Introduction
Gastric cancer (GC) is one of the most common types
of cancer worldwide and the second leading cause of cancer-related
death globally (1,2). Despite remarkable advance in
diagnostic techniques, such as endoscopic detection, and
improvement in therapeutic modalities (3), including novel chemotherapeutic
interventions and target therapy, the long-term survival of GC
patients remains unsatisfactory due to the high rates of local
invasion and distal metastasis (4). Therefore, it is critical to identify
the potential molecular mechanisms underlying the progression and
metastasis in GC and thus, provide novel therapeutic targets for
cancer treatment (5).
MicroRNAs (miRNAs) are a group of endogenous and
conserved non-coding RNAs that modulate the specific protein
expression through binding to the 3′-untranslated region (3′-UTR)
of target mRNAs based on sequence complementarity, and function as
post-transcriptional regulators of gene expression (6,7).
Increasing evidence has confirmed that miRNAs are abnormally
expressed in various cancers, including GC (8), and participate in different
biological progress including cell growth, apoptosis,
differentiation and metastasis (9,10).
Therefore, miRNAs have been proposed as promising prognostic
markers for GC patients (11).
Among numerous cancer-related miRNAs, miR-379, which
is located on chromosome 14q32.31, was recently found to be a novel
cancer-related miRNA (12,13). It was downregulated in breast
cancer (12,14), glioblastoma (15), hepatocellular carcinoma (16) and malignant pleural mesothelioma
(17). miR-379 was decreased in
breast cancer and inhibited cell proliferation by regulating cyclin
B1 expression (12). miR-379
regulated IL-18 and contributed to drug resistance in malignant
pleural mesothelioma (17).
However, in prostate cancer (13)
and papillary renal cell carcinoma (18), miR-379 was found to be upregulated.
Elevated miR-379 in prostate cancer facilitated tumor growth,
epithelial to mesenchymal transition (EMT) and bone metastasis.
Therefore, the functional significance of miR-379 in cancer
development and progression seem to be cancer-type specific.
However, the expression and functional role of miR-379 in GC have
not been elucidated before.
Epithelial-to-mesenchymal transition (EMT) has been
recognized as a physiological process in the invasion and
metastasis of various cancers through transformation of adherent
and polarized epithelial cells into an invasive mesenchymal cell
phenotype (19–21). Moreover, cancer cells undergoing
the EMT usually decreased the cell adhesion molecule E-cadherin,
which is an important determinant of epithelial cell-cell adhesion,
while increased the vimentin and N-cadherin expression (22). Accumulating evidence has revealed
that EMT can mediate both GC invasion and metastasis (23–25).
However, the association between miR-379 and EMT in GC has remained
elusive.
In the present study, we investigated the effects of
miR-379 on the FAK/AKT signaling in GC cells. Our data showed that
miR-379 was downregulated in the GC and the reduced miR-379 was
associated with poor prognostic features and poor 5-year survival
of GC patients. We also confirmed that miR-379 could regulate the
migration, invasion and EMT phenotype of GC by targeting FAK/AKT
signaling in vitro and in vivo. These data identify
the underlying mechanism by which miR-379 inhibits migration and
invasion of GC and indicates miR-379 as a novel prognostic
biomarker for GC patients.
Materials and methods
Clinical specimens
Ninety-six GC tissues and paired adjacent
non-cancerous tissues were obtained from the Traditional Chinese
Medicine Hospital of Jingshan County during January 2005 to
December 2010. Pathological diagnosis was performed according to
the World Health Organization (WHO) criteria. None of the patients
received chemotherapy or radiotherapy before surgery. All patients
gave written informed consent and this study was approved by the
Ethics Committee of the Traditional Chinese Medicine Hospital of
Jingshan County.
The human GC cell lines SGC7901, MGC803, HGC27,
MKN45 and the normal gastric epithelial cell line GES-1 were
obtained from the Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences, Shanghai, China) and were cultured in
RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10%
fetal bovine serum (FBS; Invitrogen), 1% penicillin-streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere at
37° with 5% CO2.
Quantitative reverse transcriptase
polymerase chain reaction (qRT-PCR)
Total RNA from GC tissues and cells was isolated
using TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. cDNA was reverse-transcribed from 1 µg total RNA
using a Reverse Transcription kit (Takara Bio, Shiga, Japan). cDNA
was then amplified with a SYBR® Premix Ex Taq™ II
(Perfect real-time) kit (Takara Bio). The gene expression levels
were calculated using the ΔΔCt method with U6 or GAPDH as an
internal control. Hsa-miR-379 primer (HmiRQP0476), snRNA U6 qPCR
Primer (HmiRQP9001), FAK (HQP015639) and GAPDH (HQP006940) were
purchased from GeneCopoeia (Guangzhou, China).
Cell transfection
miRNA vectors, including miR-379 expression vector
(HmiR0219), the control vector for miR-374 (CmiR0001), miR-379
inhibitor (HmiR-AN0476) and the negative control (CmiR-AN0001-AM04)
were obtained from GeneCopoeia. The FAK overexpression plasmid and
specific siRNA against FAK and a scramble siRNA were synthesized by
Sangon Biotech, Co., Ltd. (Shanghai, China). Cells were transfected
with above vectors using Lipofectamine 2000 reagent
(Invitrogen/Life Technologies) in accordance with the
manufacturer's protocol.
Western blot analysis
The whole proteins were harvested in RIPA buffer
supplemented with protease and phosphatase inhibitors (Roche) and
the concentrations were quantified with BCA protein assay kit
(Tiangen Biotech, Co., Ltd., Beijing, China), and an equal amount
of 30 µg protein was separated by 10% SDS-PAGE gel and then
transferred onto PVDF membranes (Millipore, Billerica, MA, USA).
The membranes were blocked with 5% non-fat milk in TBST for 2 h at
room temperature and incubated overnight with specific primary
antibodies (1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) at 4°C. Then the membranes were washed three times by TBST and
incubated with HRP-conjugated secondary antibody for 2 h at room
temperature (SGB-Bio, Beijing, China). Detection was performed by
enhanced chemiluminescence kit (Amersham, Little Chalfont, UK).
GAPDH was used as protein loading control. The antibodies against
FAK, E-cadherin, N-cadherin, vimentin, AKT and p-AKT were purchased
from Cell Signaling Technology.
Immunohistochemical staining (IHC)
Briefly, 4 µm sections were deparaffinized in
xylene, rehydrated through graded ethanols, followed by blocking of
endogenous peroxidase activity in 3% hydrogen peroxide for 10 min
at room temperature. The corresponding antibody (1:300; Cell
Signaling Technology) was applied as the primary antibody by a
streptavidin peroxidase-conjugated (SP-IHC) method. The staining
results were semi-quantitatively evaluated by the multiply of
staining intensity and the percentage of positive staining cells.
The percentage of positive cells was given into four grades: 0 for
<5%; 1 for 6–25%; 2 for 26–50%; 3 for 51–75% and 4 for >75%.
Staining intensity was assessed by four degrees: 0, negative; 1,
weak; 2, moderate; and 3, strong. Each section was assayed for ten
independent high magnification (×400) fields to get the average
scores.
Cell migration and invasion analyses
Matrigel-uncoated and -coated Transwell inserts (8
µm pore size; Millipore) were used to evaluate cell
migration and invasion. Briefly, 2×104 transfected cells
were suspended in 150 µl serum-free RPMI-1640 medium into
the upper chamber, and 700 µl RPMI-1640 medium containing
20% FBS was placed in the lower chamber. After 24-h incubation,
cells were fixed in 4% paraformaldehyde for 20 min and stained with
0.1% crystal violet dye for 15 min. The cells on the inner layer
were softly removed with a cotton swab and counted at five randomly
selected views, and the average cell number per view was
calculated.
