Introduction
Breast cancer is the most commonly diagnosed
malignant cancer in women and the leading cause of death among
women with cancers. The formation of a new vascular network known
as tumour angiogenesis plays a key role in the progression of this
tumour. The process was first described in 1971 by Folkman, who
postulated that the tumour growth and metastasis depends on the
number of newly formed blood vessels (1). This hypothesis became the basis for
further research demonstrating that the 'angiogenic switch' is an
integral part of most cancer progression (2). The administration of adjuvant
anti-angiogenic therapy had positive results in the case of
colorectal, breast, kidney and pancreatic cancers, and non-small
cell lung carcinoma (3,4).
The most commonly used method to quantify
intratumoural angiogenesis in histological specimens is the
assessment of MVD. The MVD can be assessed by counting
immunolabelled vessels stained with antibodies against endothelial
cell antigens, e.g., CD31 and CD34 (5). This method was developed by Weidner
et al who also confirmed that in the case of invasive ductal
carcinoma (IDC) high MVD is associated with the incidence of
regional lymph node and distant metastases (6). The results of the meta-analysis
confirmed the prognostic value of MVD assessment in breast cancer
(5). However, the evaluation of
tumour angiogenesis with MVD has some limitations. One of them is
the fact that commonly used markers are expressed not only in newly
forming vessels but also in mature vessels present in body tissues
and organs (7).
Nestin is a type VI intermediate filament (IF)
protein and participates in the cytoskeleton organization (8). Its expression is typical of
neuroepithelial stem cells (8),
but it is also present in progenitor (9–11)
muscle (9), endothelial (12,13)
and cancer cells (14,15). In the cell, nestin expression is
transient and following cell differentiation it is downregulated
and replaced by tissue-specific IFs (16). It is suggested that nestin
expression in blood vessels is mainly limited to the proliferating
and newly forming vessels, which makes nestin a more specific
marker of angiogenesis than commonly used CD34 and CD31 (17–20).
Due to nestin progenitor features and proven
expression in blood vessels we suspected that it may be a reliable
marker for angiogenesis evaluation in IDC patients. Hence, the aim
of the present study was to assess nestin-positive microvessel
density (Nes+MVD) and to determine its prognostic value
in patients with IDC. We also aimed at determining whether nestin
is a marker of newly forming and poorly differentiated blood
vessels. Accordingly, we compared Nes+MVD with the
density of newly forming CD34+ vessels
(CD34+MVD) and mature CD31+ vessels
(CD31+MVD). To confirm whether the expression of nestin
is increased in endothelial progenitor cells (EPCs) we developed an
in vitro model consisting of human endothelial cell lines
representing a different level of maturity. HUVEC-SVT and HMEC-1
cells represent the endothelial cells (EC) of mature venous vessels
and capillaries, whereas HEPC-CB.1 cells are the early EPCs.
Materials and methods
Tissue samples
The study was conducted on archival
paraffin-embedded ductal breast carcinoma samples (n=137) and NBTLs
(n=19) collected during resection procedures at The Maria
Sklodowska Curie Memorial Cancer Centre and Institute of Oncology
in Cracow and at The Lower Silesian Oncology Centre in Wroclaw from
1999 to 2013. From 137 investigated cancer patients, 26 developed
pre-invasive in situ carcinoma (DCIS), whereas 111 developed
invasive cancer (IDC). The investigated cases were categorized into
4 groups according to their invasiveness (Table I): NBTLs, ductal carcinoma in
situ (DCIS), lymph node-negative invasive ductal carcinoma (IDC
N−) and lymph node-positive invasive ductal carcinoma
(IDC N+). The clinical, pathological and survival data
were obtained only for IDC patients (n=111) from the archives of
the hospital and are listed in Table
II. From these 111 invasive cases, 23 patients died from the
disease and 25 patients had recurrence (Table II). The study was approved by the
Commission of Bioethics of the Wroclaw Medical University,
Poland.
 | Table IGroups of patients according to
tumour invasiveness. |
Table I
Groups of patients according to
tumour invasiveness.
| Histological
type | n |
|---|
| Non malignant
breast tissue lesions | 19 |
| Ductal carcinoma
in situ | 26 |
| Invasive ductal
carcinoma - lymph node negative | 62 |
| Invasive ductal
carcinoma - lymph node positive | 49 |
 | Table IIClinicopathological characteristics
of IDC patients. |
Table II
Clinicopathological characteristics
of IDC patients.
| Parameters | n | % |
|---|
| Age | | |
| ≤50 | 34 | 30.6 |
| >50 | 77 | 69.4 |
| Menopausal
status | | |
| Pre- | 36 | 32.4 |
| Post- | 73 | 65.8 |
| No data | 2 |
1.8 |
| Tumour size | | |
| T1 | 61 | 55.0 |
| T2 | 37 | 33.3 |
| T3 | 9 |
8.1 |
| T4 | 2 |
1.8 |
| No data | 2 |
1.8 |
| Lymph nodes | | |
| Negative | 62 | 55.9 |
| Positive | 49 | 44.1 |
| Grade | | |
| 1 | 11 |
9.9 |
| 2 | 60 | 54.1 |
| 3 | 40 | 36.0 |
| pTNM | | |
| I + II | 92 | 82.9 |
| III + IV | 17 | 15.3 |
| No data | 2 |
1.8 |
| ER | | |
| Negative | 28 | 25.2 |
| Positive | 83 | 74.8 |
| PR | | |
| Negative | 38 | 34.2 |
| Positive | 73 | 65.8 |
| HER2 | | |
| Negative | 67 | 60.4 |
| Positive | 44 | 39.6 |
|
Triple-negative | | |
| Yes | 17 | 15.3 |
| No | 94 | 84.7 |
| Overall
survival | | |
| Deaths | 23 | 20.7 |
| Alive | 86 | 77.5 |
| No data | 2 |
1.8 |
| Event-free
survival | | |
| Recurrence | 25 | 22.5 |
| No recurrence | 83 | 74.8 |
| No data | 3 |
2.7 |
Human endothelial cell lines
To determine the expression level of nestin human
immortalized ECs (HUVEC-SVT, HMEC-1) and EPCs (HEPC-CB.1) were
selected. The HMEC-1 cells (ATCC, Washington, CO, USA) were
cultured in MCDB 131 (Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO, USA), 10
ng/ml epidermal growth factor (EGF, Invitrogen), 1 µg/ml
hydrocortisone (Sigma) and 10 mM L-glutamine (Invitrogen).
