Introduction
Gastric cancer is the fourth most common cancer and
second leading cause of cancer-related deaths worldwide (1–4).
Although great efforts have been made, the current treatments for
gastric cancer are still not efficacious (5). Increasing evidence has shown that the
microenvironment plays important roles in gastric cancer
progression (6,7). Mesenchymal stem cells (MSCs) are a
critical component of cancer microenvironment. We have previously
reported the isolation of MSCs from gastric cancer tissues
(GC-MSCs) and have demonstrated that these cells could promote
gastric cancer growth and metastasis (8–10).
However, the underlying mechanisms responsible for the promoting
role of GC-MSCs in gastric cancer progression remain unknown.
The Hippo pathway plays a critical role in organ
size control, tissue homeostasis and early embryonic cell
specification (11).
Yes-associated protein 1 (YAP) is the central component of this
pathway. YAP has been recently identified as an oncoprotein that is
overexpressed in many cancers, including gastric cancer, liver
cancer, lung cancer, breast cancer, and ovarian cancer among others
(12–14). The knockdown of YAP inhibits
gastric cancer cell proliferation, migration, invasion, and
metastasis (15,16), suggesting that YAP plays important
roles in the development and progression of gastric cancer.
In addition to tumor cells, YAP could also regulate
the microenvironmental cells. For instance, YAP could modulate
cell-cell and cell-matrix interactions (17) as well as the production of
secretory proteins such as amphiregulin [AREG; an epidermal growth
factor (EGF) family member], cysteine-rich angiogenic inducer 61
(CYR61), and connective tissue growth factor (CTGF) (18–20).
Moreover, YAP expression in the microenvironmental cells also
affect tumor progression. YAP is required for the tumor-promoting
role of CAFs in matrix remodeling and angiogenesis (17,21),
indicating that YAP pathway may affect tumor progression by
regulating both tumor cells and microenvironmental cells.
Mesenchymal stem cells (MSCs) have been suggested as
a key player in the tumor microenvironment. We have previously
shown that MSCs isolated from gastric cancer tissues have a strong
promoting role in gastric cancer progression (8–10).
However, the detailed molecular mechanism is not clear. In this
study, we aimed to investigate whether YAP is involved in the
promoting effect of GC-MSCs on gastric cancer progression. We found
that YAP silencing significantly suppressed the promoting effects
of GC-MSC on gastric cancer growth in vitro and in
vivo, which may be associated with the decreased activation of
β-catenin in gastric cancer cells.
Materials and methods
Subjects
The gastric cancer tissues were collected from 15
patients with gastric adenocarcinoma between August 2015 and
February 2016 in the Affiliated Hospital of Jiangsu University.
There were 8 male and 7 female patients with ages ranging from 48
to 71 years old (median, 59 years old). The patients were diagnosed
by pathological analyses according to the UICC (International Union
Against Cancer) criteria. The use of clinical sample was approved
by the Ethics Committee of Jiangsu University and written informed
consent was obtained from all the patients.
Isolation and culture of MSCs from
gastric cancer tissues
GC-MSCs were isolated as previously described
(22,23). Fresh gastric cancer tissues were
washed with phosphate-buffered saline (PBS) to remove the blood.
Then, the tissues were cut into 1–3 mm3-sized pieces and
floated in Dulbecco's modified Eagle's medium with low glucose
(LG-DMEM, Invitrogen, Carlsbad, CA, USA) containing 15% fetal
bovine serum (FBS, Invitrogen), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The pieces of gastric cancer
tissues were cultured at 37°C in humidified air with 5%
CO2. After culturing for 10 days, the colonies of
fibroblast-like cells appeared. When the cells reached ~80%
confluence, they were trypsinized and re-plated into larger culture
flasks at a 1:3 split ratio. The GC-MSCs at passage 4 were used for
subsequent experiments.
Cell culture
GC-MSCs were cultured in LG-DMEM with 15% FBS. The
human GC cell lines SGC-7901 (moderately differentiated), HGC-27
(undifferentiated) and MGC-803 (poorly differentiated) were
purchased from the Cell Bank of the China Academy of Sciences
(Beijing, China) and maintained in the RPMI-1640 medium (Gibco,
Grand Island, NY, USA) supplemented with 10% FBS. Human umbilical
vein endothelial cell line EA.hy926 was purchased from the Cell
Bank of the China Academy of Sciences and maintained in
high-glucose DMEM supplemented with 10% FBS. All the cells were
cultured at 37°C in humidified atmosphere with 5%
CO2.
Lentiviral knockdown of YAP
The lentiviral expression vector containing the
shRNA sequence (Sigma) was selected for target-specific gene
silencing. The shRNA sequences targeting Yap were as follows:
forward,
CCGGGCCACCAAGCTAGATAAAGAACTCGAGTTCTTTATCTAGCTTGGTGGCTTTTTG;
reverse,
AATTCAAAAAGCCACCAAGCTAGATAAAGAACTCGAGTTCTTTATCTAGCTTGGTGGC. Control
shRNAs were constructed using scrambled sequences. The shRNA
lentiviral vectors were generated by ligating the vector
Tet-pLKO-puro; these lentiviral vectors were produced using a
lentivirus packaging mix (ViraPower, Invitrogen). In addition,
stable cell line was obtained after selection with 1 µg/ml
of puromycin (Invitrogen) for 5 days. The efficiency of YAP
knockdown was evaluated by using real-time quantitative RT-PCR and
western blotting.
Generation of conditioned medium
Control and YAP knockdown GC-MSCs were plated in
6-well plates at a density of 1×105 cells/well and
cultured in 1.6 ml complete LG-DMEM with 15% FBS. After 72 h, the
conditioned medium (CM) was collected, centrifuged to remove
cellular debris (800 g for 5 min) and passed through a
0.22-µm filter (Millipore, Billerica, MA, USA) and stored in
−20°C until use. Gastric cancer cell-derived CM was generated in a
similar manner.
RNA extraction and real-time RT-PCR
Total RNA was isolated from cells and tissues using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions, and equal amount of RNA was used for RT-PCR. The
StepOne Real-Time system was used for quantitative mRNA detection.
The expression of target genes was normalized to that of β-actin.
The expression of each gene was measured by formula
2−ΔΔCt. The primers used in this study were produced by
Invitrogen (Shanghai, China) and the sequences are listed in
Table I.
 | Table IThe sequences of the primers. |
Table I
The sequences of the primers.
| Genes | The sequences of
the primers |
|---|
| β-actin | Forward
5′-GACCTGTACGCCAACACAGT-3′ |
| Reverse
5′-CTCAGGAGGAGCAATGATCT-3′ |
| E-cadherin | Forward
5′-CGCATTGCCACATACACTCT-3′ |
| Reverse
5′-TTGGCTGAGGATGGTGTAAG-3′ |
| N-cadherin | Forward
5′-AGTCAACTGCAACGTCT-3′ |
| Reverse
5′-AGCGTTCCTGTTCCACTCAT-3′ |
| Bax | Forward
5′-CACCAGCAGATCAT-3′ |
| Reverse
5′-GATCAGTTCCGGCACCTTG-3′ |
| BCL2 | Forward
5′-CTGGGAGAACAGGGTACGATAA-3′ |
| Reverse
5′-CCCACCGAACTCAAAGAAGG-3′ |
| MMP2 | Forward
5′-CTCGAATCCATGATGGAGAG-3′ |
| Reverse
5′-TACTTCACACGGACCACTTG-3′ |
| MMP9 | Forward
5′-ACGTCTTCCAGTACCGAGAG-3′ |
| Reverse
5′-GGCACTGCAGGATGTCATAG-3′ |
| Slug | Forward
5′-CCTGGTTGCTTCAAGGACAC-3′ |
| Reverse
5′-TCCATGCTCTTGCAGCTCTC-3′ |
| Oct4 | Forward
5′-TTGAGGCTCTGCAGCTTAG-3′ |
| Reverse
5′-GCCGGTTACAGAACCACAC-3′ |
| SOX2 | Forward
5′-ACACCAATCCCATCCACACT-3′ |
| Reverse
5′-GCAAACTTCCTGCAAAGCTC-3′ |
| Nanog | Forward
5′-CCTGATTCTTCCACCAGTCC-3′ |
| Reverse
5′-TGCTATTCTTCGGCCAGTTG-3′ |
| CD44 | Forward
5′-TCACAGTGGAAGAAGAGAC-3′ |
| Reverse 5′-CAT TG
CATGTTGTCACT-3′ |
| PDGF | Forward
5′-CTCAGGCGAGATGACTTGTA-3′ |
| Reverse
5′-CCACACCATCGTCCTCTAAT-3′ |
| VEGF | Forward
5′-CCTTGCTGCTCTACCTCCAC-3′ |
| Reverse
5′-ATCTGCATGGTGATGTTGGA-3′ |
| IL-8 | Forward
5′-GCTCTGTGTGAAGGTGCAGTTT-3′ |
| Reverse
5′-TTCTGTGTTGGCGCAGTGT-3′ |
Western blotting
GC-MSCs and gastric cancer cells were homogenized
and lysed in RIPA buffer supplemented with proteinase inhibitors.