Luciferase reporter assay
The 3′-UTR sequence of FAK predicted to interact
with miR-379, together with a corresponding mutated sequence within
the predicted target sites, were synthesized and inserted into the
pmiR-GLO Dual-luciferase miRNA target expression vector (Promega,
Madison, WI, USA) called wt-FAK 3′-UTR and mt-FAK 3′-UTR.
Subsequently, MGC803 cells that were plated into 24-well plate and
were transfected with miR-379 inhibitor or negative control. Cells
were co-transfected with the wild-type or mutant 3′-UTR of FAK
vector using the Lipofectamine 2000 reagent (Invitrogen). After 48
h, cells were harvested and measured according to the
manufacturer's instructions (Dual-luciferase assay system;
Promega). pRL-TK expressing Renilla luciferase was
cotransfected as an internal control to correct the differences in
both transfection and harvest efficiencies.
Statistical analysis
Data are presented as the mean ± SD and performed at
least three independent replicates. SPSS software, 16.0 (SPSS,
Inc., Chicago, IL, USA) and Graphpad Prism 6.0 (GraphPad Software,
Inc., La Jolla, CA, USA) were used for a two-tailed Student's
t-test, Pearson's correlation analysis, Kaplan-Meier method and the
log-rank test to evaluate the statistical significance. Differences
were defined as P<0.05.
Results
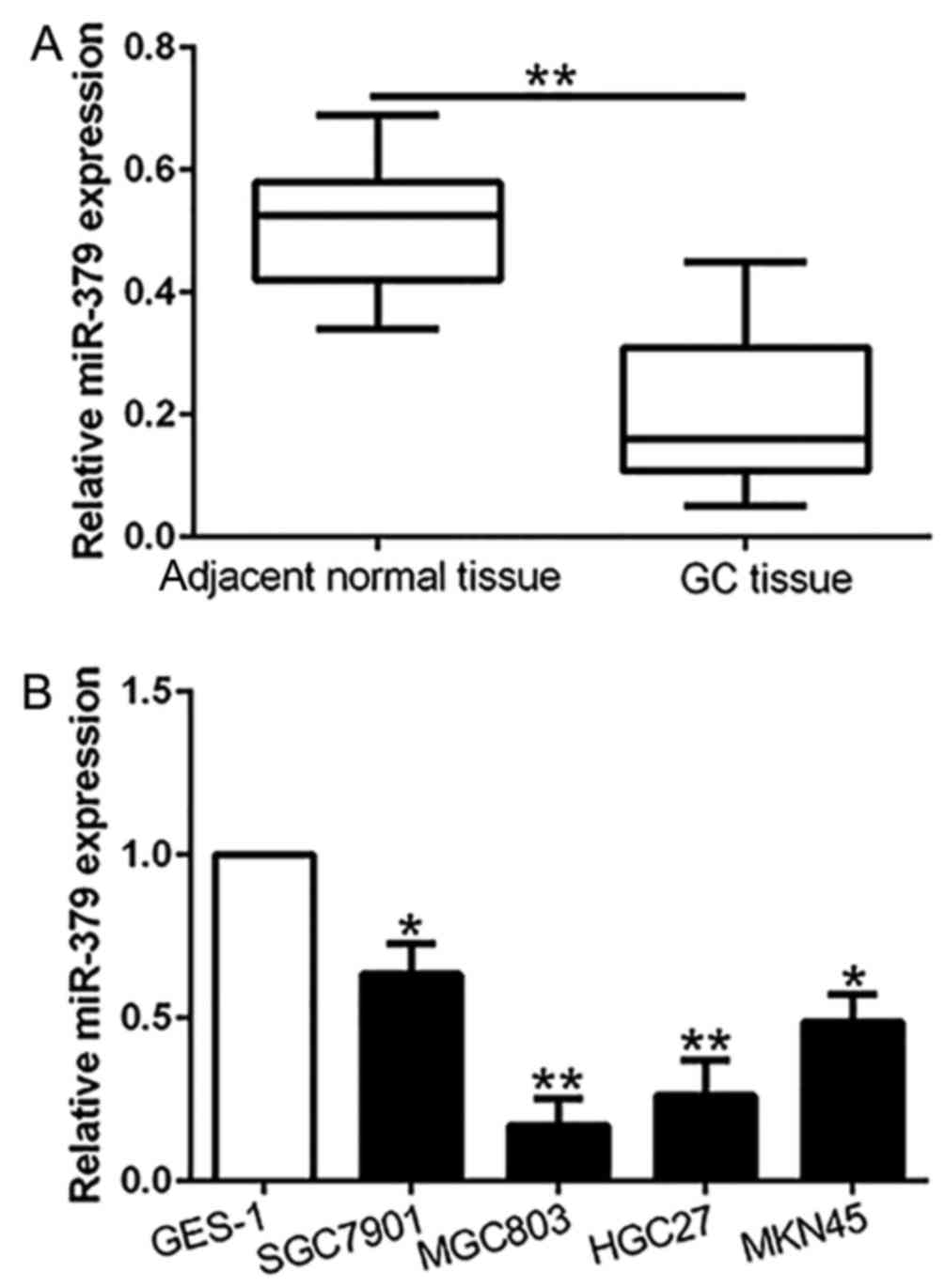
The expression of miR-379 is decreased in
gastric cancer tissues and cell lines
To investigate whether miR-379 was involved in
gastric carcinogenesis, we first examined the expression of miR-379
in 96 pairs of GC tissues and the paired normal gastric mucosa. Our
results showed that miR-379 expression in GC tissues was
significantly downregulated compared with the paired non-cancerous
tissues (P<0.01; Fig. 1A).
Moreover, similar result was found in GC cell lines. The data
revealed that miR-379 was remarkably reduced in a panel of GC cell
lines compared to the normal gastric epithelial cell line GES-1
(P<0.05; Fig. 1B). These
results confirmed that miR-379 was downregulated in gastric cancer
tissues and cell lines.
