Introduction
Considerable scientific interest in the anticancer
therapy has been focused, for long time, on the identification of
compounds able to efficiently commit cells to apoptosis. Apoptosis
plays a crucial role in eliminating the mutated hyperproliferating
cells from the system. Thus, induction of apoptosis in tumor cells
may be considered as a protective mechanism against development and
progression of cancer. Reactive oxygen species (ROS) play a major
role in carcinogenesis (1). It has
been shown that the formation of ROS contributes to the anti-tumor
activity of several chemotherapeutic drugs. However, pro-oxidant
molecules can act as selective cytotoxic agents against cancer
cells by achieving toxic levels of ROS (1).
The validation and utilization of dietary
components, natural products, or their synthetic analogs as
chemopreventive agents in the form of foods or nutraceutical has
become an important issue in health- and cancer-related research
and a growing body of evidence also suggests that many natural
compounds may cooperate in enhancing the therapeutic efficacy of
chemotherapeutic drugs, help to by-pass cancer drug resistance or
reduce side-effects of chemotherapy (2–4).
Polyphenols represent one of the most interesting and investigated
class of nutraceutical compounds because of their therapeutic
properties for several common diseases, including cancer (5). It is reported that flavonoids, a
class of polyphenolic compounds show several anticancer effects
including apoptosis induction, cell cycle arrest,
antiproliferative, antioxidative, antiangiogenic and antimetastatic
action against many human cancer cell lines. Interestingly, an
important aspect of the chemopreventive action of polyphenols is
their differential activity in targeting cancer cells but not
normal cells (6,7). Most of the advantageous effects of
natural polyphenols have been ascribed to their ability to scavenge
free radicals endogenously generated or formed by radiation and
xenobiotics. However, emerging evidence indicates that the
anticancer and chemopreventive properties of plant-derived
polyphenols are mainly related to their pro-oxidant activity
(8). The main problem related to
the use of polyphenols as anticancer agents is their poor
bioavailability, which might hamper the in vivo effects of
the single compound (9) and it has
been suggested that combinations of polyphenols naturally found in
fruits and vegetables could be most favorable for cancer prevention
owing to synergic or additive biological effects (10).
Apples are widely consumed and represents one of the
main sources of polyphenols in the Western diet (11). The anti-proliferative properties of
apple extracts have been described extensively by in vitro
studies and products extracted from apple skins have been shown to
be effective in preventing tumor formation in different types of
cancer, including colorectal, breast, prostate, liver, cervical and
lung (12–14).
Among the different varieties, great interest has
been paid to Annurca apple, one of the most important
cultivars of southern Italy with a 'Protected Geographical
Indication' of the Campania region (15). The Annurca apple is
characterized by an extremely high content of polyphenols, mainly
catechin, epicatechin, and chlorogenic acid and displays a stronger
antioxidant activity compared with other varieties (16). Many studies have recently
investigated the polyphenol content of Annurca apple peel
and have described its higher ability in comparison to other
species in reducing cell cholesterol and glucose uptake (17) and its activity in chemoprevention
of colorectal cancers (18,19).
Moreover, the antiproliferative effect of Annurca apple
polyphenol extract has been described in human HaCaT keratinocytes
where it induced p53-independent extrinsic apoptosis (20).
Breast cancer is the most common malignancy in women
with increased incidence worldwide. Poor prognosis of breast cancer
is partially attributed to multiple-drug resistance and
anti-apoptosis activity of cancer cells (21). Although antitumor activity of
polyphenols in breast cancer has been shown, its molecular
mechanism is yet to be clarified (22).
In the present study, the pro-oxidant,
antiproliferative, and pro-apoptotic effects of Annurca APE
in human breast cancer MCF-7 cells were investigated and the
potential underlying molecular mechanisms were explored. We found
that Annurca APE is able to inhibit cell proliferation by
inducing cell cycle arrest at G2/M and the activation of apoptosis
via a Bcl2- Bax-dependent mitochondrial signaling pathway.
Finally, we report that the antiproliferative effects of APE
involve downregulation of cyclin D1, upregulation of p53 and p21,
and inhibition of extracellular signal-regulated kinase 1/2
(ERK1/2) activation.
Materials and methods
Materials
Roswell Park Memorial Institute medium RPMI-1640
medium (RPMI-1640), bovine serum albumin (BSA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Folin-Ciocalteu reagent, propidium iodide (PI), thiobarbituric
acid, and RIPA buffer were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Phosphate-buffered saline (PBS) and trypsin-EDTA were
from Lonza (Milan, Italy). Fetal bovine serum (FBS) was purchased
from Gibco (Grand Island, NY, USA). Tissue culture dishes were
purchased from Microtech (Naples, Italy). Annexin V-fluorescein
isothiocyanate (V-FITC) Apoptosis Detection kit was purchased from
eBioscience (San Diego, CA, USA). Monoclonal antibodies to
caspase-9, poly(ADP ribose) polymerase (PARP), Bax, Bcl-2, cyclin
D1, p21, p53, β-actin, and polyclonal antibodies to caspases 6 and
7, ERK1/2, and pERK1/2 were purchased from Cell Signaling
Technology (Beverly, MA, USA). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit and HRP-conjugated goat
anti-mouse secondary antibodies were obtained from ImmunoReagents,
Inc., Raleigh, NC, USA. All buffers and solutions were prepared
with ultra-high quality water. All reagents were of the purest
commercial grade.
Apple samples
Annurca (Malus pumila Miller cv.
Annurca) apple fruits (each weighing approximately 100 g)
were collected in Giugliano (Napoli, Italy) in October, when fruits
had just been harvested (green peel). Fruits were reddened
following the typical treatment for approximately 30 days and then
analyzed (15).
Polyphenol extraction
APE extraction from Annurca apple was carried
out as previously reported by D'Angelo et al (20). Briefly, 40 grams of Annurca
apple flesh were homogenized for 5 min by a Tefal Rondo 500
homogenizer using 40 ml of 80% methanol and 20% water plus 0.18 N
HCl. After centrifugation (18,000 × g for 25 min), the slurry was
dried under vacuum by using the Univapor Concentrator Centrifuge,
model Univapo 100 H (Uni Equip). The dried extracts were dissolved
in 10 ml of PBS and frozen at −80°C until use.
Polyphenolic content and HPLC
analysis
The total polyphenolic content of apple extract was
assessed approximately by using the Folin-Ciocalteu phenol reagent
as described by Singleton et al (23). The extracts (100 µl) were
mixed with the Folin-Ciocalteu phenol reagent (0.5 ml), deionized
water (0.9 ml), and Na2CO3 (7.5% w/v, 4 ml).