Equal amounts of proteins were separated on a 12% SDS-PAGE gel.
Following electrophoresis, the proteins were transferred to the
PVDF membrane, blocked in 5% (w/v) non-fat milk and incubated with
the primary antibodies at 4°C overnight. The sources of antibodies
were as follows: anti-GAPDH (Kangcheng, China); anti-YAP,
anti-vimentin, anti-Bcl2, and anti-Bax (Bioworld Technology, Louis
Park, MN, USA); anti-β-catenin (Cell Signaling Technology, Beverly,
MA, USA); anti-E-cadherin, and anti-N-cadherin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA); anti-Ki67 (BOSTER,
China). The membrane was washed with Tris-buffered saline/Tween 3
times and incubated with the secondary antibodies (Bioworld
Technology) at 37°C for 1 h. The signals were visualized by using a
Lumina crescendo western horseradish peroxidase substrate
(Millipore). The dilution factor for the primary and secondary
antibodies was 1:1,000, respectively.
Colony formation assay
GC-MSCs and gastric cancer cells were seeded into
6-well plates (2×103 cells/well) and incubated at 37°C
in a 5% CO2-humidified incubator for 8 days. SGC-7901
cells were treated with 2 ml complete medium and GC-MSCs derived CM
(1:1, v/v). The medium was changed at 3-day intervals. At the end
of the incubation period, the cultures were fixed with 4%
paraformaldehyde and stained with crystal violet. The results are
the mean values of three independent experiments.
Cell migration and invasion assays
GC-MSCs (5×104 cells/well), gastric
cancer cells treated with GC-MSCs derived CM (1×105
cells/well), and EA.hy926 treated with CM of gastric cancer cells
(5×104 cells/well) were plated into the top chamber of
Transwell (8.0-µm pore sized) with serum-free L-DMEM, and
medium containing 10% FBS was placed into the bottom chamber. After
incubation at 37°C in a 5% CO2-humidified incubator for
12 h, the cells that remained at the upper surface of the membrane
were removed with a cotton swab. The filters were fixed in 4%
paraformaldehyde for 30 min, stained with crystal violet for 15
min, and photographed. The cells were observed under a microscope,
and at least five fields of cells were assayed for each group. Each
assay was repeated in triplicate. For cell invasion assay, the
Transwell chambers were coated with 200 µl of Matrigel at a
dilution of 1:5 in serum-free medium and the incubation time was
extended to 24 h. The remaining procedure was the same as that of
cell migration assay. The number of migrated and invaded cells was
counted under a microscope (Ti-S, Nikon) and five fields were
randomly selected.
Endothelial tube formation assay
The endothelial tube-formation assay was conducted
according to the manufacturer's protocol (BD Biosciences, Franklin
Lakes, NJ, USA). Matrigel (50 µl) was added to each well of
a 96-well plate and allowed to polymerize. EA.hy926 cells
(2×104) plated on Matrigel was treated with the
conditional medium of gastric cancer cells. After incubation for 12
h at 37°C, the cells were viewed under a microscope and
photographed. The number of formed tubes was counted under a
microscope and five fields were randomly selected.
Immunohistochemistry
Immunohistochemistry was used to detect protein
expression in tumor tissues sections. The antibodies used were
anti-β-catenin (1:50; Cell Signaling Technology), CD31 (1:50;
Bioworld Technology), anti-E-cadherin, anti-N-cadherin (1:50; Santa
Cruz Biotechnology), and anti-Ki67 (1:100; Boster, China). Ki67
staining is usually evaluated by counting positive cells of the
total cell count (nucleus is stained by Ki67 antibody). Both
cytosol and nucleus of the cells were positively stained for
β-catenin protein.
Cell apoptosis assay
For cell apoptosis assay, a FITC Annexin V apoptosis
detection kit (Vazyme, China) was used. SGC-7901 cells treated with
GC-MSCs derived CM were trypsinized, washed in PBS and stained
according to the manufacturer's instructions. The stained cells
were analyzed by using flow cytometry (BD Accuri C6).
Animal model
Eighteen male BALB/c nu/nu mice (Laboratory Animal
Center of Shanghai, Academy of Science, China) aged 4–6 weeks were
randomly divided into 3 groups (6 mice/group). The animals were
injected subcutaneously with untreated SGC-7901 cells (blank
group), control GC-MSCs-CM treated SGC-7901 cells (sh-Ctrl CM
group), and YAP knockdown GC-MSCs-CM treated SGC-7901 cells (sh-YAP
CM group) (1.5×106 cells in 200 µl PBS) into the
right back side of mice. Tumor volumes were measured every 2 days
using calipers according to the modified ellipsoidal formula:
(length x width2)/2. The tumors were removed after
injection for 28 days. The experiment protocols were approved by
the Animal Use Committee of Jiangsu University.
Statistical analyses
All the data are presented as mean ± standard
deviation (SD). The statistically significant differences between
groups were assessed by using analysis of variance (ANOVA) or
t-test by Prism software (GraphPad, San Diego, USA). P-value
<0.05 was considered statistically significant.
Results
YAP knockdown inhibits the proliferation
of GC-MSCs
As one of the key components of tumor
microenvironment (TME), MSCs have been found to play critical roles
in tumor progression (24,25). We have previously demonstrated that
GC-MSCs promote gastric cancer growth more efficiently than
adjacent non-cancerous gastric tissue-derived MSCs (GCN-MSCs) and
bone marrow-derived MSCs (BM-MSCs) (10,23).
We hypothesized that YAP overexpression and activation is involved
in the superior promoting role of GC-MSCs in gastric cancer growth.
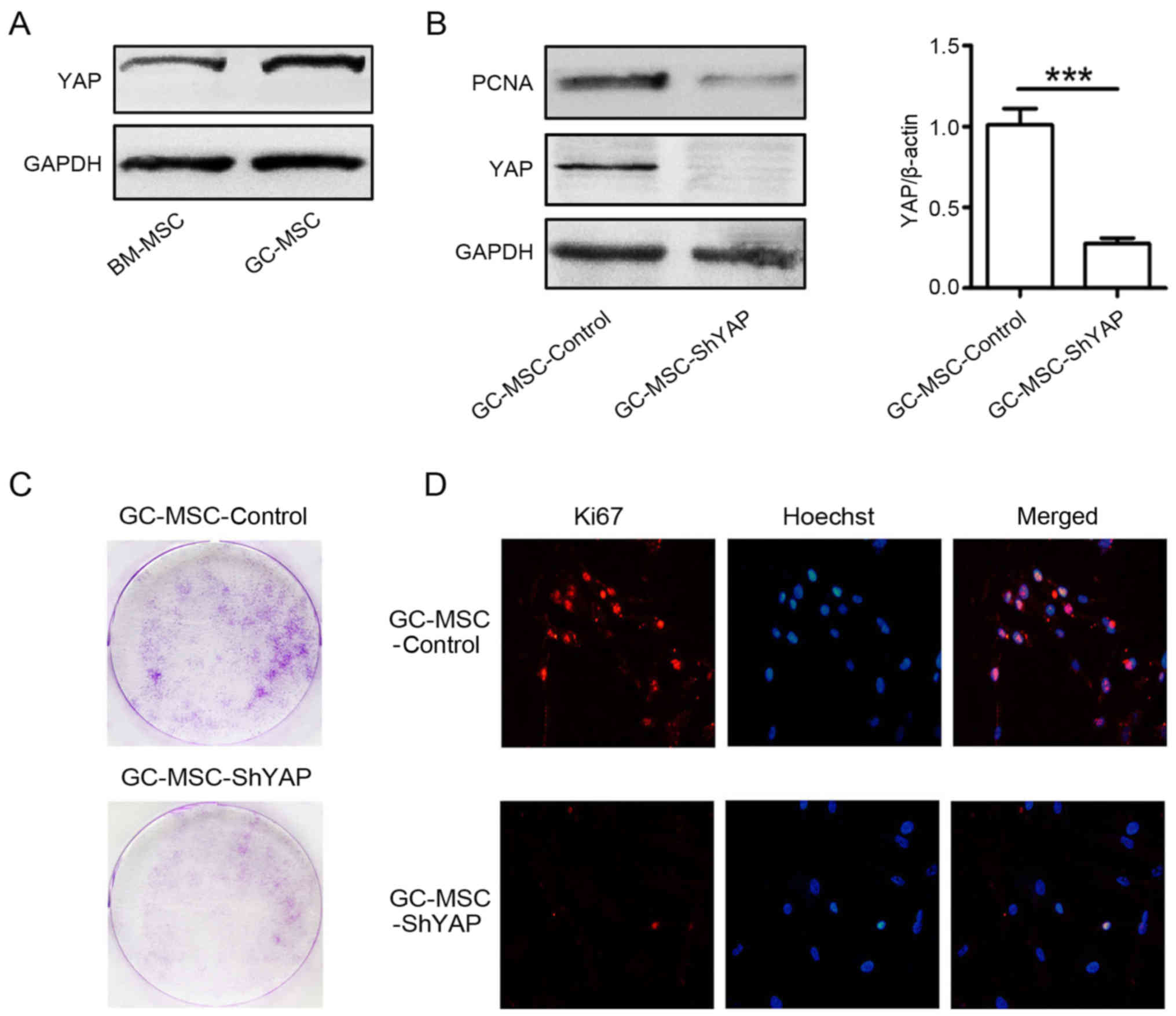
Thus, we compared YAP expression between GC-MSCs and BM-MSCs. The
results of western blotting showed that the expression level of YAP
in GC-MSCs was higher than that in BM-MSCs (Fig. 1A). Then, we used shRNA to knock
down YAP in GC-MSCs and explored the effects of YAP knockdown on
the proliferation of GC-MSCs (Fig.