HUVEC-SVT and HEPC-CB.1 cells (both courtesy of Dr M. Paprocka,
Ludwik Hirszfeld Institute of Immunology and Experimental Therapy
of the Polish Academy of Sciences, Wroclaw, Poland) were grown in
Dulbecco's modified Eagle's medium (DMEM, Lonza, Basel,
Switzerland) with 4.5 g/l glucose, 25 mM HEPES sodium pyruvate
without L-glutamine supplemented with 10% fetal bovine serum
(Merck, Millerica, MA, USA), 2 mM L-glutamine and antibiotics
(Sigma). All of the studies utilized cell passages 18–26. The
investigated cell lines exhibit different phenotypes of endothelial
cells. Human umbilical vein endothelial cells (HUVEC-SVT) are
immortalised cells isolated from the large vessel representing
macrovascular phenotype (21).
Immortalised human microvascular endothelial cells (HMEC-1)
originate in dermal microvasculature and show the characteristics
similar to ECs present in tumour environment (21). HEPC-CB.1 cells are immortalized
early human EPCs isolated from the cord blood (22).
Immunohistochemistry (IHC)
Immunohistochemical reactions were performed on
4-µm thick paraffin sections using Autostainer Link48 (Dako,
Glostrup, Denmark) with a panel of mouse-anti human monoclonal
antibodies against: nestin (dilution 1:100, OBT1610, Bio-Rad,
Hercules, CA, ISA), CD31 (ready-to-use, IR610, Dako), CD34
(ready-to-use, IR632, Dako) estrogen receptor (ER), clone 1D5
(ready-to-use, IR654; Dako) and progesterone receptor (PR) clone
636 (ready-to-use, IR068; Dako). The sections were boiled in
EnVision FLEX Target Retrieval Solution (pH 9.0, 97°C, 20 min)
using Pre-Treatment Link Platform (both from Dako). Activity of
endogenous peroxidase was blocked by 5 min incubation with EnVision
FLEX Peroxidase-Blocking Reagent (Dako). The samples were incubated
with primary antibodies for 20 min at room temperature (RT) and
then incubated with EnVision FLEX/HRP for 20 min (Dako).
3,3′-diaminobenzidine (DAB, Dako) was utilized as the peroxidase
substrate and the sections were incubated for 10 min. Finally, all
slides were counter-stained with EnVision FLEX Hematoxylin (Dako)
for 5 min. After dehydration in graded ethanol concentrations (70,
96 and 99.8%) and in xylene, slides were closed with coverslips in
Dako mounting medium (Dako). Human epidermal growth factor receptor
2 (HER2) expression status was determined using HercepTest and HER2
FISH pharmDx kit (both from Dako), according to the manufacturer's
instructions.
Examination of IHC reactions
The IHC reactions were evaluated with a BX-41 light
microscope (Olympus, Tokyo, Japan). The MVD was assessed for each
investigated antigen i.e., nestin, CD31 and CD34 according to the
Weidner method (6). Firstly, the
slides were examined under low magnification (×100) to identify
three areas with the highest vascular density (hot-spots). Then,
under magnification ×400 stained vessels were counted. The final
score for each slide was presented as a mean number of vessels per
mm2. Any stained EC or ECs clusters were counted as a
single microvessel, even in the absence of vessel lumen (6). The status of ER and PR was scored 0–3
points, depending on the percentage of positive cells (0 points, no
reactions; 1 point, 1–10%; 2 points, 11–50%; 3 points, 51–100%
stained cells) (23). The
expression of HER2 was evaluated using a scale that considers both
the intensity of the membrane reaction and the percentage of
positive tumour cells (24).
Immunocytochemistry (ICC) and
immunofluorescence (IF)
Investigated endothelial cell lines were grown on
glass cover-slips for 24 h at 37°C. After 24 h, cells were washed
with phosphate-buffered saline, fixed in 4% paraformaldehyde and
permabilised with 0.2% Triton X. The IHC of fixed cells was
performed with anti-nestin antibody in Dako Autostainer Link48
(Dako) according to the procedure described above. The slides were
incubated with primary antibodies for 20 min at RT, and then
incubated with EnVision FLEX (Dako) to visualize the antigens. For
IF the cell lines were cultured and fixed as stated above. All
slides were incubated at 4°C overnight with monoclonal antibody
against nestin (Bio-Rad) and resolved in antibody diluent (Dako) in
the concentration of 1:100. As a secondary antibody donkey
anti-mouse antibody conjugated with rhodamine (1:2,000, polyclonal;
715-025-151 Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) was applied. The slides were covered with
ProLong® Gold Antifade Mountant mounting medium
(Molecular Probes, Eugene, OR, USA) with
4′,6-diamidino-2-phenylindole (DAPI) and viewed and imaged with a
BX51 fluorescence microscope (Olympus).
RNA isolation and real-time PCR
reactions
Total RNA from HUVEC-SVT, HMEC-1 and HEPC-CB.1 cell
lines was isolated using the RNeasy Mini kit (Qiagen, Hilden,
Germany) according to the manufacturer's instructions. To eliminate
the genomic DNA, the protocol included on-column DNase digestion.