The absorbance at 765 nm was measured 1 h after incubation at room
temperature using a Cary ultraviolet-visible spectrophotometer
(Varian). Since APE is a mixture of different phenolic compounds,
to give an arbitrary APE molar concentration the measurement was
compared to a standard curve of prepared catechin solutions and
expressed as milligrams of catechin equivalent (EqC) per 100 g of
Annurca flesh fresh weight. The chemical characterization
was performed in HPLC as reported by D'Angelo et al
(24). All experiments were
carried out in triplicate. HPLC separation of polyphenols was
performed by reversed-phase chromatography on a 5 µm column
Kromasil C18 column (150×4.6 mm), using a Beckman
Apparatus (Gold-126) equipped with a UV detector fixed at 278 nm.
The column was eluted at a flow rate of 1.0 ml/min with 0.2% acetic
acid, pH 3.1 (A) and methanol (B) as the mobile phase; the gradient
was changed as follows: 95% A/5% B for 1 min, 85% A/15% B in 1 min,
75% A/25% B in 20 min, 0% A/100% B in 15 min, and 95%A/5%B in 3
min. The main o-diphenols were identified on the basis of
the retention times of authentic standard references: (+)-catechin,
(−)-epicatechin, chlorogenic acid, quercetin, and quercetin
glysosides.
Cell culture
Human breast carcinoma cell line (MCF-7) was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA) and cultured in RPMI-1640 supplemented with 10%
heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml
streptomycin, and 1% L-glutamine. The cells were grown in a
humidified atmosphere of 95% air/5% CO2 at 37°C.
Cell viability
MCF-7 cells were seeded in 96-well plates at the
density of 3×103 cells/well in RPMI complete medium.
After 24 h incubation, cells were treated with 100, 250, and 500
µM APE EqC for 24 and 48 h. Cell viability was assessed by
adding MTT solution in PBS to a final concentration of 0.5 mg/ml.
Cells were then incubated at 37°C for 4 h and the MTT-formazan
crystals were solubilized in a solution of 4% 1 N
isopropanol/hydrochloric acid on a shaking table for 20 min. The
absorbance values of the solution in each well were measured at 595
nm using a Bio-Rad iMark microplate reader (Bio-Rad Laboratories,
Milan, Italy). Cell viability was expressed as a percentage of
absorbance values in treated samples respect to that of control
(100%). All MTT experiments were performed in quadruplicate.
Thiobarbituric acid-reactive species
Thiobarbituric acid-reactive species (TBARS) were
determined on aliquots (250 µl) of cell culture medium of
not treated cells (control) or after 48 h treatment with 100, 250
and 500 µM EqC APE. TBARS were measured as previously
described (25). Briefly, samples
were incubated with a solution consisting of 0.5 ml of 20% acetic
acid, and 0.5 ml of 0.78% aqueous solution of thiobarbituric acid
(pH 3.5), heated at 95°C for 45 min, and then centrifuged at 4000
rpm for 5 min. TBARS content was quantified at 532 nm with
reference to malondialdehyde (extinction coefficient at 532 nm,
1.56×105 M−1 cm−1). Result is the
average of triplicate measurements of each individual experiment
performed in duplicate.
Evaluation of morphological changes by
phase-contrast microscopy
MCF-7 cells were cultured in 6-well tissue culture
plates (Becton-Dickinson, Franklin Lanes, NJ, USA) at a seeding
density of 9.0×104 cells. After overnight attachment,
the cells were treated with different concentrations (100, 250, and
500 µM EqC) of APE and the untreated cells were used as
control. The cells were cultured for up to 48 h under standard
culture conditions and their morphological changes were imaged
using a phase-contrast microscope (Axiovert-10 Zeiss
microscope).
Western blot analysis
MFC-7 cells were cultured in 10-cm culture dishes at
a seeding density of 5.5×105 cells. After overnight
attachment, the cells were treated for 48 h with 100, 250, and 500
µM EqC APE and the untreated cells used as control. After
the treatment, the cells were collected by centrifugation, washed
twice with ice-cold PBS, and the pellets were lysed using 100
µl of RIPA buffer. After incubation on ice for 30 min, the
samples were centrifuged at 18,000 × g in an Eppendorf
microcentrifuge for 10 min a 4°C, and the supernatants were
quantified for protein content. Aliquots containing approximately
30 µg of protein were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrotransferred to nitrocellulose membranes by Trans blot turbo
(Bio-Rad Laboratories). The blots were blocked with 5% non-fat dry
milk in 20 mM Tris/HCl, pH 7.5, 150 mM NaCl plus 0.5% Tween-20
(TBS-T). The membranes were subsequently incubated at 4°C overnight
in the 5% non-fat dry milk-TBS-T buffer, containing, for each
experiment, one of the specific primary antibodies. After three
times washing with TBS-T, the blots were incubated for 1 h at room
temperature with the HRP-conjugated secondary antibodies. After
four times washing, the blots were developed using enhanced
chemiluminescence detection reagents ECL (Millipore Corp.,
Billerica, MA, USA) and exposed to X-ray film. The film was scanned
by using ImageJ software (National Institutes of Health, Bethesda,
MD, USA).
Flow cytometry analysis of the cell
cycle
Cell cycle distribution was studied by PI staining
and flow cytometry analysis using a FACScalibur (Becton-Dickinson,
San Jose, CA, USA) interfaced with a Hewlett-Packard computer
(model 310) for data analysis. Briefly, MCF-7 cells were plated in
6-multiwell plates at the density of 9.0×104 cells/well.
After 24 h, the medium was changed and APE was added at
concentrations of 100, 250, or 500 µM EqC; conversely fresh
medium was added to the control well. After 48 h treatment, the
cells were recovered by incubation with trypsin-EDTA, washed in
PBS, collected by centrifugation, fixed by resuspension in 70%
ice-cold methanol/PBS and incubated overnight at 4°C. After fixing,
samples were centrifuged at 400 × g for 5 min, washed twice with
ice-cold PBS, resuspended in 0.5 ml DNA staining solution (50
µg/ml PI and 25 µg/ml RNaseA in PBS), and incubated
at room temperature for 1 h in the dark. Samples were then
transferred to 5-ml Falcon tubes and stored until assayed. PI
fluorescence was collected as FL2-A (linear scale) by the ModiFIT
Cell Cycle Analysis software (Becton-Dickinson). For each sample,
at least 20,000 events for each point were analyzed in at least
three different experiments giving an SD of <5%.
Flow cytometry analysis of apoptosis
Annexin V-FITC binds phosphatidylserine residues
which are translocated from the inner to the outer leaflet of the
plasma membrane during the early stages of apoptosis. A flow-based
Annexin V-FITC/PI double staining was used to distinguish apoptotic
(Annexin V-FITC-positive, PI-positive) from necrotic (Annexin
V-FITC-negative, PI-positive) cells (26). MCF-7 cells were plated in
6-multiwell plates at the density of 9.0×104 cells/well.