1B). The results of colony formation assay showed that there
were less colonies in YAP shRNA (shYAP) group than that in control
group (Fig. 1C). The results of
immunofluorescent staining revealed that there were more
Ki67-positive cells in control group than that in shYAP group
(Fig. 1D). The expression of PCNA
was decreased in shYAP GC-MSCs compared to that in control GC-MSCs
(Fig. 1B). Together, these results
suggest that YAP knockdown inhibited the proliferation of
GC-MSCs.
YAP knockdown inhibits the migration and
invasion of GC-MSCs
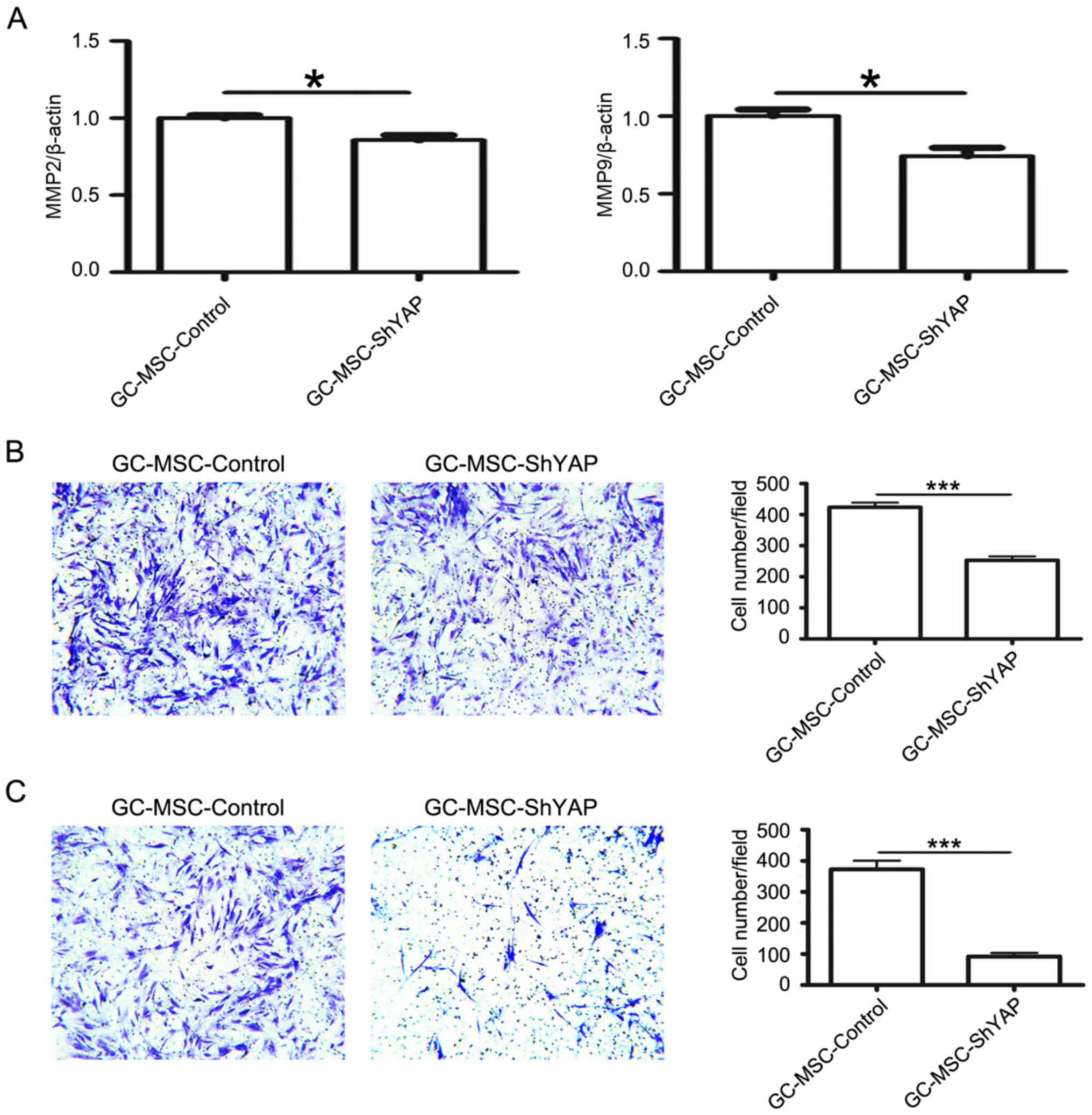
We next investigated the effects of YAP knockdown on
the migratory and invasive abilities of GC-MSCs. The expression of
MMP2 and MMP9 was detected by using quantitative RT-PCR. YAP shRNA
reduced the expression of MMP2 and MMP9 in GC-MSCs (Fig. 2A). Consistently, the number of
cells migrating towards the lower surface of the chamber in the
Transwell migration assay was lower in shYAP group than that in
control group (Fig. 2B). Similar
change was also observed in the matrigel invasion assay (Fig. 2C). Thus, these data suggest that
YAP knockdown reduces the migratory and invasive abilities of
GC-MSCs.
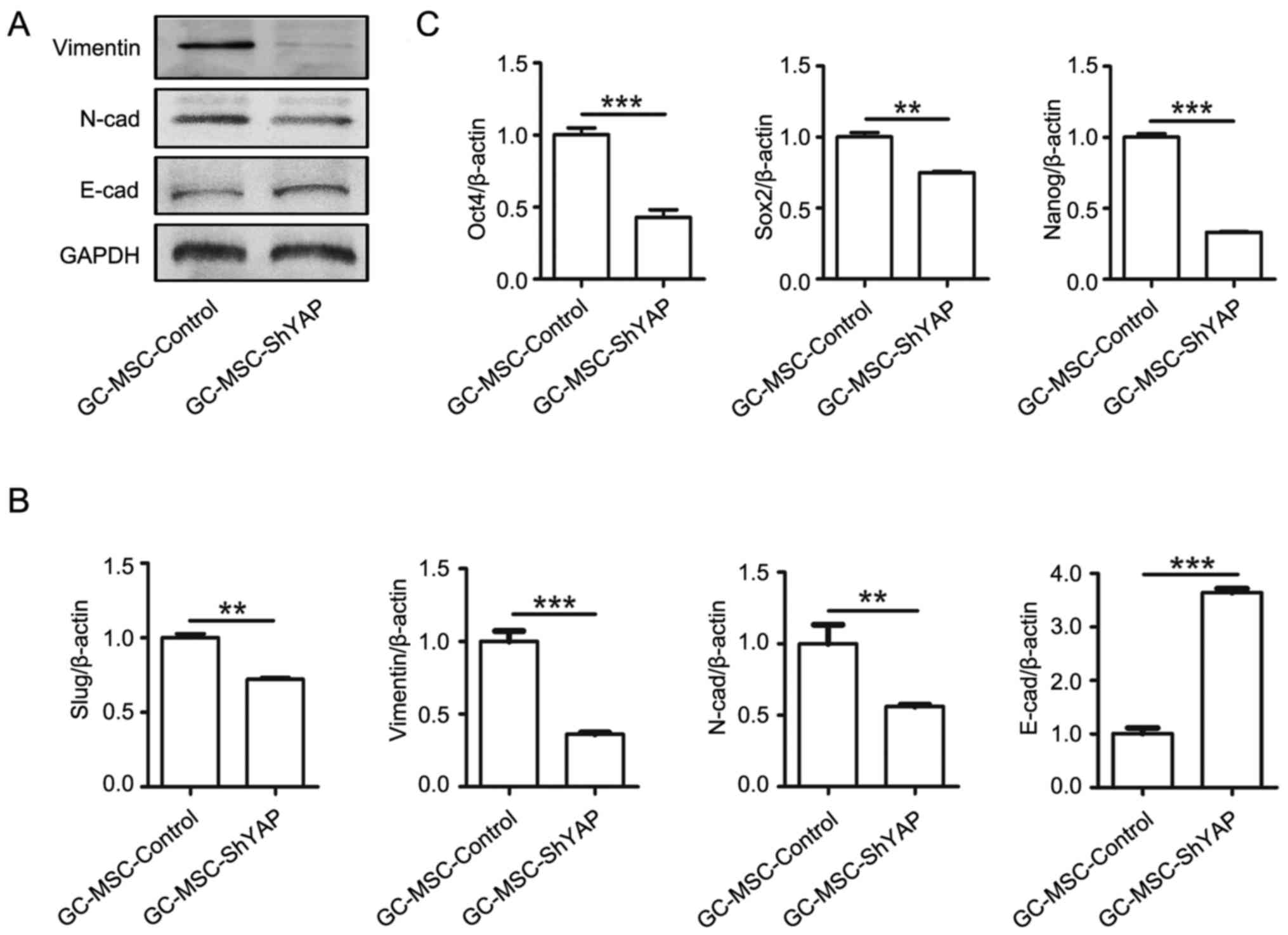
The knockdown of YAP suppresses EMT and
stemness of GC-MSCs
We further investigated whether YAP affects
mesenchymal-epithelial transition (MET) and stem cell properties of
GC-MSCs. As shown in Fig. 3, YAP
knockdown led to an increased expression of E-cadherin and a
decreased expression of N-cadherin, vimentin and slug in GC-MSCs
(Fig. 3A and B).
