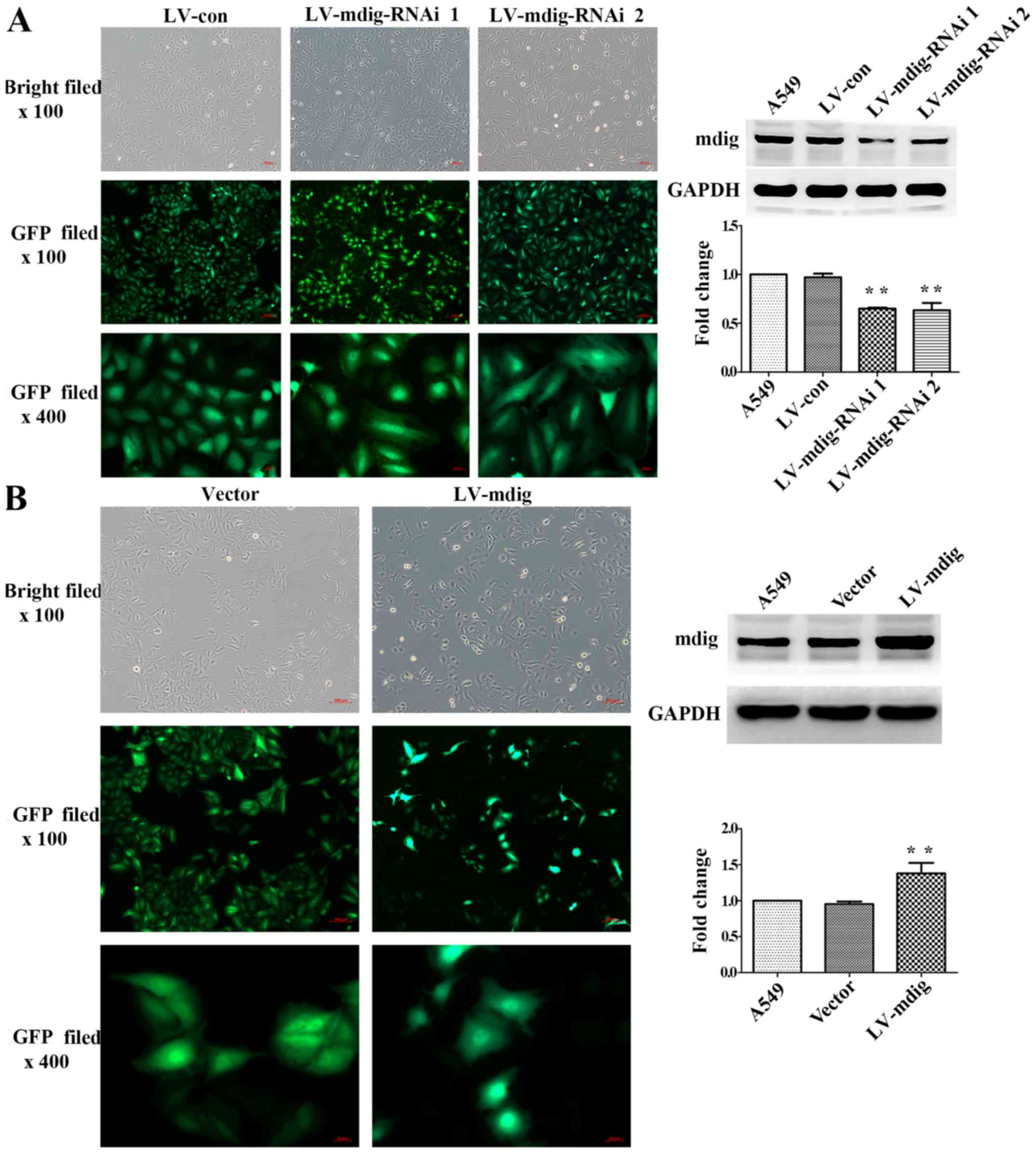
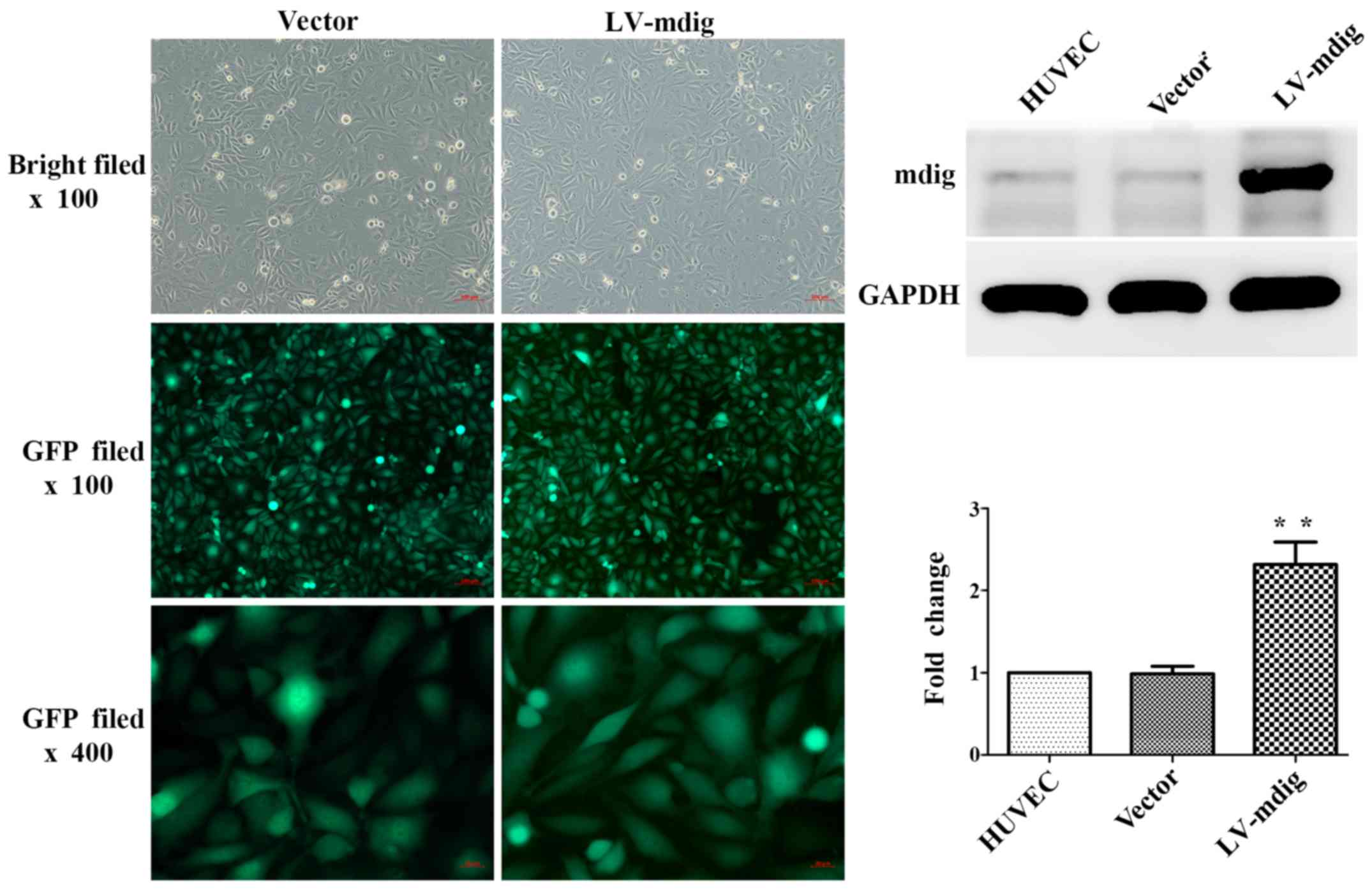
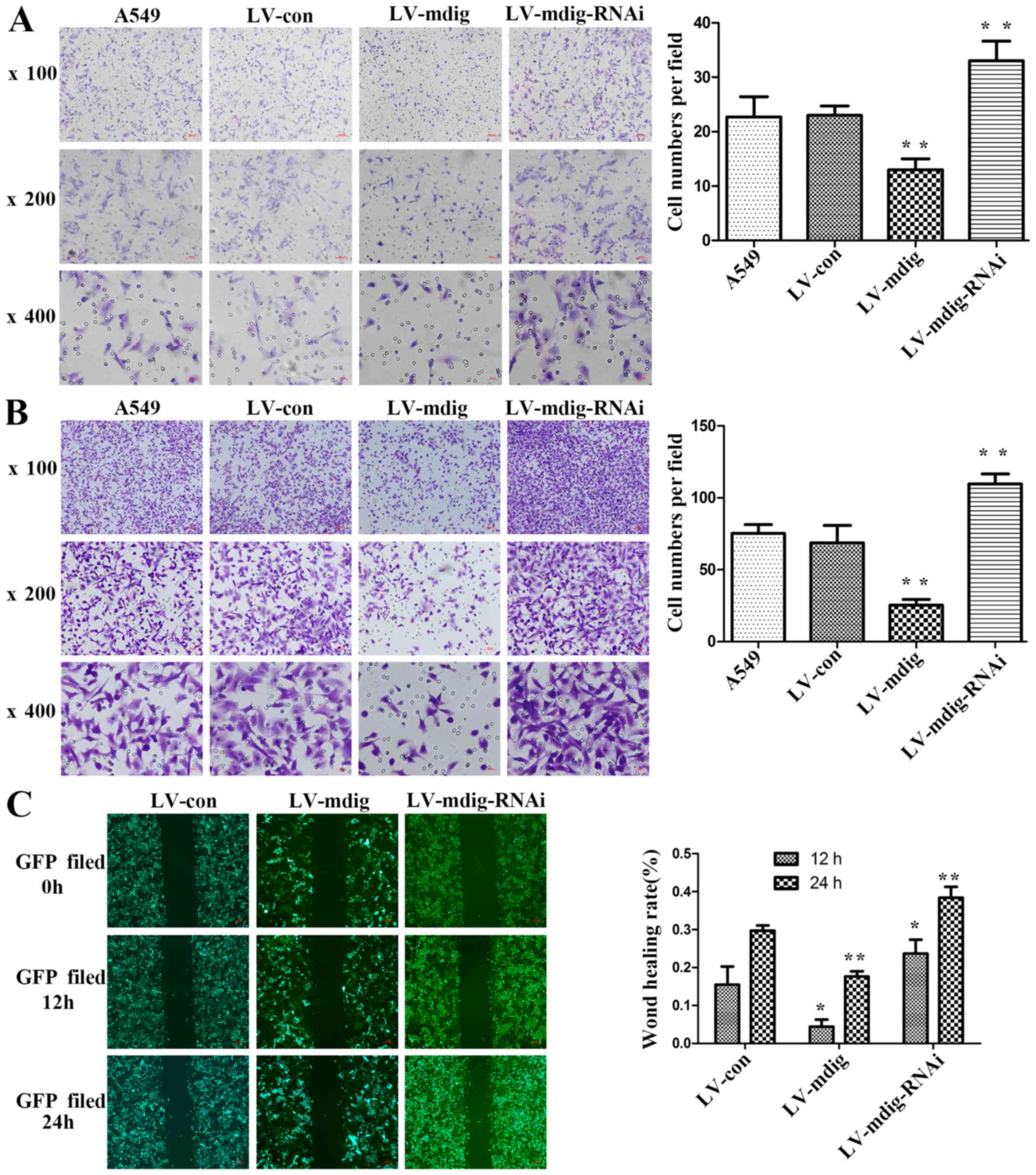
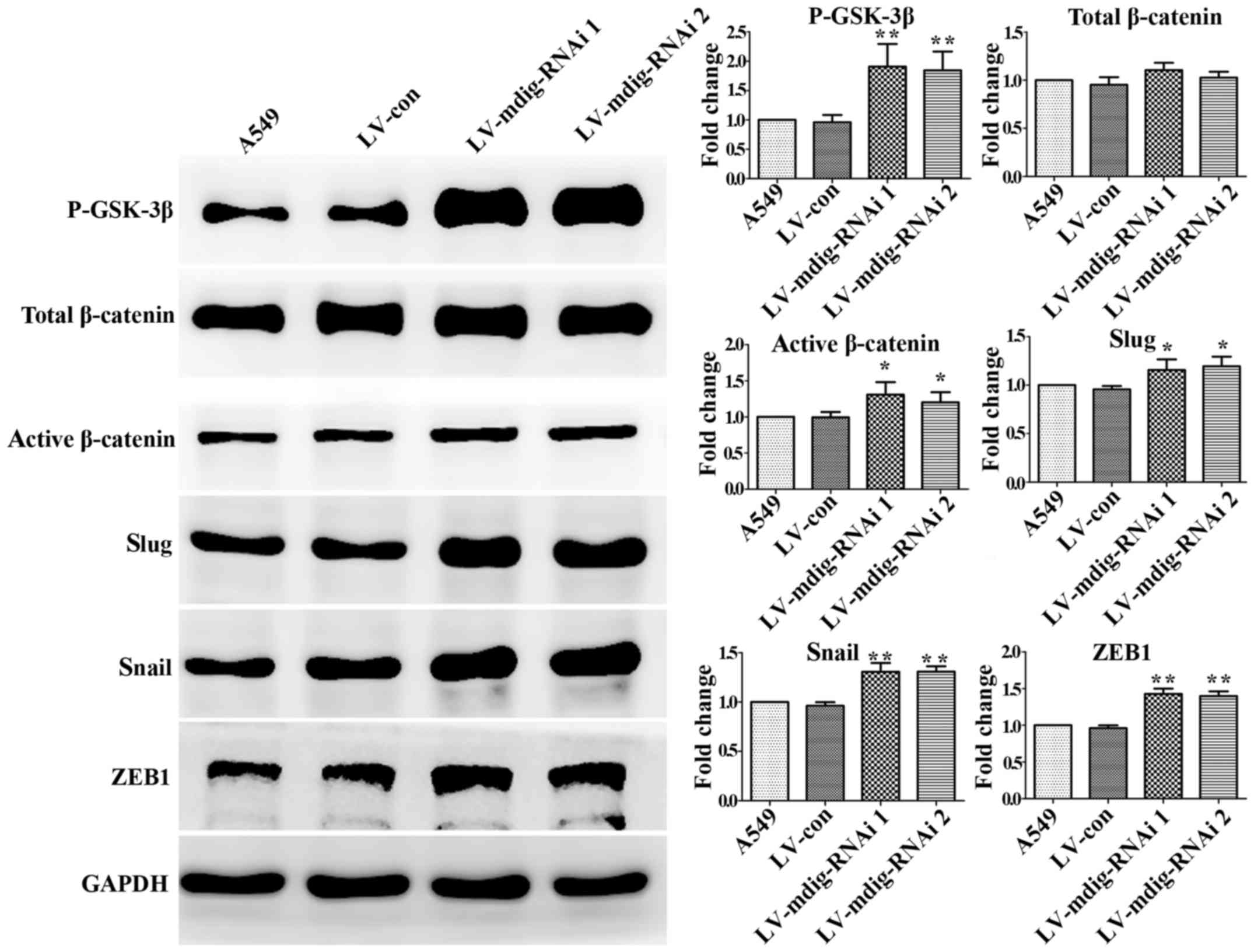
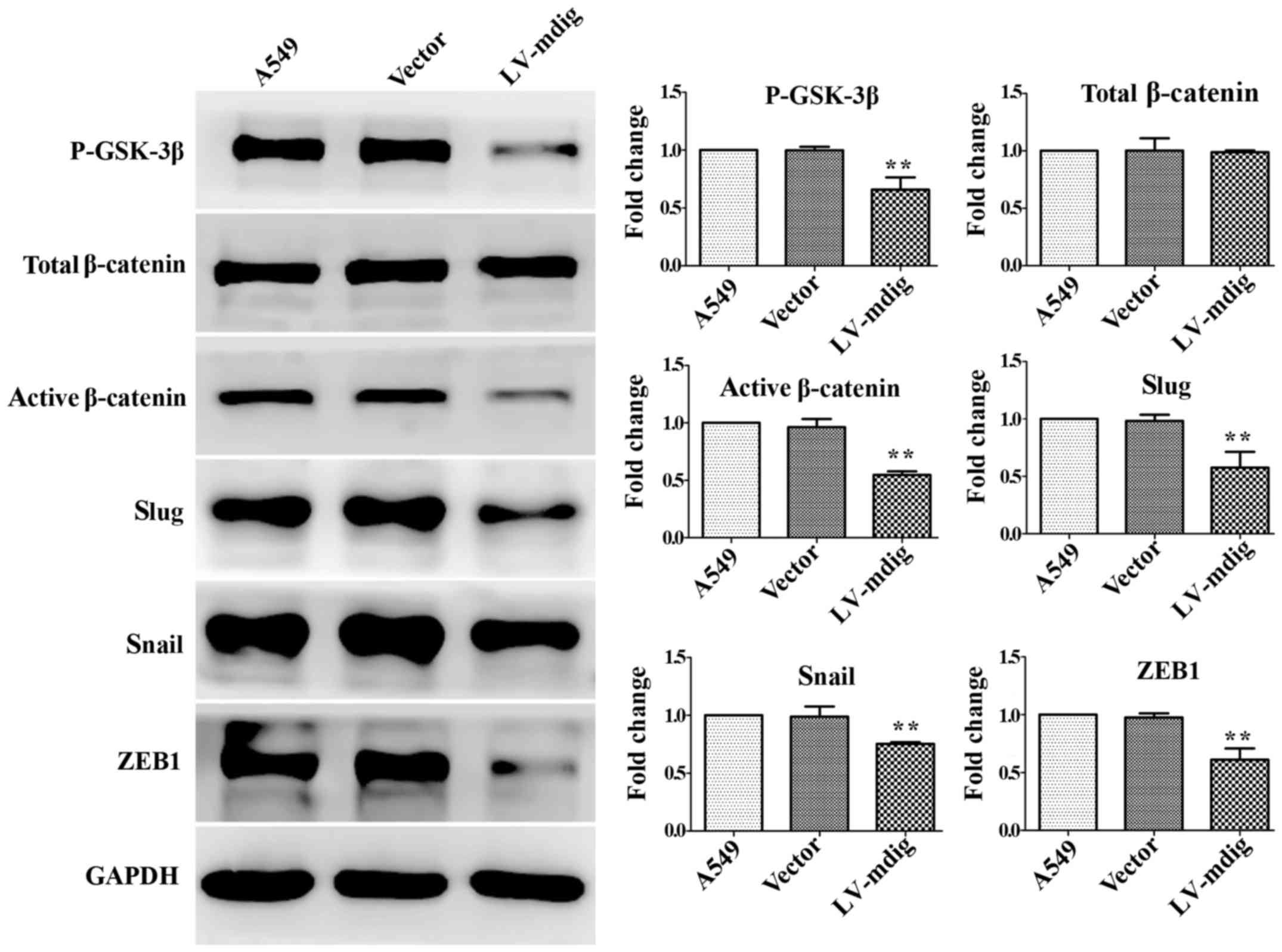
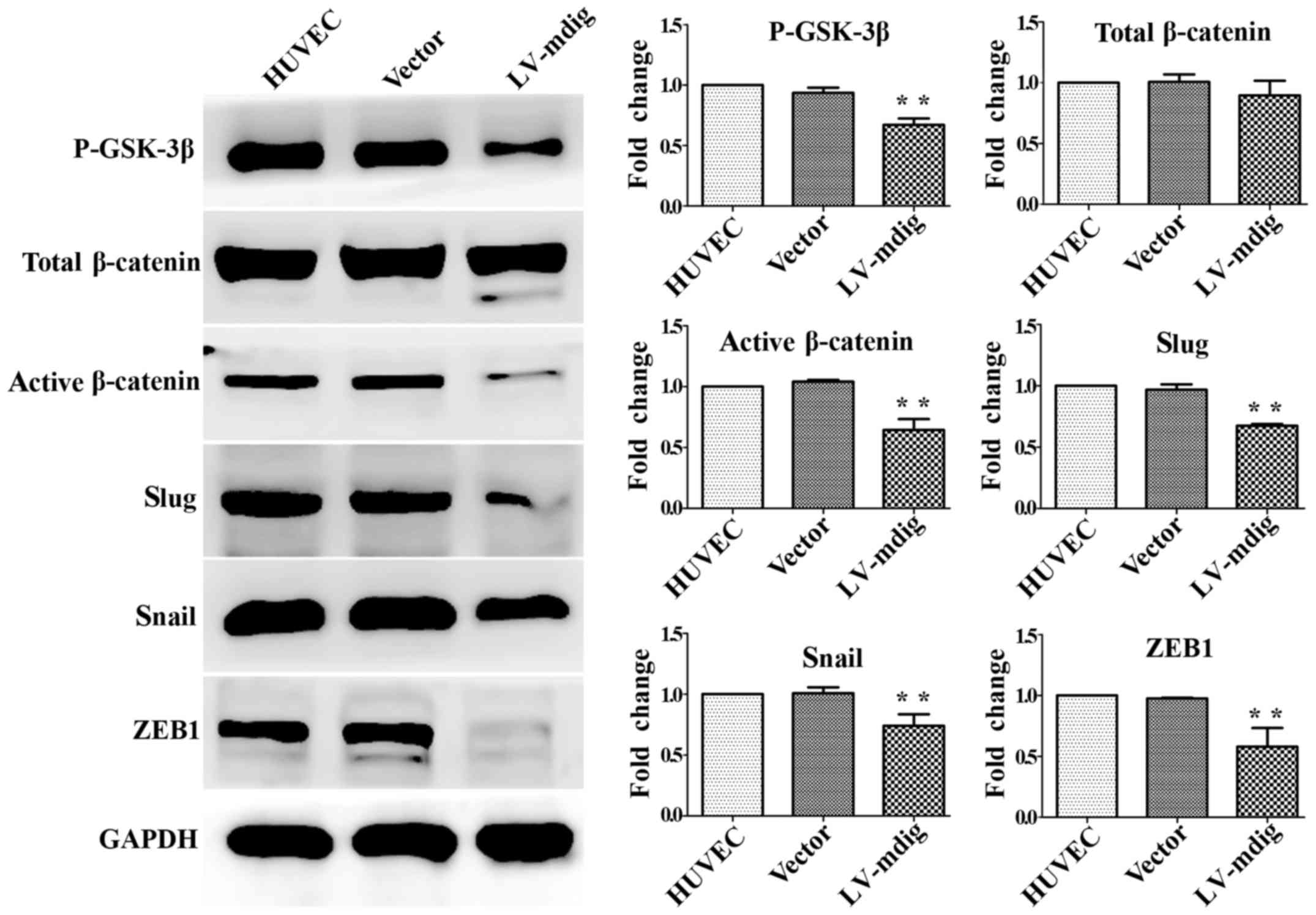
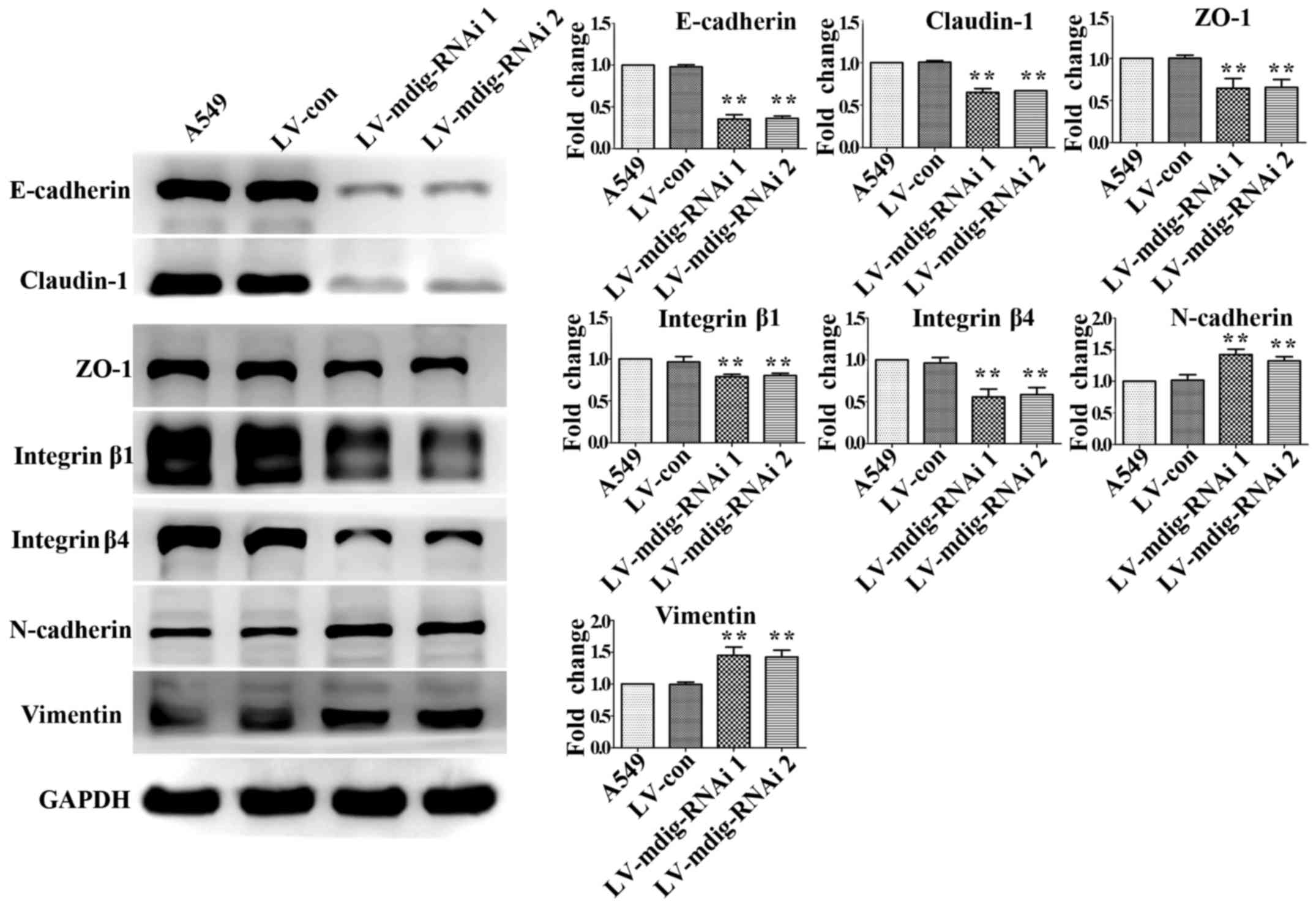
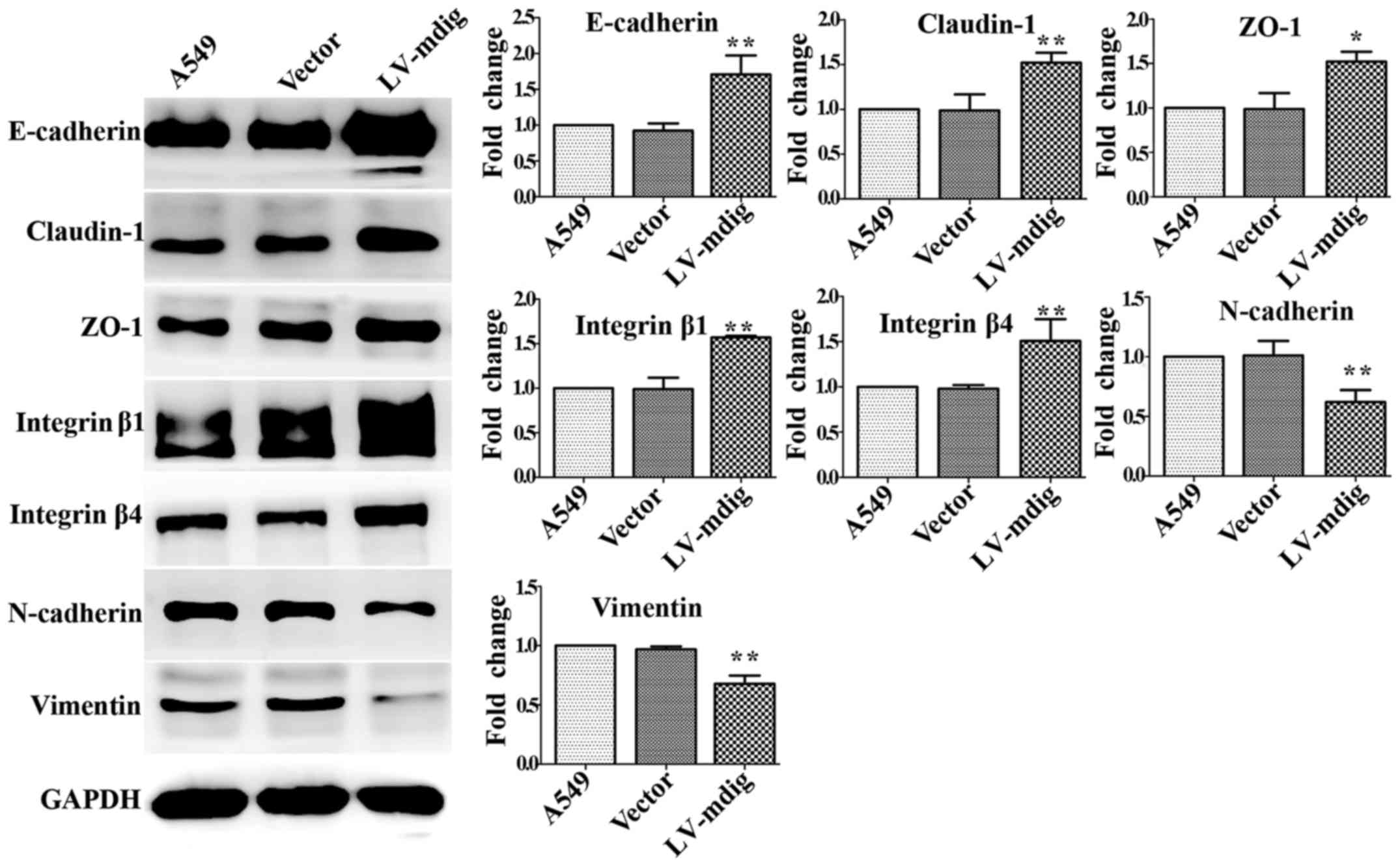