After 24 h, the medium was changed and APE was added at
concentrations of 100, 250, or 500 µM EqC while fresh medium
was added to the control well. After 48 h of treatment, the cells
were detached by incubation with trypsin-EDTA, washed in PBS,
collected by centrifugation, resuspended in binding buffer (10 mM
HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), and then
stained with 5 µl of Annexin V-FITC and 10 µl PI (20
µg/ml) for 30 min in the dark, according to the
manufacturer's instructions. Analyses were performed with flow
cytometry apparatus (Becton-Dickinson). Apoptosis was detected in
red and green fluorescence channels with excitation wavelength of
488 nm. For each sample, 2×104 events were acquired.
Analysis was carried out by triplicate determination on at least
three separate experiments.
Statistical analysis
All experiments were performed three times with
replicate sample. Data are expressed as mean ± standard deviation
(SD). Comparisons between treated samples and control were
performed using analysis of variance (ANOVA) plus Bonferroni's
t-test. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Total polyphenolic content of APE
The total polyphenolic content of the Annurca
apple extract measured by Folin-Ciocalteu phenol reagent resulted
in 125.2±7.1 mg of catechin per 100 g of sample and is similar to
that evaluated in other studies (24,25).
The HPLC analysis of APE identified (+)-catechin, (−)-epicathechin,
chlorogenic acid, quercetin, and quercetin glycosides as the main
Annurca apple o-diphenols (data not shown). This
profile confirms the results already described in the literature
(16,24).
APE inhibits cell proliferation and
induces lipid peroxidation
To evaluate the effect of APE on cell growth, MCF-7
cells were incubated for 24 and 48 h with increasing concentrations
of the extract ranging from 100 to 500 µM EqC corresponding
to 29–145 µg EqC/ml, and cell proliferation was then
assessed by MTT assay. The cells appeared to be quite resistant to
APE treatment. The results obtained evidenced that up to 100
µM EqC no appreciable changes in cell viability were
observed at any time of incubation. On the other hand, at higher
doses, the treatment with APE significantly reduced cell
proliferation in a dose- and time-dependent manner (Fig. 1). It has to be noted that after 24
h incubation, 250 µM EqC and 500 µM EqC APE exerted
only a poor cell growth inhibitory effect reducing cell
proliferation to approximately 98 and 83%, respectively, compared
to control cells. In contrast, a prolonged incubation of 48 h
resulted in a severe loss of cell viability that decreases to
43.5%, a value near the IC50, in the presence of APE 500
µM EqC. Parallel direct cell counting provided similar
results (data not shown). Based on these findings, we selected for
further investigations an incubation time of 48 h.
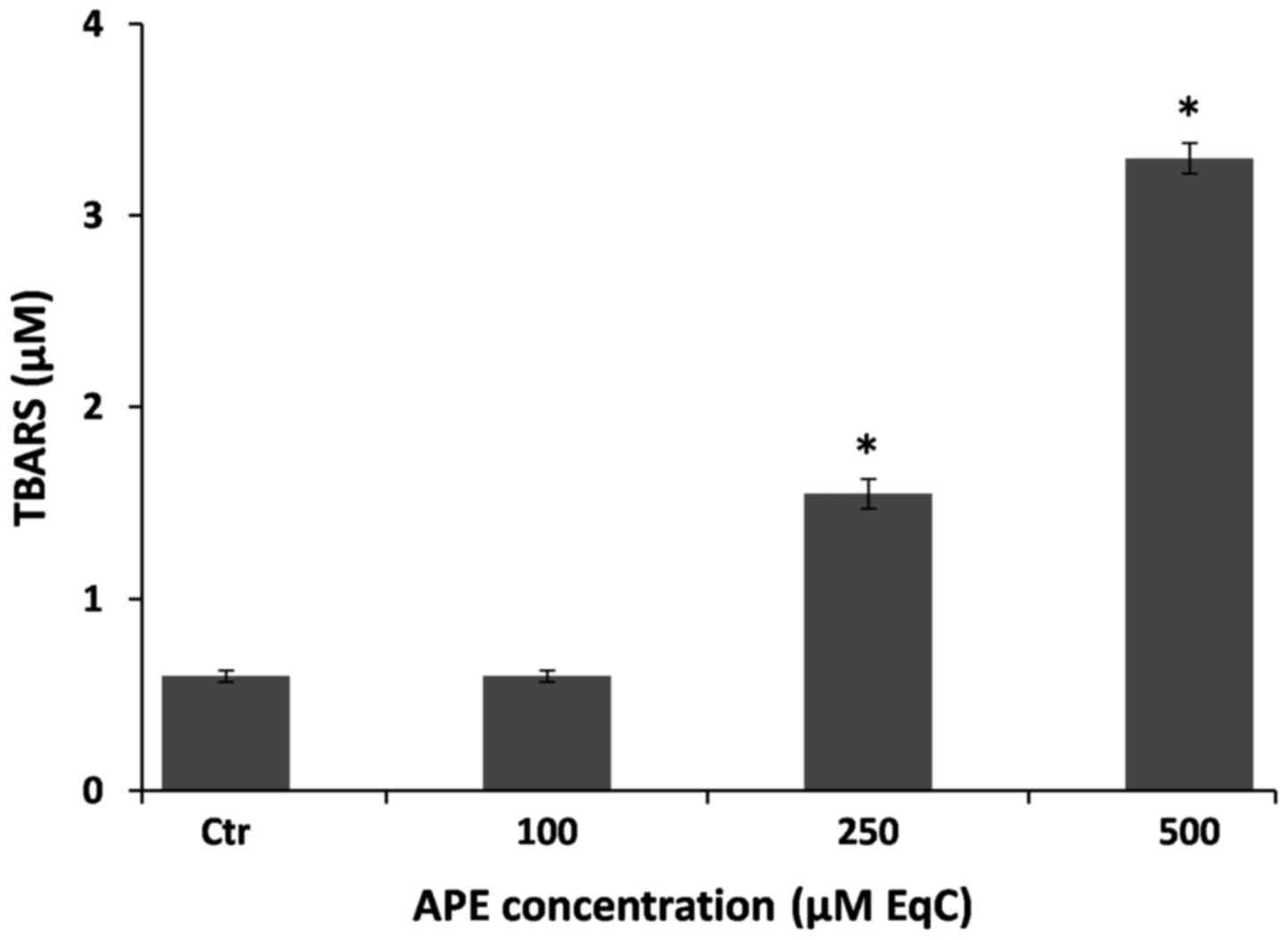
Free radicals are involved in cell death induction
in a number of systems and membrane phospholipids represent one of
the major target of oxidative stress in cells. It has been shown
that lipid hydroperoxides as well as lipid peroxidation initiators,
such as ROS, are involved in signaling pathways that control cell
proliferation, differentiation, maturation, and apoptosis (1,27).
Moreover, it has been proposed that lipid peroxidation may
represent a protective mechanism in breast cancer (27).
