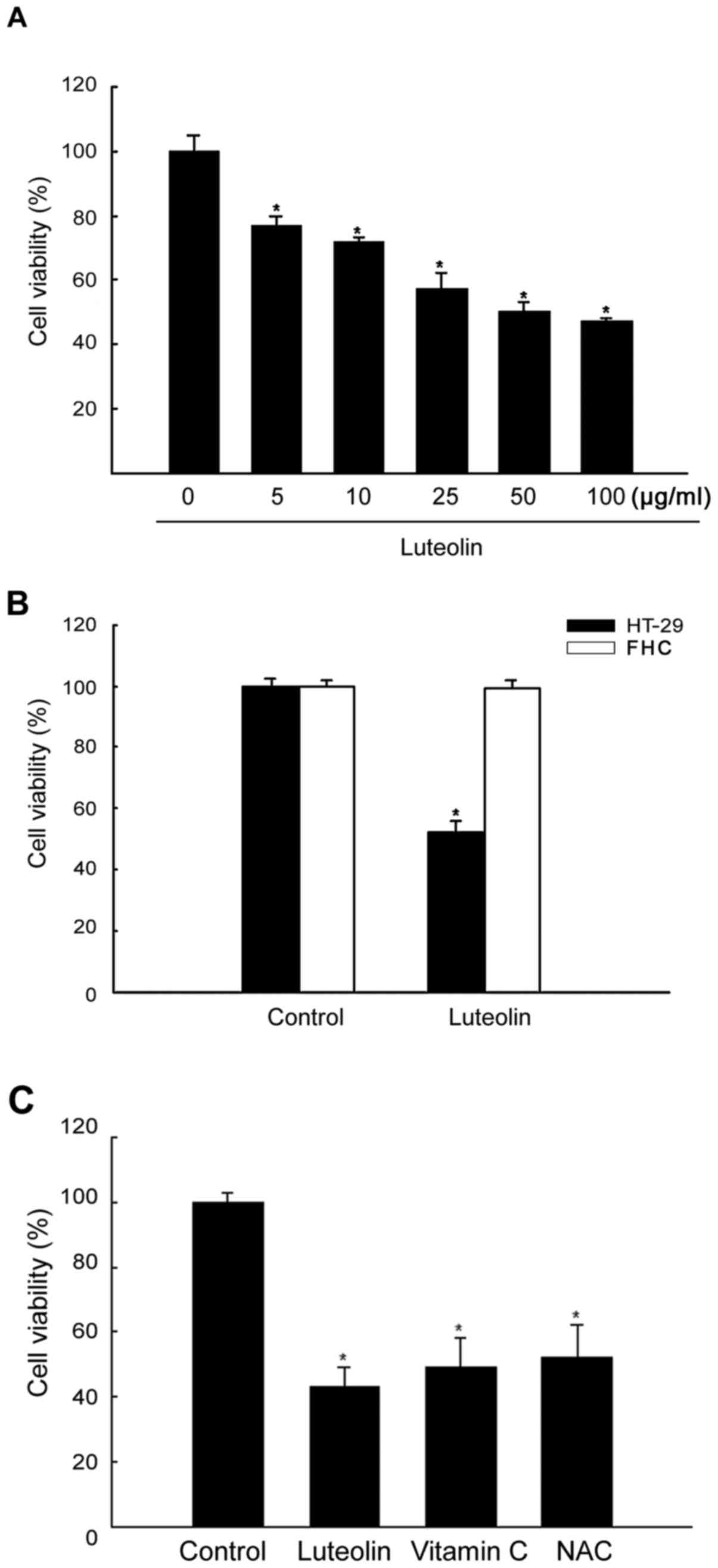
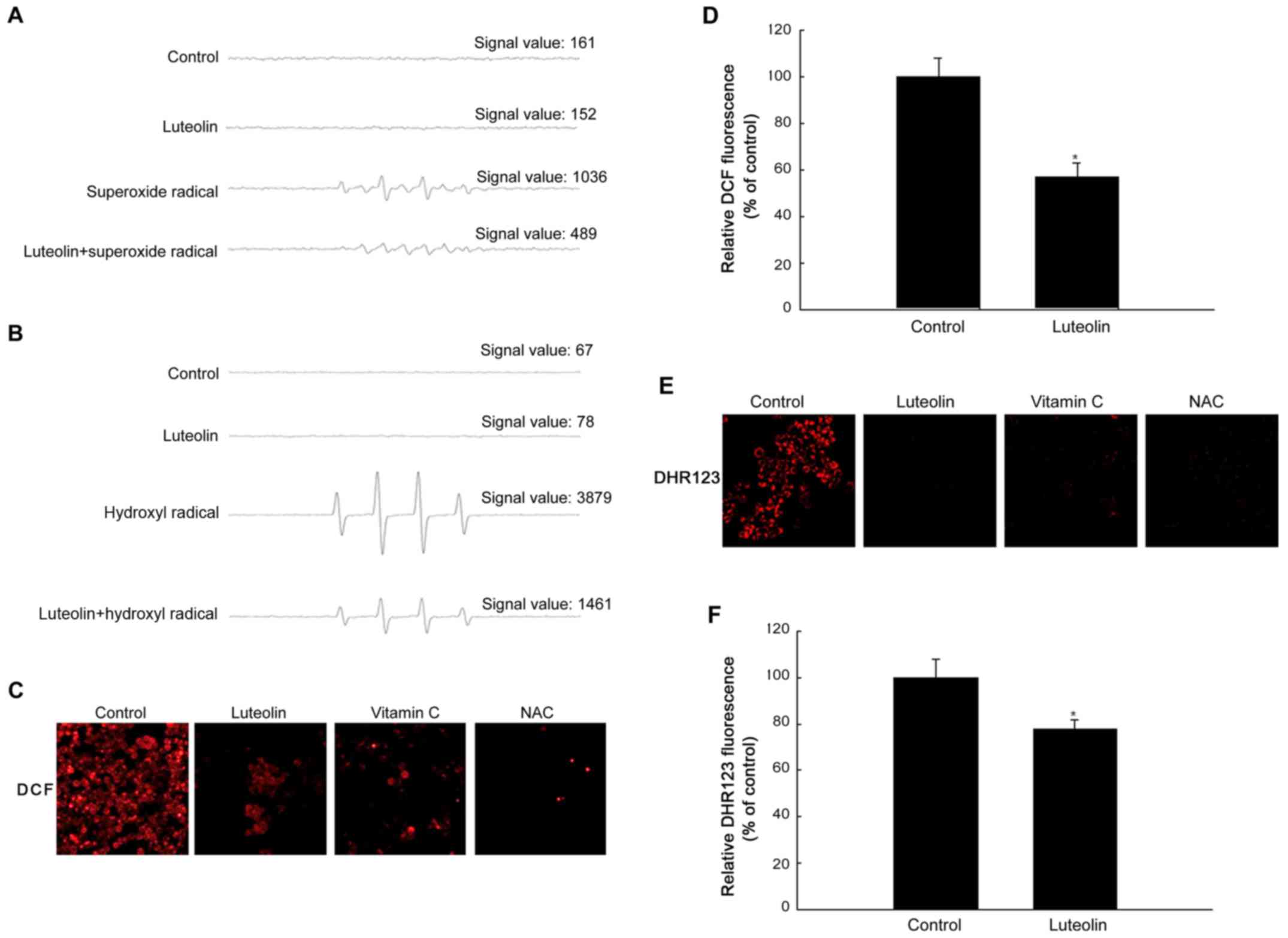