RNA concentration and purity were measured using the NanoDrop1000
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The
absorbance was measured at 260–280 nm. The first-strand cDNA was
synthesized using the High-Capacity cDNA Reverse Transcription kits
(Applied Biosystems, Foster City, CA, USA). The relative nestin
gene (NES) mRNA expression level was determined by quantitative
real-time PCR using the 7500 Real-Time PCR system and the iTaq
Universal Probes Supermix (Bio-Rad), according to the
manufacturer's protocol. We applied the following human Taqman Gene
Expression Assays: NES Hs04187831_g1 for nestin and ACTB
Hs99999903_m1 for β-actin (Applied Biosystems). Since β-actin is a
housekeeping gene, it was used as a reference for determining NES
expression in the analyzed human endothelial cell lines. The
reactions were carried out in triplicate in the following
conditions: initial denaturation at 95°C for 10 min, followed by 45
cycles of denaturation at 95°C for 15 sec, and annealing and
elongation at 60°C for 60 sec. The relative mRNA expression level
of the NES gene was calculated with the ΔΔCt method (25).
Flow cytometry (FC)
For flow cytometric immunophenotyping of
intercellular nestin, cells were fixed and permeabilized with BD
Cytofix/Cytoperm™ (Becton-Dickinson, CA, USA) according to the
manufacturer's instructions. Subsequently, the cells were stained
with monoclonal antibody against human nestin (1:100; OBT1610) for
20 min/RT and immunolabelled with sheep anti-mouse secondary
antibody conjugated with FITC (1:200, polyclonal; P8547 Sigma). The
cells were analyzed by flow cytometry using FACSCalibur
(Becton-Dickinson) equipped with a 488-nm laser and filter for FITC
analysis (530 BP). As single labelling was performed, no
compensation setting was required. Data were recorded for 10,000
events using CellQuest version 3.3 software (Becton-Dickinson),
analyzed on the ungated population (except for debris) and
presented without any transformation as histograms using WINMDI 2.7
(Scripps Institute, CA, USA) software. Mean fluorescence intensity
(MFI) for nestin is shown as a difference between MFI for samples
incubated with primary and secondary antibodies and MFI for isotype
control.
Statistical analysis
The data were analyzed with Prism 5.0 (GraphPad, La
Jolla, CA, USA) software. The Kolmogorov-Smirnov test was applied
to determine whether sample data are normally distributed. To
evaluate the relationships and correlations between the examined
markers and with clinicopathological factors, Student's t-test and
Spearman rank correlation test were utilized. The Kaplan-Meier
method and the Mantel-Cox test were used to determine the
significance of patient OS and event-free survival (EFS). A Cox
proportional hazards model with forward stepwise selection was used
to calculate univariate and multivariate hazard ratio for the study
variables. Differences were considered statistically significant at
p<0.05.
Results
IHC
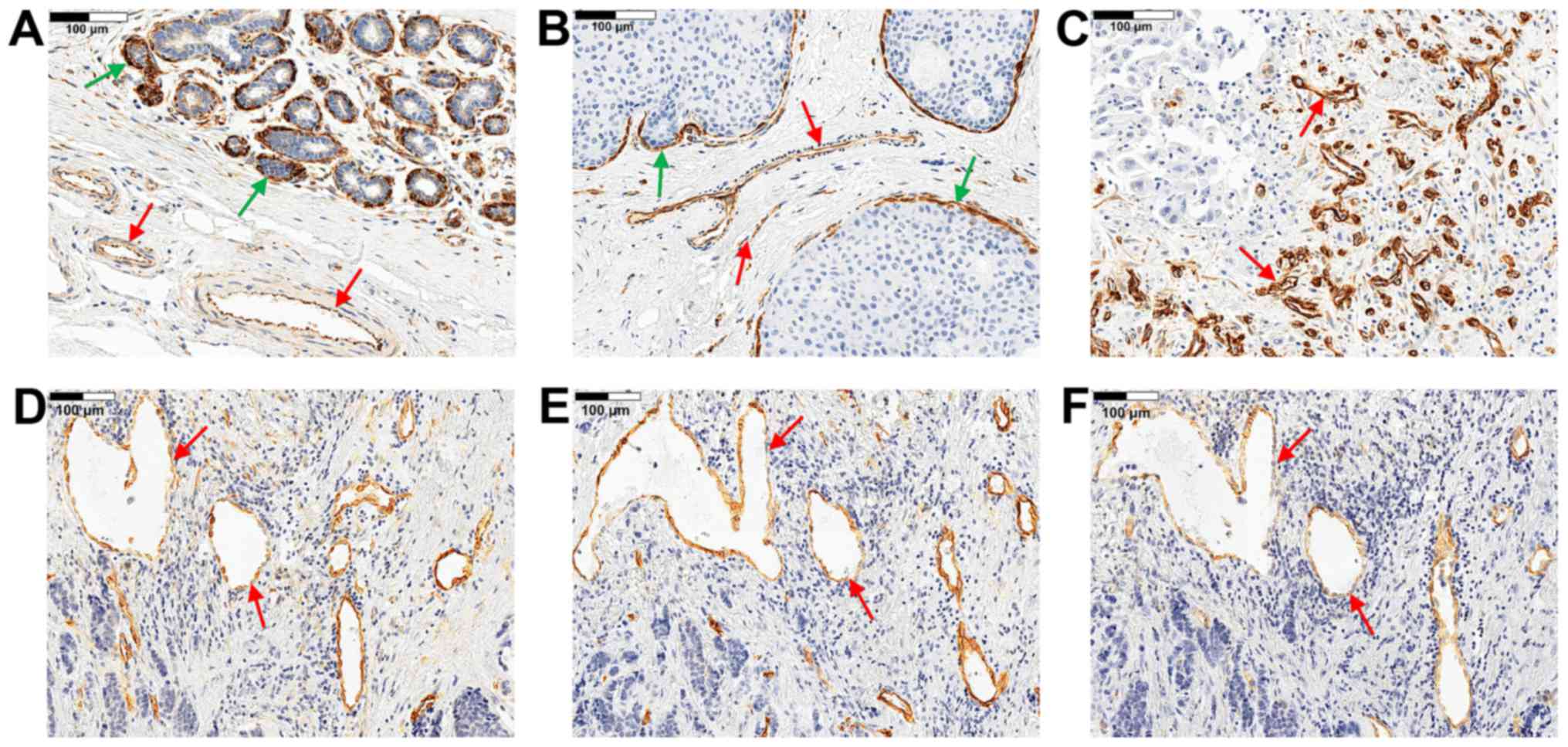
Nestin expression was observed in the cytoplasm of
ECs in all study cases (Fig.