In order to investigate whether an oxidative stress
could be produced under our experimental conditions, we evaluated
lipid peroxidation by measuring the extent of lipid degradation
products such as, malondyaldehyde and other aldehydes reactive to
thiobarbituric acid after 48 h incubation of MCF-7 cells in the
presence of increasing amounts of APE. It is interesting to note
that, in analogy with the effect exerted on cell viability, APE is
able to cause lipid peroxidation only at concentration >100
µM EqC inducing, at 250 µM EqC and 500 µM EqC
concentration, respectively, an increase of TBARS cell content of
3- and 6-fold higher than the untreated cells (Fig. 2). These results indicate that, at
elevated concentrations, apple polyphenols may act as pro-oxidants
and may induce growth inhibition probably enhancing intracellular
ROS oxidative stress.
APE induces morphological changes in
MCF-7 cells
MCF-7-cells were incubated for 48 h with 100, 250,
and 500 µM EqC APE, and the morphology of treated cells was
then analyzed with a phase-contrast microscope and compared with
the untreated cells (Fig. 3). The
results obtained indicated that the cells treated with APE 100
µM EqC (Fig. 3B) maintained
the characteristic epithelial morphology and prolific growth as a
monolayer, quite similar to untreated MCF-7 cells (Fig. 3A). On the contrary, the cells
treated with APE 250 µM EqC (Fig. 3C) and 500 µM EqC (Fig. 3D) displayed morphological
alterations such as, shrinkage and cytoplasmic condensation, cell
rounding, poor adherence, and cell detachment. Altogether the
evidence suggests that APE is able to commit MCF-7 cells to a type
of death that mimics apoptosis.
APE induces cell cycle arrest at G2/M by
downregulation of cyclin D1 and upregulation of p21 and p53
Recent literature data have highlighted the
chemopreventive and anticancer properties of polyphenolic compounds
and polyphenol-rich nutritional sources. Moreover, several recent
investigations have elucidated the mechanisms by which polyphenols
are able to modulate some cellular events such as, cell cycle
arrest by decreasing cyclin levels, and apoptosis induction
(28).
To verify whether APE caused cell cycle perturbation
in MCF-7 cells, we evaluated the cell distribution in the cell
cycle phases by flow cytometric analysis. In addition, the severe
morphological changes observed in APE-treated MCF7-cells under the
inverted phase-contrast microscope prompted us to further examine
whether the APE-induced cell death was a consequence of activation
of apoptosis. Therefore, we also looked at the proportion of cells
with hypodiploid DNA content (sub-G1 population), characteristic of
cells having undergone DNA fragmentation, a biochemical hallmark of
apoptosis.
MCF-7 cells were exposed to increasing
concentrations of APE (100, 250, and 500 µM EqC), harvested
at 48 h, and examined for DNA content after proper staining with
PI. Fig. 4 shows that the
treatment of cells with 100 and 250 µM EqC APE does not
cause any evident effect on cell proliferation while 500 µM
EqC APE induces a cell cycle arrest at G2/M, as evidenced by the
significant increase of cell percentage in this phase of the cell
cycle (26%) respect to untreated cells (11%). Moreover, a
dose-dependent increase of sub-G1 population can be observed that
becomes clearly evident at 500 µM EqC APE (34%) suggesting
that the cytotoxic activity of APE in MCF7-cells occurs via
apoptosis.
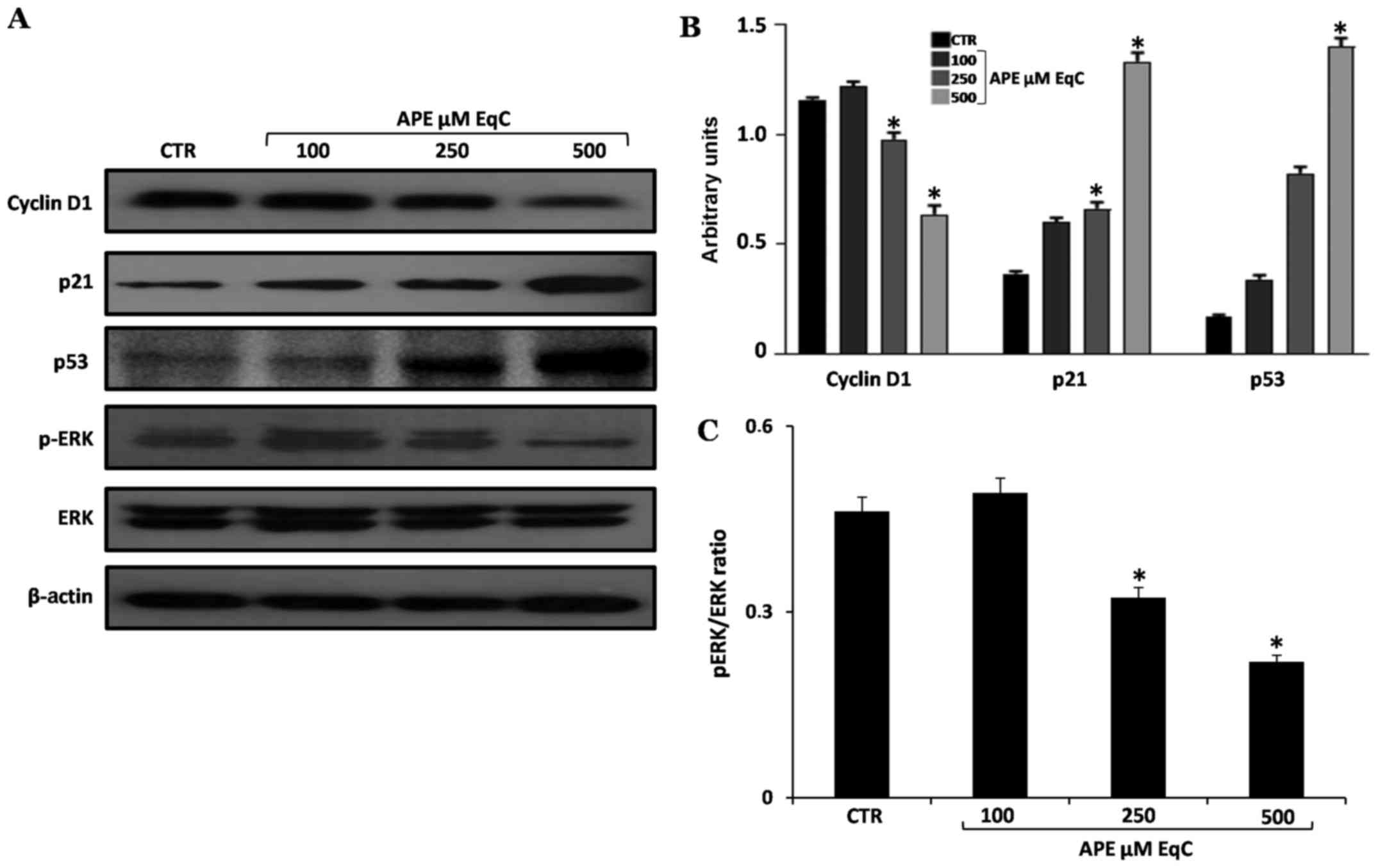
To gain further insight into the molecular mechanism
of cell cycle arrest induced by APE, we analyzed the expression
level of some relevant cell proliferation- and cell
cycle-regulators such as, cyclin D1, cyclin-dependent kinase
inhibitor 1 (p21) and p53 transcription factor. Thus, MCF-7 cells
were exposed to 100, 250, and 500 µM EqC APE for 48 h, after
which cell extracts were analyzed by western blotting. As shown in
Fig. 5, the treatment for 48 h
with APE induced a significant dose-dependent increase in the level
of p53 and p21 proteins. It has to be pointed out that the tumor
suppressor p53 is a transcription factor that regulates a broad
range of processes among which cell cycle arrest, senescence, and
apoptosis are the best characterized. In vitro and in
vivo studies have shown that polyphenols such as,
epigallocatechin, resveratrol, and curcumin as well as nutritional
sources of polyphenols are able to upregulate p53 protein in
several cancer cell lines (28–32)
and that the polyphenol-induced stabilization and expression of p53
is often associated with a G1 or G2/M phase cell cycle arrest
(33). It has also been reported
that tumor suppressors, like p53 and its analogs, are key molecular
targets of polyphenols responsible for their pro-apoptotic effect
in human and animal cancer models through the transcriptional
regulation of target genes such as p21, Bax, and PUMA (32).