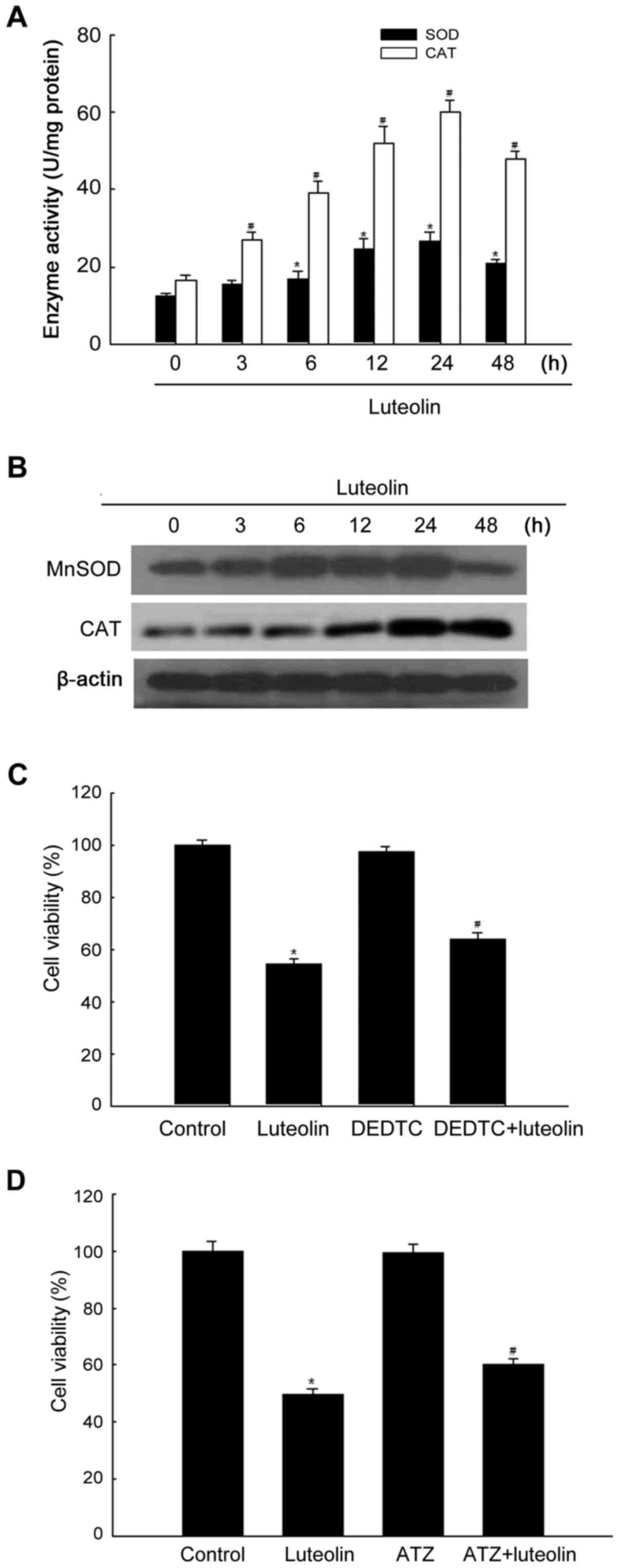
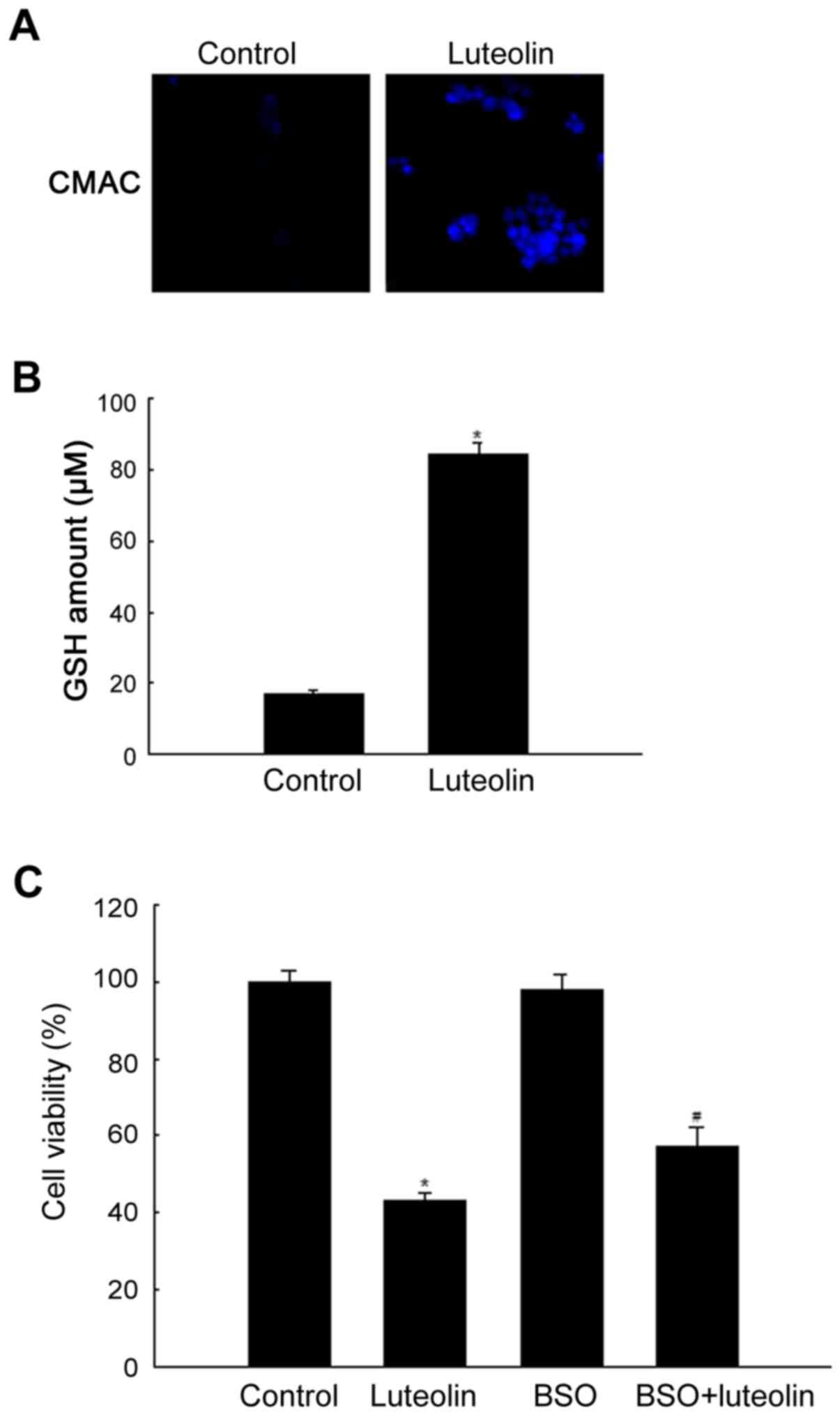
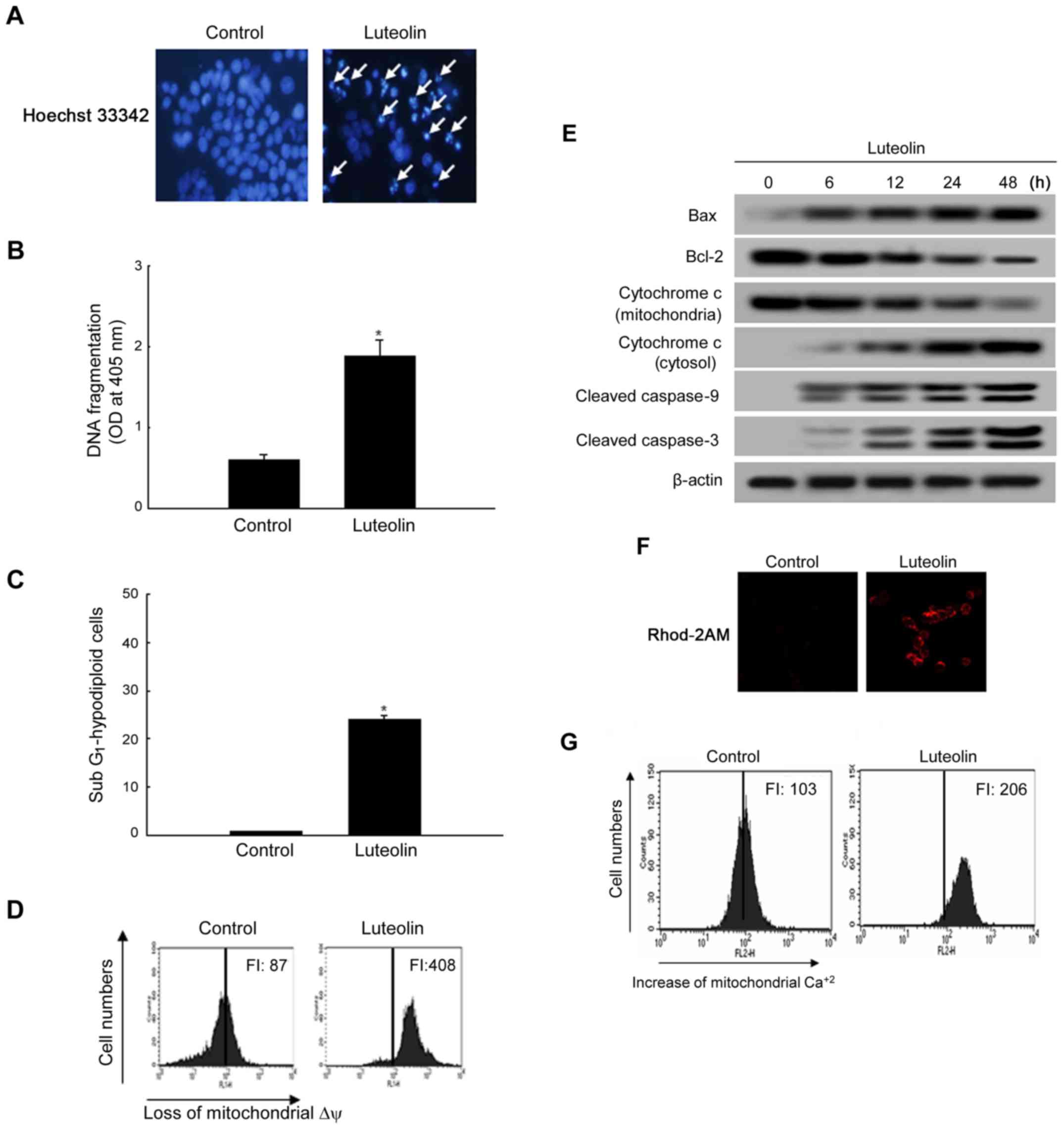
