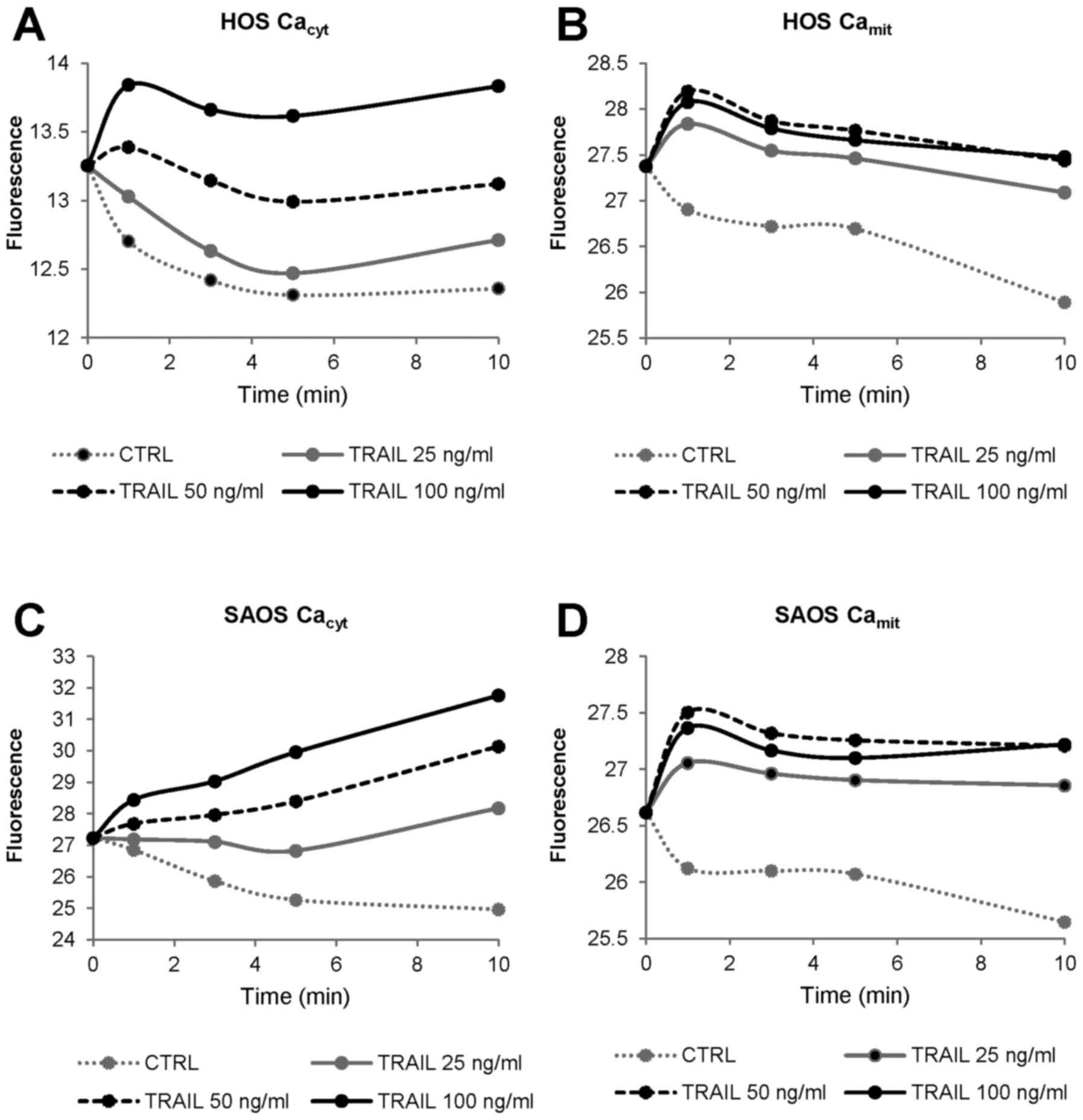
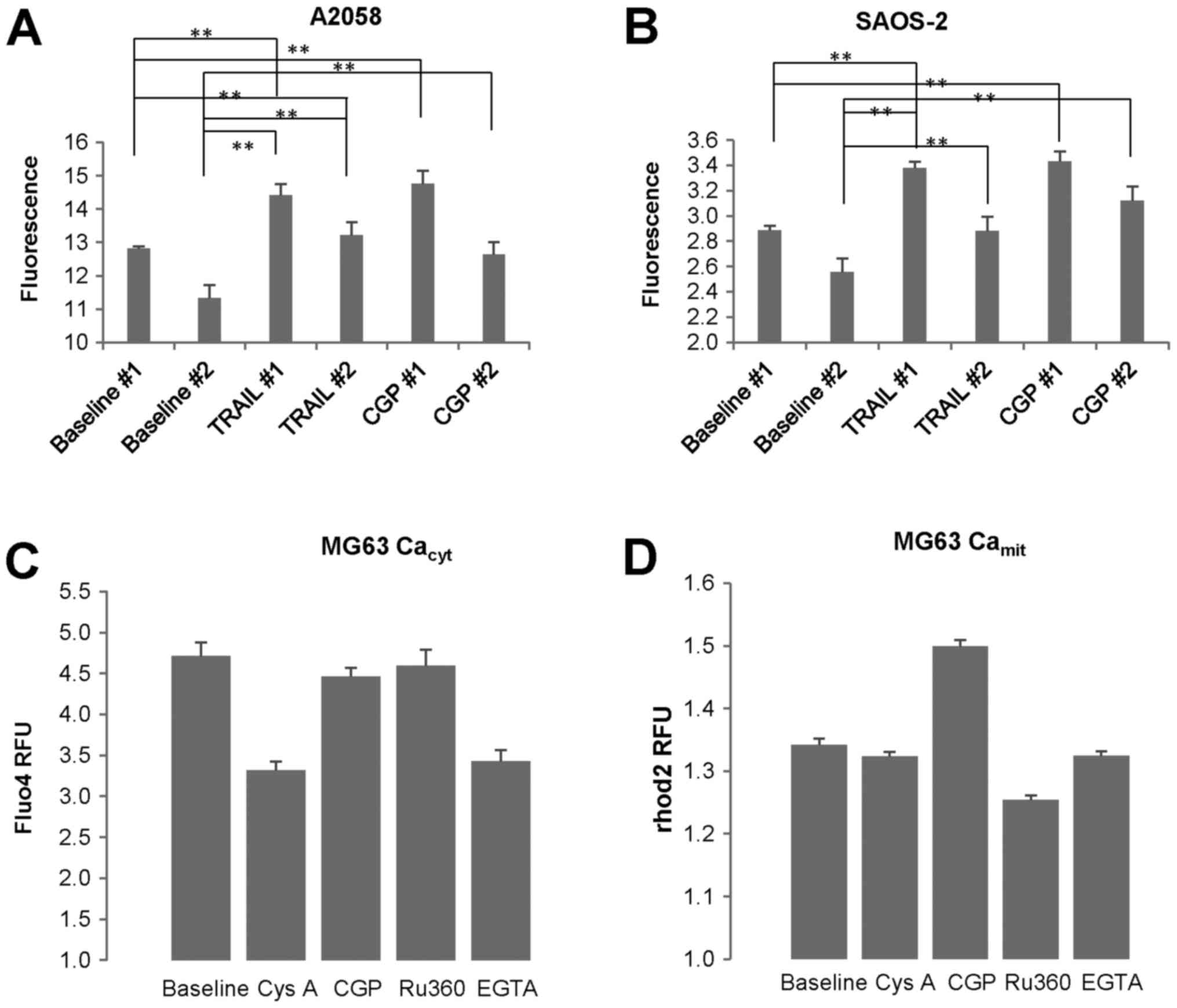
