Tran MA, Pitruzzello MC, Wen
W, Loeza J, Dellinger TH, Mor G and Glackin CA: TWIST1 drives
cisplatin resistance and cell survival in an ovarian cancer model,
via upregulation of GAS6, L1CAM, and Akt signalling. Sci Rep.
6:376522016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YJ, Lim T, Han MS, Lee SH, Baek SH,
Nan HY and Lee C: Anticancer effect of luteolin is mediated by
downregulation of TAM receptor tyrosine kinases, but not
interleukin-8, in non-small cell lung cancer cells. Oncol Rep.
37:1219–1226. 2017.
|
|
38
|
Bagli E, Stefaniotou M, Morbidelli L,
Ziche M, Psillas K, Murphy C and Fotsis T: Luteolin inhibits
vascular endothelial growth factor-induced angiogenesis; inhibition
of endothelial cell survival and proliferation by targeting
phosphatidylinositol 3′-kinase activity. Cancer Res. 64:7936–7946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yadav L, Puri N, Rastogi V, Satpute P and
Sharma V: Tumour angiogenesis and angiogenic inhibitors: A review.
J Clin Diagn Res. 9:XE01–XE05. 2015.PubMed/NCBI
|
|
40
|
Keating GM: Bevacizumab: A review of its
use in advanced cancer. Drugs. 74:1891–1925. 2014. View Article : Google Scholar : PubMed/NCBI
|