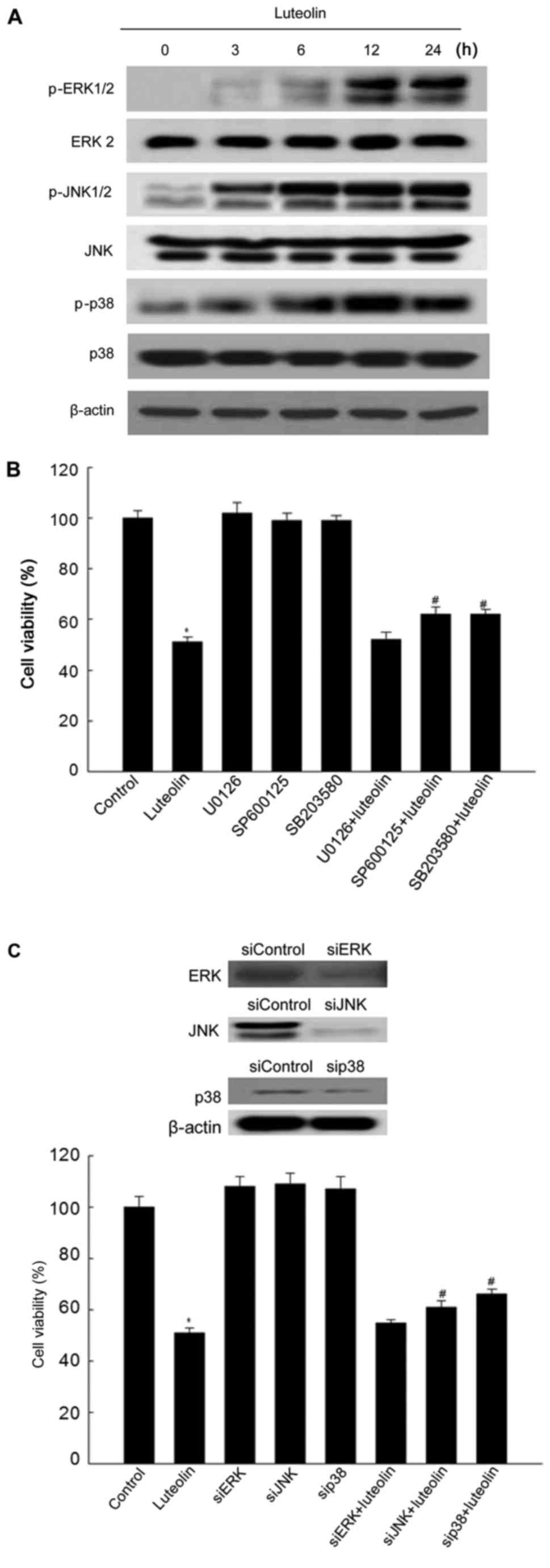
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Lee HH, Kim SK, Choi HH, Kim HK, Kim SS,
Chae HS, Cho H and Cho YS: Post-colonoscopy colorectal cancers in
average-risk Korean subjects with a normal initial colonoscopy.