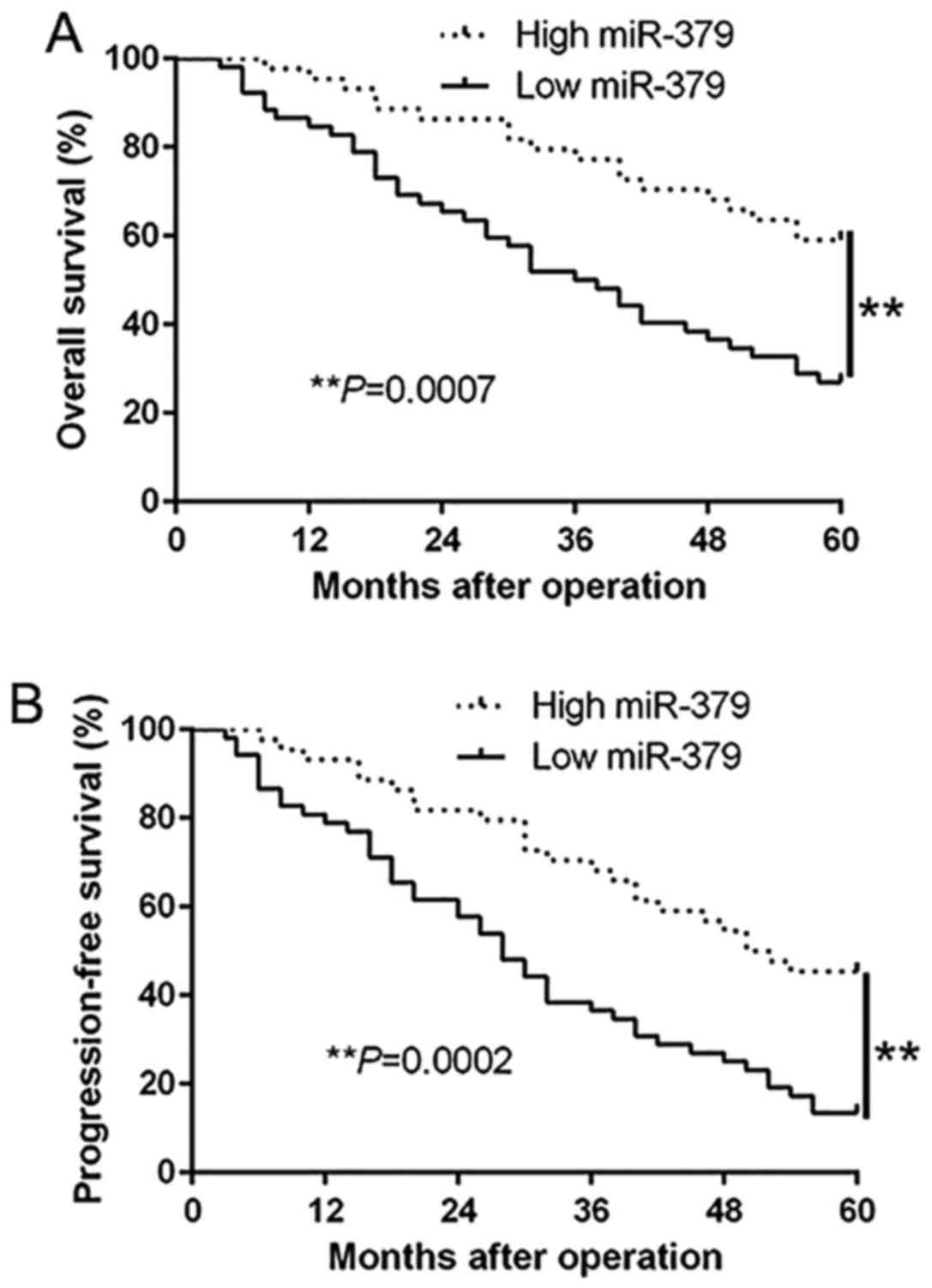
Clinical significance of the
downregulated miR-379 expression in GC tissues
To further investigate the role of miR-379 in the
progression of GC, we determined the relationship between miR-379
expression and the clinicopathological features and prognosis of GC
patients. With the median level of miR-379 as the cut-off, the low
miR-379 expression was obviously associated with lymph node
metastasis (P<0.001) and advanced TNM stage (P<0.001)
(Table I). Moreover, Kaplan-Meier
analysis revealed that the downregulation of miR-379 was
prominently correlated with shorter overall survival (P=0.0007;
Fig. 2A) and shorter
progression-free survival (P=0.0002; Fig. 2B) in GC patients. Furthermore,
miR-379 expression was an independent factor for predicting both
5-year overall and progression-free survival in GC patients
(P=0.012, P=0.014, respectively; Table II). These results indicate that
miR-379 may act as a potent biomarker for predicting prognosis of
GC patients.
 | Table IThe relationship between miR-379
expression and clinicopathological features in GC patients
(n=96). |
Table I
The relationship between miR-379
expression and clinicopathological features in GC patients
(n=96).
| Clinical
parameters | Cases
(n) | Expression level
| P-value |
|---|
miR-379high
(n=44) |
miR-379low
(n=52) |
|---|
| Age (years) | | | | 0.986 |
| <60 | 35 | 16 | 19 | |
| ≥60 | 61 | 28 | 33 | |
| Sex | | | | 0.439 |
| Male | 66 | 32 | 34 | |
| Female | 30 | 12 | 18 | |
| Tumor size
(cm) | | | | 0.156 |
| <5 | 72 | 36 | 36 | |
| ≥5 | 24 | 8 | 16 | |
| Histological
type | | | | 0.238 |
| Intestinal | 78 | 38 | 40 | |
| Diffuse | 18 | 6 | 12 | |
| TNM stage | | | | <0.001a |
| I+II | 40 | 28 | 12 | |
| III+IV | 56 | 16 | 40 | |
| Lymph
metastasis | | | | <0.001a |
| Present | 58 | 18 | 40 | |
| Absent | 38 | 26 | 12 | |
 | Table IIMultivariate Cox regression analysis
of 5-year OS and PFS of 96 GC patients. |
Table II
Multivariate Cox regression analysis
of 5-year OS and PFS of 96 GC patients.
| Variables | Overall survival
| Progression-free
survival
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| miR-379
expression | 0.212 | 0.056–0.892 | 0.012a | 0.232 | 0.072–0.726 | 0.014a |
| TNM stage | 2.563 | 1.317–5.982 | 0.007a | 2.223 | 1.113–4.889 | 0.009a |
| Lymph
metastasis | 3.243 | 1.572–6.238 | 0.003a | 3.027 | 1.476–6.193 | 0.004a |
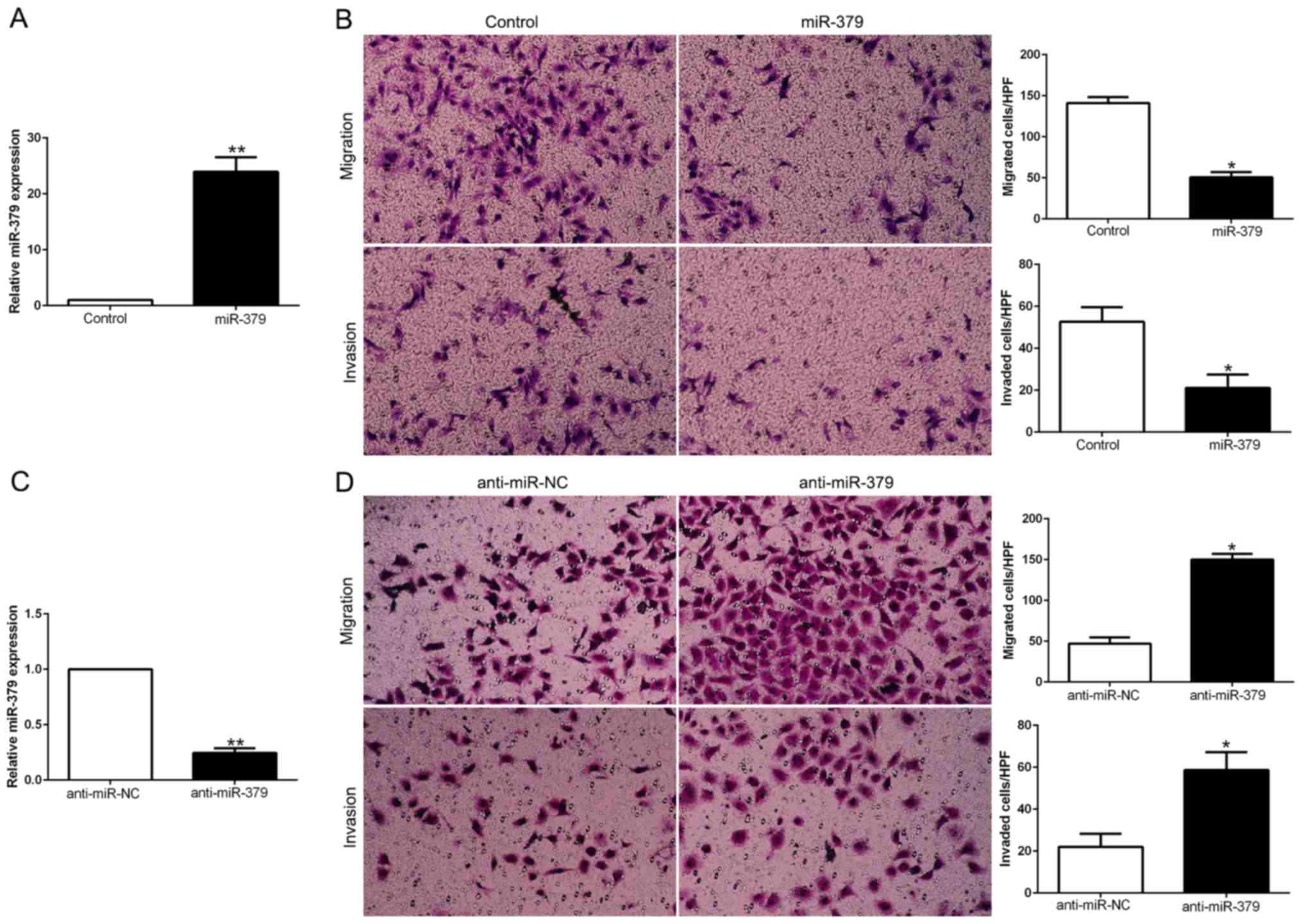
miR-379 inhibits GC cell migration and
invasion
To explore the biological function of miR-379 in
HCC, we transduced GC cell lines with miR-379 expression vector or
anti-miR-379 vector which contained different endogenous miR-379
levels. As determined by qRT-PCR, we confirmed that miR-379
effectively upregulated miR-379 in MGC803 (P<0.05; Fig. 3A) or downregulated miR-379 in
SGC7901 cells (P<0.05; Fig.