To explore whether YAP maintains stem cell
properties in GC-MSCs, we detected the expression of stem cell
markers including Sox-2, Oct-4, and Nanog. As shown in Fig. 3C, YAP knockdown led to the reduced
expression of Sox-2, Oct-4, and Nanog. Collectively, these results
suggest that YAP may regulate the stem cell properties of
GC-MSCs.
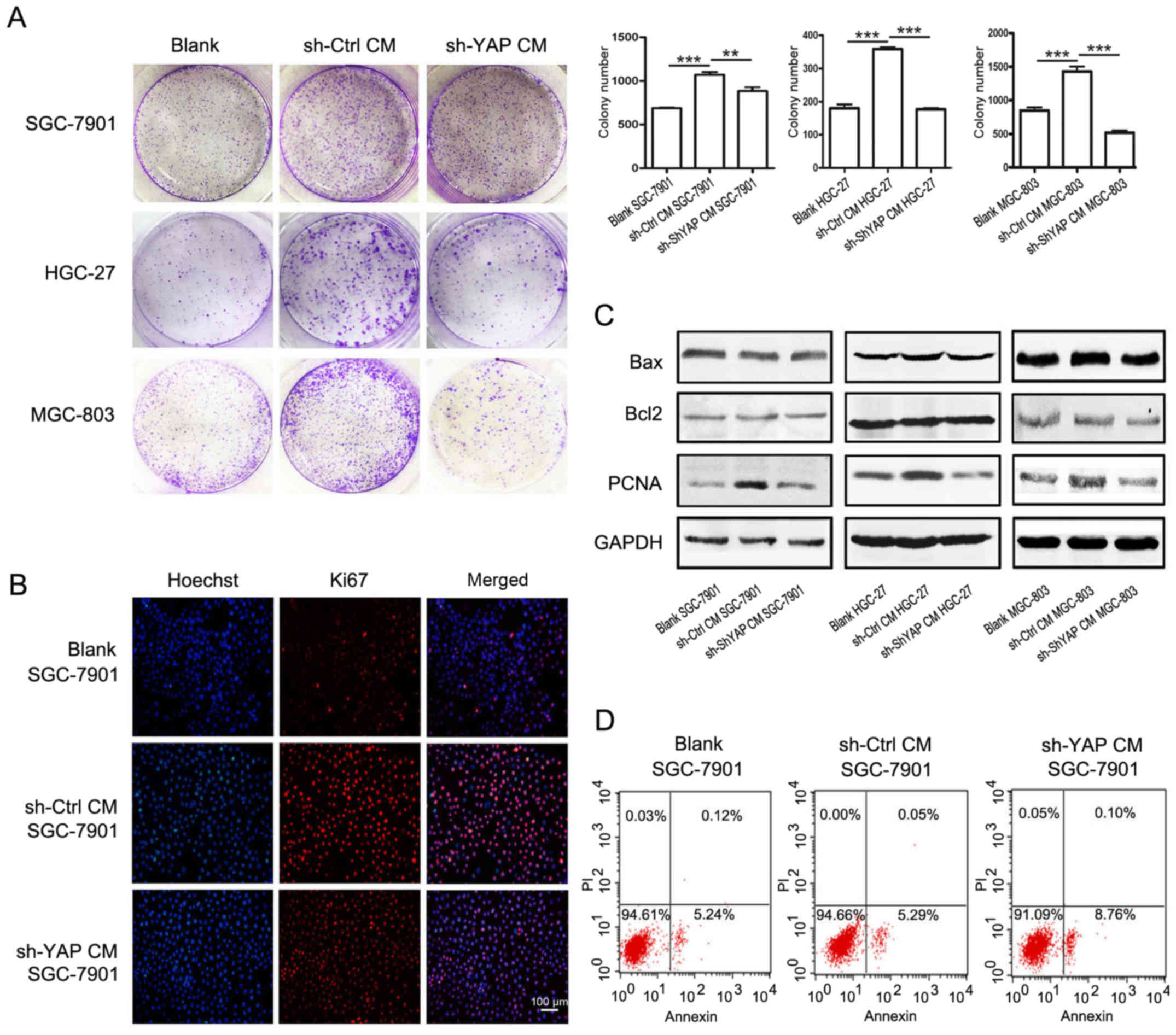
YAP knockdown in GC-MSCs reduced its
promoting effect on gastric cancer cell growth in vitro
We further investigated whether YAP knockdown could
affect the promoting role of GC-MSCs in the proliferation of
gastric cancer cells. The results of cell colony formation assay
showed that treatment with the conditioned media (CM) from control
GC-MSCs increased the number of colonies of SGC-7901, HGC-27 and
MGC-803 cells (Fig. 4A). However,
when cultured with CM from shYAP GC-MSCs, the number of cell
colonies was significantly decreased. The number of Ki67-positive
cells was increased in SGC-7901 cells treated with CM from control
GC-MSCs (sh-Ctrl CM group) but was decreased when treated with CM
from shYAP GC-MSCs (sh-YAP CM group) (Fig. 4B). The results of western blotting
showed that the expression of PCNA was increased in sh-Ctrl CM
group but was decreased in sh-YAP CM group (Fig. 4C). There was no significant change
in the expression of the anti-apoptotic protein Bcl-2 and the
pro-apoptotic protein Bax in gastric cancer cells when treated with
CM from either control GC-MSCs or shYAP GC-MSCs (Fig. 4C). In addition, YAP knockdown had
minimal effects on the apoptosis of SGC-7901 cells (Fig. 4D). In summary, YAP knockdown in
GC-MSCs reduced its promoting role in the proliferation of gastric
cancer cells.
YAP knockdown in GC-MSCs inhibits its
promoting role in the migration and invasion of gastric cancer
cells
We investigated the effects of YAP knockdown on the
promoting role of GC-MSCs in gastric cancer cell migration and
invasion. The gastric cancer cells in sh-YAP CM group exhibited
lower migratory and invasive capacities than that in sh-Ctrl CM
grou (Fig. 5A–D). We further
investigated whether YAP knockdown in GC-MSCs affected its inducing
effect on the EMT of gastric cancer cells. Sh-YAP CM group showed
an increased expression of E-cadherin and a reduced expression of
Slug, Vimentin and N-cadherin compared with sh-Ctrl CM group
(Fig. 5E and F). These results
indicate that YAP knockdown in GC-MSCs inhibited its promoting role
in the migration and invasion of gastric cancer cells.