|
1
|
Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi
X, Stauffer JL, Demers LM and Chen F: The Human mineral
dust-induced gene, mdig, is a cell growth regulating gene
associated with lung cancer. Oncogene. 24:4873–4882. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu
P, Zhao H and Chen F: Carcinogenic metalloid arsenic induces
expression of mdig oncogene through JNK and STAT3 activation.
Cancer Lett. 346:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu K, Li L, Thakur C, Lu Y, Zhang X, Yi Z
and Chen F: Proteomic characterization of the World Trade Center
dust-activated mdig and c-myc signaling circuit linked to multiple
myeloma. Sci Rep. 6:363052016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma D, Guo D, Li W and Zhao H: Mdig, a lung
cancer-associated gene, regulates cell cycle progression through
p27(KIP1). Tumour Biol. 36:6909–6917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan XP, Dong WG, Zhang Q, Yang ZR, Lei XF
and Ai MH: Potential effects of Mina53 on tumor growth in human
pancreatic cancer. Cell Biochem Biophys. 69:619–625. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu M, Sun J, Thakur C, Chen B, Lu Y, Zhao
H and Chen F: Paradoxical roles of mineral dust induced gene on
cell proliferation and migration/invasion. PLoS One. 9:e879982014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma
D, Yi Z and Chen F: Mdig de-represses H19 large intergenic
non-coding RNA (lincRNA) by down-regulating H3K9me3 and
heterochromatin. Oncotarget. 4:1427–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Mina53, a novel c-Myc target gene, is frequently
expressed in lung cancers and exerts oncogenic property in NIH/3T3
cells. J Cancer Res Clin Oncol. 136:465–473. 2010. View Article : Google Scholar
|
|
10
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cuyàs E, Corominas-Faja B and Menendez JA:
The nutritional phenome of EMT-induced cancer stem-like cells.