3C). As examined by Matrigel-coated (for invasion) and
-uncoated (for migration) Transwell assays, miR-379 overexpression
significantly inhibited the migration and invasion of MGC803 cells
(P<0.05; Fig. 3B), whereas
miR-379 knockdown obviously increased the number of migrated and
invaded SGC7901 cells (P<0.05; Fig.
3D). In conclusion, these data suggested that miR-379 could
regulate the GC cell migration and invasion and may exert an
anti-metastatic effect on GC.
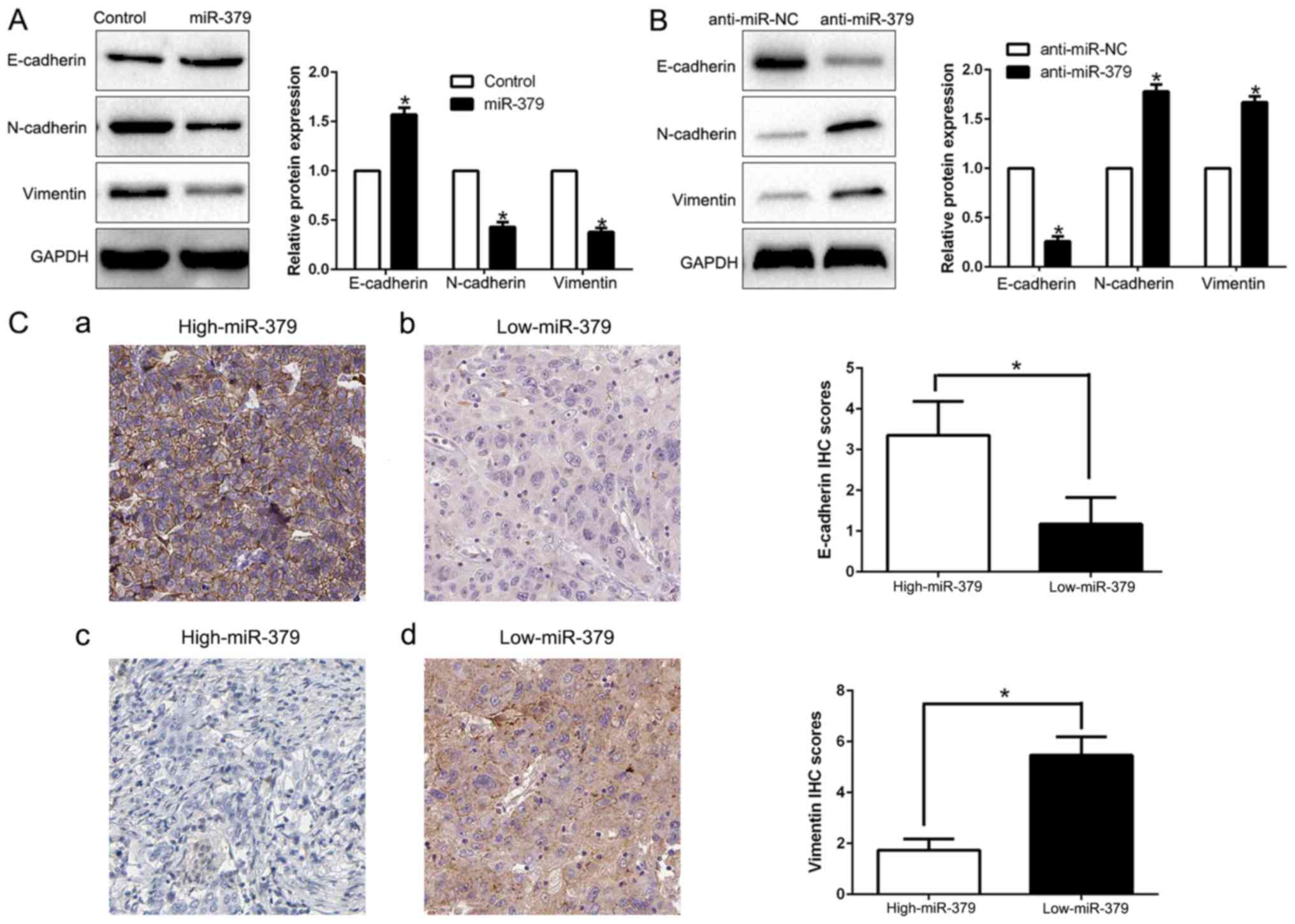
miR-379 suppresses
epithelial-to-mesenchymal transition in GC cells
EMT has been proposed as a critical role in the
initiation of metastasis progression of cancer. To gain a
mechanistic illustration of the potential role of miR-379 in
modulating GC metastasis, the EMT markers were measured. We found
that miR-379 overexpression facilitated the epithelial marker
E-cadherin and suppressed N-cadherin and vimentin expression
(P<0.05; Fig. 4A). In contrast,
miR-379 knockdown decreased E-cadherin expression and increased
N-cadherin and vimentin expression (P<0.05; Fig. 4B). In addition, we further explored
the correlation between miR-379 expression and EMT marker in GC
tissues. We found that the E-cadherin expression in high miR-379
group was higher than that in low miR-379 group. Conversely, the
expression level of vimentin in the high miR-379 group was markedly
lower than that in low miR-379 group (P<0.05; Fig. 4C). Taken together, these results
suggest that miR-379 function as a suppressor of EMT in GC
cells.