1A–D). In addition, nestin expression was observed in
myoepithelial cells of the ducts and lobular acinar units (Fig. 1A and B) and in some cases in tumour
cells (data not shown). A significantly higher Nes+MVD
was observed in both groups of IDC patients i.e., IDC N−
and IDC N+ in relation to the control group comprising
NBTLs (Fig. 2A; 72.32±25.01;
86.12±31.60 vs. 33.85±14.83; p<0.0001, p<0.0001,
respectively, Student's t-test). It was also demonstrated that
Nes+MVD was higher in both groups with IDC (IDC
N+ and IDC N−) as compared to the group with
DCIS (Fig. 2A, 86.12±31.60;
72.32±25.01 vs. 43.52±18.21; p<0.0001, p<0.0001, Student's
t-test). We also showed that Nes+MVD was significantly
higher in the group of patients with IDC N+ than with
IDC N− (Fig. 2A,
86.12±31.60 vs. 72.32±25.01; p=0.0132, Student's t-test).
Additionally, in the group of IDC, Nes+MVD was
significantly higher in the case of G3 and G2 tumours than in G1
tumours (Fig. 2B, 86.63±32.96;
78.84±28.88 vs. 57.14±20.87; p=0.0072, p=0.0203, Student's t-test).
A lower value of Nes+MVD was found in patients with
early-stage disease (I and II) than in patients with advanced-stage
disease (III and IV; Fig. 2C,
75.99±27.62 vs. 92.02±35.05; p=0.0377, Student's t-test).
Among patients with IDC, a high Nes+MVD
was also related to the TN phenotype of breast cancer characterized
by a lack of ER, PR and HER2 expression (Fig. 2D, 76.91±28.97 vs. 93.83±39.58;
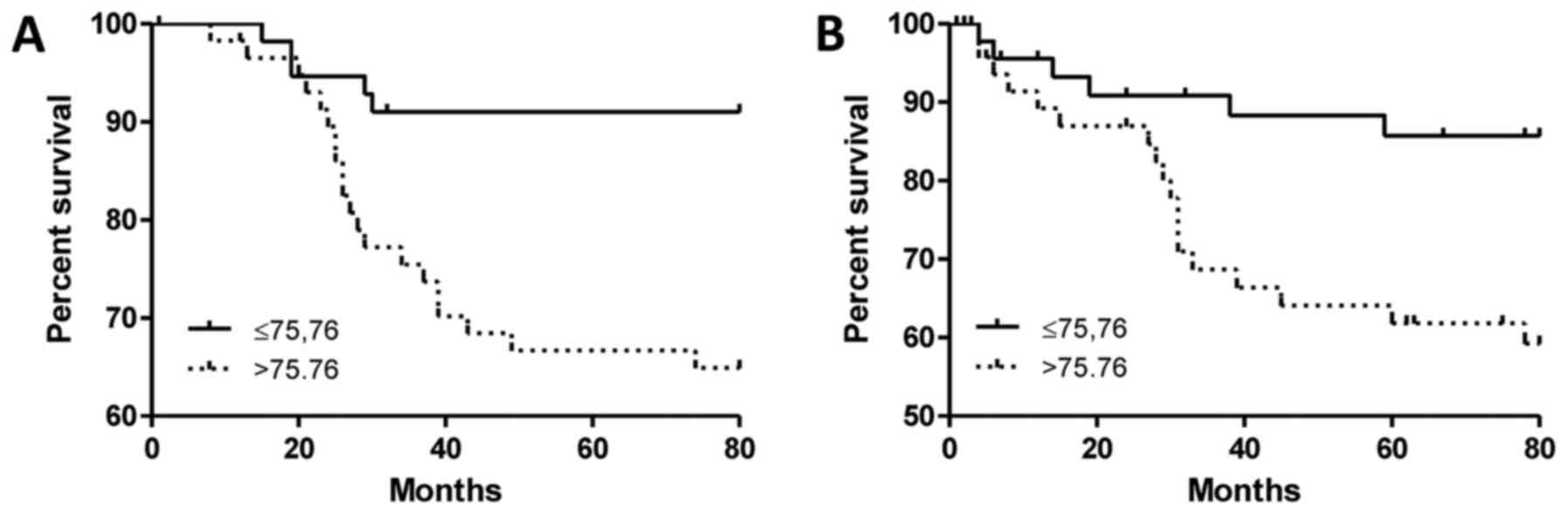
p=0.0357 Student's t-test). The analysis of survival data in the
group of IDC patients showed that a high value of
Nes+MVD was associated with shorter OS (Fig. 3A, p=0.0013, Mantel-Cox) and shorter
EFS (Fig. 3B, p=0.0091; median
75.76; Mantel-Cox). Moreover, in the case of OS, nestin turned out
to be an independent prognostic factor (Table III, p=0.007, multivariate Cox
analysis). Additionally, the correlation analysis showed that
Nes+MVD in IDC correlates with the density of
CD34+ vessels (Fig. 4A,
r=0.3280; p=0.0032, Spearman rank test) whereas no correlation was
noted between Nes+MVD and the density of
CD31+ vessels (Fig. 4B,
r=0.1563; p=0.1304, Spearman rank test).