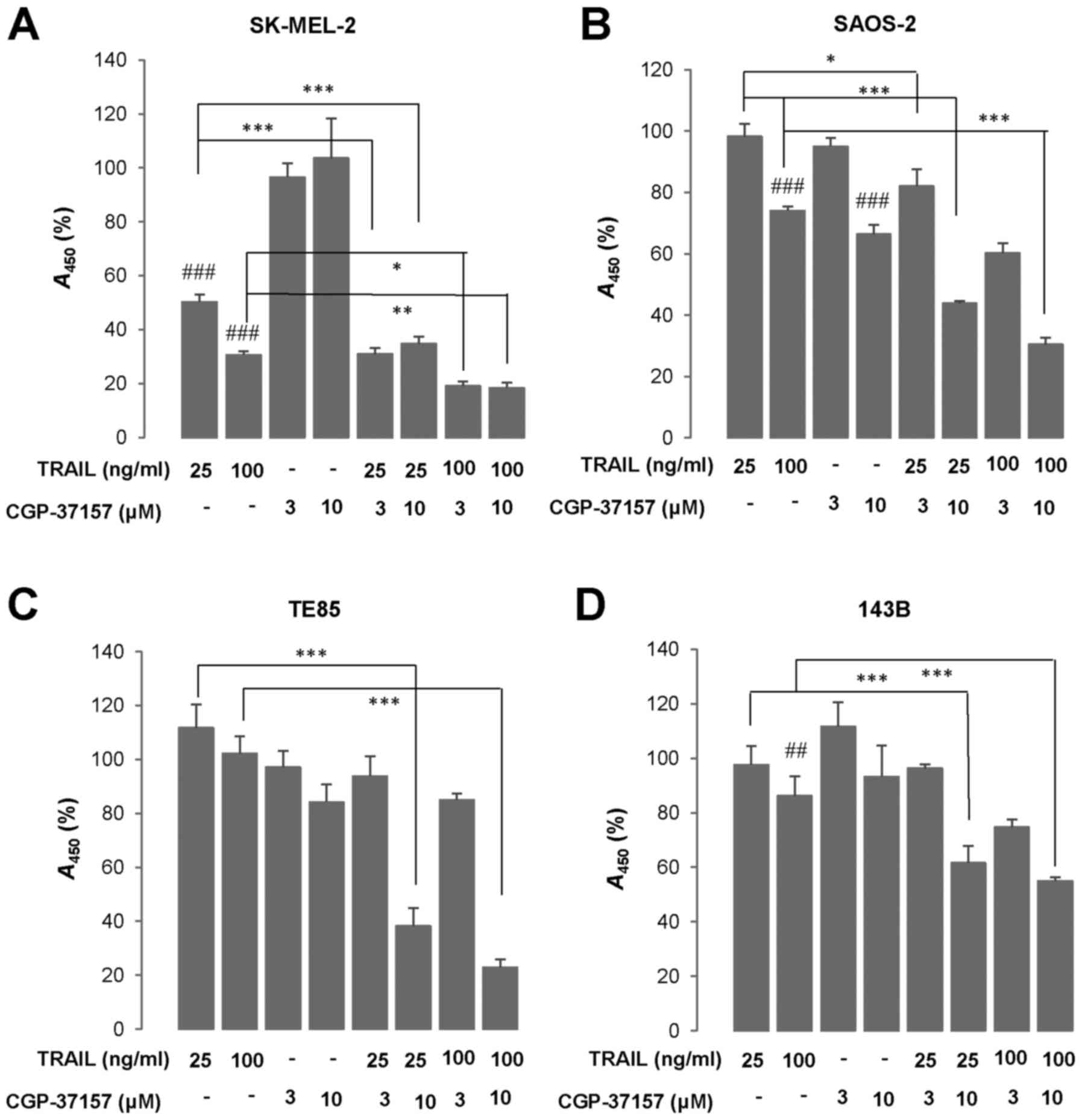
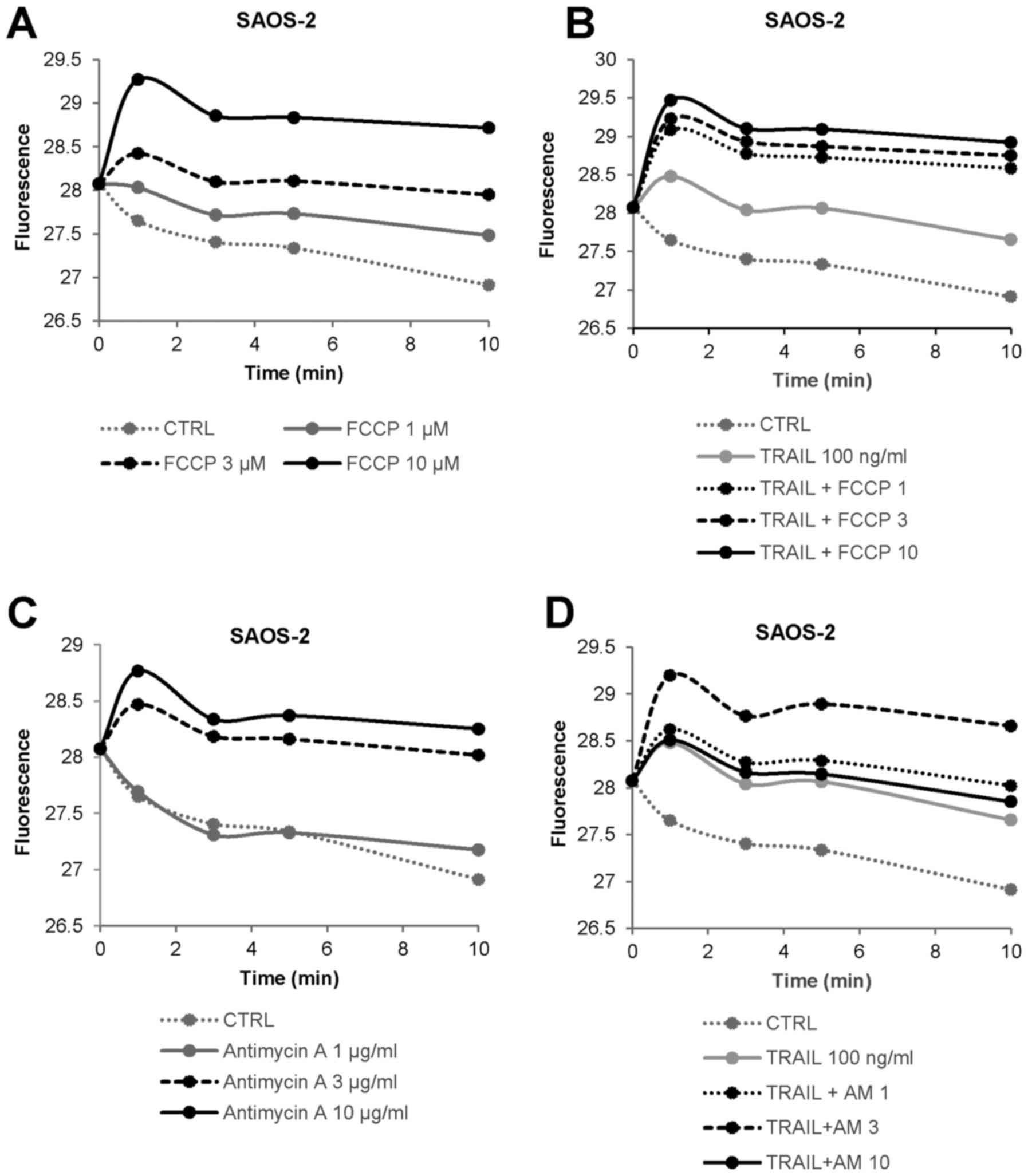