FAK is a direct downstream target of
miR-379 in GC cells
To elucidate the molecular mechanisms responsible
for the functional influence of miR-379 in GC cells, we searched
the publically available database TargetScan to explore the
candidate target genes. Among them, FAK was known to play an
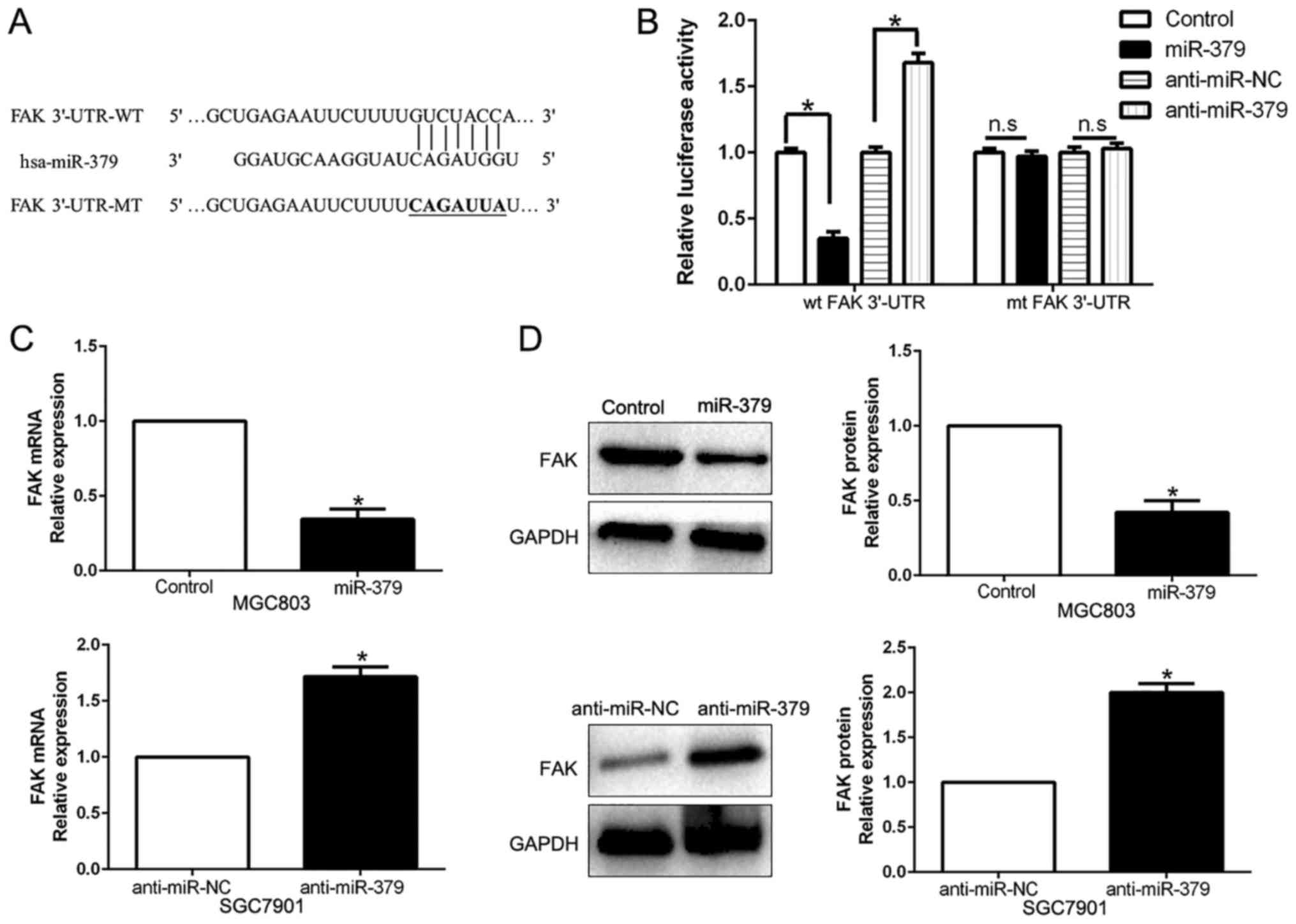
important role in GC invasion and metastasis (26). As shown in Fig. 5A, the sequence complementary to the
binding sites of miR-379 was revealed in the 3′-UTR of FAK. We
performed a luciferase reporter assay to verify that miR-379 could
bind to the 3′-UTR of FAK. The results showed that miR-379
overexpression significantly decreased the luciferase activity of
wild-type (wt) FAK 3′-UTR while had no influence on that of mutant
(mt) FAK 3′-UTR (P<0.05; Fig.
5B). On the contrary, miR-379 knockdown increased the
luciferase activity of wt FAK 3′-UTR (P<0.05; Fig. 5B) but did not affect the luciferase
activity of mt FAK 3′-UTR constructs. In addition, miR-379
overexpression markedly reduced the mRNA and protein levels of FAK
in MGC803 cells (P<0.05, respectively; Fig. 5C and D). By contrast, the
expression of FAK mRNA and protein were significantly increased by
the downregulation of miR-379 in SGC7901 cells (P<0.05,
respectively; Fig. 5C and D).
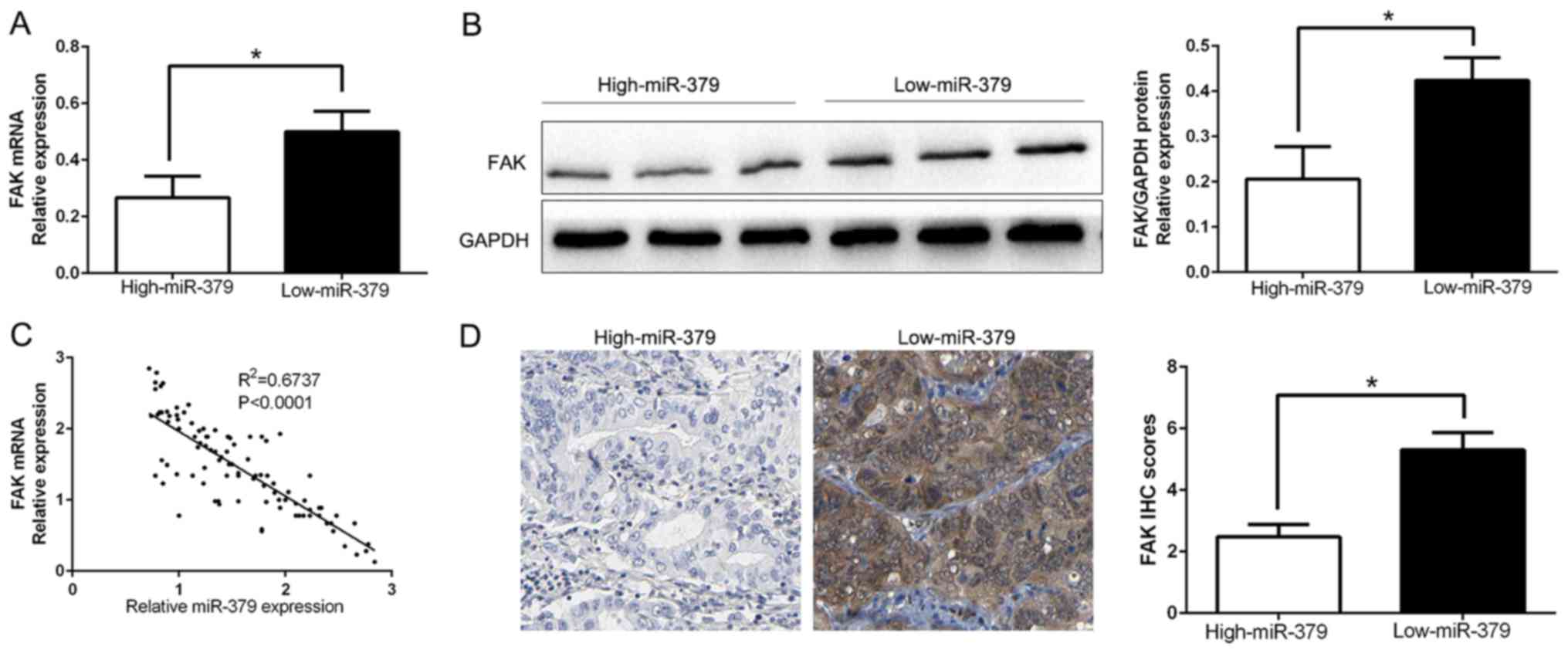
miR-379 correlates negatively with the
FAK expression in GC samples
To further evaluate the relationship between miR-379
and FAK in GC tissues, we measured the FAK mRNA and protein
expression in two groups of miR-379. As expected, our data showed
that both FAK mRNA and protein expression level in high miR-379
group were significantly lower than that in low miR-379 group in GC
(P<0.05; Fig. 6A and B).