Oncotarget. 5:3970–3982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Godde NJ, Galea RC, Elsum IA and Humbert
PO: Cell polarity in motion: Redefining mammary tissue organization
through EMT and cell polarity transitions. J Mammary Gland Biol
Neoplasia. 15:149–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren ZX, Yu HB, Li JS, Shen JL and Du WS:
Suitable parameter choice on quantitative morphology of A549 cell
in epithelial-mesenchymal transition. Biosci Rep. 35:352015.
|
|
17
|
Yost C, Torres M, Miller JR, Huang E,
Kimelman D and Moon RT: The axis-inducing activity, stability, and
subcellular distribution of beta-catenin is regulated in Xenopus
embryos by glycogen synthase kinase 3. Genes Dev. 10:1443–1454.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komiya K, Sueoka-Aragane N, Sato A,
Hisatomi T, Sakuragi T, Mitsuoka M, Sato T, Hayashi S, Izumi H,
Tsuneoka M, et al: Expression of Mina53, a novel c-Myc target gene,
is a favorable prognostic marker in early stage lung cancer. Lung
Cancer. 69:232–238. 2010. View Article : Google Scholar
|
|
23
|
Thakur C, Lu Y, Sun J, Yu M, Chen B and
Chen F: Increased expression of mdig predicts poorer survival of
the breast cancer patients. Gene. 535:218–224. 2014. View Article : Google Scholar :
|
|
24
|
Huo Q, Ge C, Tian H, Sun J, Cui M, Li H,
Zhao F, Chen T, Xie H, Cui Y, et al: Dysfunction of IKZF1/MYC/MDIG
axis contributes to liver cancer progression through regulating
H3K9me3/p21 activity. Cell Death Dis. 8:e27662017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xing J, Wang K, Liu PW, Miao Q and Chen
XY: Mina53, a novel molecular marker for the diagnosis and
prognosis of gastric adenocarcinoma. Oncol Rep. 31:634–640. 2014.
View Article : Google Scholar
|
|
26
|
Ogasawara S, Komuta M, Nakashima O, Akiba
J, Tsuneoka M and Yano H: Accelerated expression of a Myc target
gene Mina53 in aggressive hepatocellular carcinoma. Hepatology Res.
40:330–336. 2010. View Article : Google Scholar
|
|
27
|
Fukahori S, Yano H, Tsuneoka M, Tanaka Y,
Yagi M, Kuwano M, Tajiri T, Taguchi T, Tsuneyoshi M and Kojiro M:
Immunohistochemical expressions of Cap43 and Mina53 proteins in
neuroblastoma. J Pediatr Surg. 42:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishizaki H, Yano H, Tsuneoka M, Ogasawara
S, Akiba J, Nishida N, Kojiro S, Fukahori S, Moriya F, Matsuoka K,
et al: Overexpression of the myc target gene Mina53 in advanced
renal cell carcinoma. Pathol Int. 57:672–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teye K, Arima N, Nakamura Y, Sakamoto K,
Sueoka E, Kimura H and Tsuneoka M: Expression of Myc target gene
mina53 in subtypes of human lymphoma. Oncol Rep. 18:841–848.
2007.PubMed/NCBI
|
|
30
|
Tsuneoka M, Fujita H, Arima N, Teye K,
Okamura T, Inutsuka H, Koda K, Shirouzu K and Kimura H: Mina53 as a
potential prognostic factor for esophageal squamous cell carcinoma.
Clin Cancer Res. 10:7347–7356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuratomi K, Yano H, Tsuneoka M, Sakamoto
K, Kusukawa J and Kojiro M: Immunohistochemical expression of
Mina53 and Ki67 proteins in human primary gingival squamous cell
carcinoma. Kurume Med J. 53:71–78. 2006. View Article : Google Scholar
|
|
32
|
Thakur C and Chen F: Current understanding
of mdig/MINA in human cancers. Genes Cancer. 6:288–302.
2015.PubMed/NCBI
|