 | Table IIIUni- and multivariate Cox analysis of
overall survival. |
Table III
Uni- and multivariate Cox analysis of
overall survival.
| Univariate Cox
analysis p-value
| Multivariate Cox
analysis HR
|
|---|
|
Characteristics | p-value | HR | 95% CI lower | 95% CI upper | p-value | HR | 95% CI lower | 95% CI upper |
|---|
Age
≤50 vs >50 | 0.714 | 0.978 | 0.869 | 1.101 | | | | |
Menopausal
status
Pre vs post | 0.687 | 0.832 | 0.340 | 2.034 | | | | |
| G | 0.032 | 2.220 | 1.073 | 4.595 | 0.042 | 2.417 | 1.034 | 5.649 |
Stage
I–II vs III–IV | 0.0001 | 7.675 | 3.357 | 17.549 | 0.004 | 6.971 | 1.865 | 26.063 |
| pT | 0.0001 | 3.787 | 2.281 | 6.288 | 0.041 | 2.045 | 1.029 | 4.062 |
pN
N0 vs N1-3 | 0.129 | 1.015 | 0.995 | 1.035 | | | | |
ER
Negative vs positive | 0.0118 | 0.346 | 0.152 | 0.790 | 0.446 | 0.582 | 0.145 | 2.338 |
PR
Negative vs positive | 0.039 | 0.423 | 0.187 | 0.959 | 0.14 | 0.384 | 0.108 | 1.368 |
HER2
Negative vs positive | 0.209 | 1.816 | 0.716 | 4.609 | | | | |
|
Triple-negative | 0.069 | 2.376 | 0.936 | 6.031 | 0.03 | 0.182 | 0.039 | 0.849 |
CD34+MVD
<95.44 vs >95.44 | 0.315 | 1.655 | 0.62 | 4.421 | | | | |
CD31+MVD
<48.42 vs >48.42 | 0.244 | 1.718 | 0.691 | 4.272 | | | | |
Nes+MVD
<75.76 vs >75.76 | 0.002 | 4.960 | 1.84 | 13.37 | 0.007 | 4.303 | 1.478 | 12.52 |
Real-time PCR
The real-time PCR was performed to evaluate NES
expression level in human endothelial cell lines. The relative
NES expression was assessed in relation to the HUVEC-SVT
cell line. The analysis showed significant differences in NES
expression between all the investigated cell lines i.e., HUVEC-SVT,
HMEC-1 and HEPC-CB.1 (Fig. 5,
p<0.0001, Student's t-test). The highest NES expression
was observed in the progenitor HEPC-CB.1 cells isolated from human
umbilical cord blood, and a trace expression in the HMEC-1 line
isolated from dermal microvessels.
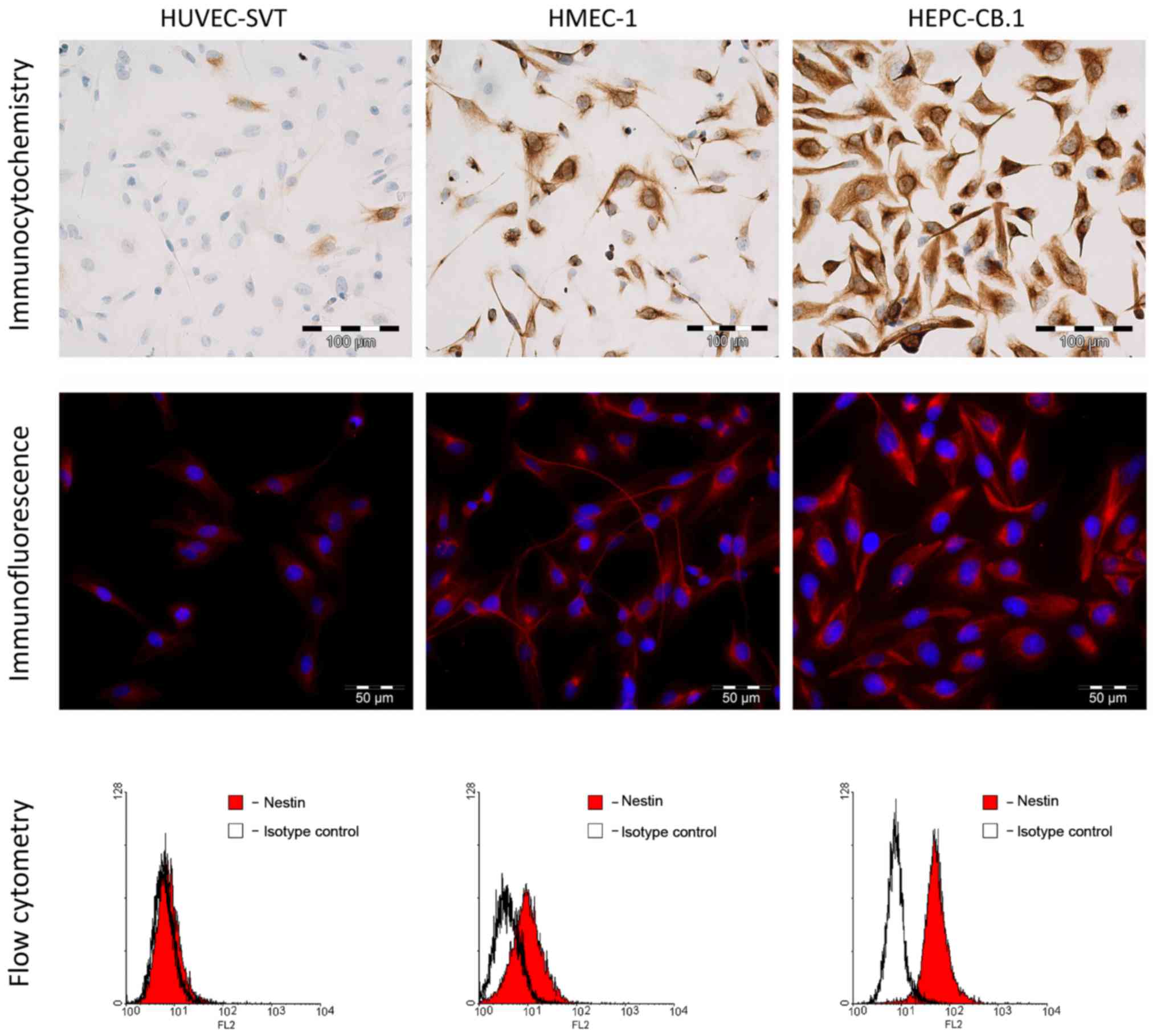
IF and ICC
Experiments using ICC and IF showed a cytoplasmic
expression of nestin in all the examined cell lines (Fig. 6). The most intense reaction was
observed in the progenitor HEPC-CB.1 cells, mildly intense in the
HMEC-1 cells and the weakest in the HUVEC-SVT cells.