Moreover, we demonstrated that the mRNA level of FAK in the GC
tissues was inversely correlated with miR-379 expression
(R2=0.6737, P<0.0001; Fig. 6C). Consistently, as assessed by IHC
assay, FAK protein expression in miR-379 high-expressing tumors was
obviously lower than miR-379 low-expressing tumors (P<0.05;
Fig. 6D), which was similar with
previous studies. In conclusion, these data suggest that FAK was a
direct downstream target of miR-379 in GC.
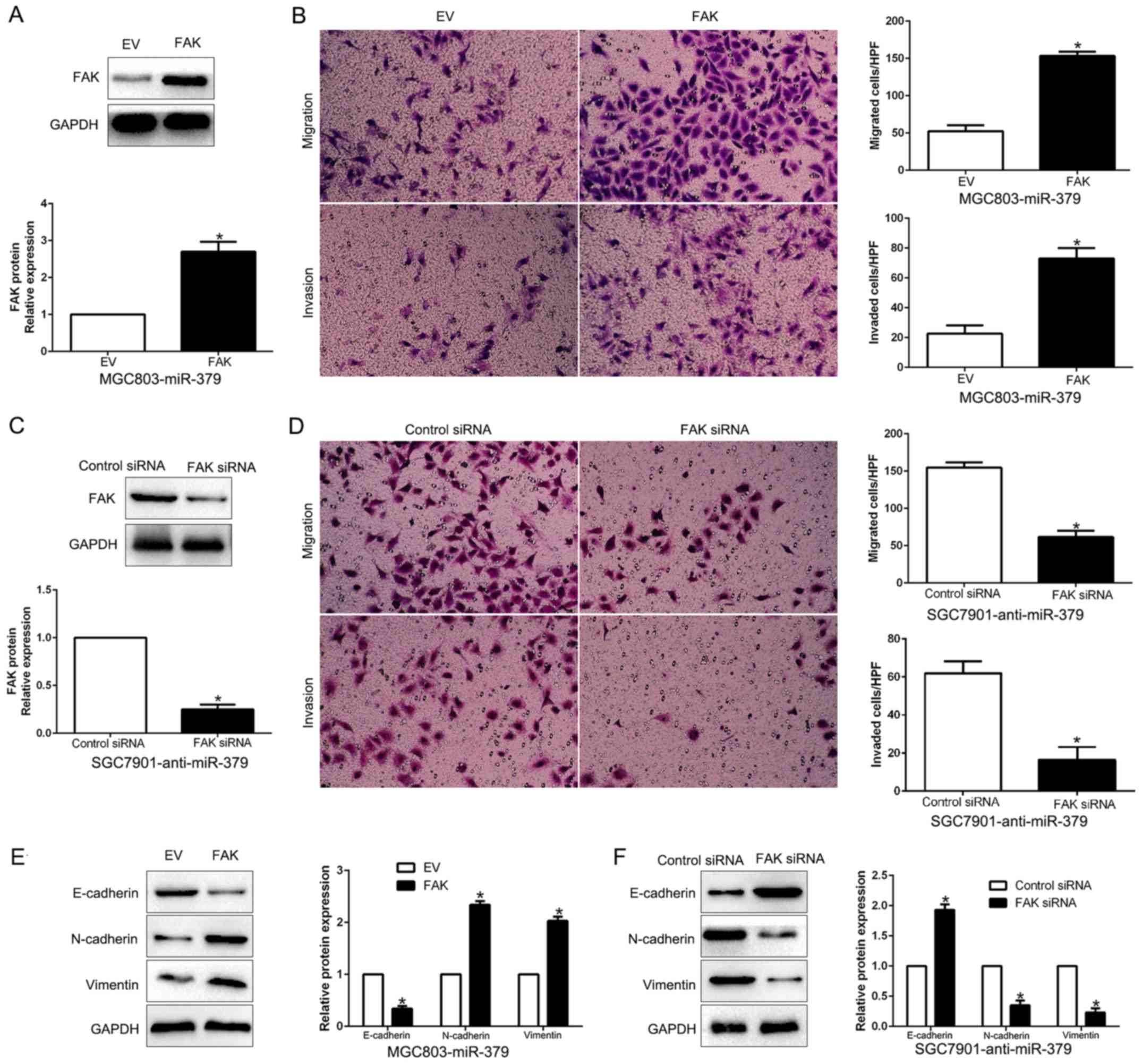
FAK is essential for the miR-379-mediated
inhibition of cell migration, invasion and EMT in HCC cells
To clarify that FAK is a functional target of
miR-379, FAK was overexpressed by a plasmid vector in
miR-379-overexpressing MGC803 cells (P<0.05; Fig. 7A). Furthermore, FAK overexpression
increased cell migration, and invasion (P<0.05, respectively;
Fig. 7B) and promoted EMT progress
(P<0.05; Fig. 7E). Similarly,
FAK knockdown by a specific siRNA in miR-379-suppressive SGC7901
cells (P<0.05; Fig. 7C)
significantly inhibited cell migration, invasion (P<0.05,
respectively; Fig. 7D) and EMT
progress (P<0.05; Fig. 7F).
These data demonstrated that FAK is a downstream mediator in the
function of miR-379 in GC.