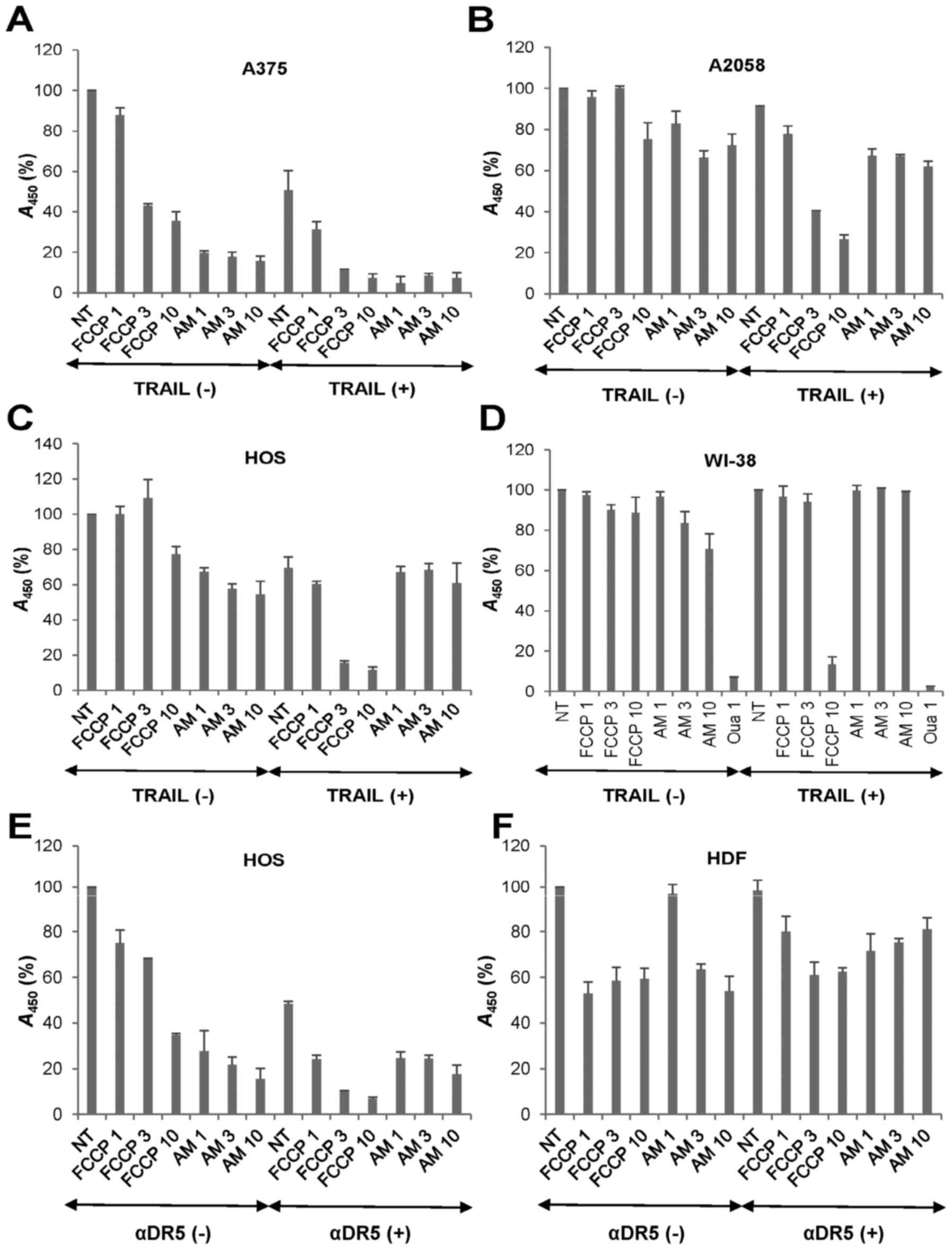