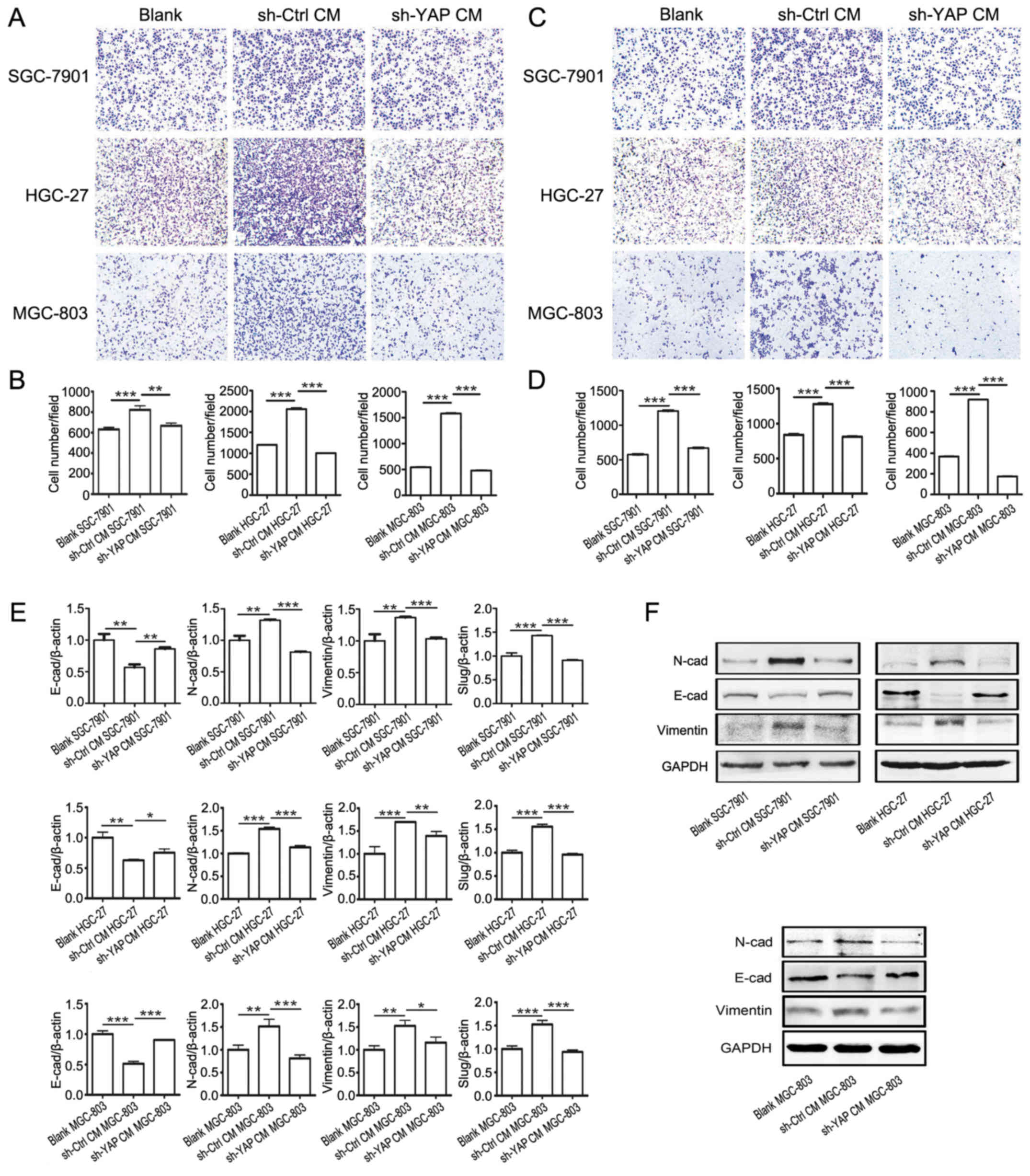 | Figure 5YAP knockdown in GC-MSCs inhibits its
promoting role in the migration and invasion of gastric cancer
cells. (A) Transwell migration assay for blank group, sh-Ctrl CM
group and sh-YAP CM group gastric cancer cells. (B) Cells that
migrated to the bottom were counted. ***P<0.001,
**P<0.01. (C) Matrigel invasion assay for blank
group, sh-Ctrl CM group and sh-YAP CM group gastric cancer cells.
(D) Cells that invaded to the bottom were counted.
***P<0.001. (E) RT-PCR analysis of Slug, Vimentin,
N-cadherin, E-cadherin and Slug expression in blank group, sh-Ctrl
CM group and sh-YAP CM group gastric cancer cells.
***P<0.001, **P<0.01,
*P<0.05. (F) Western blotting for protein levels of
N-cadherin, E-cadherin and Vimentin in blank group, sh-Ctrl CM
group and sh-YAP CM group gastric cancer cells. |
YAP knockdown in GC-MSCs inhibits its
promoting role in the pro-angiogenic ability of gastric cancer
cells
Angiogenesis is considered as a critical step for
cancer development and progression. MSCs can favor the formation of
tumor blood vessels and thus promote tumor growth and metastasis
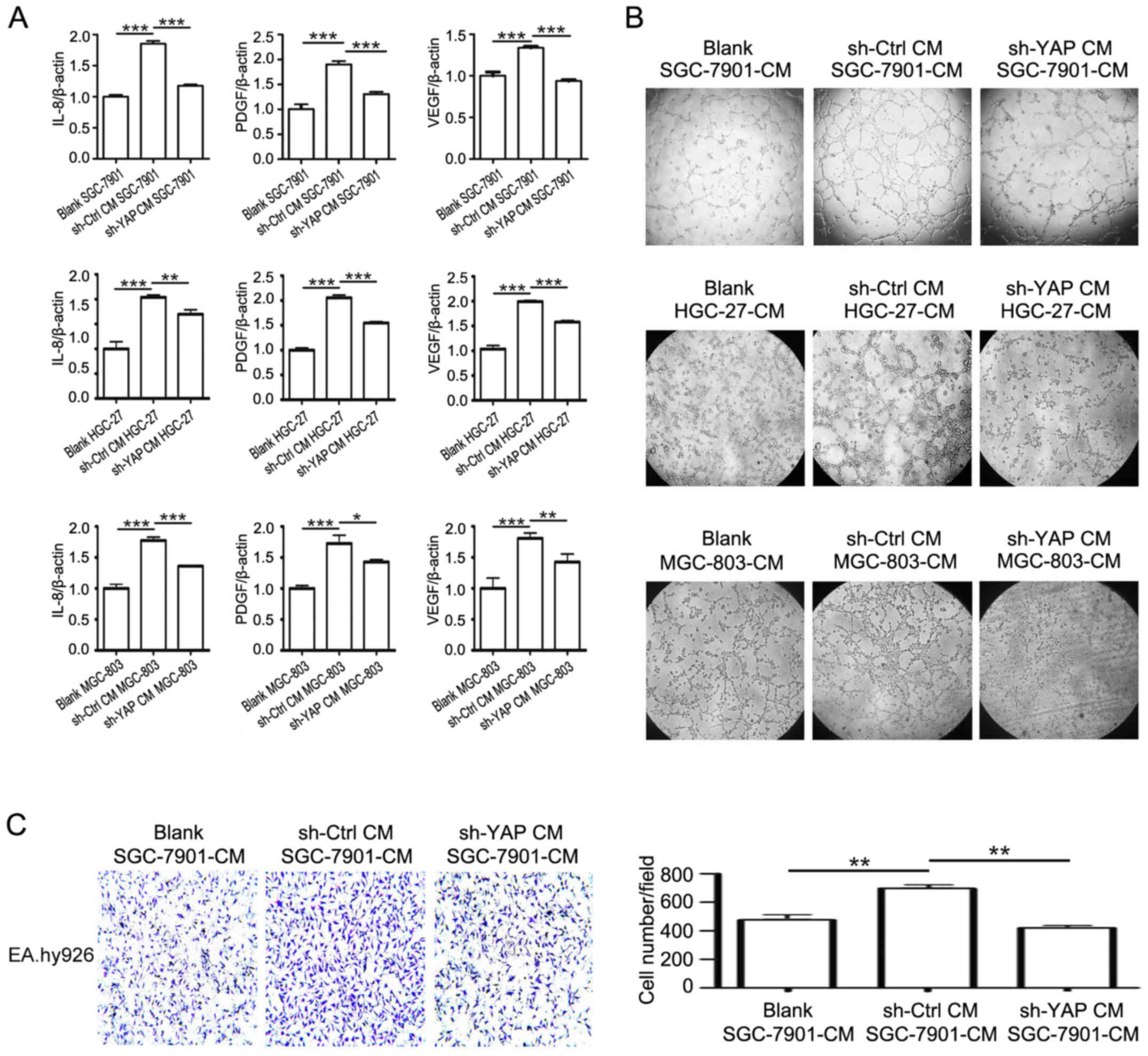
(26). As shown in Fig. 6, the incubation with CM from shYAP
GC-MSCs dramatically decreased the expression of VEGF, PDGF, and
IL-8 in gastric cancer cells compared to incubation with CM from
control GC-MSCs (Fig. 6A). The
results of tube formation assay demonstrated that the supernatant
of sh-YAP CM group had reduced ability to promote endothelial cell
tube formation than that of sh-Ctrl CM group (Fig. 6B). Endothelial cell migration is
critical for angiogenesis. The results of Transwell migration assay
showed that the CM from sh-YAP CM group had decreased ability to
promote the migration of endothelial cells compared to the CM from
sh-Ctrl CM group (Fig. 6C). Taken
together, these results reveal that YAP knockdown in GC-MSCs
inhibits its promoting role in the pro-angiogenic ability of
gastric cancer cells.