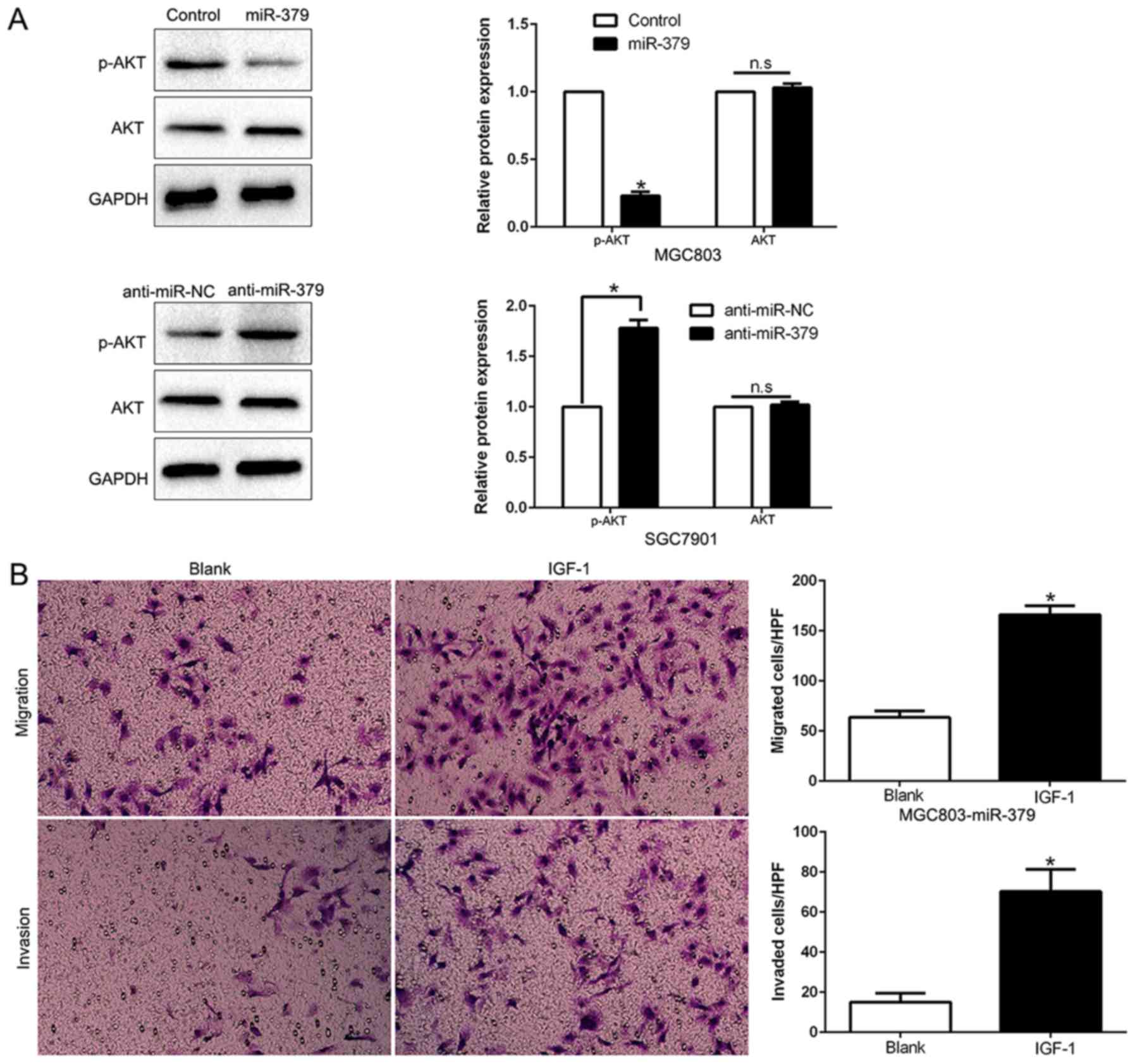
PI3K/AKT signaling is essential for the
biological function of miR-379 in GC
Previous studies demonstrated that FAK could induce
the activation of PI3K/AKT signaling and play a critical role in
the invasion and metastasis of GC and EMT (27,28).
As shown in Fig. 8A, ectopic
expression of miR-379 significantly decreased, while miR-379
knockdown increased the AKT phosphorylation in GC cells (P<0.05;
Fig. 8A). However, the total AKT
protein had no change (P<0.05; Fig.
8A). These data indicate that miR-379 suppressed the PI3K/AKT
pathway in GC cells. To determine whether AKT phosphorylation
mediated miR-379-induced inhibition of cell migration, invasion and
EMT process in GC cells, we treated miR-379-overexpressing MGC803
cells with insulin-like growth factor 1 (IGF-1), which is an
activator of PI3K/AKT pathway. We found that IGF-1 at least
partially rescued the miR-379-induced inhibition of cell migration,
invasion (P<0.05; Fig. 8B) and
EMT process (P<0.05; Fig. 8D).
Conversely, the restraint of the PI3K/AKT pathway by MK2206
abrogated the effects of miR-379 inhibition to induce cell
migration, invasion (P<0.05; Fig.
8C) and EMT progress (P<0.05; Fig. 8D) in miR-379-suppressive SGC7901
cells. Taken together, our results demonstrate that PI3K/AKT
signaling plays an essential function during miR-379-induced GC
cell migration, invasion and EMT progression
Discussion
Systemic metastasis of GC is the major cause of the
tumor recurrence and patient mortality. Increasing evidence has
demonstrated that miRNAs were involved in GC invasion and
metastasis (29,30). Therefore, miRNAs have been regarded
as potential biomarkers and therapeutic targets for GC. In previous
studies, Chen et al (16)
demonstrated that microRNA-379-5p inhibited tumor invasion and
metastasis by targeting FAK/AKT signaling in hepatocellular
carcinoma (HCC), moreover, miR-379 suppressed HCC metastasis and
EMT in vivo. In addition, Khan et al (12) confirmed that miR-379 was decreased
in breast cancer and could be a novel regulator of cyclin B1.
However, on the contrary, miR-379 in the DLK1-DIO3 miRNA
mega-cluster regulated EMT and bone metastasis of prostate cancer
(13). Moreover, miR-379 was
downregulated in papillary renal cell carcinoma and significantly
associated with patient survival (18). These data indicated that the
expression level and biological effect was dependent on the cancer
type.
In the present study, we found that miR-379 was
significantly downregulated in 96 GC tissues compared with the
corresponding non-cancerous tissues. Similarly, the expression
level of miR-379 in gastric cancer cell lines were significantly
decreased. Reduced miR-379 expression was obviously correlated with
malignant clinicopathological characteristics of GC patients,
including advanced TNM stage and lymph node metastasis. Moreover,
we found that low miR-379 group had a significantly worse 5-year OS
and PFS for GC patients. Multivariate Cox repression analysis
indicated that miR-379 was an independent prognostic factor for
predicting survival of GC patients. Taken together, these results
suggest that miR-379 is critical for prognosis outcome of GC
patients. Importantly, gain- and loss-function experiment
demonstrated that miR-379 inhibited cell migration, invasion and
EMT, at least partially by targeting FAK mediated PI3K/AKT
signaling pathway. Furthermore, miR-379 was inversely correlated
with FAK expression, which was elevated in GC tissues (31). In addition, miR-379 could
negatively modulate FAK accumulation in GC cells. Taken together,
these results demonstrated that miR-379 functions as a tumor
suppressor in the migration, invasion and EMT of GC by directly
inhibiting FAK/AKT pathway.
FAK, a non-receptor tyrosine kinase, plays a
critical role in integrin signaling and promotes cancer
progression, invasion and metastasis (32). Increased FAK expression was
positively associated with poor survival and cancer progression in
different cancers, including GC (33). FAK/PI3K/AKT signaling was found to
promote EMT progression, which was proposed as a vital mechanism
that regulates the initial steps of Figure 8. Continued. (C) Quantification of
migration and invasion of SGC7901 cells stably expressing miR-379
inhibitor treated with 1 µM MK2206 for 24 h. (D) Western
blot analysis of indicated proteins in MGC803 cells stably
expressing miR-379 treated for 24 h with 100 ng/ml IGF-1 or SGC7901
cells stably expressing miR-379 inhibitor treated with 1 µM
MK2206 for 24 h. *P<0.05. metastatic progression of
cancer (34). Our results showed
that AKT pathway abolished the inhibitory or stimulatory effect of
miR-379 on GC cells. Taken together, these data demonstrated the
suppressive effect of miR-379 was mediated by targeting FAK to
inhibit AKT phosphorylation pathway in GC.