From the western blot analysis reported in Fig. 5 we can observe that concomitantly
with the increase of p53 and p21, the level of cyclin D1 was
decreased in a dose-dependent manner. In agreement with our results
it has been recently reported that epigallocatechin-3-gallate, the
major polyphenolic component of green tea, induces apoptosis and
cell cycle arrest in colorectal cancer cells by downregulating
cyclin D1 and upregulating p21 (34).
Cyclin D1 is a key factor regulating cell
proliferation, this cyclin links the extracellular signals to cell
cycle advancement (35). Cyclin D1
levels must be elevated during G1 phase to start DNA synthesis, but
then they must be suppressed during S phase to allow DNA synthesis.
If the cell has to continue to proliferate, cyclin D1 levels must
be induced once again during G2 phase. This induction depends upon
the activity of proliferative signaling molecules, and ensures that
the extracellular environment continues to be conducive for growth
(36). For its prominence, cyclin
D1 is an intriguing target in anticancer agent development. Many
anticancer drugs induce cyclin D1 degradation in several tumor cell
lines. Moreover, increased cyclin D1 degradation in cancer cells,
is achieved also by various natural compounds such as polyphenols
(37). Altogether our results
indicate that cyclin D1 degradation and p53 and p21 overexpression
contribute to APE-induced growth inhibition in MCF-7 cells.
APE inhibits ERK1/2 phosphorylation
MAPK cascade is a major cell signaling pathway
triggered by ROS, leading to activation of numerous transcription
factors which control DNA repair, cell cycle, apoptosis, or the
immune system, among other processes. Major MAPKs can be grouped
into four subfamilies: extracellular signal-regulated kinases
(ERKs), c-Jun amino terminal kinase (JNK), p38 MAPK, and big-1
kinases (BMAPK-1). The whole pathway is regulated by various
extracellular signals and, in this manner, it regulates distinct
and even opposing cellular phenomena, such as proliferation,
differentiation, survival and apoptosis (38). Moreover, in human hepatocellular
carcinoma cell lines, multiple anticancer effects such as
inhibition of cellular proliferation as well as induction of cell
cycle arrest and apoptosis have been achieved by blocking ERK
signaling (39).
In order to investigate whether the
antiproliferative effect induced by APE in MCF-7 cells was related
to ERK, we analyzed the expression and activation (phosphorylation)
of ERK by western blotting. As reported in Fig. 5, APE caused a strong dose-dependent
inhibition of ERK phosphorylation and a decrease of pERK/ERK ratio
while no variations in the total amount of ERK1/2 protein levels