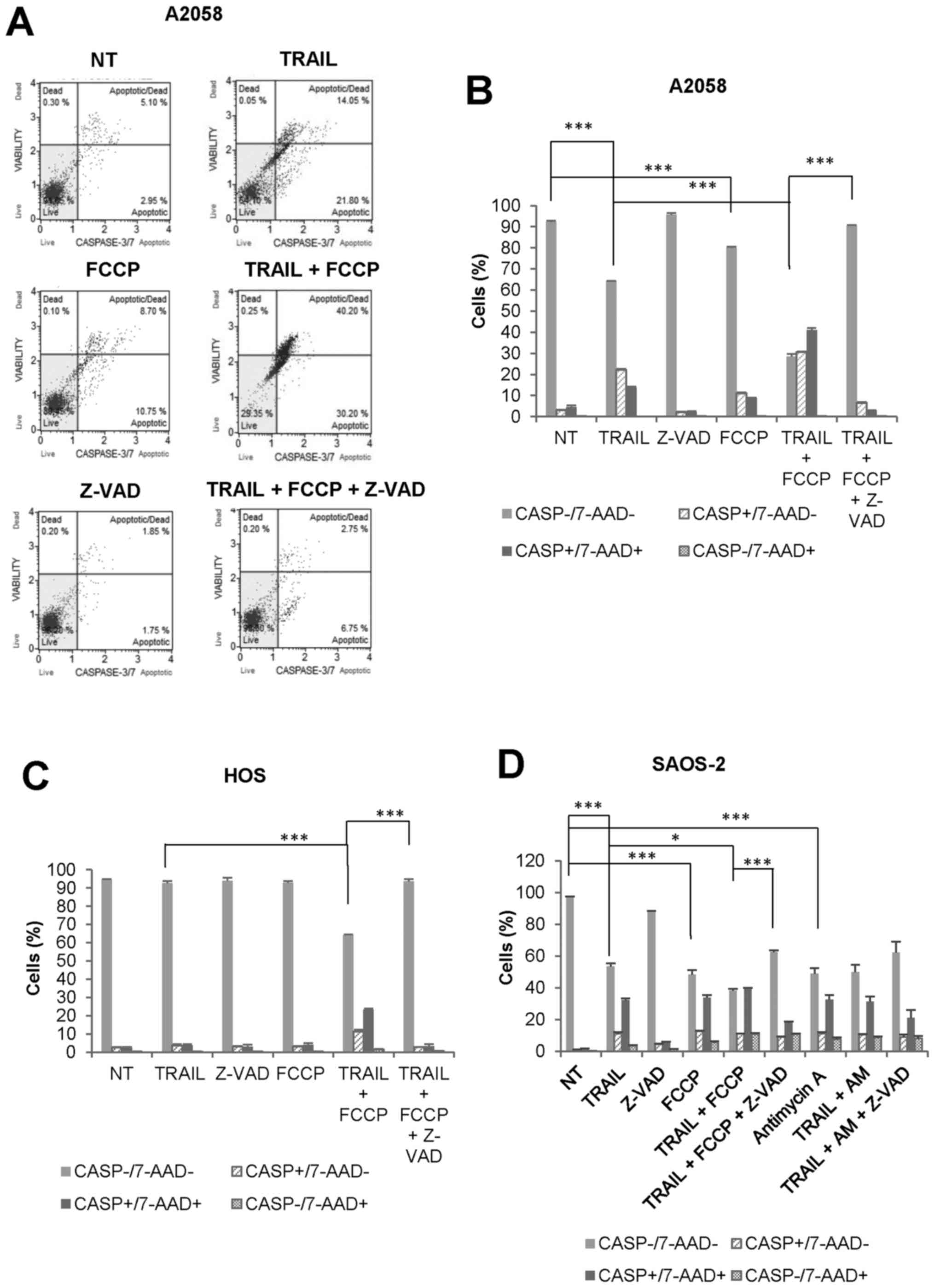
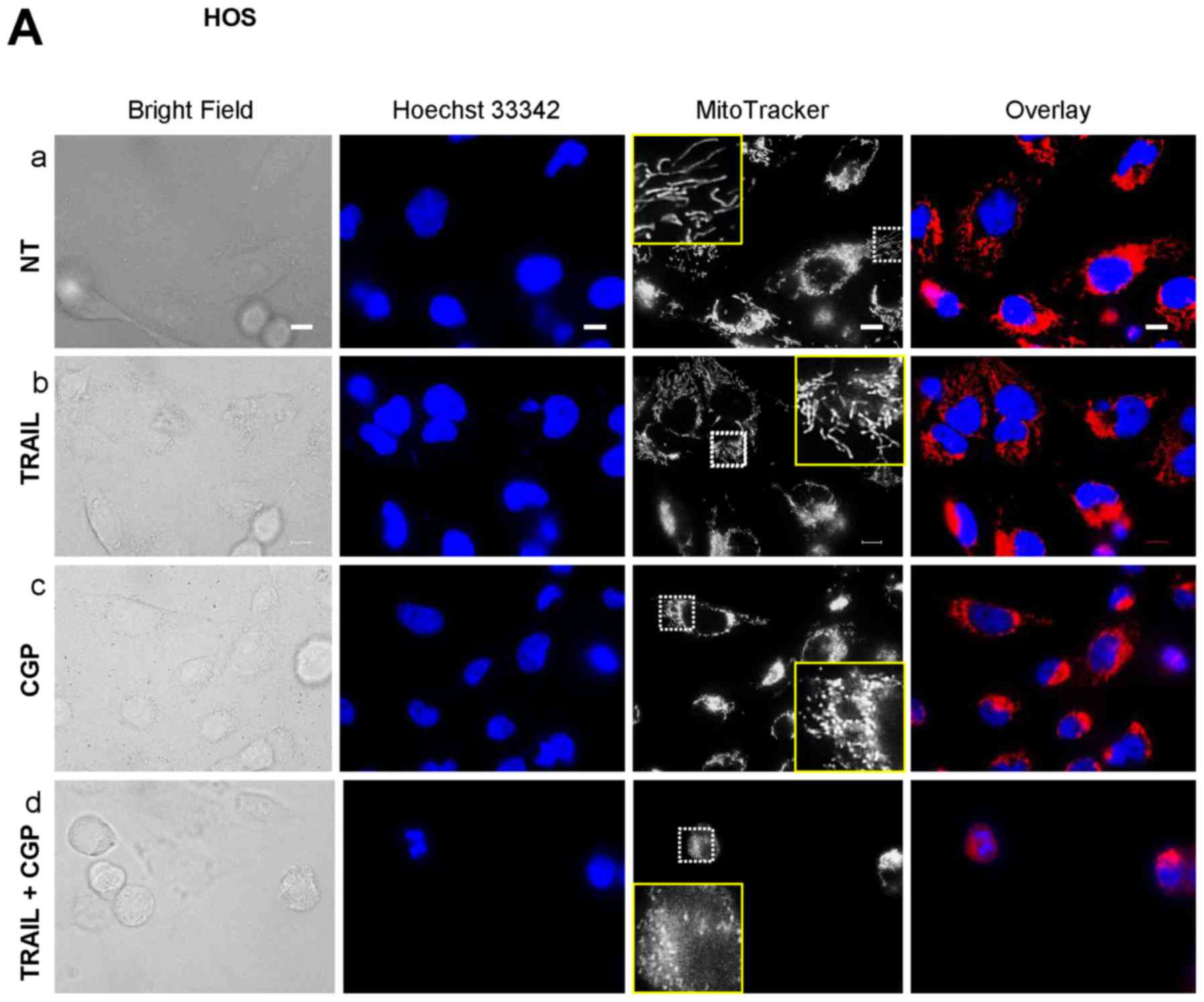