YAP knockdown in GC-MSCs reduced its
promoting effect on the activation of β-catenin in gastric cancer
cells
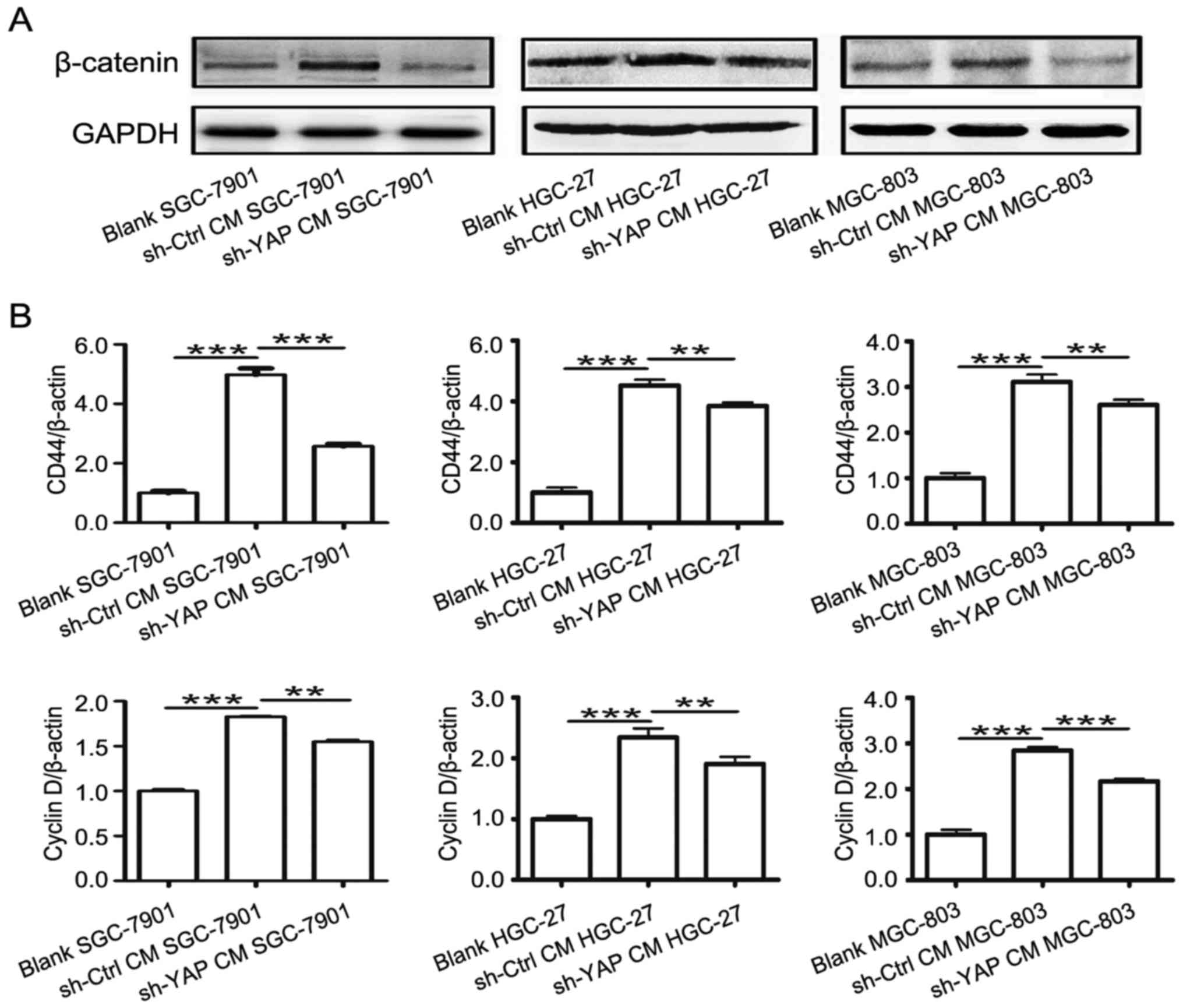
β-catenin is an important pathway in gastric
carcinogenesis (27–31). MSCs enhanced the activation of
β-catenin signaling in cancer. We then examined the role of YAP in
MSC-mediated regulation of β-catenin signaling in gastric cancer
cells. As shown in Fig. 7A, the
expression of β-catenin was lower in sh-YAP CM group compared to
that in sh-Ctrl CM group. Moreover, the expression of β-catenin
downstream genes including cyclin D and CD44 was lower in sh-YAP CM
group than that in sh-Ctrl CM group (Fig. 7B). Collectively, these results
suggest that YAP knockdown in GC-MSCs reversed their activating
role in β-catenin signaling in gastric cancer cells.
YAP knockdown in GC-MSCs inhibits its
promoting role in gastric cancer growth in vivo
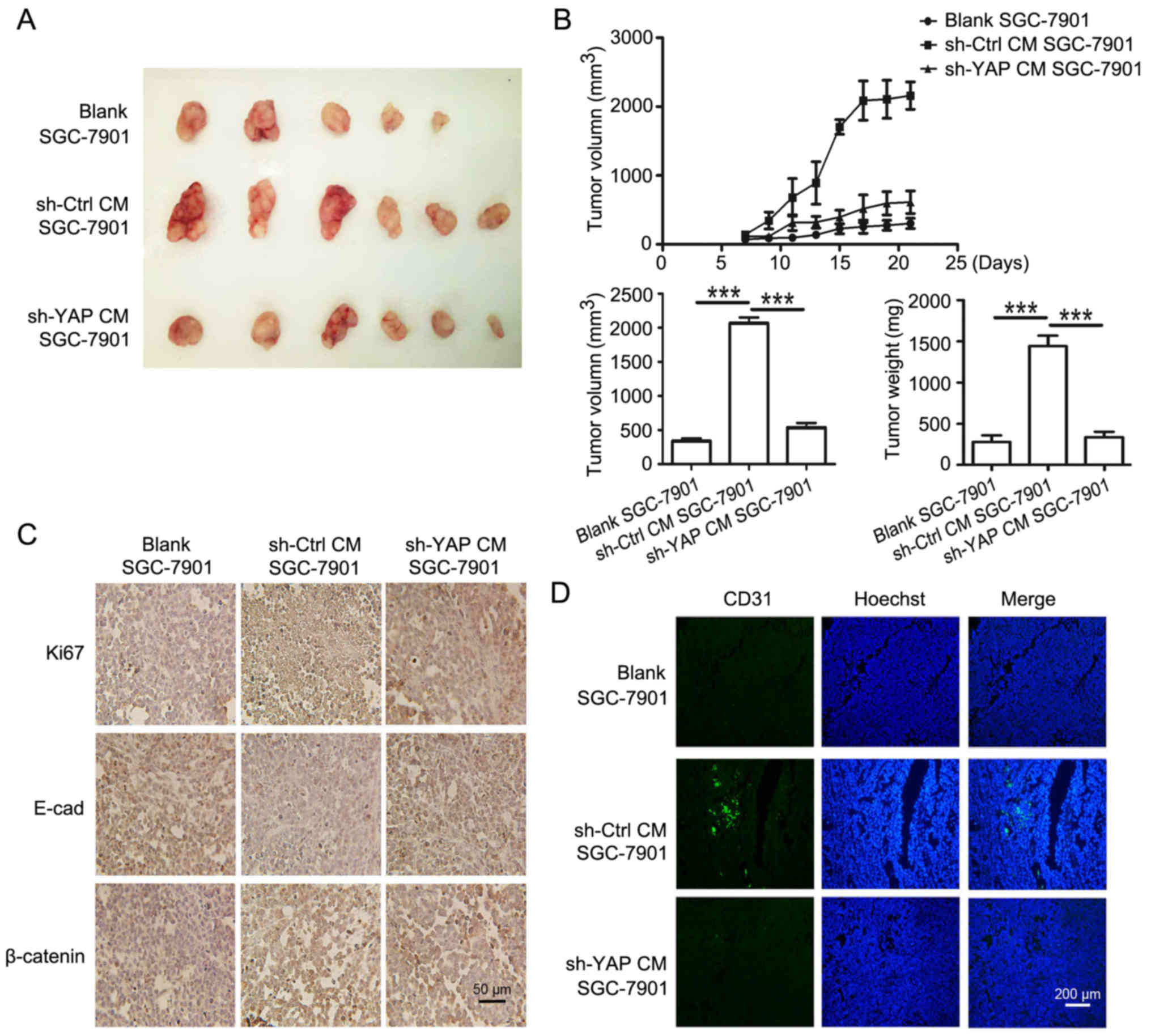
To confirm the in vitro results, SGC-7901
cells treated with CM from control and shYAP GC-MSCs were used to
establish mouse xenograft tumor models. The images of xenograft
tumors are shown in Fig. 8A. The
tumors in sh-YAP CM group grew slower and were smaller, and the
average tumor weight was lower than that in sh-Ctrl CM group
(Fig. 8B). The expression of Ki67
and β-catenin in tumor tissues was determined by using
immunohistochemistry. We found that the percentage of Ki67-positive
cells was 24.0% in sh-YAP CM group and was 92.1% in sh-Ctrl CM
group. The expression of β-catenin in the nucleus was stronger in
sh-Ctrl CM group than that in sh-YAP CM group (Fig. 8C). The decreased expression of
E-cadherin observed in sh-Ctrl CM group were reversed in sh-YAP CM
group (Fig. 8C). Moreover, CD31
expression in sh-YAP CM group was significantly lower than that in
sh-Ctrl CM group (Fig. 8D). Taken
together, these results suggest that YAP knockdown in GC-MSCs
reversed its promoting role in gastric cancer growth in
vivo.