occur at any of the concentrations tested. The obtained results
suggest that ERK inactivation may contribute to the G2/M cell cycle
arrest and to the antiproliferative activity of APE even if the
detailed molecular mechanisms underlying the APE-induced ERK
signaling modulation needs to be further investigated. In agreement
with our data it has been reported that red wine polyphenols
inhibit the proliferation of colon carcinoma cells by modulating
MAPK intracellular signal transduction pathways (40) and that quercetin may induce
apoptosis in HepG2 cells by direct activation of caspase cascade
and by inhibiting survival signaling (41).
APE induces apoptosis through the
mitochondrial pathway
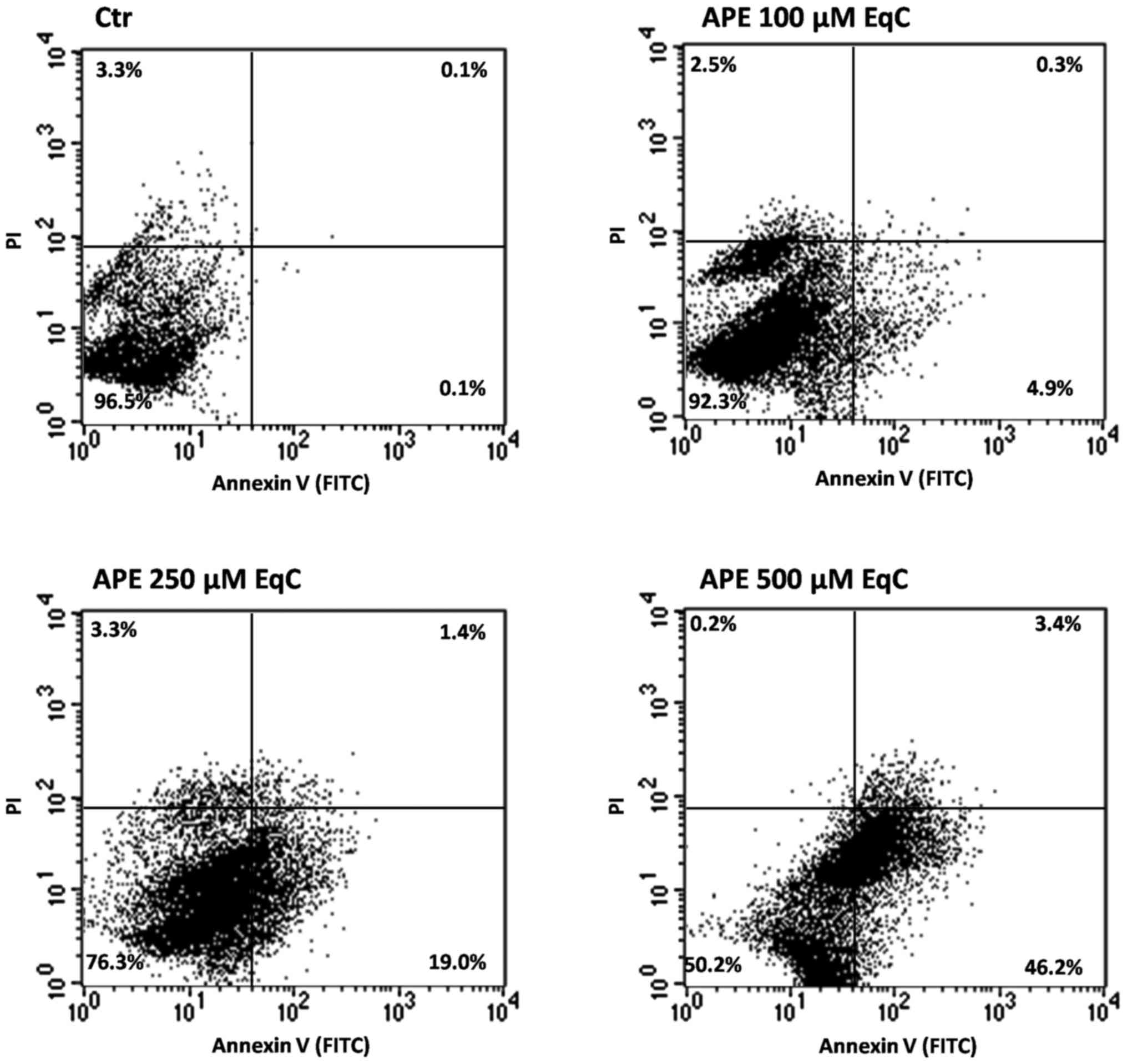
The occurrence of apoptosis in MCF-7 cells upon APE
treatment was assessed by FACS analysis after double labeling of
cells with Annexin V and PI. Representative pictures are shown in
Fig. 6 together with the
percentage of apoptotic cells. The exposure of cells for 48 h to
Annurca apple polyphenols caused a dose-dependent increase
of apoptotic cells respect to the control. A low percentage of
early apoptotic cells (4.9%) is already evident after exposure to
100 µM EqC APE. This value significantly increases in the
cells treated with APE 250 µM EqC (19%) and 500 µM
EqC (46.2%).
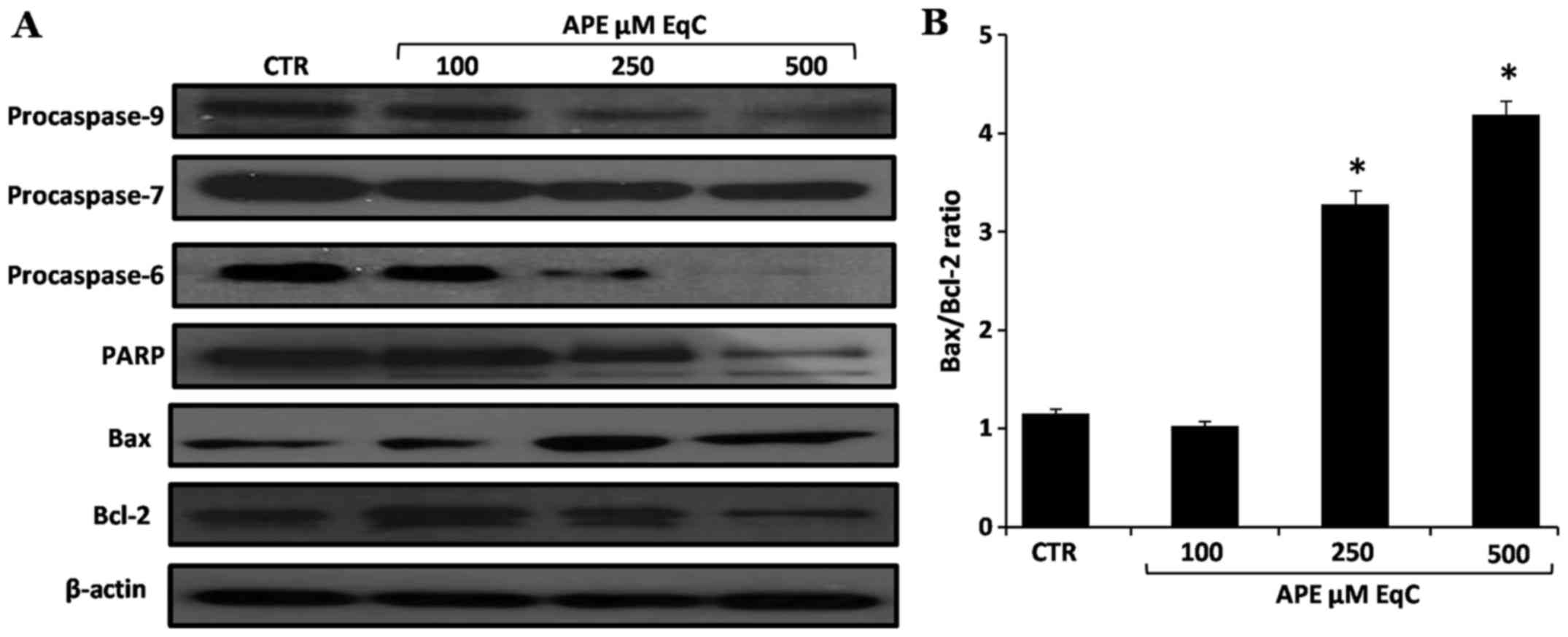
The APE-induced lipid peroxidation and the highly
increased expression levels of p53 after cell incubation with APE
led us to hypothesize that the apoptotic process in MCF-7 cells
occurs through a ROS-dependent mitochondrial pathway.
The balance of the expression of anti- and
pro-apoptotic members of the Bcl-2 gene family is one of the major
mechanisms that regulate this type of apoptotic process in
mammalian cells (42). Therefore,
to determine whether APE-induced apoptosis in MCF-7 cells was
associated with the modulation of members of this protein family,
we examined the expression of Bcl-2 and Bax proteins. The results
obtained, reported in Fig. 7, show
that APE treatment resulted in the increased expression of
pro-apoptotic Bax while the level of the anti-apoptotic Bcl-2 was
decreased. We then tested the effect of APE on the cascade of
caspases that are crucial initiators or effectors in this cell
death pathway. Western blot analysis shows that the increased
Bax/Bcl-2 ratio coincides with the activation of the initiator
caspase-9 and of the executioner caspase-6 and -7 and with the
increased PARP cleavage (Fig. 7).
Altogether these results indicate that the activation of the
mitochondrial pathway is involved in the apoptosis of MCF-7 cells
induced by APE.