FC
To confirm the different expression level of nestin
in the examined cell lines, we used flow cytometric assay.
Measurements of the MFI showed a lack of nestin expression in the
HUVEC-SVT cells and a very weak nestin expression in HMEC-1 cells
(Table IV and Fig. 6). A high MFI of nestin was observed
in the progenitor HEPC-CB.1 cells (Table IV and Fig. 6).
 | Table IVMean values of fluorescence for
isotype control and nestin in HUVEC-SVT, HMEC-1 and HEPC-CB.1 cell
lines. |
Table IV
Mean values of fluorescence for
isotype control and nestin in HUVEC-SVT, HMEC-1 and HEPC-CB.1 cell
lines.
| Cell line | Isotype
control | I+II antibody | Nestin |
|---|
| HUVEC-SVT |
6.08 |
7.20 |
1.12 |
| HMEC-1 |
3.93 |
9.86 |
5.93 |
| HEPC-CB.1 |
7.10 | 51.20 | 44.10 |
Discussion
Initially, nestin expression in the proliferating
endothelium of blood vessels in human tumours was reported in the
tumours of the central nervous system (26) and rhabdomyosarcoma (27). Further studies on animal models
showed that nestin expression in cerebellar blood vessels can be
induced by pro-angiogenic factors (28). Then Mokry et al confirmed
that nestin undergoes expression in blood vessels in many human
tissues and organs in which angiogenesis occurs (13). The first attempt at determining the
density of Nes+ vessels and comparing it with the
density of CD34+ vessels was assessed in patients with
gastric adenocarcinoma (17).
However, this study demonstrated no prognostic value for either
antigen, but in the case of large tumours nestin showed some
prognostic value. Further studies revealed that the high density of
proliferating microvessels co-expressing nestin and Ki-67 antigen
was associated with a worse prognosis in patients with prostate
(29) and breast cancers (30). Previous studies on nestin
expression in breast cancer tumour cells revealed that nestin
correlates with TN subtype and worse prognosis (15,31,32).
However, Kruger et al demonstrated in a large
population-based study that nestin expression in tumour cells
strongly correlates with basal-like molecular subtype of TN breast
cancers, with BRCA1-related breast cancer and with reduced survival
(33) In our study, we also noted
nestin expression in some of tumour cells (data not shown).
In the study, we assessed the prognostic value of
Nes+MVD in patients with ductal breast carcinoma. Our
results suggest that in ductal breast carcinoma Nes+MVD
increases with tumour invasiveness. The lowest value of
Nes+MVD was observed in non-malignant lesions and in
in situ carcinomas, while in invasive cancers it was
significantly higher. These results are consistent with the Folkman
hypothesis, according to which increased tumour vascularity is
necessary for its transition from in situ to invasive cancer
(1). When tumour cells become
invasive, they start to migrate in the extracellular matrix and
invade blood and lymphatic vessels, thus resulting in the formation
of metastases. Increased blood and lymphatic vessel density within
the tumour area increases the probability of vessel invasion, and
thus the probability of invading lymph nodes and developing
metastases (34). In this study,
we demonstrated that Nes+MVD in IDC was higher in
patients with lymph node metastases than in cases without lymph
node invasion. Similar results for MVD, but for different
endothelial antigens (i.e., CD31 and CD34) were obtained by Weidner
et al (6), Popiela et
al (35) and Xie et al
(36). Furthermore, we
demonstrated that Nes+MVD increases with the
histological grade of IDC and achieves the highest value in the G3
and the lowest in G1 tumours. Similar results, but with the use of
Nes+Ki-67+ vascular proliferation index (VPI)
were reported in breast cancers by Kruger et al (30). In their study, VPI was calculated
as the ratio between the number of Nes+ microvessels
containing Ki-67+ proliferating endothelial cells
(Nes+Ki-67+MVD) and the total number of
Nes+ microvessels (Nes+MVD) expressed as a
percentage
(Nes+Ki-67+MVD/Nes+MVD). They
shown that high value of VPI but not Nes+MVD itself,
correlates with aggressive features and poor outcome of breast
cancer (30). Additionally, we
found significantly higher Nes+MVD values in patients
with the advanced-stage disease as compared to patients with the
early-stage disease. Interestingly, we also noted that the number
of Nes+ vessels was associated with molecular subtype of
breast cancer. A high Nes+MVD was observed in patients
with TN breast cancer. TN is an extremely aggressive cancer subtype
with a poor prognosis. Similarly to our results, Kruger et
al demonstrated that the highest MVD of both Nes+
and Nes+Ki-67+ vessels and a higher VPI were
noted in TN and basal-like cancers (30). Furthermore, in contrast to their
results, we found that in IDC Nes+MVD is an independent
prognostic factor. In our study, high Nes+MVD was
associated with a shorter OS and earlier relapse. On the contrary,
Kruger et al (30) did not
demonstrate statistically significant relationship between
Nes+MVD and patient survival. This might be due to the
use of a more restrictive cut-off values determining high
Nes+MVD values and that they obtained higher median
value of Nes+ microvessels (84.3 >75.8
v/mm2). However, the same authors showed that in the
case of nestin VPI might be a valuable prognostic factor (30). The different results might be due
to the fact, that in our study we took into account only ductal
carcinoma cases (from 0.4 to 8 cm in diameter), whereas Kruger
et al (30) selected both
ductal and lobular histological types and tumours oscillating ~2 cm
in diameter during screening mammography. Finally, we are the first
to report that the number of Nes+ microvessels is
noticeably higher in invasive tumours than in pre-invasive lesions
and that Nes+MVD correlates with immature
CD34+ vessels but not with mature CD31+
vessels.