Turk J Gastroenterol. 27:17–22. 2016. View Article : Google Scholar
|
|
3
|
Vargas AJ and Thompson PA: Diet and
nutrient factors in colorectal cancer risk. Nutr Clin Pract.
27:613–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang SY, Kim YS, Lee JE, Seol J, Song JH,
Chung GE, Yim JY, Lim SH and Kim JS: Dietary protein and fat intake
in relation to risk of colorectal adenoma in Korean. Medicine
(Baltimore). 95:e54532016. View Article : Google Scholar
|
|
5
|
Mehta RS, Nishihara R, Cao Y, Song M, Mima
K, Qian ZR, Nowak JA, Kosumi K, Hamada T, Masugi Y, et al:
Association of dietary patterns with risk of colorectal cancer
subtypes classified by fusobacterium nucleatum in tumor tissue.
JAMA Oncol. 6374:20162017.
|
|
6
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris IS, Treloar AE, Inoue S, Sasaki M,
Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA,
et al: Glutathione and thioredoxin antioxidant pathways synergize
to drive cancer initiation and progression. Cancer Cell.
27:211–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schumacker PT: Reactive oxygen species in
cancer: A dance with the devil. Cancer Cell. 27:156–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho BY, Wu YM, Chang KJ and Pan TM:
Dimerumic acid inhibits SW620 cell invasion by attenuating
H2O2-mediated MMP-7 expression via JNK/C-Jun
and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol
Sci. 7:869–880. 2011. View Article : Google Scholar :
|
|
10
|
Impei S, Gismondi A, Canuti L and Canini
A: Metabolic and biological profile of autochthonous Vitis vinifera
L. ecotypes. Food Funct. 6:1526–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Potze L, di Franco S, Kessler JH, Stassi G
and Medema JP: Betulinic acid kills colon cancer stem cells. Curr
Stem Cell Res Ther. 11:427–433. 2016. View Article : Google Scholar
|
|
12
|
Pandurangan AK and Esa NM: Luteolin, a
bioflavonoid inhibits colorectal cancer through modulation of
multiple signaling pathways: A review. Asian Pac J Cancer Prev.
15:5501–5508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gismondi A, Di Marco G, Canuti L and
Canini A: Antiradical activity of phenolic metabolites extracted
from grapes of white and red Vitis vinifera L. cultivars. Vitis.
56:19–26. 2017.
|
|
14
|
Kumar S and Pandey AK: Chemistry and
biological activities of flavonoids: An overview. Scientific World
Journal. 2013:1627502013. View Article : Google Scholar
|
|
15
|
Zhang YC, Gan FF, Shelar SB, Ng KY and
Chew EH: Antioxidant and Nrf2 inducing activities of luteolin, a
flavonoid constituent in Ixeris sonchifolia Hance, provide
neuroprotective effects against ischemia-induced cellular injury.