Discussion
Over the past decades, the relation between MSCs and
tumor has attracted increasing attention (8,25,32).
Although the previous studies have shown an important role of MSCs
in cancer (33–35), the detailed mechanisms responsible
for the regulation of tumor-resident MSCs are not clear. The
Hippo/YAP pathway has recently been reported to play important
roles in human cancers (36–38).
As a critical component of Hippo pathways, YAP could exert
oncogenic activities with its paralog transcriptional co-activator
with a PDZ-binding motif (TAZ) (39). The expression of YAP in gastric
cancer tissues is closely associated with poorer overall survival
of patients. RUNX3 is reported to function as a tumor suppressor by
downregulating YAP in the progression of cancer (40). Moreover, VGLL4 could inhibit the
expression of YAP, and a peptide is found to act as a YAP
antagonist therapy against gastric cancer by mimicking VGLL4
function (41).
The interaction between MSCs and tumor cells is
critical for tumor progression (21,42).
GC-MSCs enhanced the proliferation and migration of gastric cancer
cells as well as facilitate tumor angiogenesis (10). In this study, we identified that
YAP signaling was critical for the promoting roles of GC-MSCs in
gastric cancer progression. We reported that YAP knockdown led to
the inhibition of the growth, migration, and invasion of GC-MSCs,
indicating that YAP plays an important role in the phenotype and
function of GC-MSCs.
The oncogenic roles of YAP in cancer has recently
been a research focus (43–45).
In this study, we reported that gastric cancer cells grow slower
when incubated with CM from YAP knockdown GC-MSCs. However, YAP
knockdown in GC-MSCs have no effects on the apoptosis of gastric
cancer cells. It was reported that YAP could promote the growth of
cholangiocarcinoma by interacting with TEAD transcription factors
(49). Sun and colleagues found
that YAP could enhance the proliferation, migration, and invasion
of gastric cancer cells in vitro and in vivo
(16). The decreased YAP signaling
inhibited tumor growth and metastasis by reducing the expression of
PCNA, MMP-2, MMP-9, and cyclin D1 (45). In the present study, we found that
YAP knockdown in GC-MSCs abrogated its promoting roles in gastric
cancer cell proliferation, migration, and invasion, indicating an
important role of YAP signaling in the tumor-promoting effect of
GC-MSCs in gastric cancer. Moreover, YAP could also promote
angiogenesis in human cancer (46). We observed that endothelial cells
exposed to the supernatant from sh-YAP CM-treated gastric cancer
cells showed decreased tube formation and migration abilities,
which may be associated with the decreased expression of
pro-angiogenic factors including VEGF, PDGF, and IL-8 in gastric
cancer cells. These findings suggest a potent role of YAP in
GC-MSCs in regulating tumor angiogenesis. Metastasis is associated
with increased cell migration and invasion. The β-catenin pathway
is reported to affect the migration and invasion of cancer cells
(47). In our study, YAP knockdown
in GC-MSCs inhibited its promoting role in the activation of
β-catenin and the migration and invasion of gastric cancer cells.
Thus, YAP signaling in GC-MSCs may promote gastric cancer
metastasis through an indirect activation of β-catenin pathway in
gastric cancer cells.
The β-catenin pathway contributes to cancer
progression by regulating the proliferation, invasion, and
metastasis of cancer cells (47–50).
Our results revealed that the increased expression of β-catenin in
sh-Ctrl CM group was abrogated in the sh-YAP CM group. In addition,
the expression of β-catenin downstream genes CD44 and cyclin D1 was
also decreased in sh-YAP CM group compared to sh-Ctrl CM group.
These findings suggest that YAP signaling modulates GC-MSC-mediated
activation of β-catenin in gastric cancer cells. We have recently
reported that YAP critically regulates the activity of β-catenin
(51). YAP knockdown may affect
the components of CM from GC-MSCs, which thus abrogates the
activation of β-catenin signaling in tumor cells. However, the
exact factors responsible for this role need to be identified in
future studies.
In conclusion, we demonstrated that YAP knockdown in
GC-MSCs not only inhibits their proliferation, migration and
invasion, but also suppresses their promoting roles in the
proliferation, migration, invasion and pro-angiogenesis of gastric
cancer cells in vitro and in vivo. Disturbing the
expression of YAP in GC-MSCs inhibits its derived CM-induced
activation of β-catenin in gastric cancer cells. In conclusion, YAP
expression in GC-MSCs plays an important role in promoting gastric
cancer progression, which may provide a novel avenue for gastric
cancer therapy.
Acknowledgments
This study was supported by the Major Research Plan
of the National Natural Science Foundation of China (grant no.
91129718), the National Natural Science Foundation of China (grant
nos. 81572075, 81672416 and 816702883), the Project of Major
Research and Development, Jiangsu Province (grant no. BE2015667),
the Doctoral Program Foundation of China (grant nos. 2016M591791
and 2016M591792), the Doctoral Program Foundation, Jiangsu Province
(grant no. 1501067C), Jiangsu Province for Outstanding Sci-Tech
Innovation Team in Colleges and Universities (grant no.
SJK2013-10), and Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions.
References
|
1
|
Tan YK and Fielding JW: Early diagnosis of
early gastric cancer. Eur J Gastroenterol Hepatol. 18:821–829.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pornsuksiri K, Chewatanakornkul S,
Kanngurn S, Maneechay W, Chaiyapan W and Sangkhathat S: Clinical
outcomes of gastrointestinal stromal tumor in southern Thailand.
World. J Gastrointest Oncol. 4:216–222. 2012. View Article : Google Scholar
|
|
5
|
Yu B and Xie J: Identifying therapeutic
targets in gastric cancer: The current status and future direction.
Acta Biochim Biophys Sin (Shanghai). 48:90–96. 2016.
|
|
6
|
Kasashima H, Yashiro M, Nakamae H,
Kitayama K, Masuda G, Kinoshita H, Fukuoka T, Hasegawa T, Nakane T,
Hino M, et al: CXCL1-chemokine (C-X-C motif) receptor 2 signaling
stimulates the recruitment of bone marrow-derived mesenchymal cells
into diffuse-type gastric cancer stroma. Am J Pathol.
186:3028–3039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang F, Wang M, Yang T, Cai J, Zhang Q,
Sun Z, Wu X, Zhang X, Zhu W, Qian H, et al: Gastric cancer-derived
MSC-secreted PDGF-DD promotes gastric cancer progression. J Cancer
Res Clin Oncol. 140:1835–1848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Zhou Y, Yang J, Zhang X, Zhang H,
Zhang T, Zhao S, Zheng P, Huo J and Wu H: Gastric cancer-derived
mesenchymal stem cells prompt gastric cancer progression through
secretion of interleukin-8. J Exp Clin Cancer Res. 34:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hua G, Lv X, He C, Remmenga SW, Rodabough
KJ, Dong J, Yang L, Lele SM, Yang P, Zhou J, et al: YAP induces
high-grade serous carcinoma in fallopian tube secretory epithelial
cells. Oncogene. 35:2247–2265. 2016. View Article : Google Scholar :
|
|
15
|
Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z
and Chen WX: Expression of Yes-associated protein in gastric
adenocarcinoma and inhibitory effects of its knockdown on gastric
cancer cell proliferation and metastasis. Int J Immunopathol
Pharmacol. 25:583–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun D, Li X, He Y, Li W, Wang Y, Wang H,
Jiang S and Xin Y: YAP1 enhances cell proliferation, migration, and
invasion of gastric cancer in vitro and in vivo. Oncotarget.