Discussion
The potential of dietary components, in particular
polyphenols, as antiproliferative agents has been evidenced in the
literature, and growing scientific interest is focused on
identifying the biological mechanisms and the signal transduction
pathways related to the chemopreventive activities of these
compounds. Polyphenols, a heterogeneous class of phytochemicals
with a wide range of pharmacological properties, appear quite
intringuing molecules because of their antioxidant/pro-oxidant
effects. As antioxidants, they are able to act as scavengers of
ROS. Conversely, under some conditions, they can act as
pro-oxidant, generating ROS and causing cellular oxidative stress
(43). Such pro-oxidant effect of
polyphenols seems to be responsible for apoptosis induction in
cancer cells. It is speculated that the malignant cells which have
an increased level of oxidative stress would be more vulnerable to
further ROS attack. Thus, the conventional anticancer therapeutic
strategies are based on increased ROS generation treatments which
trigger apoptotic damage (44).
In the current study we show that Annurca
apple polyphenol extract induces lipid peroxidation and inhibits
the growth of human breast cancer MCF-7 cells by altering cell
cycle kinetics and by inducing apoptosis. We also show that APE
exerts its antiproliferative effect by targeting p53 and ERK1/2
signaling pathways. The following considerations deduced from the
results obtained allowed us to hypothesize that the pro-apoptotic
effect of Annurca apple polyphenol extract is related to its
pro-oxidant activity: i) APE causes MCF-7 cells to arrest in G2/M
phase of the cell cycle, and this is accompanied by a marked
decrease of cyclin D1. It is well known that cyclin D1 is
downregulated in presence of ROS causing a cell cycle arrest in
G2/M and it has been also reported that cyclin D1 depletion is a
defense mechanism to reduce ROS-induced genotoxic damages (45) and that ROS affect the proteasomal
degradation of cyclin D1 (46);
ii) APE induces a marked increase of p53. It is well known that in
presence of oxidative stress, the tumor suppressor p53 has a dual
action increasing the synthesis of pro- or anti-oxidant enzymes
depending to high or low levels of ROS, respectively. In response
to low levels of oxidative stress, p53 exhibits antioxidant
activities and assists the survival and repair of cells with minor
injuries while in response to high levels of ROS p53 exhibits
pro-oxidant activities and induces mitochondrial apoptosis
(47); iii) APE causes a decrease
of phosphorylated ERK1/2 protein. Because the activation of ERK1/2
is necessary for cell survival and proliferation, the inhibition of
ERK1/2 by APE contributes to the increased occurrence of cell
death.
The ERK signaling pathway is activated in response
to certain situations of cellular stress, and it is implicated in
cellular death or survival signaling. Generally, the ERK signal
transduction pathway is activated by growth factors and is
important for proliferation. Nevertheless, ERK has been reported to
be inactivated by p53-regulated transcription of phosphatases MKP1,
PAC1 and DUSP5. It has been shown, that in response to oxidative
stress, MAPKs phosphorylate and activate p53 leading to
upregulation of PAC1 and DUSP5 phosphatases. The resulting
inactivation of ERK1/2 in turn causes cell cycle arrest and
apoptosis (48). The functional
interaction between p53 and ERK 1/2 suggests the existence of a
reciprocal negative regulation between these signaling pathways.
ERK 1/2-dependent p53 phosphorylation induced by different
stressful stimuli can lead to p53-dependent cell cycle arrest and
apoptosis. On the other hand, activated p53 is able to suppress
ERK1/2 signaling via the transcriptional activation of members of
the dual specificity phosphatase family thus facilitating
p53-induced apoptosis. On the basis of these considerations, it is
possible to assume that the balance between the p53 and MAPK
pathways will determine the final cell response to oxidative
stress.
In conclusion, our findings suggest that
APE-mediated ROS generation probably represents the central trigger
for the antiproliferative activity of these compounds in MCF-7
cells and allow us to propose the Annurca apple polyphenol
extract as a promising target for further investigations finalized
to the design of innovative adjuvant therapies in breast cancer
treatments.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
APE
|
apple polyphenol extract
|
|
RPMI
|
Roswell Park Memorial Institute
medium
|
|
ERK1/2
|
extracellular signal-regulated kinases
1/2
|
|
pERK1/2
|
phospho-extracellular signal-regulated
kinases 1/2
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
TBARS
|
thiobarbituric acid-reactive
species
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PARP
|
poly(ADP ribose) polymerase
|
|
PI
|
propidium iodide
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
|
HRP
|
horseradish peroxidase
|
|
HPLC
|
high performance liquid
chromatography
|
|
TBS-T
|
Tris-buffered saline with Tween-20
|
|
SD
|
standard deviation
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
Annexin V-FITC
|
Annexin V-fluorescein
isothiocyanate
|
|
EqC
|
catechin equivalent
|
References
|
1
|
Reczek CR and Chandel NS: The two faces of
reactive oxygen species in cancer. Annu Rev Cancer Biol. 1:79–98.
2017. View Article : Google Scholar
|
|
2
|
Wang P, Yang HL, Yang YJ, Wang L and Lee
SC: Overcome cancer cell drug resistance using natural products.
Evid Based Complement Alternat Med. 2015:7671362015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemaiswarya S and Doble M: Potential
synergism of natural products in the treatment of cancer. Phytother
Res. 20:239–249. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
León-González AJ, Auger C and Schini-Kerth
VB: Pro-oxidant activity of polyphenols and its implication on
cancer chemo-prevention and chemotherapy. Biochem Pharmacol.
98:371–380. 2015. View Article : Google Scholar
|
|
6
|
Rengarajan T and Yaacob NS: The flavonoid
fisetin as an anticancer agent targeting the growth signaling
pathways. Eur J Pharmacol. 789:8–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batra P and Sharma AK: Anti-cancer
potential of flavonoids: Recent trends and future perspectives. 3
Biotech. 3:439–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan HY, Zubair H, Ullah MF, Ahmad A and
Hadi SM: A prooxidant mechanism for the anticancer and
chemopreventive properties of plant polyphenols. Curr Drug Targets.
13:1738–1749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crozier A, Jaganath IB and Clifford MN:
Dietary phenolics: Chemistry, bioavailability and effects on
health. Nat Prod Rep. 26:1001–1043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vauzour D, Rodriguez-Mateos A, Corona G,
Oruna-Concha MJ and Spencer JP: Polyphenols and human health:
Prevention of disease and mechanisms of action. Nutrients.
2:1106–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyer J and Liu RH: Apple phytochemicals
and their health benefits. Nutr J. 3:52004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kern M, Tjaden Z, Ngiewih Y, Puppel N,
Will F, Dietrich H, Pahlke G and Marko D: Inhibitors of the
epidermal growth factor receptor in apple juice extract. Mol Nutr
Food Res. 49:317–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun J, Chu YF, Wu X and Liu RH:
Antioxidant and anti-proliferative activities of common fruits. J
Agric Food Chem. 50:7449–7454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tow WW, Premier R, Jing H and Ajlouni S:
Antioxidant and antiproliferation effects of extractable and
nonextractable polyphenols isolated from apple waste using
different extraction methods. J Food Sci. 76:T163–T172. 2011.