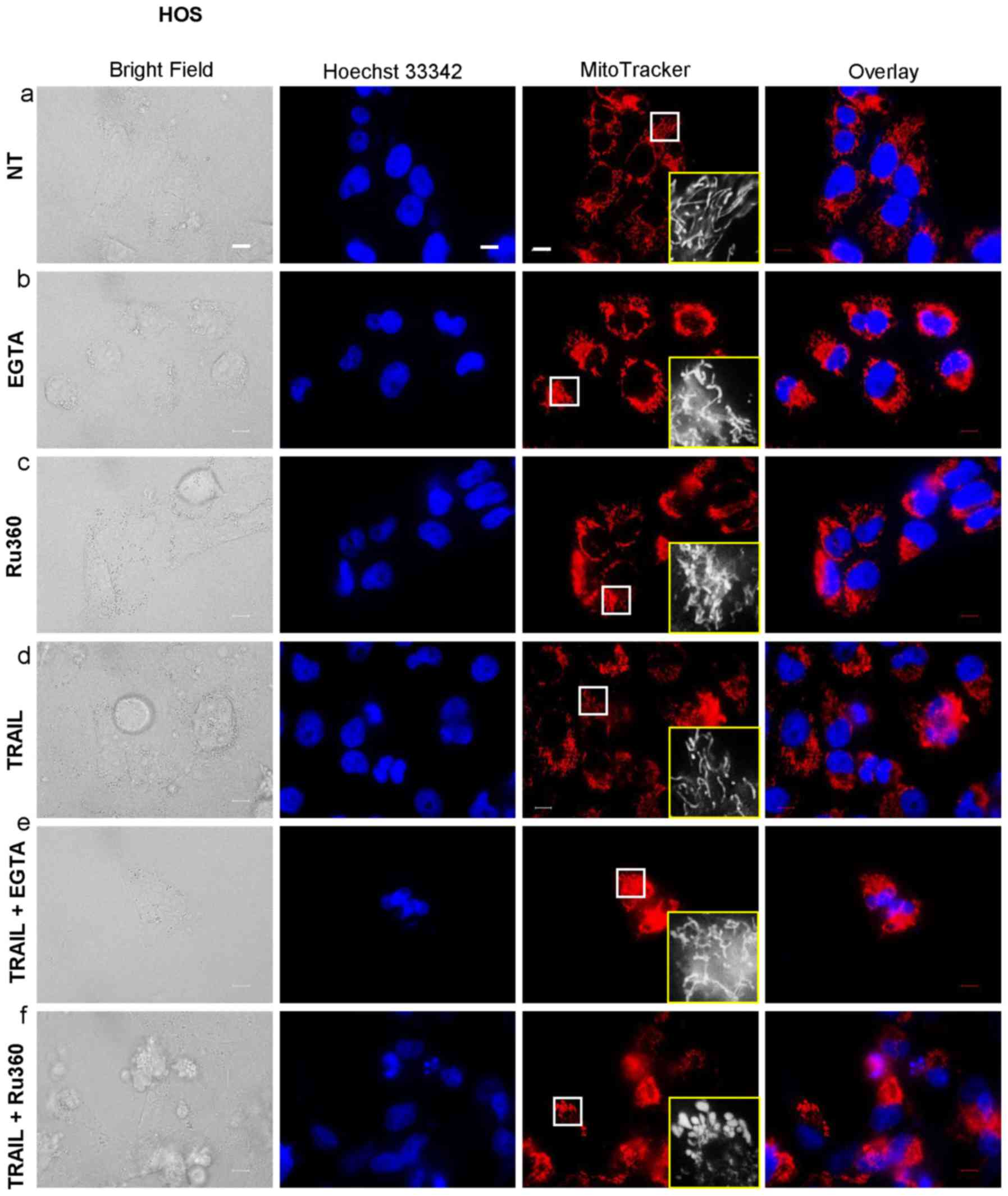
|
1
|
Almasan A and Ashkenazi A: Apo2L/TRAIL:
Apoptosis signaling, biology, and potential for cancer therapy.