7:81062–81076. 2016.PubMed/NCBI
|
|
17
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G, et al: Mechanotransduction and YAP-dependent matrix
remodelling is required for the generation and maintenance of
cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Ji JY, Yu M, Overholtzer M,
Smolen GA, Wang R, Brugge JS, Dyson NJ and Haber DA: YAP-dependent
induction of amphiregulin identifies a non-cell-autonomous
component of the Hippo pathway. Nat Cell Biol. 11:1444–1450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii M, Toyoda T, Nakanishi H, Yatabe Y,
Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E, et al:
TGF-β synergizes with defects in the Hippo pathway to stimulate
human malignant mesothelioma growth. J Exp Med. 209:479–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
21
|
Zhang T, Lee YW, Rui YF, Cheng TY, Jiang
XH and Li G: Bone marrow-derived mesenchymal stem cells promote
growth and angiogenesis of breast and prostate tumors. Stem Cell
Res Ther. 4:702013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X, Xu X, Li J, Chen Z, et al: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar
|
|
23
|
Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao
H, Wang M, Chen Y and Xu W: Isolation and comparison of mesenchymal
stem-like cells from human gastric cancer and adjacent noncancerous
tissues. J Cancer Res Clin Oncol. 137:495–504. 2011. View Article : Google Scholar
|
|
24
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barcellos-de-Souza P, Gori V, Bambi F and
Chiarugi P: Tumor microenvironment: Bone marrow-mesenchymal stem
cells as key players. Biochim Biophys Acta. 1836:321–335.
2013.PubMed/NCBI
|
|
26
|
Li GC, Zhang HW, Zhao QC, Sun LI, Yang JJ,
Hong L, Feng F and Cai L: Mesenchymal stem cells promote tumor
angiogenesis via the action of transforming growth factor β1. Oncol
Lett. 11:1089–1094. 2016.PubMed/NCBI
|
|
27
|
Su YJ, Lin WH, Chang YW, Wei KC, Liang CL,
Chen SC and Lee JL: Polarized cell migration induces cancer
type-specific CD133/integrin/Src/Akt/GSK3β/β-catenin signaling
required for maintenance of cancer stem cell properties.
Oncotarget. 6:38029–38045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MJ, Wu DW, Wang YC, Chen CY and Lee
H: PAK1 confers chemoresistance and poor outcome in non-small cell
lung cancer via β-catenin-mediated stemness. Sci Rep. 6:349332016.
View Article : Google Scholar
|
|
29
|
Lettini G, Sisinni L, Condelli V, Matassa
DS, Simeon V, Maddalena F, Gemei M, Lopes E, Vita G, Del Vecchio L,
et al: TRAP1 regulates stemness through Wnt/β-catenin pathway in
human colorectal carcinoma. Cell Death Differ. 23:1792–1803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu D, Du L, Chen D, Ye Z, Duan H, Tu T,
Feng J, Yang Y, Chen Q and Yan X: Reduced CD146 expression promotes
tumorigenesis and cancer stemness in colorectal cancer through
activating Wnt/β-catenin signaling. Oncotarget. 7:40704–40718.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Xu J, Jiang T, Liu G, Wang D and
Lu Y: MicroRNA-195 suppresses colorectal cancer cells proliferation
via targeting FGF2 and regulating Wnt/β-catenin pathway. Am J
Cancer Res. 6:2631–2640. 2016.
|
|
32
|
Gabrielyan A, Knaak S, Gelinsky M, Arnhold
S and Rösen-Wolff A: Hypoxia-conditioned media allows
species-specific attraction of bone marrow stromal cells without
need for recombinant proteins. BMC Vet Res. 10:562014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bergfeld SA, Blavier L and DeClerck YA:
Bone marrow-derived mesenchymal stromal cells promote survival and
drug resistance in tumor cells. Mol Cancer Ther. 13:962–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han I, Yun M, Kim EO, Kim B, Jung MH and
Kim SH: Umbilical cord tissue-derived mesenchymal stem cells induce
apoptosis in PC-3 prostate cancer cells through activation of JNK
and down-regulation of I3K/AKT signaling. Stem Cell Res Ther.
5:542014. View Article : Google Scholar
|
|
35
|
Zhu M, Wang M, Yang F, Tian Y, Cai J, Yang
H, Fu H, Mao F, Zhu W, Qian H, et al: miR-155-5p inhibition
promotes the transition of bone marrow mesenchymal stem cells to
gastric cancer tissue derived MSC-like cells via NF-κB p65
activation. Oncotarget. 7:16567–16580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Azzolin L, Panciera T, Soligo S, Enzo E,
Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V,
et al: YAP/TAZ incorporation in the β-catenin destruction complex
orchestrates the Wnt response. Cell. 158:157–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y,
Yu T, Gong L, Li S, Xiao B and Zou QM: MicroRNA-141 inhibits tumor
growth and metastasis in gastric cancer by directly targeting
transcriptional co-activator with PDZ-binding motif, TAZ. Cell
Death Dis. 6:e16232015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qiao Y, Lin SJ, Chen Y, Voon DC, Zhu F,
Chuang LS, Wang T, Tan P, Lee SC, Yeoh KG, et al: RUNX3 is a novel
negative regulator of oncogenic TEAD-YAP complex in gastric cancer.
Oncogene. 35:2664–2674. 2016. View Article : Google Scholar
|
|
41
|
Jiao S, Wang H, Shi Z, Dong A, Zhang W,
Song X, He F, Wang Y, Zhang Z, Wang W, et al: A peptide mimicking
VGLL4 function acts as a YAP antagonist therapy against gastric
cancer. Cancer Cell. 25:166–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu C, Liu Y, Xu XX, Guo X, Sun GW and Ma
XJ: Mesenchymal stem cells enhance the metastasis of 3D-cultured
hepatocellular carcinoma cells. BMC Cancer. 16:5662016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ehmer U and Sage J: Control of
proliferation and cancer growth by the Hippo signaling pathway. Mol
Cancer Res. 14:127–140. 2016. View Article : Google Scholar :
|
|
44
|
Yagi H, Asanoma K, Ohgami T, Ichinoe A,
Sonoda K and Kato K: GEP oncogene promotes cell proliferation
through YAP activation in ovarian cancer. Oncogene. 35:4471–4480.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Wang G, Chu SJ, Zhu JS, Zhang R,
Lu WW, Xia LQ, Lu YM, Da W and Sun Q: Loss of large tumor
suppressor 1 promotes growth and metastasis of gastric cancer cells
through upregulation of the YAP signaling. Oncotarget.
7:16180–16193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhuang K, Yan Y, Zhang X, Zhang J, Zhang L
and Han K: Gastrin promotes the metastasis of gastric carcinoma
through the β-catenin/TCF-4 pathway. Oncol Rep. 36:1369–1376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo F, Ren X, Dong Y, Hu X, Xu D, Zhou H,
Meng F, Tian W and Zhao Y: Constitutive expression of PPARγ
inhibits proliferation and migration of gastric cancer cells and
down-regulates Wnt/β-catenin signaling pathway downstream target
genes TERT and ENAH. Gene. 584:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan D, Ren B, Yang X, Liu J and Zhang Z:
Upregulation of miR-501-5p activates the wnt/β-catenin signaling
pathway and enhances stem cell-like phenotype in gastric cancer. J
Exp Clin Cancer Res. 35:1772016. View Article : Google Scholar
|
|
49
|
Marti P, Stein C, Blumer T, Abraham Y,
Dill MT, Pikiolek M, Orsini V, Jurisic G, Megel P, Makowska Z, et
al: YAP promotes proliferation, chemoresistance, and angiogenesis
in human cholangiocarcinoma through TEAD transcription factors.
Hepatology. 62:1497–1510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao
Y, Cheng Y, Yang M, Wang Q, Feng X, et al: MiRNA-194 activates the
Wnt/β-catenin signaling pathway in gastric cancer by targeting the
negative Wnt regulator, SUFU. Cancer Lett. 385:117–127. 2017.
View Article : Google Scholar
|
|
51
|
Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang
H, Fu H, Yan Y, Zhang X, Wang M, et al: HucMSC exosome-delivered
14-3-3ζ orchestrates self-control of the Wnt response via
modulation of YAP during cutaneous regeneration. Stem Cells.
34:2485–2500. 2016. View Article : Google Scholar : PubMed/NCBI
|