View Article : Google Scholar
|
|
15
|
Lo Scalzo R, Testoni A and Genna A:
'Annurca' apple fruit, a southern Italy apple cultivar: Textural
properties and aroma composition. Food Chem. 73:333–343. 2001.
View Article : Google Scholar
|
|
16
|
Napolitano A, Cascone A, Graziani G,
Ferracane R, Scalfi L, Di Vaio C, Ritieni A and Fogliano V:
Influence of variety and storage on the polyphenol composition of
apple flesh. J Agric Food Chem. 52:6526–6531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tenore GC, Campiglia P, Stiuso P, Ritieni
A and Novellino E: Nutraceutical potential of polyphenolic
fractions from Annurca apple (M. pumila Miller cv Annurca). Food
Chem. 140:614–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fini L, Piazzi G, Daoud Y, Selgrad M,
Maegawa S, Garcia M, Fogliano V, Romano M, Graziani G, Vitaglione
P, et al: Chemoprevention of intestinal polyps in
ApcMin/+ mice fed with western or balanced diets by
drinking Annurca apple polyphenol extract. Cancer Prev Res (Phila).
4:907–915. 2011. View Article : Google Scholar
|
|
19
|
Fini L, Selgrad M, Fogliano V, Graziani G,
Romano M, Hotchkiss E, Daoud YA, De Vol EB, Boland CR and
Ricciardiello L: Annurca apple polyphenols have potent
demethylating activity and can reactivate silenced tumor suppressor
genes in colorectal cancer cells. J Nutr. 137:2622–2628.
2007.PubMed/NCBI
|
|
20
|
D'Angelo S, La Porta R, Napolitano M,
Galletti P, Quagliuolo L and Boccellino M: Effect of Annurca apple
polyphenols on human HaCaT keratinocytes proliferation. J Med Food.
15:1024–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Bai L, Lu J, Liu L, Yang CY and
Sun H: Targeting inhibitors of apoptosis proteins (IAPs) for new
breast cancer therapeutics. J Mammary Gland Biol Neoplasia.
17:217–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou QP: Molecular mechanisms of green tea
polyphenols. Nutr Cancer. 61:827–835. 2009. View Article : Google Scholar
|
|
23
|
Singleton VL, Orthofer R and
Lamuela-Raventos RM: Analysis of total phenols and other oxidation
substrates and antioxidants by means of Folin-Ciocalteu reagent.
Methods Enzymol. 299:152–178. 1999. View Article : Google Scholar
|
|
24
|
D'Angelo S, Cimmino A, Raimo M, Salvatore
A, Zappia V and Galletti P: Effect of reddening-ripening on the
antioxidant activity of polyphenol extracts from cv. 'Annurca'
apple fruits. J Agric Food Chem. 55:9977–9985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Angelo S and Sammartino D: Protective
effect of Annurca apple extract against oxidative damage in human
erythrocytes. Curr Nutr Food Sci. 11:248–256. 2015. View Article : Google Scholar
|
|
26
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gago-Dominguez M, Jiang X and Castelao JE:
Lipid peroxidation, oxidative stress genes and dietary factors in
breast cancer protection: A hypothesis. Breast Cancer Res.
9:201–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fantini M, Benvenuto M, Masuelli L,
Frajese GV, Tresoldi I, Modesti A and Bei R: In vitro and in vivo
antitumoral effects of combinations of polyphenols, or polyphenols
and anticancer drugs: Perspectives on cancer treatment. Int J Mol
Sci. 16:9236–9282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Min NY, Kim JH, Choi JH, Liang W, Ko YJ,
Rhee S, Bang H, Ham SW, Park AJ and Lee KH: Selective death of
cancer cells by preferential induction of reactive oxygen species
in response to (−)-epigallocatechin-3-gallate. Biochem Biophys Res
Commun. 421:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casanova F, Quarti J, da Costa DC, Ramos
CA, da Silva JL and Fialho E: Resveratrol chemosensitizes breast
cancer cells to melphalan by cell cycle arrest. J Cell Biochem.
113:2586–2596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HP, Li TM, Tsao JY, Fong YC and Tang
CH: Curcumin induces cell apoptosis in human chondrosarcoma through
extrinsic death receptor pathway. Int Immunopharmacol. 13:163–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Etienne-Selloum N, Dandache I, Sharif T,
Auger C and Schini-Kerth VB: Polyphenolic compounds targeting
p53-family tumor suppressors: current progress and challenges.
Future Aspects of Tumor Suppressor Gene. Cheng Y: InTech; pp.
129–166. 2013, https://doi.org/10.5772/56102.
|
|
33
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Min KW, Wimalasena J and Baek SJ:
Cyclin D1 degradation and p21 induction contribute to growth
inhibition of colorectal cancer cells induced by
epigallocatechin-3-gallate. J Cancer Res Clin Oncol. 138:2051–2060.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sherr CJ: D-type cyclins. Trends Biochem
Sci. 20:187–190. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang K, Hitomi M and Stacey DW: Variations
in cyclin D1 levels through the cell cycle determine the
proliferative fate of a cell. Cell Div. 1:322006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar
|
|
39
|
Chaparro M, González Moreno L,
Trapero-Marugán M, Medina J and Moreno-Otero R: Review article:
Pharmacological therapy for hepatocellular carcinoma with sorafenib
and other oral agents. Aliment Pharmacol Ther. 28:1269–1277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Briviba K, Pan L and Rechkemmer G: Red
wine polyphenols inhibit the growth of colon carcinoma cells and
modulate the activation pattern of mitogen-activated protein
kinases. J Nutr. 132:2814–2818. 2002.PubMed/NCBI
|
|
41
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
42
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:pii: a008722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Babich H, Schuck AG, Weisburg JH and
Zuckerbraun HL: Research strategies in the study of the pro-oxidant
nature of polyphenol nutraceuticals. J Toxicol. 2011:4673052011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Trachootham D, Lu W, Ogasawara MA, Nilsa
RD and Huang P: Redox regulation of cell survival. Antioxid Redox
Signal. 10:1343–1374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Burch PM and Heintz NH: Redox regulation
of cell-cycle re-entry: Cyclin D1 as a primary target for the
mitogenic effects of reactive oxygen and nitrogen species. Antioxid
Redox Signal. 7:741–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fasanaro P, Magenta A, Zaccagnini G,
Cicchillitti L, Fucile S, Eusebi F, Biglioli P, Capogrossi MC and
Martelli F: Cyclin D1 degradation enhances endothelial cell
survival upon oxidative stress. FASEB J. 20:1242–1244. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Budanov AV: The role of tumor suppressor
p53 in the antioxidant defense and metabolism. Subcell Biochem.
85:337–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu GS: The functional interactions between
the p53 and MAPK signaling pathways. Cancer Biol Ther. 3:156–161.
2004. View Article : Google Scholar : PubMed/NCBI
|