Cytokine Growth Factor Rev. 14:337–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gonzalvez F and Ashkenazi A: New insights
into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrero-Martín G, Høyer-Hansen M,
García-García C, Fumarola C, Farkas T, López-Rivas A and Jäättelä
M: TAK1 activates AMPK-dependent cytoprotective autophagy in
TRAIL-treated epithelial cells. EMBO J. 28:677–685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He W, Wang Q, Xu J, Xu X, Padilla MT, Ren
G, Gou X and Lin Y: Attenuation of TNFSF10/TRAIL-induced apoptosis
by an autophagic survival pathway involving TRAF2- and
RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 8:1811–1821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jouan-Lanhouet S, Arshad MI,
Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van
Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D,
Vandenabeele P, et al: TRAIL induces necroptosis involving
RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ.
19:2003–2014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sosna J, Philipp S, Fuchslocher Chico J,
Saggau C, Fritsch J, Föll A, Plenge J, Arenz C, Pinkert T, Kalthoff
H, et al: Differences and similarities in TRAIL- and tumor necrosis
factor-mediated necroptotic signaling in cancer cells. Mol Cell
Biol. 36:2626–2644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanov VN, Bhoumik A and Ronai Z: Death
receptors and melanoma resistance to apoptosis. Oncogene.
22:3152–3161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dyer MJ, MacFarlane M and Cohen GM:
Barriers to effective TRAIL-targeted therapy of malignancy. J Clin
Oncol. 25(25): 4505–4506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dimberg LY, Anderson CK, Camidge R,
Behbakht K, Thorburn A and Ford HL: On the TRAIL to successful
cancer therapy? Predicting and counteracting resistance against
TRAIL-based therapeutics. Oncogene. 32:1341–1350. 2013. View Article : Google Scholar
|
|
14
|
Guiho R, Biteau K, Heymann D and Redini F:
TRAIL-based therapy in pediatric bone tumors: How to overcome
resistance. Future Oncol. 11:535–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Miguel D, Lemke J, Anel A, Walczak H
and Martinez-Lostao L: Onto better TRAILs for cancer treatment.
Cell Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elustondo PA, Nichols M, Robertson GS and
Pavlov EV: Mitochondrial Ca(2+) uptake pathways. J Bioenerg
Biomembr. 49:113–119. 2017. View Article : Google Scholar
|
|
17
|
Bonora M, Wieckowski MR, Chinopoulos C,
Kepp O, Kroemer G, Galluzzi L and Pinton P: Molecular mechanisms of
cell death: Central implication of ATP synthase in mitochondrial
permeability transition. Oncogene. 34:1475–1486. 2015. View Article : Google Scholar
|
|
18
|
Izzo V, Bravo-San Pedro JM, Sica V,
Kroemer G and Galluzzi L: Mitochondrial permeability transition:
New findings and persisting uncertainties. Trends Cell Biol.
26:655–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galluzzi L, Bravo-San Pedro JM, Kepp O and
Kroemer G: Regulated cell death and adaptive stress responses. Cell
Mol Life Sci. 73:2405–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Calcium and mitochondria in the regulation of cell death. Biochem
Biophys Res Commun. 460:72–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danese A, Patergnani S, Bonora M,
Wieckowski MR, Previati M, Giorgi C and Pinton P: Calcium regulates
cell death in cancer: Roles of the mitochondria and
mitochondria-associated membranes (MAMs). Biochim Biophys Acta.
1858:615–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marchi S and Pinton P: Alterations of
calcium homeostasis in cancer cells. Curr Opin Pharmacol. 29:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monteith GR, Prevarskaya N and
Roberts-Thomson SJ: The calcium-cancer signalling nexus. Nat Rev
Cancer. 17:367–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takata N, Ohshima Y, Suzuki-Karasaki M,
Yoshida Y, Tokuhashi Y and Suzuki-Karasaki Y: Mitochondrial
Ca2+ removal amplifies TRAIL cytotoxicity toward
apoptosis-resistant tumor cells via promotion of multiple cell
death modalities. Int J Oncol. 51:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elgass K, Pakay J, Ryan MT and Palmer CS:
Recent advances into the understanding of mitochondrial fission.
Biochim Biophys Acta. 1833:150–161. 2013. View Article : Google Scholar
|
|
26
|
Chang CR and Blackstone C: Dynamic
regulation of mitochondrial fission through modification of the
dynamin-related protein Drp1. Ann NY Acad Sci. 1201:34–39. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Senft D and Ronai ZA: Regulators of
mitochondrial dynamics in cancer. Curr Opin Cell Biol. 39:43–52.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Jeong SY, Karbowski M, Smith CL
and Youle RJ: Roles of the mammalian mitochondrial fission and
fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell.