In summary, we demonstrated that miR-379 was
down-regulated in GC tissues and cell lines, and its decreased
expression was correlated with malignant clinicopathological
features. Furthermore, we confirmed that miR-379 inhibited cell
migration, invasion and EMT by inhibiting FAK mediated PI3K/AKT
signaling pathway. These results suggest that miR-379 is a
potential metastasis-associated tumor suppressor in GC.
Collectively, the deregulation of miR-379 may play an important
role in tumor metastasis and may be a novel prognostic factor and
potential therapeutic target for GC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
De Vita F, Vecchione L, Galizia G, Di
Martino N, Fabozzi T, Catalano G, Ciardiello F and Orditura M:
Perspectives in adjuvant therapy of gastric cancer. Oncology.
77(Suppl 1): 38–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kagawa S, Shigeyasu K, Ishida M, Watanabe
M, Tazawa H, Nagasaka T, Shirakawa Y and Fujiwara T: Molecular
diagnosis and therapy for occult peritoneal metastasis in gastric
cancer patients. World J Gastroenterol. 20:17796–17803.
2014.PubMed/NCBI
|
|
5
|
Bessette DC, Qiu D and Pallen CJ: PRL
PTPs: Mediators and markers of cancer progression. Cancer
Metastasis Rev. 27:231–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Song L, Zhang Z, Bai XX, Cui MF and
Ma LJ: MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal
transition in gastric cancer through up-regulating PTEN expression.
Oncotarget. 7:66989–67003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng L, Jiao W, Mei H, Song H, Li D,
Xiang X, Chen Y, Yang F, Li H, Huang K, et al: miRNA-337-3p
inhibits gastric cancer progression through repressing myeloid zinc
finger 1-facilitated expression of matrix metalloproteinase 14.
Oncotarget. 7:40314–40328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang PF, Sheng LL, Wang G, Tian M, Zhu
LY, Zhang R, Zhang J and Zhu JS: miR-363 promotes proliferation and
chemo-resistance of human gastric cancer via targeting of FBW7
ubiquitin ligase expression. Oncotarget. 7:35284–35292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan S, Brougham CL, Ryan J, Sahrudin A,
O'Neill G, Wall D, Curran C, Newell J, Kerin MJ and Dwyer RM:
miR-379 regulates cyclin B1 expression and is decreased in breast
cancer. PLoS One. 8:e687532013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gururajan M, Josson S, Chu GC, Lu CL, Lu
YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P, et al: miR-154*
and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate
epithelial to mesenchymal transition and bone metastasis of
prostate cancer. Clin Cancer Res. 20:6559–6569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pollari S, Leivonen SK, Perälä M, Fey V,
Käkönen SM and Kallioniemi O: Identification of microRNAs
inhibiting TGF-β-induced IL-11 production in bone metastatic breast
cancer cells. PLoS One. 7:e373612012. View Article : Google Scholar
|
|
15
|
Skalsky RL and Cullen BR: Reduced
expression of brain-enriched microRNAs in glioblastomas permits
targeted regulation of a cell death gene. PLoS One. 6:e242482011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JS, Li HS, Huang JQ, Dong SH, Huang
ZJ, Yi W, Zhan GF, Feng JT, Sun JC and Huang XH: MicroRNA-379-5p
inhibits tumor invasion and metastasis by targeting FAK/AKT
signaling in hepatocellular carcinoma. Cancer Lett. 375:73–83.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto K, Seike M, Takeuchi S, Soeno C,
Miyanaga A, Noro R, Minegishi Y, Kubota K and Gemma A: MiR-379/411
cluster regulates IL-18 and contributes to drug resistance in
malignant pleural mesothelioma. Oncol Rep. 32:2365–2372.
2014.PubMed/NCBI
|
|
18
|
Ge YZ, Xu LW, Xu Z, Wu R, Xin H, Zhu M, Lu
TZ, Geng LG, Liu H, Zhou CC, et al: Expression profiles and
clinical significance of MicroRNAs in papillary renal cell
carcinoma: A STROBE-Compliant Observational Study. Medicine
(Baltimore). 94:e7672015. View Article : Google Scholar
|
|
19
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
21
|
Duan F, Jia D, Zhao J, Wu W, Min L, Song
S, Wu H, Wang L, Wang H, Ruan Y, et al: Loss of GFAT1 promotes
epithelial-to-mesenchymal transition and predicts unfavorable
prognosis in gastric cancer. Oncotarget. 7:38427–38439. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Ha S, Jung CK and Lee HH:
High-mobility-group A2 overexpression provokes a poor prognosis of
gastric cancer through the epithelial-mesenchymal transition. Int J
Oncol. 46:2431–2438. 2015.PubMed/NCBI
|
|
23
|
Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu
HY, Wang JS and Duan XY: High tumor-associated macrophages
infiltration is associated with poor prognosis and may contribute
to the phenomenon of epithelial-mesenchymal transition in gastric
cancer. Onco Targets Ther. 9:3975–3983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J, Shan Z, Hu K, Ren F, Zhang W, Han M,
Li Y, Feng K, Lei L and Feng Y: miRNA-223 inhibits
epithelial-mesenchymal transition in gastric carcinoma cells via
Sp1. Int J Oncol. 49:325–335. 2016.PubMed/NCBI
|
|
25
|
Wang LL, Zhang XH, Zhang X and Chu JK:
MiR-30a increases cisplatin sensitivity of gastric cancer cells
through suppressing epithelial-to-mesenchymal transition (EMT). Eur
Rev Med Pharmacol Sci. 20:1733–1739. 2016.PubMed/NCBI
|
|
26
|
Zhang LL, Liu J, Lei S, Zhang J, Zhou W
and Yu HG: PTEN inhibits the invasion and metastasis of gastric
cancer via downregulation of FAK expression. Cell Signal.
26:1011–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R and Yang S: Effects of combining
erlotinib and RNA-interfered downregulation of focal adhesion
kinase expression on gastric cancer. J Int Med Res. 44:855–864.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen P, Zhao H, Huang J, Yan X, Zhang Y
and Gao Y: MicroRNA-17-5p promotes gastric cancer proliferation,
migration and invasion by directly targeting early growth response
2. Am J Cancer Res. 6:2010–2020. 2016.PubMed/NCBI
|
|
30
|
Sun J, Li J, Zhang W, Zhang J, Sun S, Li
G, Song H and Wan D: MicroRNA-509-3p inhibits cancer cell
proliferation and migration via upregulation of XIAP in gastric
cancer cells. Oncol Res. 25:455–461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JH, Lee BL, Yoon J, Kim J, Kim MA,
Yang HK and Kim WH: Focal adhesion kinase (FAK) gene amplification
and its clinical implications in gastric cancer. Hum Pathol.
41:1664–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Dang J, Chang KY, Yau E, Aza-Blanc
P, Moscat J and Rana TM: miR-1298 inhibits mutant KRAS-driven tumor
growth by repressing FAK and LAMB3. Cancer Res. 76:5777–5787. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo LL, He ZC, Yang CQ, Qiao PT and Yin
GL: Epigenetic silencing of olfactomedin-4 enhances gastric cancer
cell invasion via activation of focal adhesion kinase signaling.
BMB Rep. 48:630–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song G, Xu S, Zhang H, Wang Y, Xiao C,
Jiang T, Wu L, Zhang T, Sun X, Zhong L, et al: TIMP1 is a
prognostic marker for the progression and metastasis of colon
cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer
Res. 35:1482016. View Article : Google Scholar : PubMed/NCBI
|