Food Chem Toxicol. 59:272–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasala ER, Bodduluru LN, Barua CC and
Gogoi R: Antioxidant and antitumor efficacy of Luteolin, a dietary
flavone on benzo(a) pyrene-induced experimental lung
carcinogenesis. Biomed Pharmacother. 82:568–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK and Ganapasam S: Luteolin inhibits matrix metalloproteinase 9
and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp
Toxicol. 33:1176–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pandurangan AK, Ananda Sadagopan SK,
Dharmalingam P and Ganapasam S: Inhibitory effect of luteolin on
azoxymethane induced colon carcinogenesis: Involvement of iNOS and
COX-2. Pharmacogn Mag. 10:306–310. 2014. View Article : Google Scholar
|
|
19
|
Pandurangan AK, Ananda Sadagopan SK,
Dharmalingam P and Ganapasam S: Luteolin, a bioflavonoid inhibits
Azoxymethane-induced colorectal cancer through activation of Nrf2
signaling. Toxicol Mech Methods. 24:13–20. 2014. View Article : Google Scholar
|
|
20
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK, Ramar M, Munusamy A and Ganapasam S: Luteolin induces growth
arrest in colon cancer cells through involvement of
Wnt/β-catenin/GSK-3β signaling. J Environ Pathol Toxicol Oncol.
32:131–139. 2013. View Article : Google Scholar
|
|
21
|
Ashokkumar P and Sudhandiran G: Luteolin
inhibits cell proliferation during Azoxymethane-induced
experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest
New Drugs. 29:273–284. 2011. View Article : Google Scholar
|
|
22
|
Attoub S, Hassan AH, Vanhoecke B, Iratni
R, Takahashi T, Gaben AM, Bracke M, Awad S, John A, Kamalboor HA,
et al: Inhibition of cell survival, invasion, tumor growth and
histone deacetylase activity by the dietary flavonoid luteolin in
human epithelioid cancer cells. Eur J Pharmacol. 651:18–25. 2011.
View Article : Google Scholar
|
|
23
|
Pandurangan AK and Ganapasam S: Cytotoxic
effect of luteolin on human colorectal cancer cell line (HCT-15):
Crucial involvement of reactive oxygen species. Middle East J
Cancer. 4:177–182. 2013.
|
|
24
|
Safi W, Kuehnl A, Nüssler A, Eckstein HH
and Pelisek J: Differentiation of human CD14+ monocytes:
An experimental investigation of the optimal culture medium and
evidence of a lack of differentiation along the endothelial line.
Exp Mol Med. 48:e2272016. View Article : Google Scholar
|
|
25
|
Kimura S, Inoguchi T, Yamasaki T, Yamato
M, Ide M, Sonoda N, Yamada K and Takayanagi R: A novel DPP-4
inhibitor teneligliptin scavenges hydroxyl radicals: In vitro study
evaluated by electron spin resonance spectroscopy and in vivo study
using DPP-4 deficient rats. Metabolism. 65:138–145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HB and Yoo BS: Propolis inhibits
UVA-induced apoptosis of human keratinocyte HaCaT cells by
scavenging ROS. Toxicol Res. 32:345–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carrillo MC, Kanai S, Nokubo M and Kitani
K: (−) deprenyl induces activities of both superoxide dismutase and
catalase but not of glutathione peroxidase in the striatum of young
male rats. Life Sci. 48:517–521. 1991. View Article : Google Scholar
|
|
28
|
Tauskela JS, Hewitt K, Kang LP, Comas T,
Gendron T, Hakim A, Hogan M, Durkin J and Morley P: Evaluation of
glutathione-sensitive fluorescent dyes in cortical culture. Glia.
30:329–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernando PM, Piao MJ, Kang KA, Ryu YS,
Hewage SR, Chae SW and Hyun JW: Rosmarinic acid attenuates cell
damage against UVB radiation-induced oxidative stress via enhancing
antioxidant effects in human HaCaT cells. Biomol Ther (Seoul).
24:75–84. 2016. View Article : Google Scholar
|
|
30
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D and Armstrong JS: Bax and the
mitochondrial permeability transition cooperate in the release of
cytochrome c during endoplasmic reticulum-stress-induced apoptosis.