To date, CD34 and CD31 antigens have been commonly
used for the assessment of angiogenesis. However, these markers are
not selective for newly forming vessels and they do not constitute
a reliable reflection of tumour angiogenesis. During EC
differentiation, cells initially express CD34 antigen whereas the
expression of CD31 occurs at later stages of EC development
(22). The findings suggest that
the expression of CD31 antigen is typical of more mature vessels,
while CD34 expression is related to a more primary vascular
phenotype (37–40). Our results demonstrated that
Nes+MVD correlates with the density of newly forming
CD34+ vessels, whereas no correlation was found in the
case of CD31+ mature vessels. It may indicate that
nestin expression reflects a more progenitor nature of vessels and
that it is mainly limited to those undifferentiated and newly
forming ones. To confirm the obtained results, we developed an
in vitro model consisting of three endothelial cell lines
isolated from different types of vessels. Examinations of nestin
expression showed that the highest expression occurs in the
HEPC-CB.1 cell line, originating from human umbilical cord blood.
This line was characterized as early EPCs and shows the expression
of both stem cell (e.g., CD133) and endothelial antigens (e.g.,
VEGFR2, nitric oxide synthase) (22). We noted a lower nestin expression
in the HMEC-1 cells isolated from dermal microvessels and the
HUVEC-SVT cells isolated from the umbilical vein, respectively.
HUVEC-SVT and HMEC-1 cell lines are derived from different blood
vessel types, but both represent mature and differentiated
phenotypes of vessels. Similarly, Suzuki et al demonstrated
that in the bone marrow nestin is expressed exclusively in
proliferating progenitor ECs, but not in mature ECs (20). Moreover, the literature data
indicate that nestin undergoes expression not only in EPCs, but
also in mesenchymal stem cells (MSCs) which may differentiate into
pericytes and vascular smooth muscle cells (41). Thus, Nes+ cells may
participate not only in the formation of the endothelium but also
in the stabilization of the entire vessel wall.
In conclusion, we assume that nestin might be a
reliable marker for angiogenesis evaluation in IDC and higher
Nes+MVD may be related to a more aggressive course of
the disease and a poorer prognosis. Additionally, nestin seems to
be a selective marker for newly forming vessels and its expression
may reflect the process of tumour angiogenesis.
References
|
1
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih T and Lindley C: Bevacizumab: An
angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. 28:1779–1802. 2006. View Article : Google Scholar
|
|
4
|
Shaheen RM, Ahmad SA, Liu W, Reinmuth N,
Jung YD, Tseng WW, Drazan KE, Bucana CD, Hicklin DJ and Ellis LM:
Inhibited growth of colon cancer carcinomatosis by antibodies to
vascular endothelial and epidermal growth factor receptors. Br J
Cancer. 85:584–589. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: A systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis–correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meert AP, Paesmans M, Martin B, Delmotte
P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C and Sculier JP:
The role of microvessel density on the survival of patients with
lung cancer: A systematic review of the literature with
meta-analysis. Br J Cancer. 87:694–701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sejersen T and Lendahl U: Transient
expression of the intermediate filament nestin during skeletal
muscle development. J Cell Sci. 106:1291–1300. 1993.PubMed/NCBI
|
|
10
|
Terling C, Rass A, Mitsiadis TA, Fried K,
Lendahl U and Wroblewski J: Expression of the intermediate filament
nestin during rodent tooth development. Int J Dev Biol. 39:947–956.
1995.PubMed/NCBI
|
|
11
|
Fröjdman K, Pelliniemi LJ, Lendahl U,
Virtanen I and Eriksson JE: The intermediate filament protein
nestin occurs transiently in differentiating testis of rat and
mouse. Differentiation. 61:243–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lardon J, Rooman I and Bouwens L: Nestin
expression in pancreatic stellate cells and angiogenic endothelial
cells. Histochem Cell Biol. 117:535–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mokrý J, Cízková D, Filip S, Ehrmann J,
Osterreicher J, Kolár Z and English D: Nestin expression by newly
formed human blood vessels. Stem Cells Dev. 13:658–664. 2004.
View Article : Google Scholar
|
|
14
|
Krupkova O Jr, Loja T, Zambo I and
Veselska R: Nestin expression in human tumors and tumor cell lines.
Neoplasma. 57:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin
F, Xu H and Lu P: Clinical implications for nestin protein
expression in breast cancer. Cancer Sci. 101:815–819. 2010.
View Article : Google Scholar
|
|
16
|
Sjöberg G, Jiang WQ, Ringertz NR, Lendahl
U and Sejersen T: Colocalization of nestin and vimentin/desmin in
skeletal muscle cells demonstrated by three-dimensional
fluorescence digital imaging microscopy. Exp Cell Res. 214:447–458.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HS, Kang HS, Messam CA, Min KW and
Park CS: Comparative evaluation of angiogenesis in gastric
adenocarcinoma by nestin and CD34. Appl Immunohistochem Mol
Morphol. 10:121–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klein T, Ling Z, Heimberg H, Madsen OD,
Heller RS and Serup P: Nestin is expressed in vascular endothelial
cells in the adult human pancreas. J Histochem Cytochem.