15:5001–5011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Estaquier J and Arnoult D: Inhibiting
Drp1-mediated mitochondrial fission selectively prevents the
release of cytochrome c during apoptosis. Cell Death Differ.
14:1086–1094. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rehman J, Zhang HJ, Toth PT, Zhang Y,
Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C and Archer SL:
Inhibition of mitochondrial fission prevents cell cycle progression
in lung cancer. FASEB J. 26:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Westrate LM, Sayfie AD, Burgenske DM and
MacKeigan JP: Persistent mitochondrial hyperfusion promotes G2/M
accumulation and caspase-dependent cell death. PLoS One.
9:e919112014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akita M, Suzuki-Karasaki M, Fujiwara K,
Nakagawa C, Soma M, Yoshida Y, Ochiai T, Tokuhashi Y and
Suzuki-Karasaki Y: Mitochondrial division inhibitor-1 induces
mitochondrial hyperfusion and sensitizes human cancer cells to
TRAIL-induced apoptosis. Int J Oncol. 45:1901–1912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki-Karasaki Y, Fujiwara K, Saito K,
Suzuki-Karasaki M, Ochiai T and Soma M: Distinct effects of TRAIL
on the mitochondrial network in human cancer cells and normal
cells: Role of plasma membrane depolarization. Oncotarget.
6:21572–21588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nita II, Hershfinkel M, Kantor C, Rutter
GA, Lewis EC and Sekler I: Pancreatic β-cell Na+
channels control global Ca2+ signaling and oxidative
metabolism by inducing Na+ and Ca2+ responses
that are propagated into mitochondria. FASEB J. 28:3301–3312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruiz A, Alberdi E and Matute C: CGP37157,
an inhibitor of the mitochondrial Na+/Ca2+ exchanger,
protects neurons from excitotoxicity by blocking voltage-gated
Ca2+ channels. Cell Death Dis. 5:e11562014. View Article : Google Scholar
|
|
37
|
Ben-Hail D, Palty R and Shoshan-Barmatz V:
Measurement of mitochondrial Ca2+ transport mediated by
three transport proteins: VDAC1, the Na+/Ca2+
exchanger, and the Ca2+ uniporter. Cold Spring Harb
Protoc. 2014:161–166. 2014.PubMed/NCBI
|
|
38
|
Izeradjene K, Douglas L, Tillman DM,
Delaney AB and Houghton JA: Reactive oxygen species regulate
caspase activation in tumor necrosis factor-related
apoptosis-inducing ligand-resistant human colon carcinoma cell
lines. Cancer Res. 65:7436–7445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inoue T and Suzuki-Karasaki Y:
Mitochondrial superoxide mediates mitochondrial and endoplasmic
reticulum dysfunctions in TRAIL-induced apoptosis in Jurkat cells.
Free Radic Biol Med. 61:273–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki-Karasaki M, Ochiai T and
Suzuki-Karasaki Y: Crosstalk between mitochondrial ROS and
depolarization in the potentiation of TRAIL-induced apoptosis in
human tumor cells. Int J Oncol. 44:616–628. 2014. View Article : Google Scholar
|
|
41
|
Bernardi P and von Stockum S: The
permeability transition pore as a Ca(2+) release channel: New
answers to an old question. Cell Calcium. 52:22–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gutiérrez-Aguilar M and Baines CP:
Structural mechanisms of cyclophilin D-dependent control of the
mitochondrial permeability transition pore. Biochim Biophys Acta.
1850:2041–2047. 2015. View Article : Google Scholar :
|
|
43
|
Kaddour-Djebbar I, Lakshmikanthan V,
Shirley RB, Ma Y, Lewis RW and Kumar MV: Therapeutic advantage of
combining calcium channel blockers and TRAIL in prostate cancer.
Mol Cancer Ther. 5:1958–1966. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaddour-Djebbar I, Choudhary V, Brooks C,
Ghazaly T, Lakshmikanthan V, Dong Z and Kumar MV: Specific
mitochondrial calcium overload induces mitochondrial fission in
prostate cancer cells. Int J Oncol. 36:1437–1444. 2010.PubMed/NCBI
|
|
45
|
Han J, Hou W, Goldstein LA, Lu C, Stolz
DB, Yin XM and Rabinowich H: Involvement of protective autophagy in
TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem.
283:19665–19677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hou W, Han J, Lu C, Goldstein LA and
Rabinowich H: Enhancement of tumor-TRAIL susceptibility by
modulation of autophagy. Autophagy. 4:940–943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goodall ML, Fitzwalter BE, Zahedi S, Wu M,
Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD and
Thorburn A: The autophagy machinery controls cell death switching
between apoptosis and necroptosis. Dev Cell. 37:337–349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Haga N, Fujita N and Tsuruo T:
Mitochondrial aggregation precedes cytochrome c release from
mitochondria during apoptosis. Oncogene. 22:5579–5585. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haga N, Fujita N and Tsuruo T: Involvement
of mitochondrial aggregation in arsenic trioxide
(As2O3)-induced apoptosis in human
glioblastoma cells. Cancer Sci. 96:825–833. 2005. View Article : Google Scholar : PubMed/NCBI
|