Cell Death Differ. 14:703–715. 2007. View Article : Google Scholar
|
|
32
|
Gold-Smith F, Fernandez A and Bishop K:
Mangiferin and cancer: Mechanisms of action. Nutrients. 8:3962016.
View Article : Google Scholar :
|
|
33
|
Tait SW and Green DR: Mitochondrial
regulation of cell death. Cold Spring Harb Perspect Biol.
5:0087062013. View Article : Google Scholar
|
|
34
|
Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL,
Hou LH and Wang CL: ROS-Ca(2+) is associated with mitochondria
permeability transition pore involved in surfactin-induced MCF-7
cells apoptosis. Chem Biol Interact. 190:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng YY, Yang JS, Tsai SC, Liaw CC, Chung
JG, Huang LJ, Lee KH, Lu CC, Chien HC, Tsuzuki M, et al: The newly
synthesized
2-(3-hydroxy-5-methoxyphenyl)-6,7-methylenedioxyquinolin-4-one
triggers cell apoptosis through induction of oxidative stress and
upregulation of the p38 MAPK signaling pathway in HL-60 human
leukemia cells. Oncol Rep. 28:1482–1490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai
EM and Hsu YL: Arctigenin, a dietary phytoestrogen, induces
apoptosis of estrogen receptor-negative breast cancer cells through
the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol
Med. 67:159–170. 2014. View Article : Google Scholar
|
|
37
|
Schulze A and Harris AL: How cancer
metabolism is tuned for proliferation and vulnerable to disruption.
Nature. 491:364–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang P, Feng L, Oldham EA, Keating MJ and
Plunkett W: Superoxide dismutase as a target for the selective
killing of cancer cells. Nature. 407:390–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee LT, Huang YT, Hwang JJ, Lee AY, Ke FC,
Huang CJ, Kandaswami C, Lee PP and Lee MT: Transinactivation of the
epidermal growth factor receptor tyrosine kinase and focal adhesion
kinase phosphorylation by dietary flavonoids: Effect on invasive
potential of human carcinoma cells. Biochem Pharmacol.
67:2103–2114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sonoda M, Nishiyama T, Matsukawa Y and
Moriyasu M: Cytotoxic activities of flavonoids from two Scutellaria
plants in Chinese medicine. J Ethnopharmacol. 91:65–68. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar
|
|
42
|
Miar A, Hevia D, Muñoz-Cimadevilla H,
Astudillo A, Velasco J, Sainz RM and Mayo JC: Manganese superoxide
dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers
for tumor progression and metastasis in prostate, colon, and lung
cancer. Free Radic Biol Med. 85:45–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sarsour EH, Kalen AL and Goswami PC:
Manganese superoxide dismutase regulates a redox cycle within the
cell cycle. Antioxid Redox Signal. 20:1618–1627. 2014. View Article : Google Scholar :
|
|
44
|
Hart PC, Mao M, de Abreu AL,
Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A,
Diamond AM, Minshall RD, Consolaro ME, et al: MnSOD upregulation
sustains the Warburg effect via mitochondrial ROS and
AMPK-dependent signalling in cancer. Nat Commun. 6:60532015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dhar SK, Tangpong J, Chaiswing L, Oberley
TD and St Clair DK : Manganese superoxide dismutase is a
p53-regulated gene that switches cancers between early and advanced
stages. Cancer Res. 71:6684–6695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Y, St Clair DK , Xu Y, Crooks PA and
St Clair WH : A NADPH oxidase-dependent redox signaling pathway
mediates the selective radiosensitization effect of parthenolide in
prostate cancer cells. Cancer Res. 70:2880–2890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Becuwe P, Ennen M, Klotz R, Barbieux C and
Grandemange S: Manganese superoxide dismutase in breast cancer:
From molecular mechanisms of gene regulation to biological and
clinical significance. Free Radic Biol Med. 77:139–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dhar SK and St Clair DK : Manganese
superoxide dismutase regulation and cancer. Free Radic Biol Med.
52:2209–2222. 2012. View Article : Google Scholar : PubMed/NCBI
|