51:697–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teranishi N, Naito Z, Ishiwata T, Tanaka
N, Furukawa K, Seya T, Shinji S and Tajiri T: Identification of
neovasculature using nestin in colorectal cancer. Int J Oncol.
30:593–603. 2007.PubMed/NCBI
|
|
20
|
Suzuki S, Namiki J, Shibata S, Mastuzaki Y
and Okano H: The neural stem/progenitor cell marker nestin is
expressed in proliferative endothelial cells, but not in mature
vasculature. J Histochem Cytochem. 58:721–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bouïs D, Hospers GA, Meijer C, Molema G
and Mulder NH: Endothelium in vitro: A review of human vascular
endothelial cell lines for blood vessel-related research.
Angiogenesis. 4:91–102. 2001. View Article : Google Scholar
|
|
22
|
Paprocka M, Krawczenko A, Dus D, Kantor A,
Carreau A, Grillon C and Kieda C: CD133 positive progenitor
endothelial cell lines from human cord blood. Cytometry A.
79:594–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ; Panel members: Thresholds for
therapies: Highlights of the St Gallen International Expert
Consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mueller-Holzner E, Fink V, Frede T and
Marth C: Immunohistochemical determination of HER2 expression in
breast cancer from core biopsy specimens: A reliable predictor of
HER2 status of the whole tumor. Breast Cancer Res Treat. 69:13–19.
2001. View Article : Google Scholar
|
|
25
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dahlstrand J, Collins VP and Lendahl U:
Expression of the class VI intermediate filament nestin in human
central nervous system tumors. Cancer Res. 52:5334–5341.
1992.PubMed/NCBI
|
|
27
|
Kobayashi M, Sjöberg G, Söderhäll S,
Lendahl U, Sandstedt B and Sejersen T: Pediatric rhabdomyosarcomas
express the intermediate filament nestin. Pediatr Res. 43:386–392.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mokrý J and Nemecek S: Cerebral
angiogenesis shows nestin expression in endothelial cells. Gen
Physiol Biophys. 18(Suppl 1): 25–29. 1999.
|
|
29
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: Proliferation of immature tumor vessels is a novel
marker of clinical progression in prostate cancer. Cancer Res.
69:4708–4715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krüger K, Stefansson IM, Collett K, Arnes
JB, Aas T and Akslen LA: Microvessel proliferation by co-expression
of endothelial nestin and Ki-67 is associated with a basal-like
phenotype and aggressive features in breast cancer. Breast.
22:282–288. 2013. View Article : Google Scholar
|
|
31
|
Piras F, Ionta MT, Lai S, Perra MT, Atzori
F, Minerba L, Pusceddu V, Maxia C, Murtas D, Demurtas P, et al:
Nestin expression associates with poor prognosis and
triple-negative phenotype in locally advanced (T4) breast cancer.
Eur J Histochem. 55:e392011. View Article : Google Scholar
|
|
32
|
Parry S, Savage K, Marchiò C and
Reis-Filho JS: Nestin is expressed in basal-like and
triple-negative breast cancers. J Clin Pathol. 61:1045–1050. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krüger K, Wik E, Knutsvik G, Nalwoga H,
Klingen TA, Arnes JB, Chen Y, Mannelqvist M, Dimitrakopoulou K,
Stefansson IM, et al: Expression of Nestin associates with BRCA1
mutations, a basal-like phenotype and aggressive breast cancer. Sci
Rep. 7:10892017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paduch R: The role of lymphangiogenesis
and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
39:397–410. 2016. View Article : Google Scholar
|
|
35
|
Popiela TJ, Sikora J, Klimek M, Basta P,
Niemiec T, Dobrogowski J, Kotlarz A, Rudnicka-Sosin L and
Dutsch-Wicherek M: The analysis of CD34 antigen immunoreactivity
level in invasive ductal breast cancer with respect to the presence
of lymph node metastases. Neuro Endocrinol Lett. 29:443–446.
2008.PubMed/NCBI
|
|
36
|
Xie XD, Qu SX, Liu ZZ, Zhang F and Zheng
ZD: Study on relationship between angiogenesis and micrometastases
of peripheral blood in breast cancer. J Cancer Res Clin Oncol.
135:413–419. 2009. View Article : Google Scholar
|
|
37
|
Ribatti D: The discovery of endothelial
progenitor cells. An historical review Leuk Res. 31:439–444.
2007.
|
|
38
|
Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa
H, Tamamura R, Sugahara T, Sasaki A and Nagai N: Various
immunostaining patterns of CD31, CD34 and endoglin and their
relationship with lymph node metastasis in oral squamous cell
carcinomas. J Oral Pathol Med. 34:70–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walzer SM, Cetin E, Grübl-Barabas R,
Sulzbacher I, Rueger B, Girsch W, Toegel S, Windhager R and Fischer
MB: Vascularization of primary and secondary ossification centres
in the human growth plate. BMC Dev Biol. 14:362014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Corselli M, Chen CW, Crisan M, Lazzari L
and Péault B: Perivascular ancestors of adult multipotent stem
cells. Arterioscler Thromb Vasc Biol. 30:1104–1109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klein D, Meissner N, Kleff V, Jastrow H,
Yamaguchi M, Ergün S and Jendrossek V: Nestin(+) tissue-resident
multipotent stem cells contribute to tumor progression by
differentiating into pericytes and smooth muscle cells resulting in
blood vessel remodeling. Front Oncol. 4:1692014. View Article : Google Scholar : PubMed/NCBI
|